COVID-19-S-RBD virus-like particles and vaccine, and preparation methods of virus-like particles and vaccine
A COVID-19-S-RBD and vaccine preparation technology, applied in the field of biomedicine, can solve the problems of high production costs, limited vaccine development, low yield of chimeric vaccines, etc., to achieve easy purification, fast immune response, and simplified screening process Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
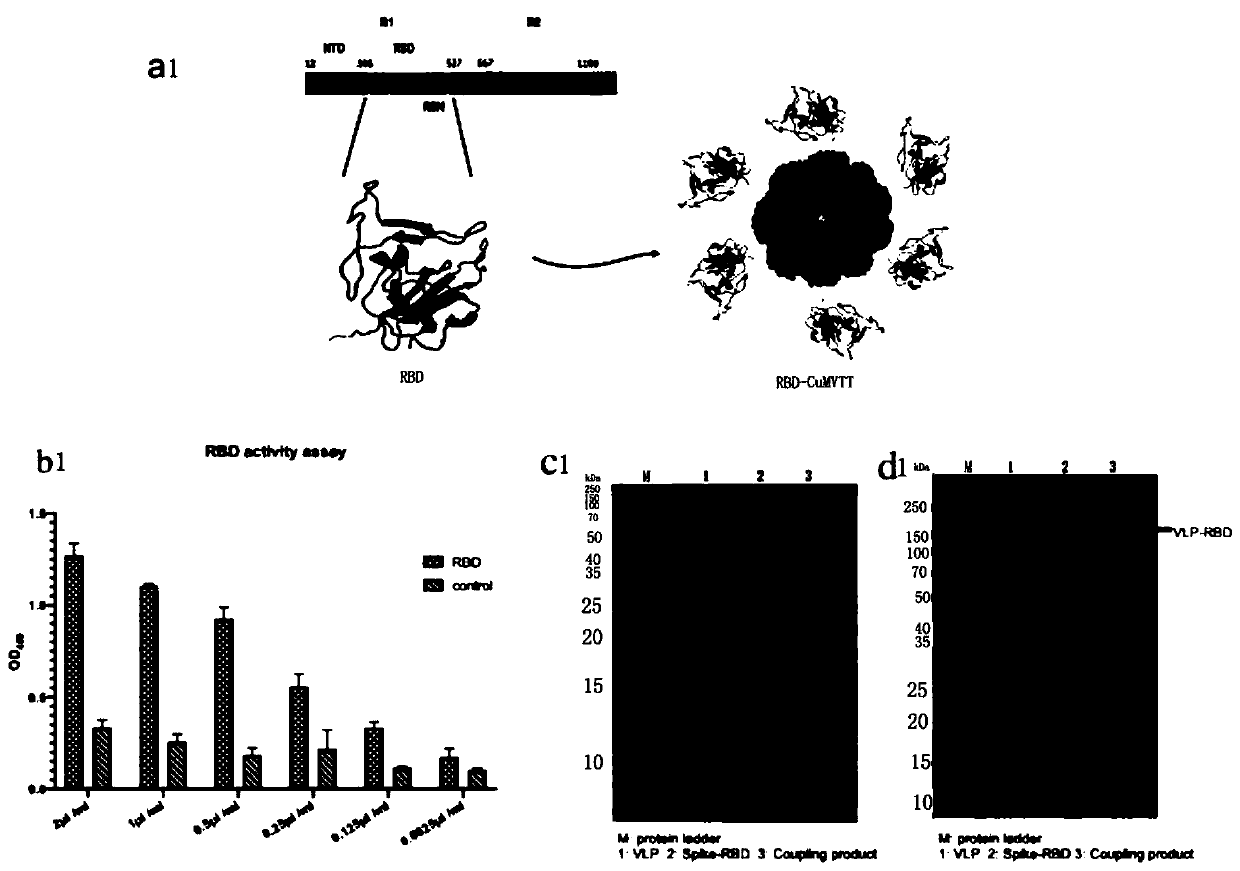
[0049] Provide a kind of COVID-19-S-RBD virus-like particle, described virus-like particle is by CuMV of cucumber mosaic virus TT The pET28a-CuMVTT recombinant plasmid formed after the gene is connected with the pET28a plasmid is obtained by expressing the expression strain.
[0050] Specifically, the cucumber mosaic virus CuMV TT Both the gene and the pET28a plasmid were digested with HindⅢ endonuclease and BamHI endonuclease, and then the CuMV was digested with T4 DNA ligase. TT Ligated with pET28a plasmid double digestion product to obtain pET28a-CuMV TT recombinant plasmid. The cucumber mosaic virus CuMV TT Gene to CuMV TT The viral nucleotide sequence was deduced back to the codon sequence. The expression strain adopts Escherichia coli T7 Shuffle expression strain. pET28a-CuMV was obtained by screening with 50ug / ml concentration of kanamycin TT T7 Shuffle recombinant expression strain.
[0051] The method for preparing the above-mentioned COVID-19-S-RBD virus-lik...
Embodiment 2
[0117] Provide a COVID-19-S-RBD vaccine, including the above-mentioned COVID-19-S-RBD virus-like particles, and also include pFUSE-COVID-19- The S-RBD recombinant plasmid expresses the COVID-19-S-RBD protein expressed by the cell line; the CuMV TT Virus-like particles and the COVID-19-S-RBD protein are coupled into COVID-19-S-RBD-CuMV by chemical coupling reagent SMPH TT After mixing Freund's complete adjuvant to obtain COVID-19-S-RBD vaccine.
[0118] Specifically, the expression cell line adopts the 293F expression cell line, and the pFUSE-COVID-19-S-RBD recombinant expression cell line is obtained after the pFUSE-COVID-19-S-RBD recombinant plasmid is transferred into the 293F expression cell line.
[0119] Above-mentioned COVID-19-S-RBD vaccine preparation method, comprises the following steps:
[0120] (21) Construction of recombinant plasmid: construct pFUSE-COVID-19-S-RBD recombinant plasmid after connecting COVID-19-S-RBD gene with pFUSE plasmid;
[0121] (22) Transf...
Embodiment 3
[0168] 1.1 Immunogen preparation
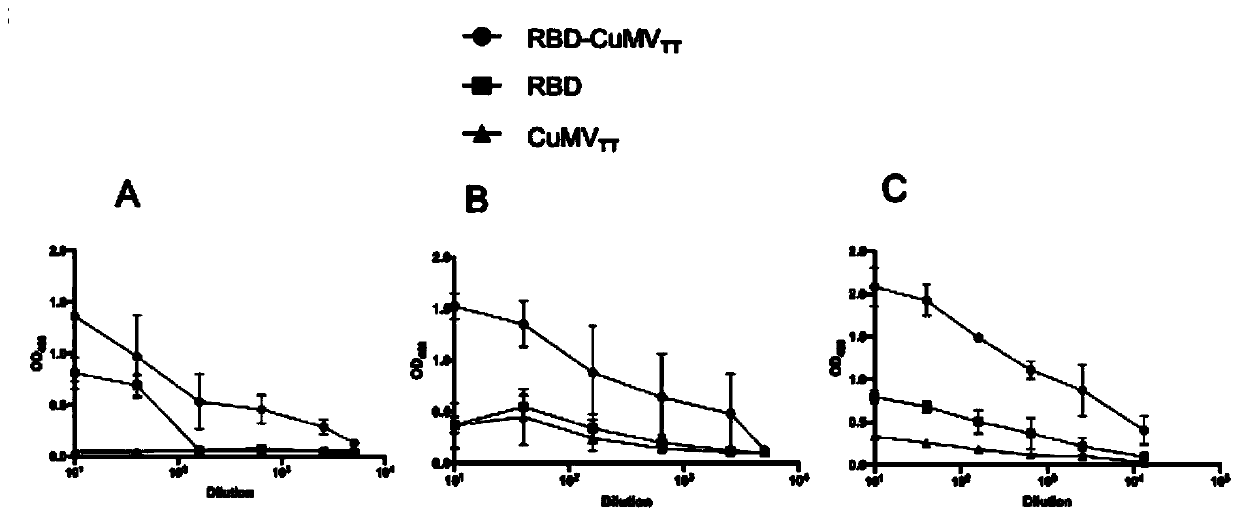
[0169] The above-mentioned COVID-19-S-RBD-CuMVTT was emulsified into a vaccine according to the injection amount of 50ug / mouse and Freund's complete adjuvant 1:1, and the control group had two groups injected with COVID-19-S-RBD and PBS respectively , are emulsified into vaccines with Freund's complete adjuvant 1:1.
[0170] 1.2 Immunization of mice
[0171] Six-week-old BALB / c mice were purchased from Beijing Life River Experimental Animal Technology Co., Ltd. and kept in the animal facility of Anhui Agricultural University. All animal experiments were performed in accordance with the National Animal Protection Guidelines and were approved by the Chinese Association for Laboratory Animal Science.
[0172] By subcutaneous injection of 50 μg COVID-19-S-RBD-CuMV TT or COVID-19-S-RBD (PBS as a control) to immunize six-week-old newborn BALB / c mice (3 in each group). Booster immunizations at weeks 2 and 3. Serum was collected 1 week after eac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com