Blood serum/blood plasma micro ribonucleic acid (miRNA) marker relevant with pancreatic cancer and application thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] Example 1 Collection of samples and arrangement of sample data
[0071] The cases are new cases of pancreatic cancer collected at Huai'an Cancer Hospital and Jiangsu Cancer Hospital from June 2007 to December 2010, all of which were confirmed by histopathology. Controls were healthy individuals who had been screened for community diseases during the same period, and were frequency-matched with cases by gender and age (±5 years). The samples used for the research were collected at the same time, and the conditions of sampling, aliquoting, and storage were uniform. After sorting out the sample data, the inventor selected 48 samples that met the following standards as the TLDA chip detection and subsequent series of qRT-PCR verification Experimental sample:
[0072] 1. New cases of pancreatic cancer
[0073] 2. No surgery, radiotherapy and chemotherapy before blood collection, no preoperative radiotherapy and chemotherapy
[0074] 3. Control of healthy people matching the age an...
Embodiment 2
[0076] Example 2 TLDA chip detection of miRNA in serum / plasma
[0077] The 24 pancreatic cancer patients and 24 healthy controls were tested by TLDA chip to obtain relevant results. The specific steps are:
[0078] 1. Take 600μl of serum from patients in the "pancreatic cancer case" group and the "healthy control" group respectively, and add 3 times the volume of Trizol reagent;
[0079] 2. Phase separation: place at room temperature for 15 minutes, add the final concentration to 10 -4 pmol / μl cel-39 (TAKARA) was used as the internal control, and then added the same volume of chloroform as the plasma, shaking for 50s, room temperature for 15min, 14,000rpm, 4℃, centrifugation for 15min;
[0080] 3. RNA precipitation: transfer the water phase to a new 15ml centrifuge tube, add 1.5 times the volume of the water phase absolute ethanol, and mix well;
[0081] 4. Use QIAGEN miRNeasy kit to enrich RNA: Pipette 700μl sample into the spin column each time, centrifuge at 14,000rpm for 15s, disca...
Embodiment 3
[0087] Example 3 qRT-PCR experiment of miRNA in serum / plasma
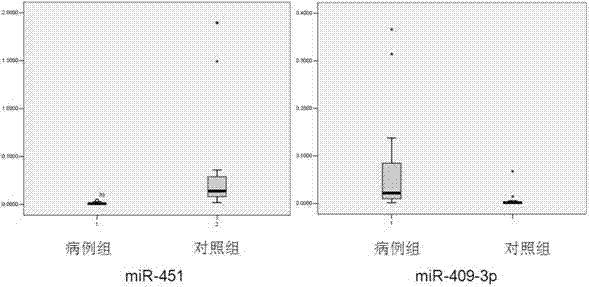
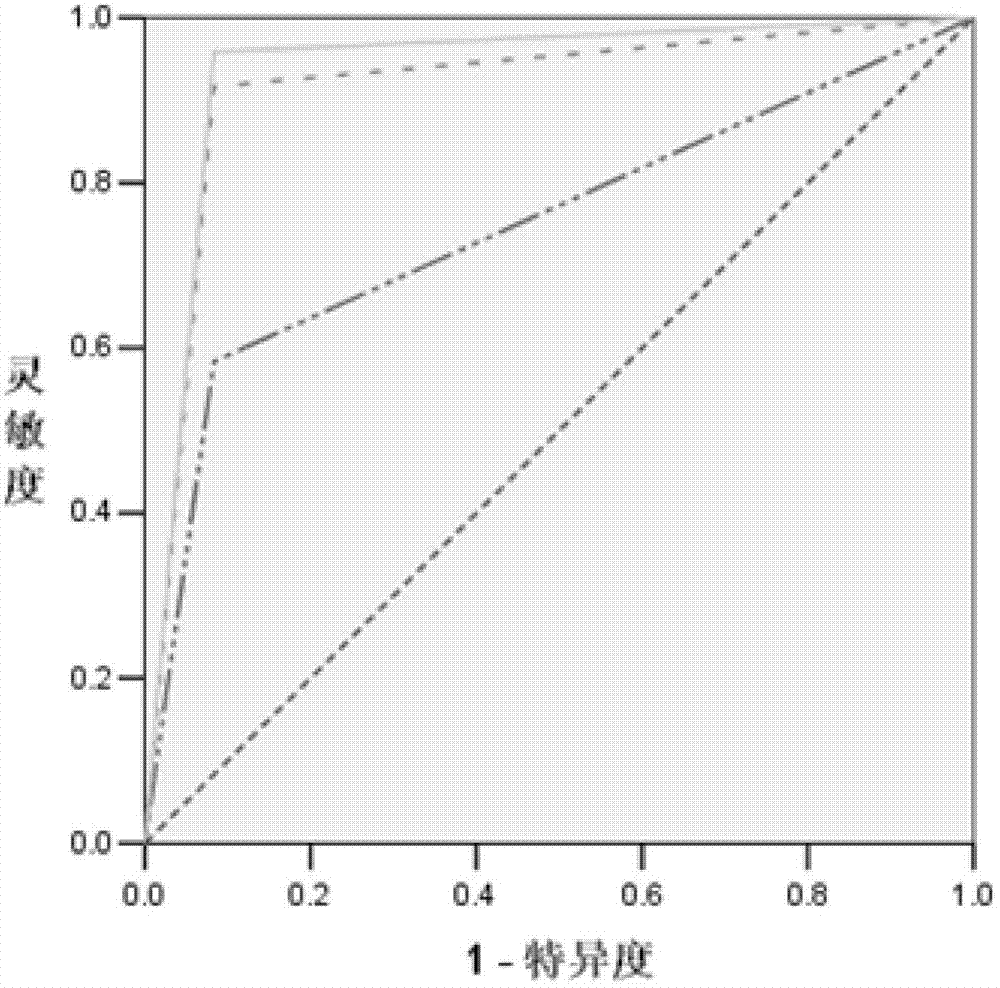
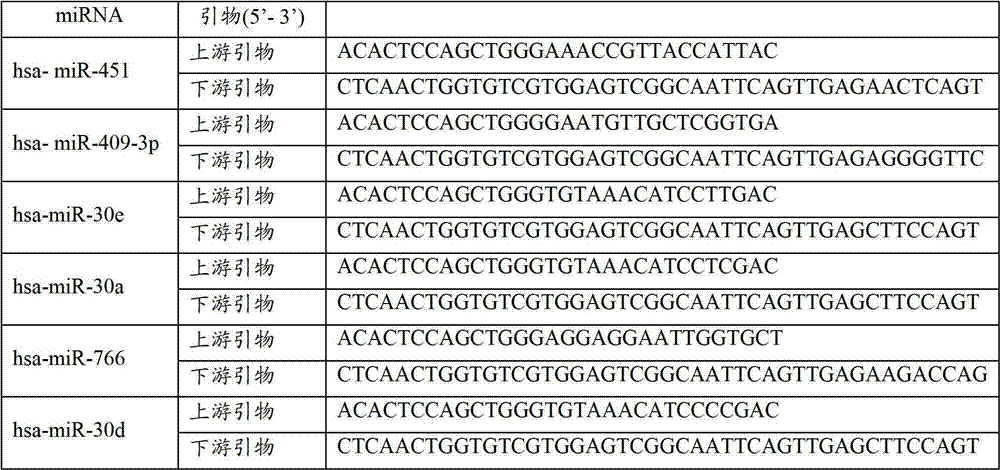
[0088] According to the above TLDA results, select miRNAs that meet the following conditions to further verify with qRT-PCR method: 1) The CT values of the two groups of subjects in the TLDA chip are not greater than 35 to improve the detection efficiency; 2) The ΔΔCT in the TLDA chip is greater than 2. Design primers for reverse transcription and qRT-PCR for the selected miR-451 and miR-409-3p miRNAs (see Table 1). The qRT-PCR detection of miRNA was performed on individual serum individuals in the "pancreatic cancer case" group and the "healthy control" group. Strict quality control was implemented throughout the research process. Each sample was tested three times in a row. All tests are blinded, that is, done without knowing the background of the sample to avoid bias. Two methods of dye method and probe method were used for qRT-PCR detection.
[0089] Table 1 Related miRNA primer information
[0090]
[0091] (1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com