Method of preparing porcine parvovirus virus-like particle subunit vaccine by using Escherichia coli expression system and application of method
A parvovirus and expression method technology, applied in the field of bioengineering, can solve the problems of research work and application barriers, low production costs, etc., achieve good immunogenicity, improve the correct folding rate, and improve the solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] The invention discloses a prokaryotic method for expressing porcine parvovirus VP2 protein. In the method, PPV VP2 protein and chaperone protein are co-expressed to obtain a recombinant expression protein with good solubility and high activity. The specific steps are as follows:
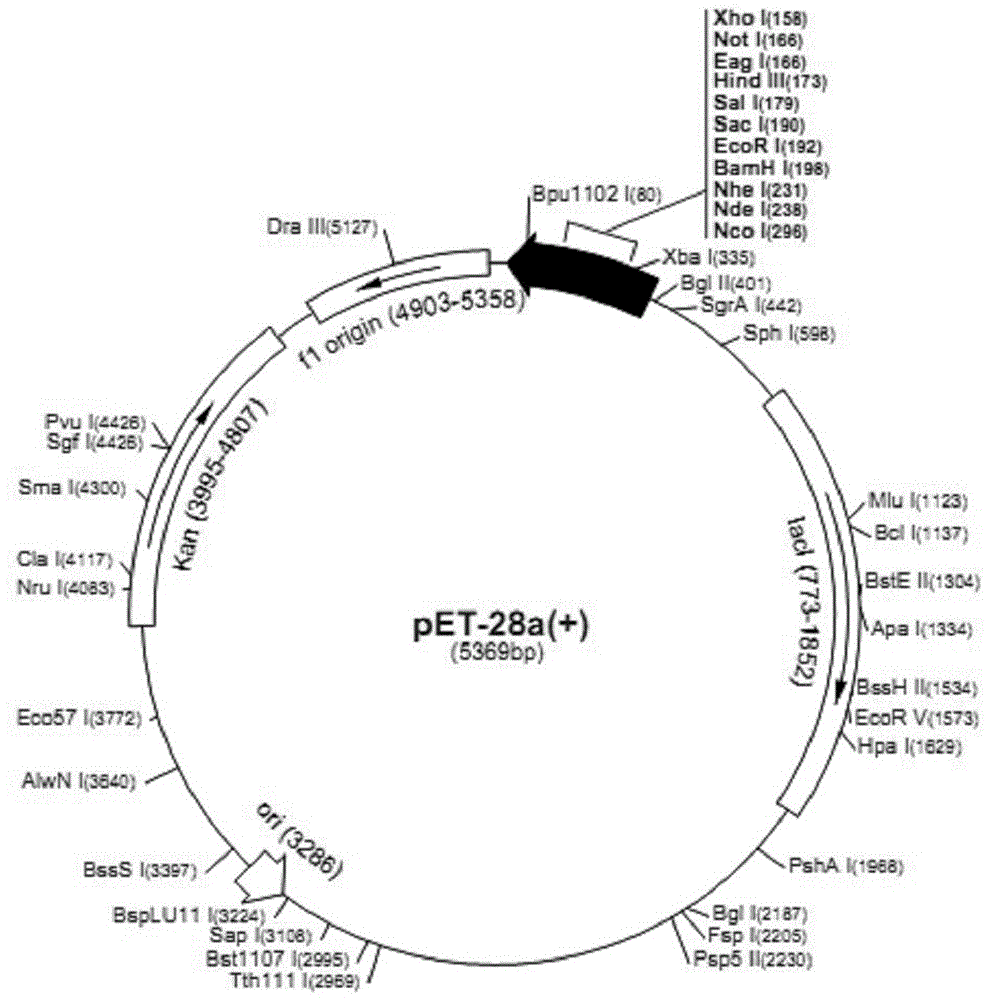
[0047] 1.1 Construction of recombinant vector pET28a-VP2
[0048] 1.1.1 Artificial modification and synthesis of PPV main immunogenic gene VP2
[0049] Referring to the VP2 gene sequence of PPV China strain (GenBank: AY583318), the sequence was optimized according to the codon preference of Escherichia coli, and the VP2 gene was artificially synthesized, and its nucleotide sequence is shown in SEQ ID NO.1.
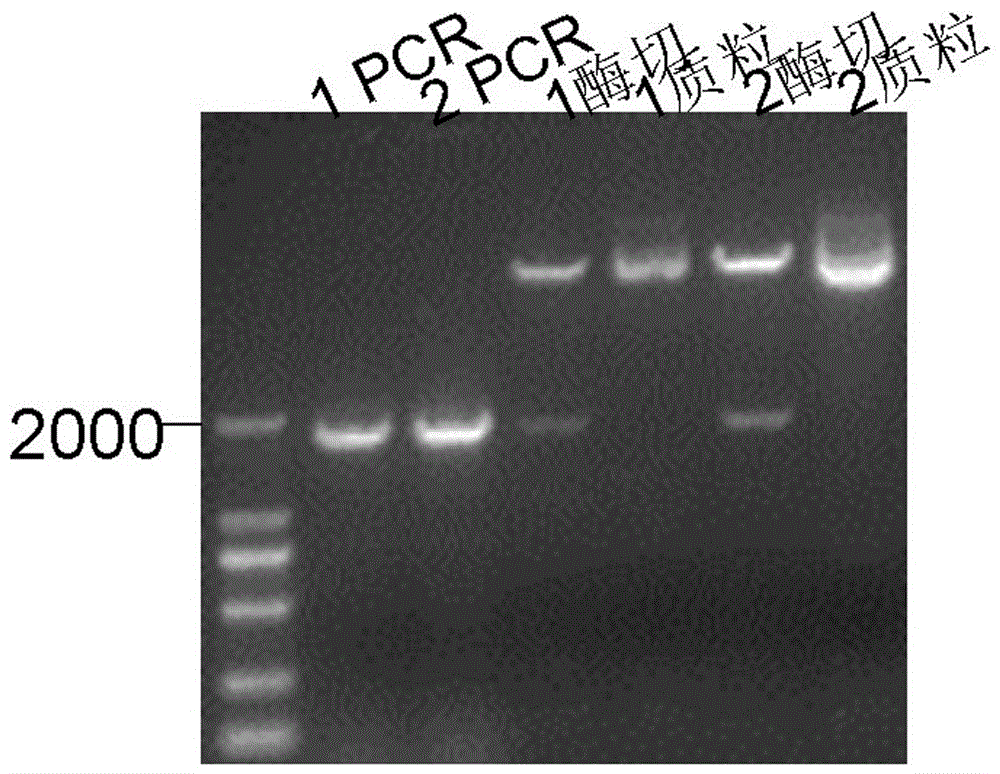
[0050] 1.1.2 Construction of pET28a-VP2 vector
[0051] The primer sequences used to amplify the VP2 protein-encoding gene are as follows:
[0052] F: 5'- GGATCC ATGTCGGAAAATGTGGAACA-3' ( Bam HI)
[0053] R: 5'- AAGCTT ATACAGTTTCCGTGGAATGA-3' ( Hind III)
[0054] Among them, t...
Embodiment 2
[0064] Example 2 Preparation of porcine parvovirus virus-like particles
[0065] 2.1 Purification of VP2 protein and determination of VLP
[0066] 2.1.1 Purification of recombinant VP2 protein
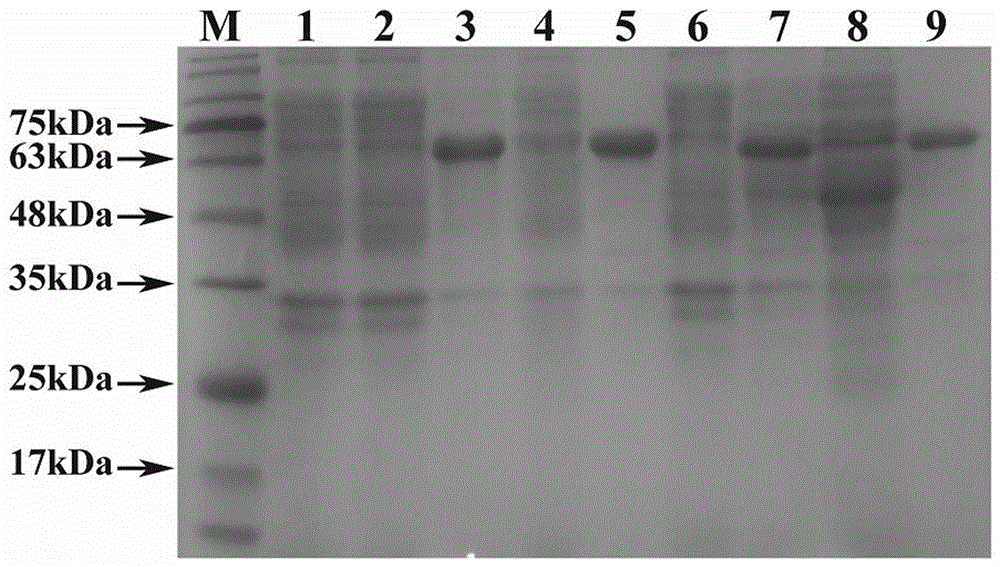
[0067] The supernatant of ultrasonic lysis was purified by Ni-NTA chromatography column (Merck). The specific process was: after Ni-NTA was equilibrated with buffer A, the sample was slowly flowed through the chromatography column, and then at least 10 times the column volume Buffer B (50mmol / L PB, 250mmol / L NaCl, 30mmol / L imidazole, pH7.0) was passed through the column to wash the impurities, and finally buffer C (50mmol / L PB, 250mmol / L NaCl, 300mmol / L imidazole , pH7.0) to elute the target protein, collect the eluted fractions, and use SDS-PAGE and Western-blot detection, the results are as follows Figure 4 , the purity of VP2 protein can reach more than 95% after purification by affinity chromatography, and it has a good reaction with His monoclonal antibody.
[0068] 2.1.2 Prot...
Embodiment 3
[0074] Example 3 PPV virus-like particle immunogenicity detection
[0075] 3.1 Determination of VP2 protein concentration and determination of endotoxin content
[0076] The concentration of the purified VP2 protein was determined with the BCA protein content assay kit (Pierce Biotechnology), and the specific steps refer to its instructions. The measured concentration of VP2 protein is 0.57mg / mL, and the expression level is relatively high, which can meet the requirements of industrial production.
[0077] Endotoxin content assay kit (ToxinSensor TM Endotoxin Detection System, GenScript) was used to determine the endotoxin content of the purified protein. For specific steps, refer to its instructions. The measured endotoxin content of the VP2 protein was less than 0.3mU / mg.
[0078] 3.2 Animal Immunization
[0079] Referring to the method in "Pharmacopoeia of the People's Republic of China" (2005 edition), the VLP obtained in Example 2 was prepared into a vaccine.
[0080...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com