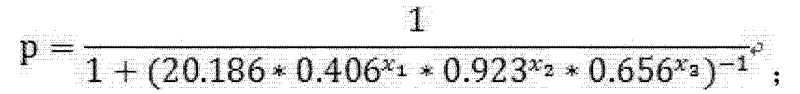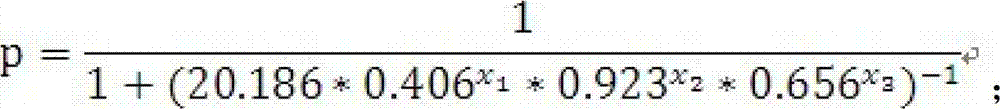Test kit of auxiliary diagnosis of non-small cell lung cancer patients
A non-small cell lung cancer and auxiliary diagnosis technology, applied in biological testing, material inspection products, measuring devices, etc., can solve the problems of failing to meet clinical needs and low diagnostic efficiency, and achieve high sensitivity and strong specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1, establishment of the inventive method
[0033] 1. Model establishment
[0034]952 plasma samples from NSCLC patients and 479 plasma samples from healthy people (normal controls) were collected from lung cancer patients diagnosed by pathology in the Department of Thoracic Surgery, Cancer Hospital, Chinese Academy of Medical Sciences, and normal people who participated in cancer screening. This experiment was approved by the Ethics Committee, and all subjects were informed and signed an informed consent form. Plasma samples were obtained before surgery, and the patients had not received chemotherapy, radiotherapy, or interventional treatment before.
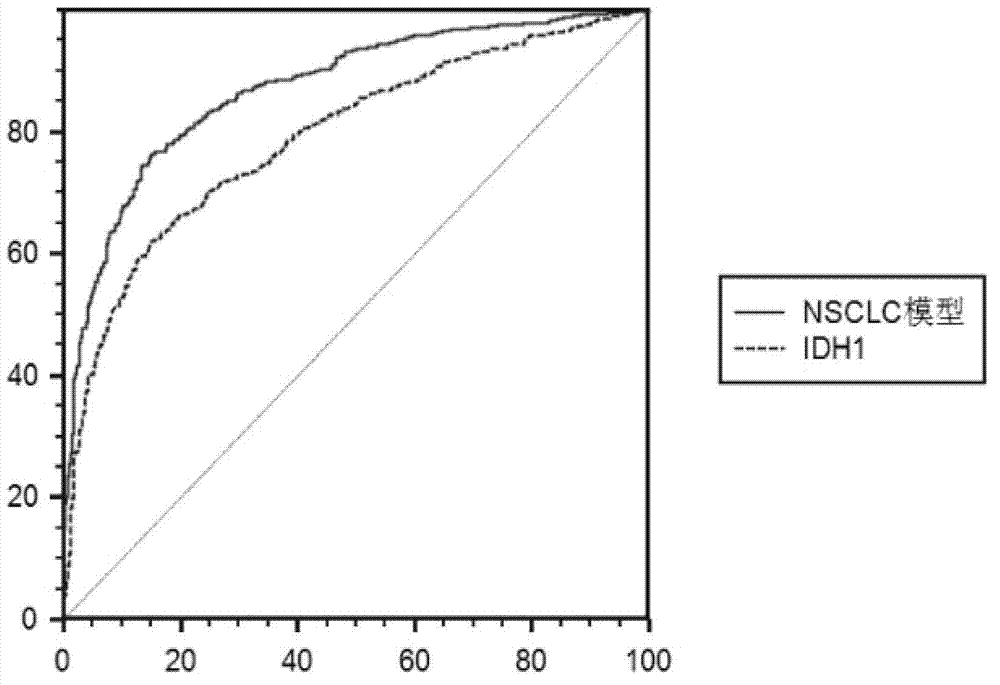
[0035] Data processing uses curve expert 1.4 to process the standard curve; GraphPad Prism 5.01 to process the Column statistical graph; SPSS 13.0 to fit the data and establish a mathematical model; SAS 9.2 to test the mathematical model; MedCalc 9.6.2.0 version to calculate the model ROC curve and cutoff valu...
Embodiment 2
[0045] Embodiment 2, verification of the inventive method
[0046] The patients used in this example were 150 clinically diagnosed patients with non-small cell lung cancer (peripheral venous blood was obtained before surgery, and the patients had not received chemotherapy, radiotherapy, intervention, etc.) and 50 healthy people (non-small cell lung cancer). cell lung cancer patients), all volunteers with informed consent.
[0047] 1. Take 5ml of peripheral venous blood from the patient to be tested on an empty stomach, let it stand at room temperature for 1h, then centrifuge at 3000r / min for 15min, take the supernatant, divide it into two 1.5mL EP tubes, and store in a -80°C refrigerator.
[0048] 2. Dissolve the sample from step 1 at room temperature for 3 hours, then centrifuge at 3000r / min for 5 minutes.
[0049] 3. Detect the concentration of the protein marker IDH1 (in U / L), the concentration of the protein marker CA125 (in U / mL), and the concentration of the protein mar...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com