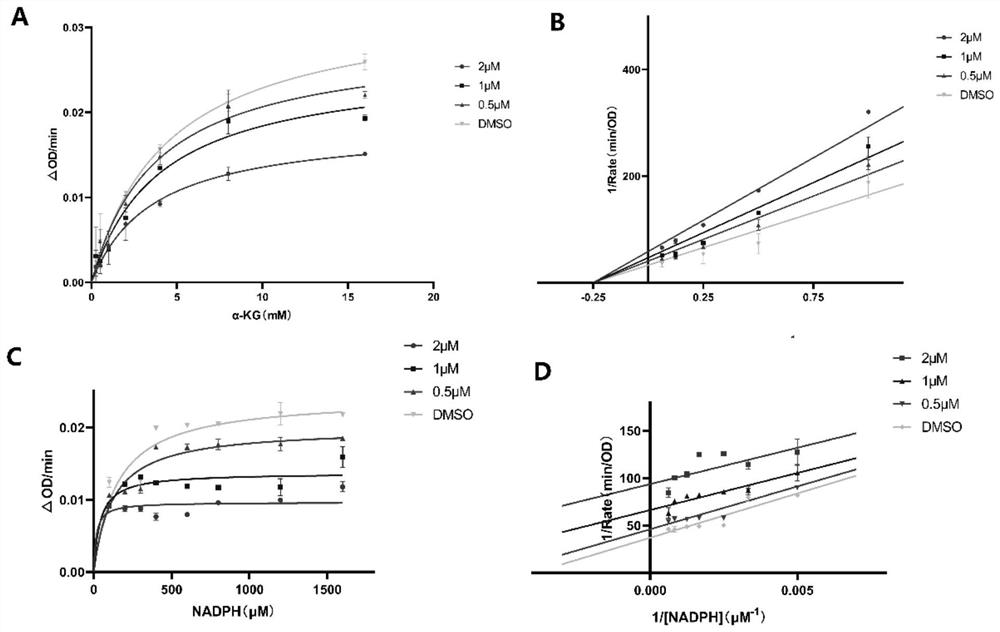
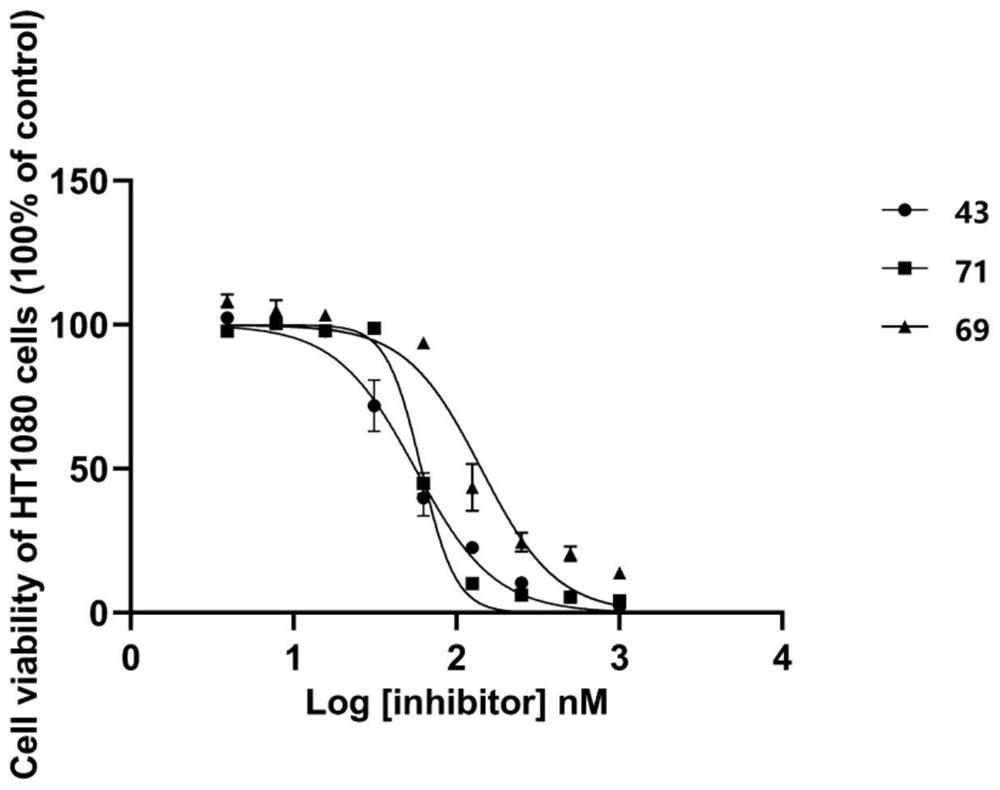
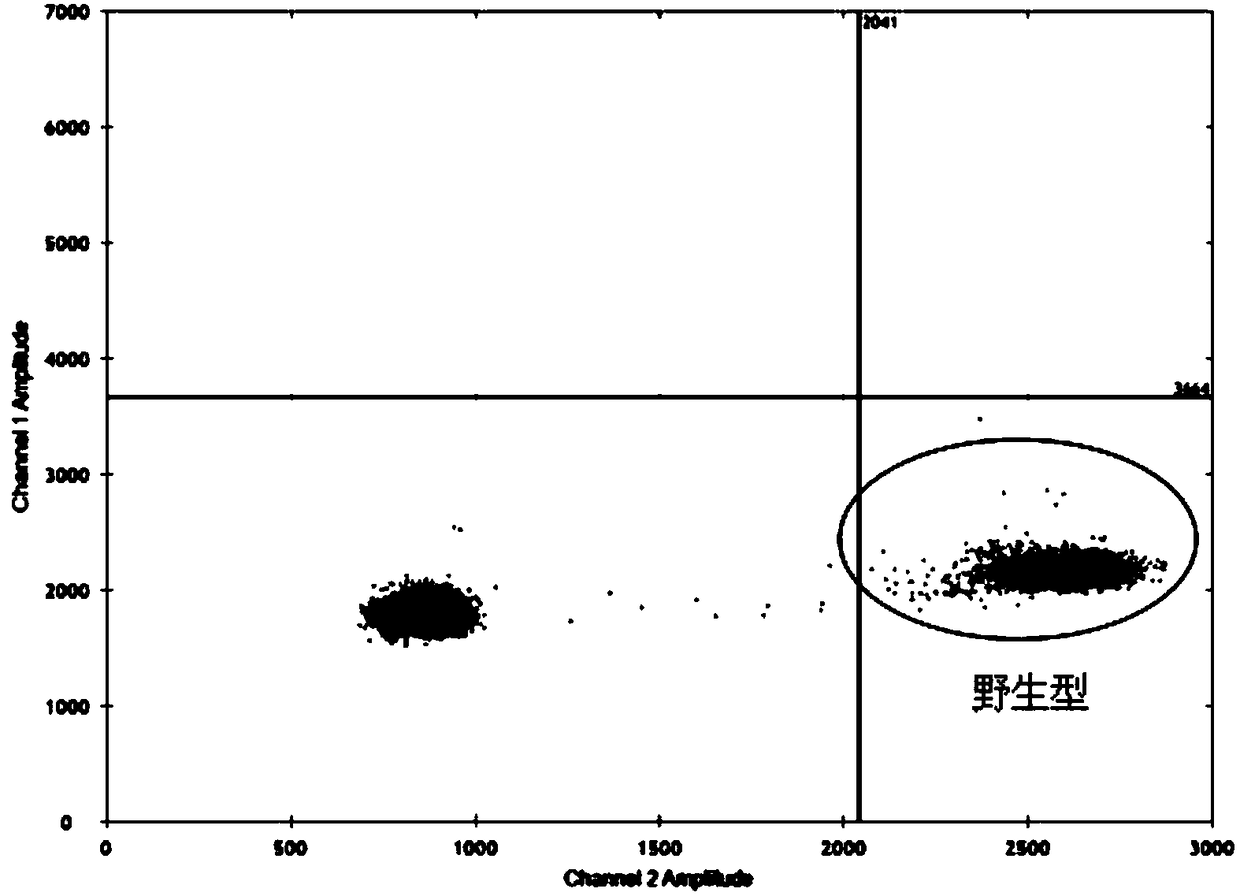
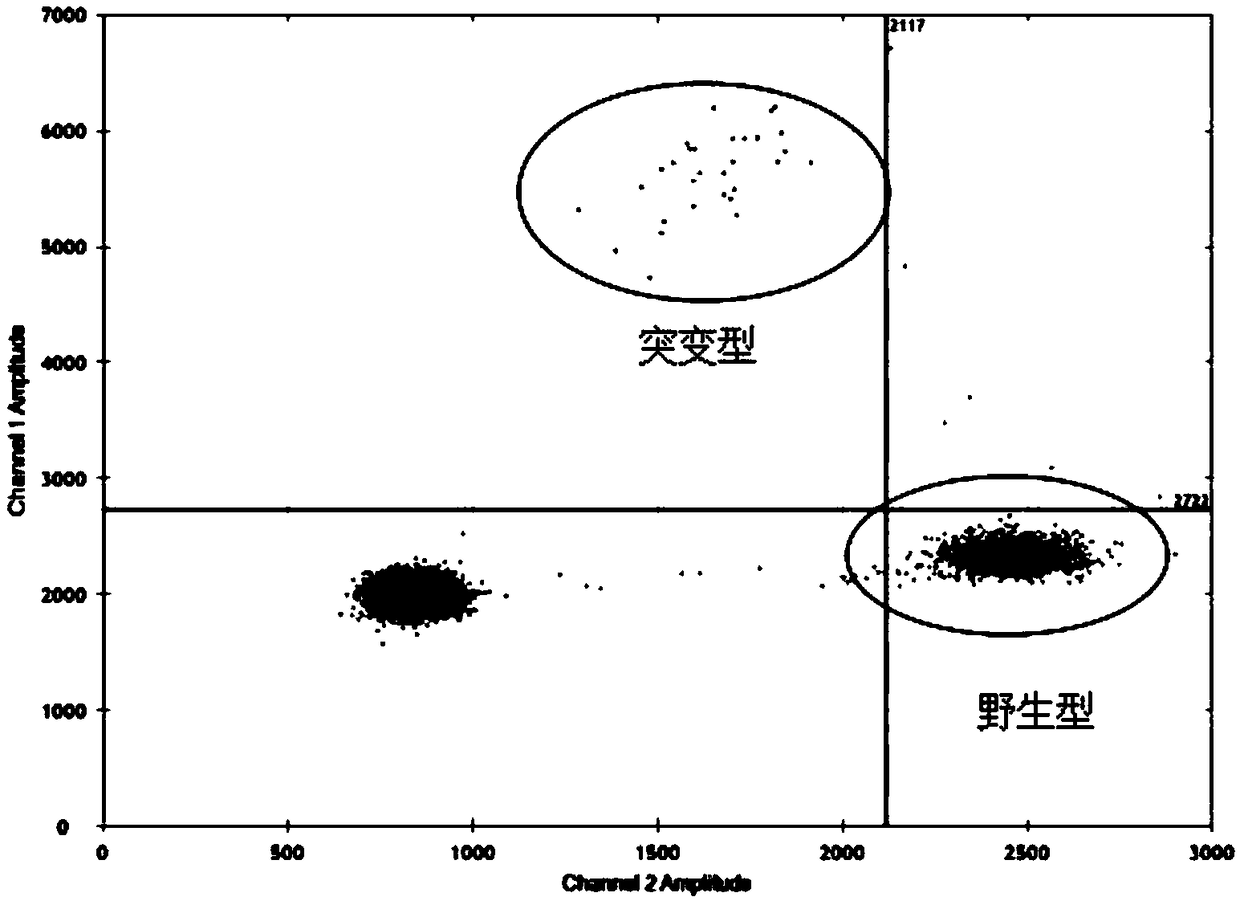
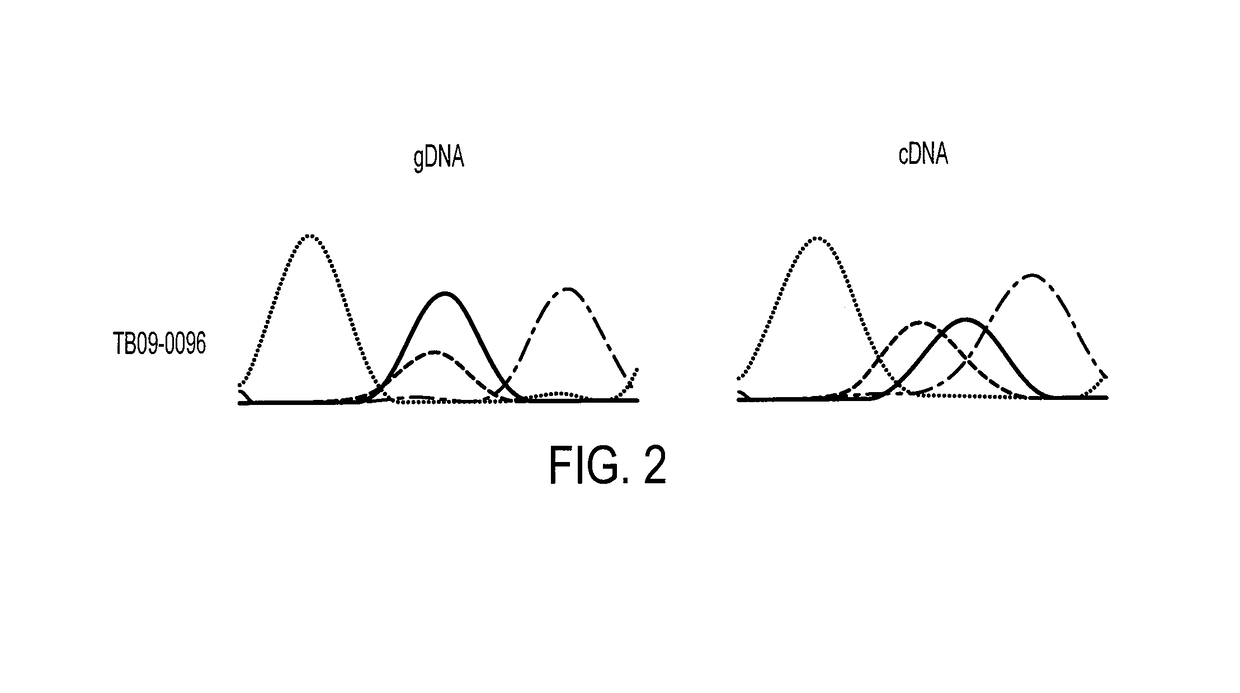
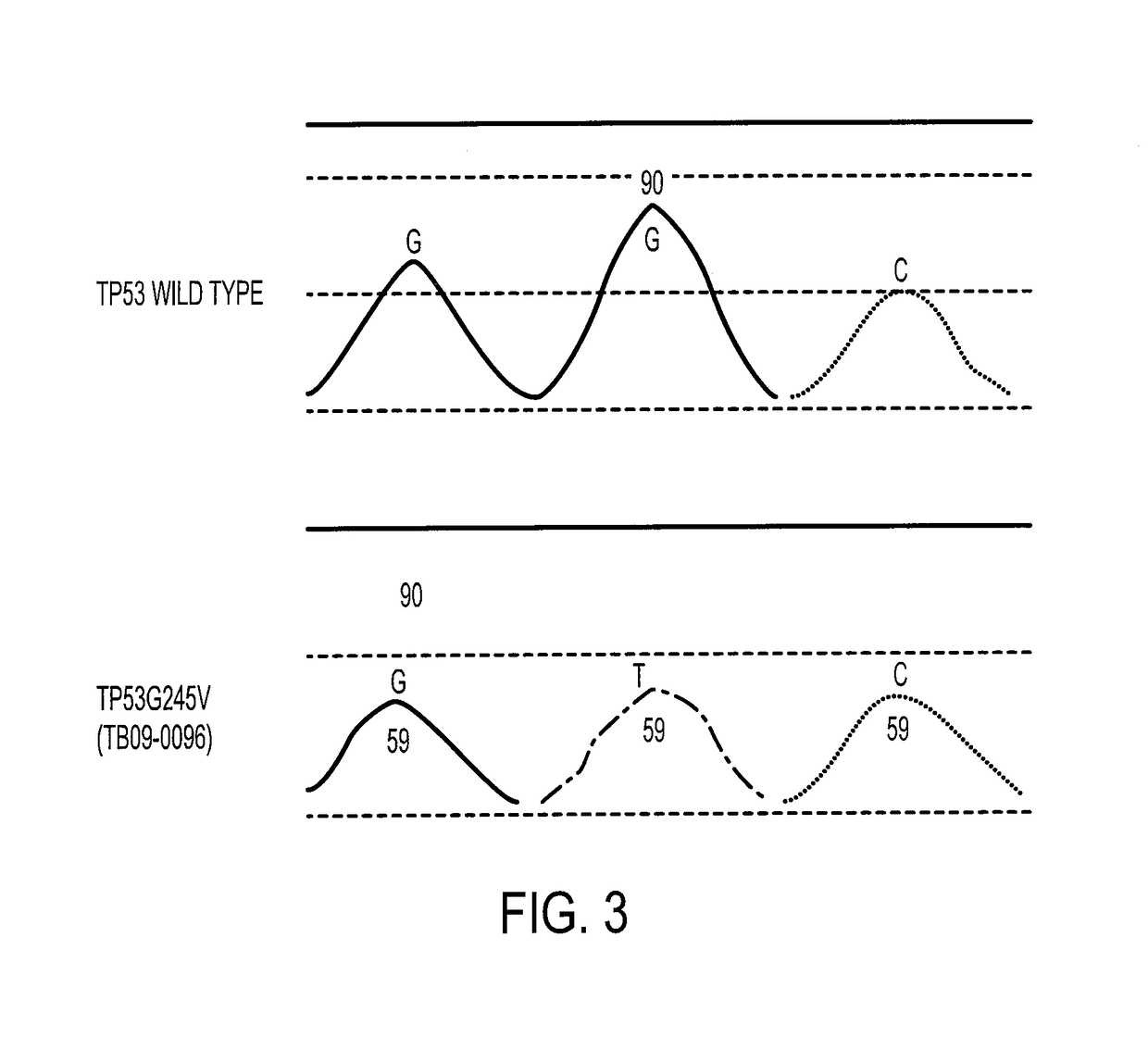
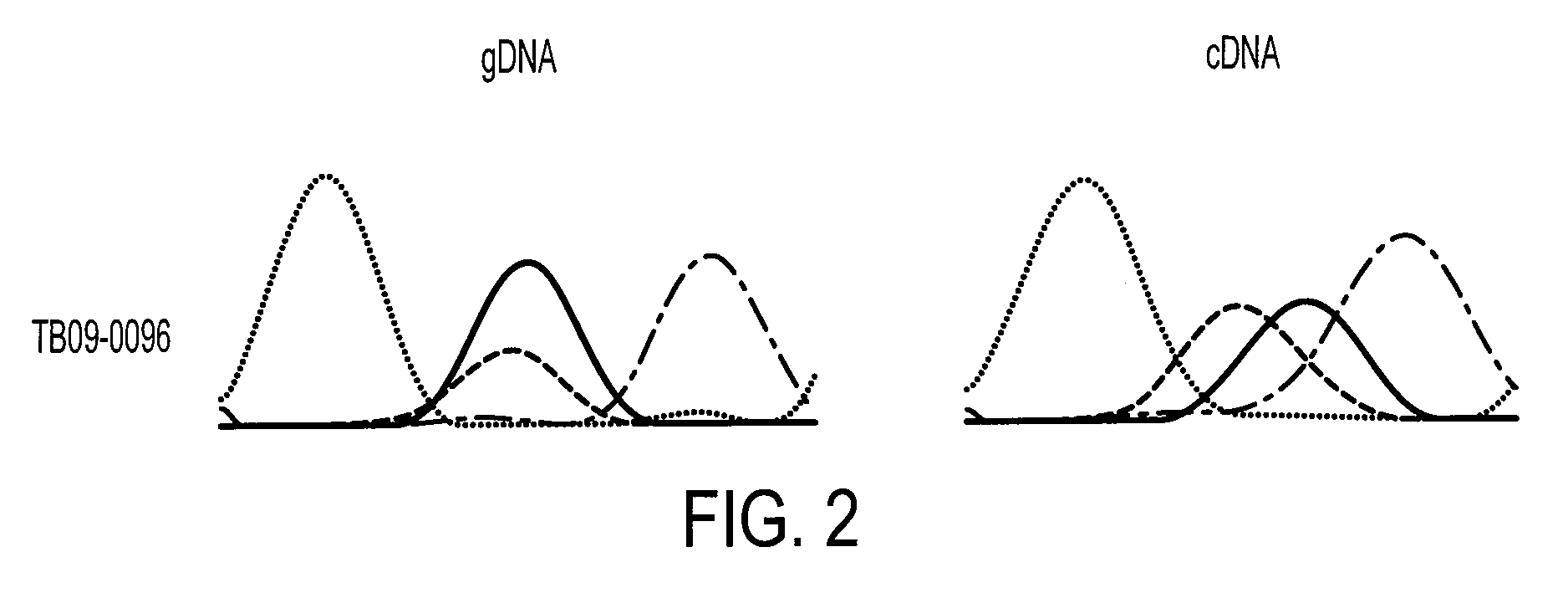
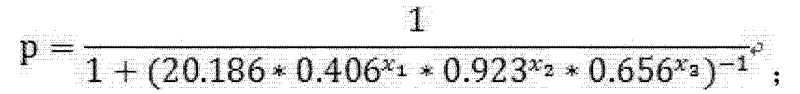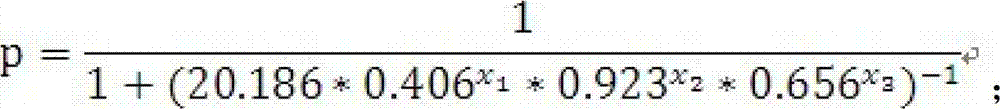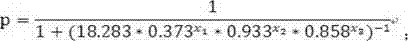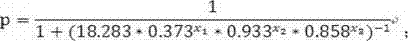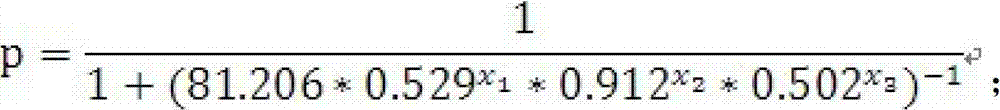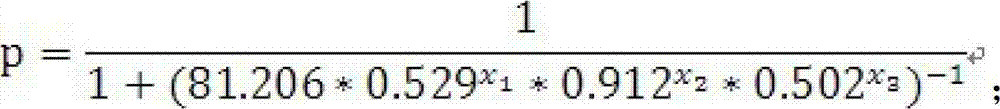Patents
Literature
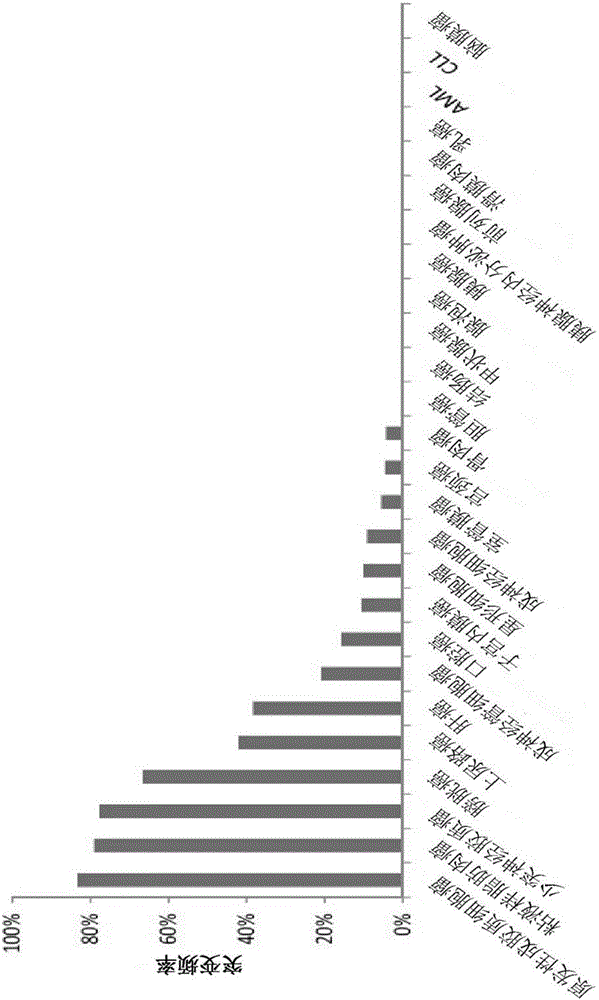
43 results about "IDH1" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
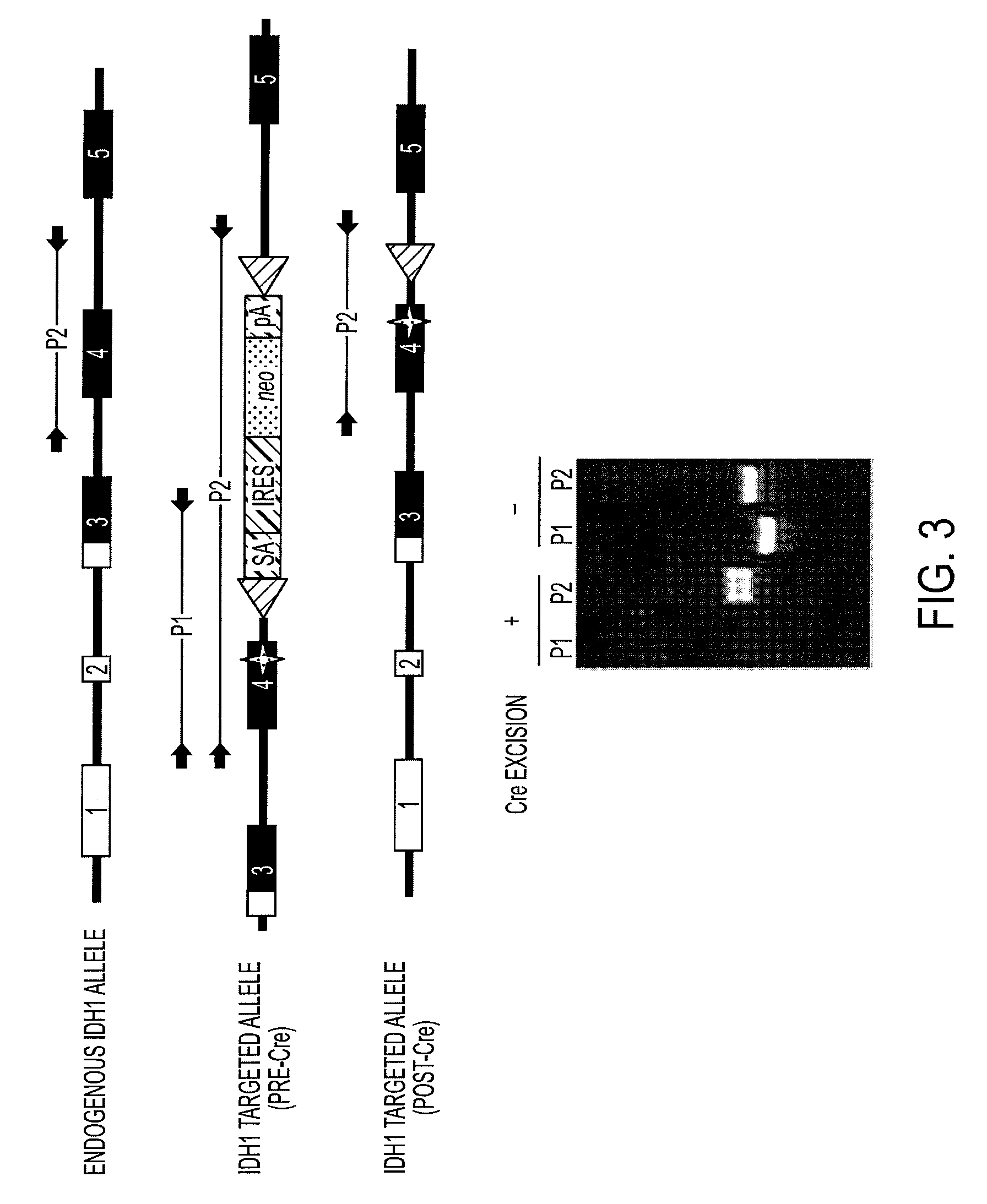
Isocitrate dehydrogenase 1 (NADP+), soluble is an enzyme that in humans is encoded by the IDH1 gene on chromosome 2. Isocitrate dehydrogenases catalyze the oxidative decarboxylation of isocitrate to 2-oxoglutarate. These enzymes belong to two distinct subclasses, one of which uses NAD⁺ as the electron acceptor and the other NADP⁺. Five isocitrate dehydrogenases have been reported: three NAD⁺-dependent isocitrate dehydrogenases, which localize to the mitochondrial matrix, and two NADP⁺-dependent isocitrate dehydrogenases, one of which is mitochondrial and the other predominantly cytosolic. Each NADP⁺-dependent isozyme is a homodimer. The protein encoded by this gene is the NADP⁺-dependent isocitrate dehydrogenase found in the cytoplasm and peroxisomes. It contains the PTS-1 peroxisomal targeting signal sequence. The presence of this enzyme in peroxisomes suggests roles in the regeneration of NADPH for intraperoxisomal reductions, such as the conversion of 2,4-dienoyl-CoAs to 3-enoyl-CoAs, as well as in peroxisomal reactions that consume 2-oxoglutarate, namely the alpha-hydroxylation of phytanic acid. The cytoplasmic enzyme serves a significant role in cytoplasmic NADPH production. Alternatively spliced transcript variants encoding the same protein have been found for this gene. [provided by RefSeq, Sep 2013]
Methods and compositons for cell-proliferation-related disorders
ActiveUS20130183281A1Lower Level RequirementsImprove matchBiocideHydroxy compound active ingredientsIDH1Gastroenterology
Owner:SERVIER PHARM LLC
Microorganism for the production of succinic acid
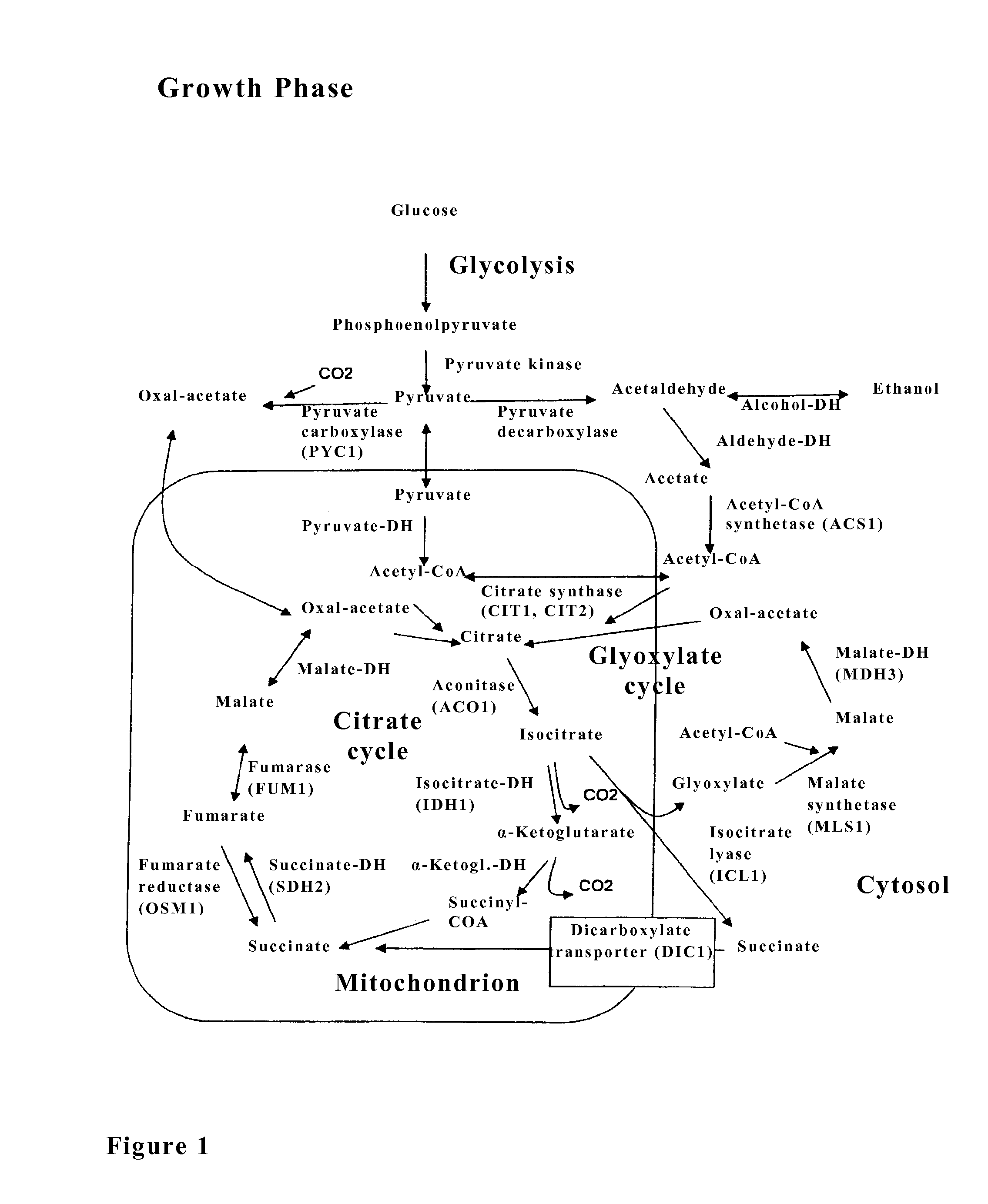
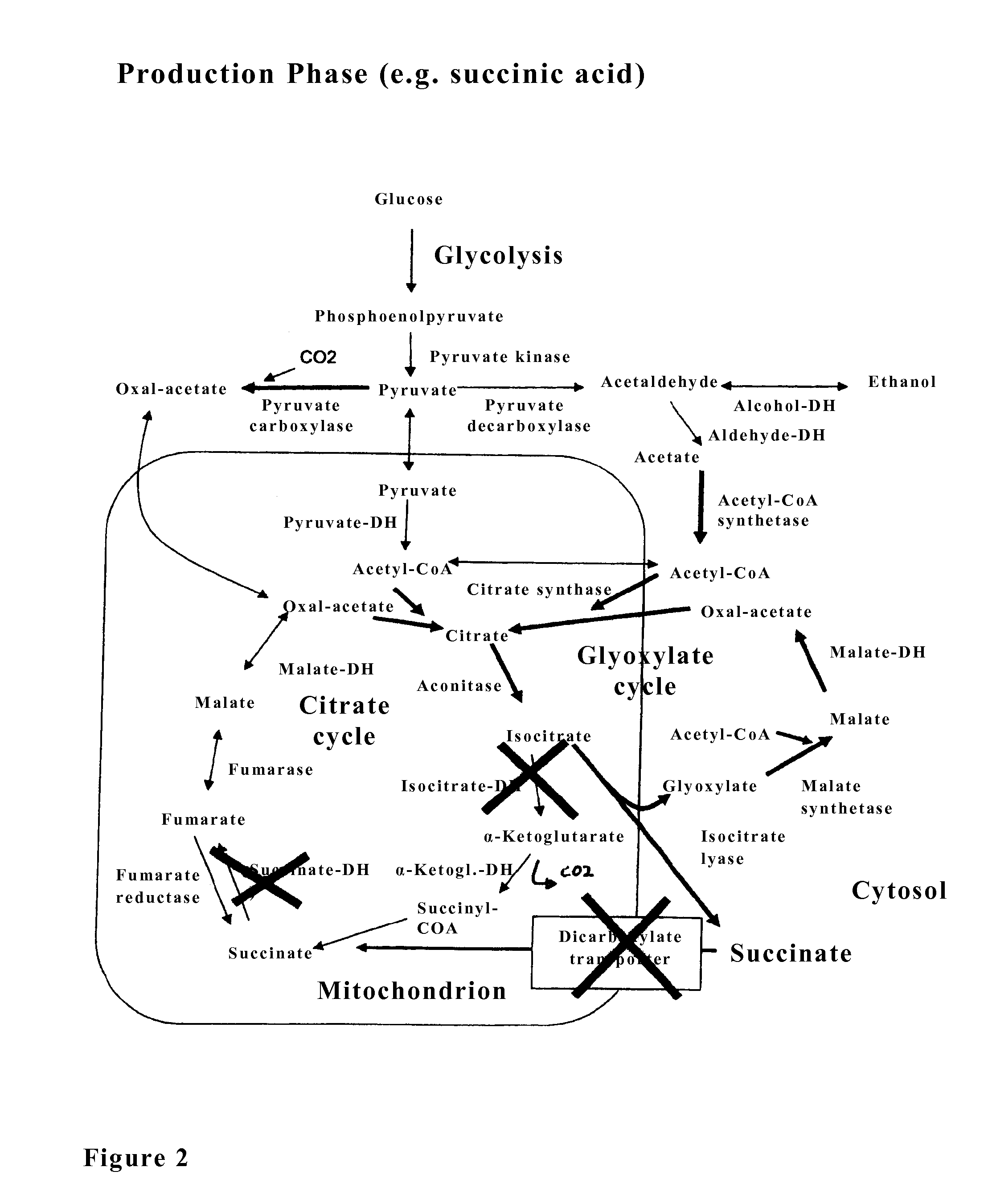
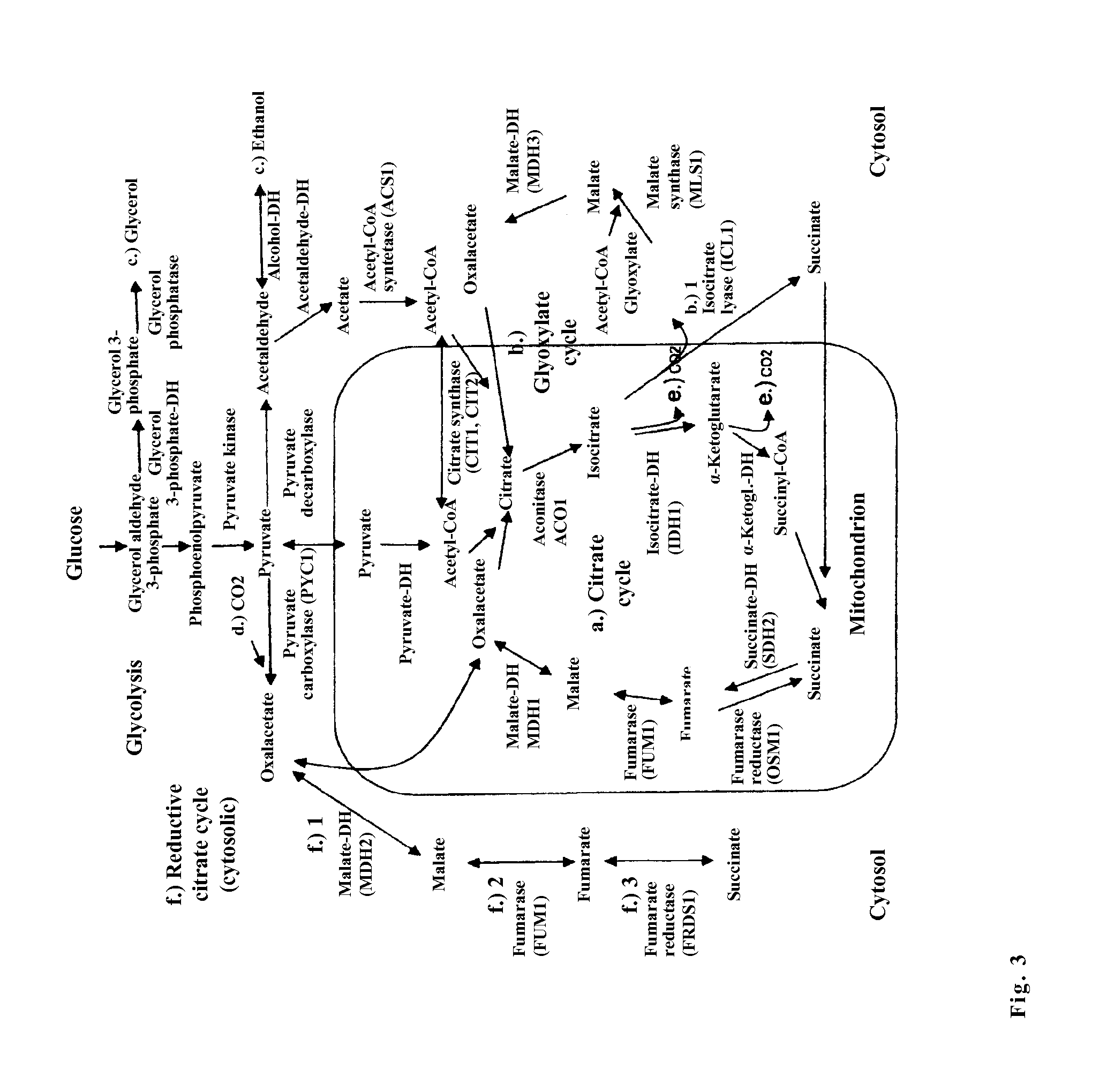
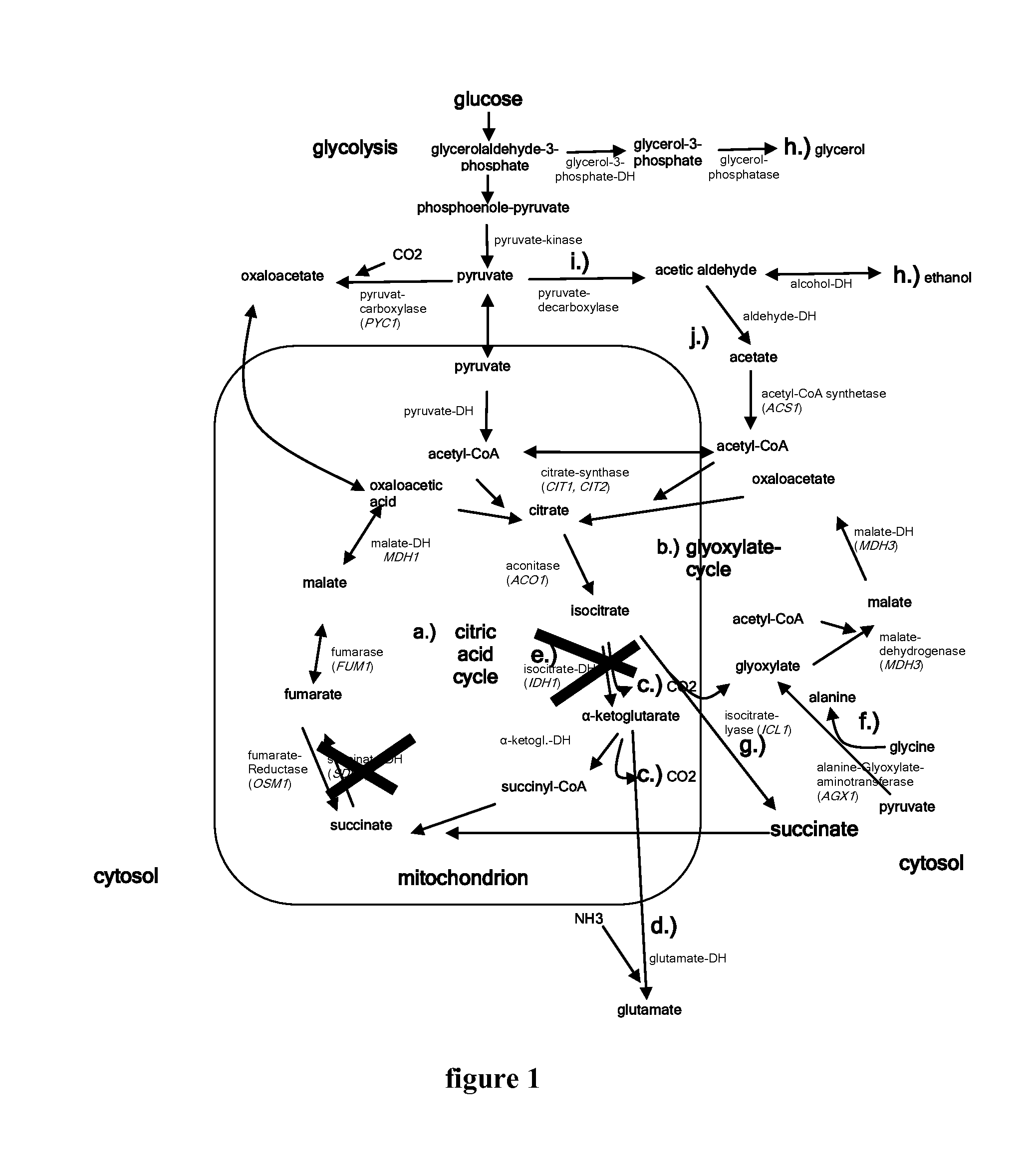
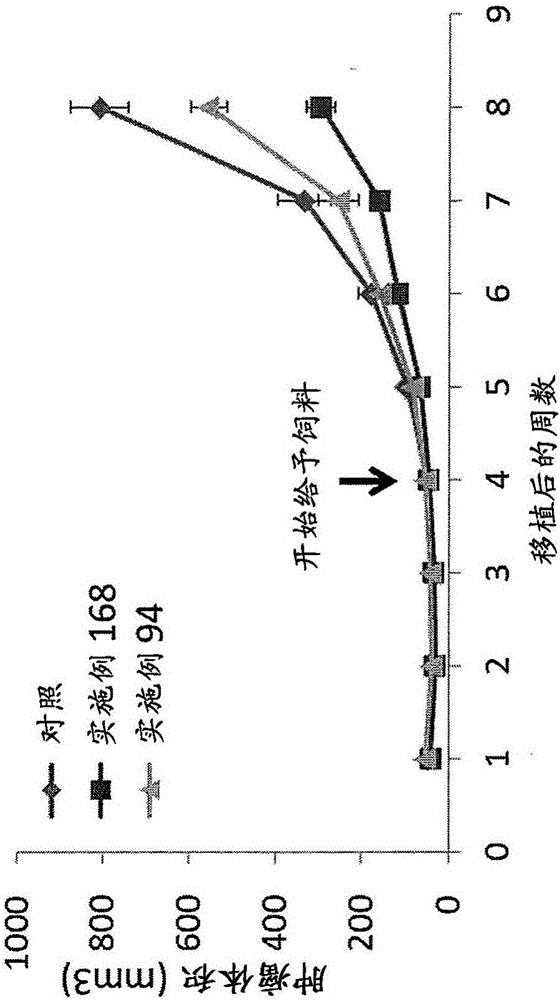
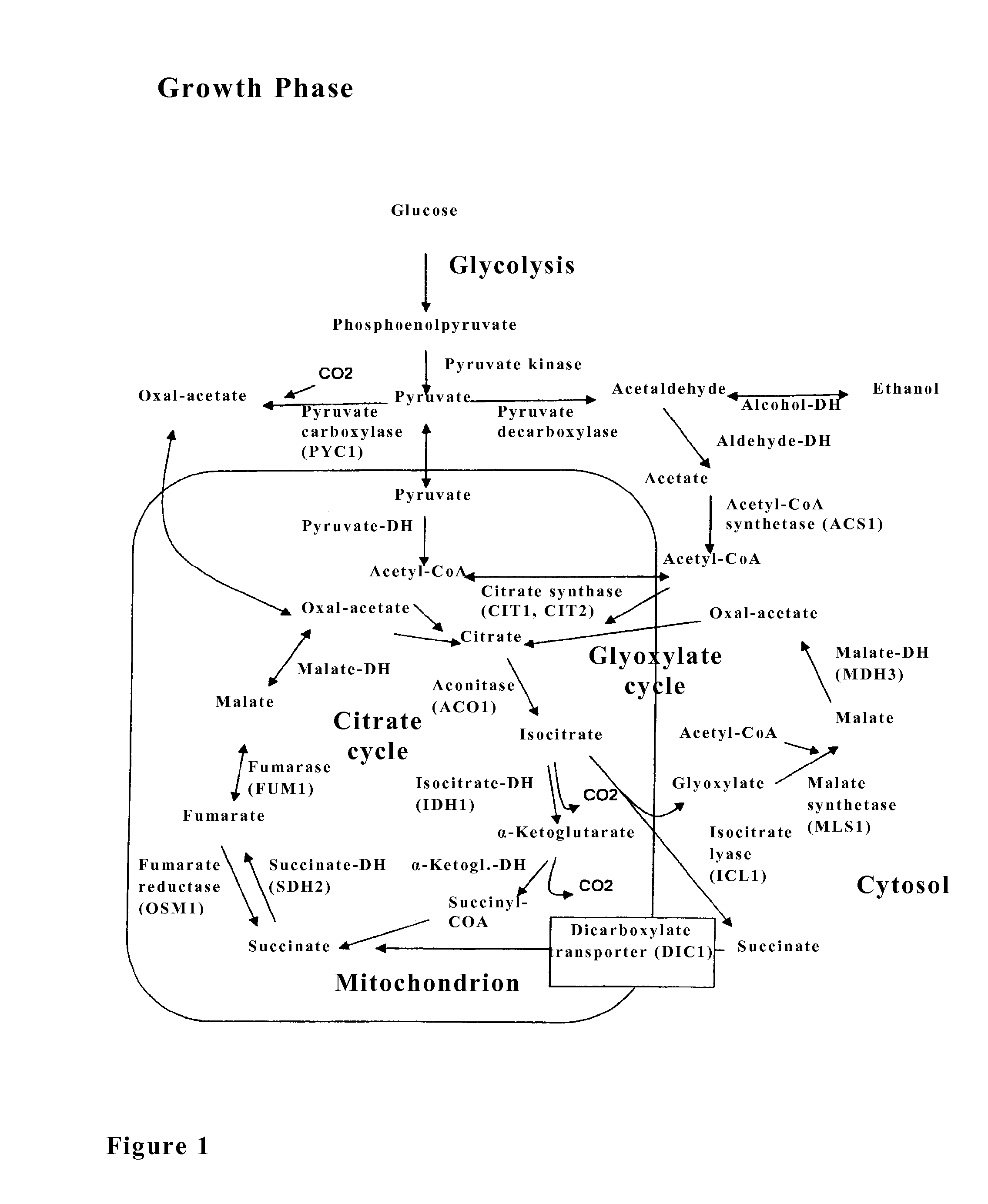
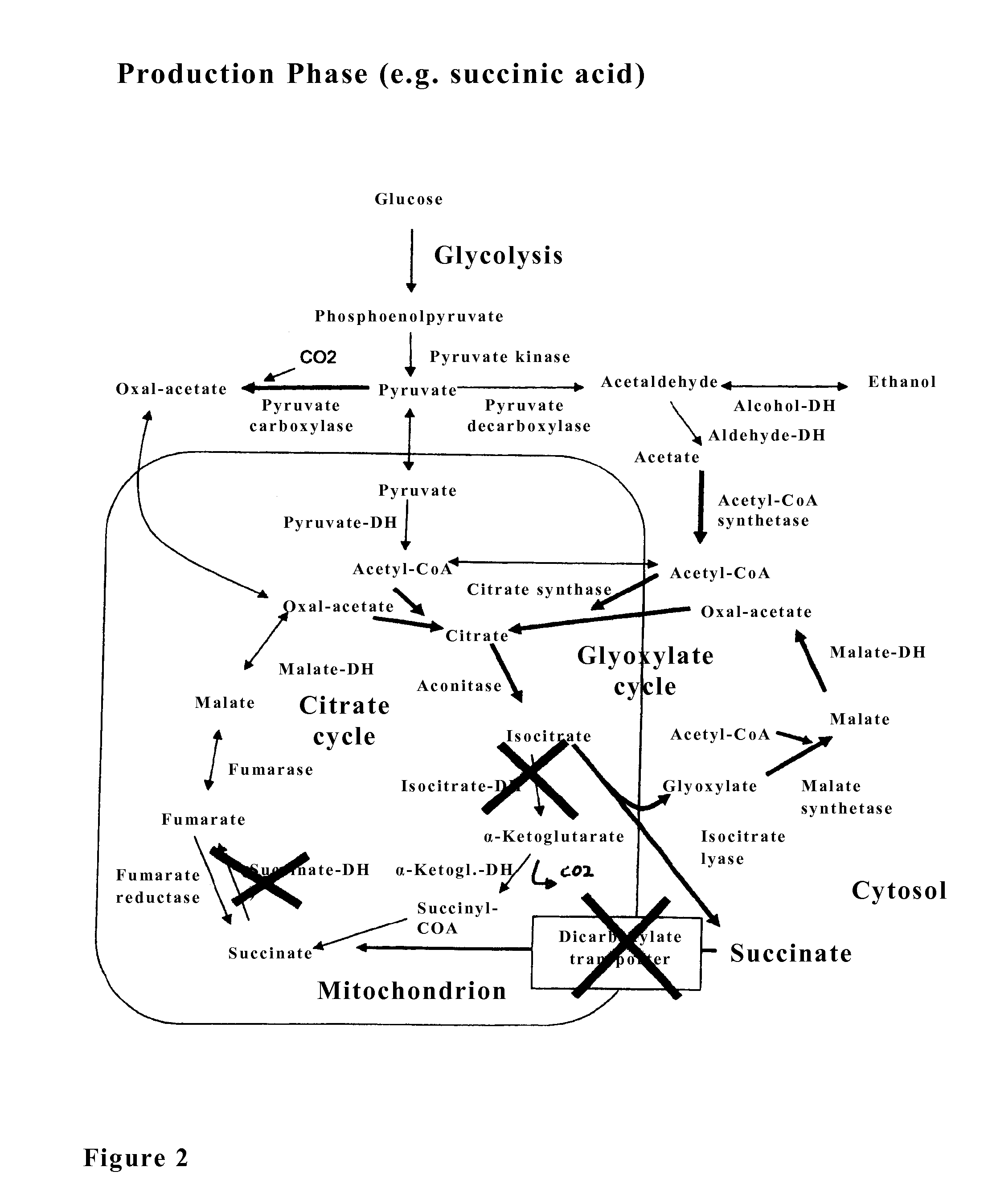
The invention relates to an isolated genetically modified microorganism in which the gene IDH1 and at least one of the genes SDH2 and DIC1 are under the control of a first promoter that is repressed to a growth culture medium by means of a cultivation additive and is active in the absence of the cultivation additive. The genes that are part of the group comprising “PYC1, ACS1, CIT1, ACO1, ICL1, MSL1, and CIT2, optionally also MDH3” are constitutively active. The invention further relates to uses of such a microorganism, especially for producing succinic acid.
Owner:NOVOZYMES AS
RNA interference mediated inhibition of isocitrate dehydrogenase (IDH1) gene expression
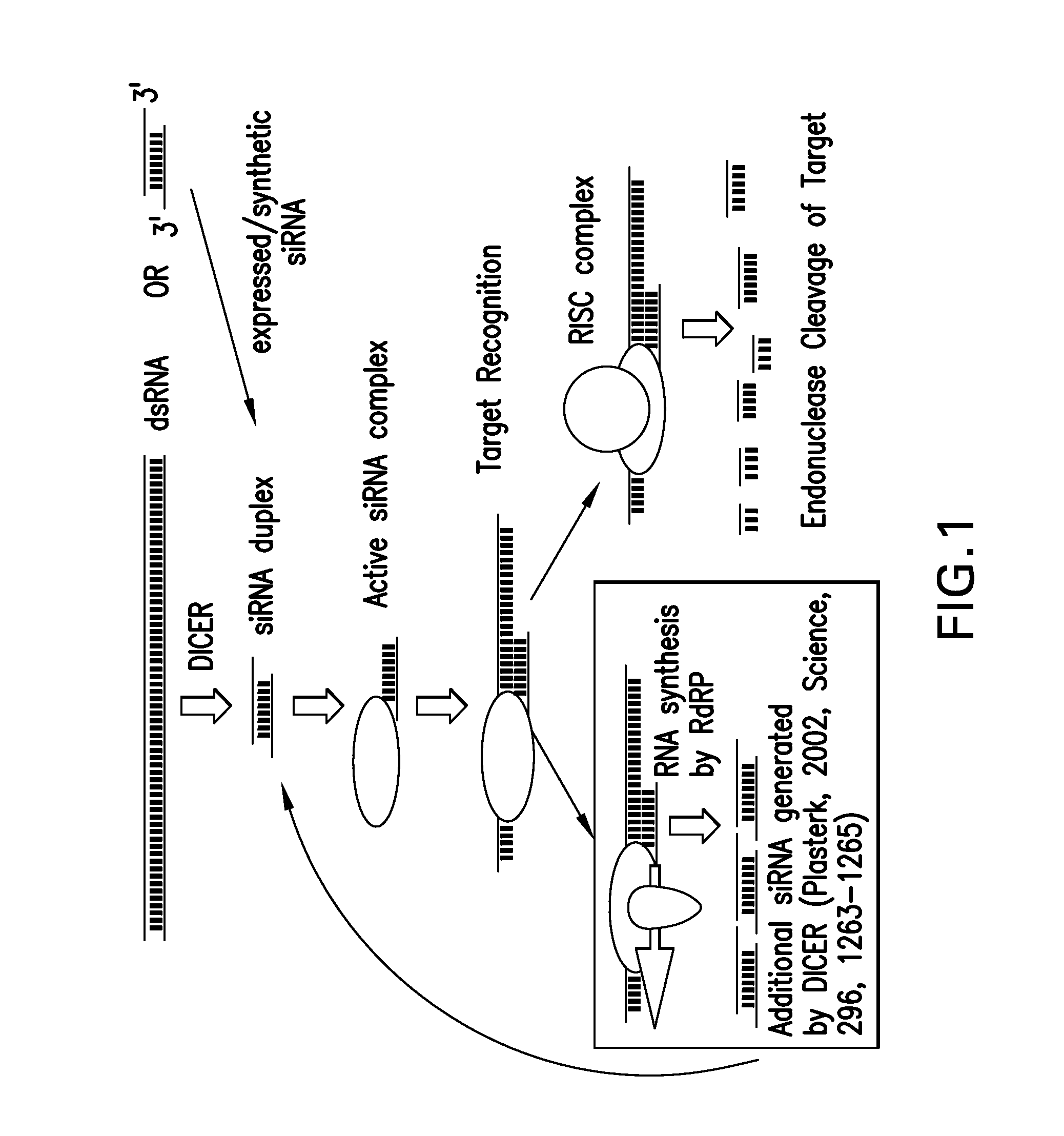
The present invention relates to compounds, compositions, and methods for the study, diagnosis, and treatment of traits, diseases and conditions that respond to the modulation of IDH1 and mutant IDH1 gene expression and / or activity, and / or modulate an IDH1 or mutant IDH1 gene expression pathway. Specifically, the invention relates to double-stranded nucleic acid molecules, including small nucleic acid molecules such as short interfering nucleic acid (siNA), short interfering RNA (siRNA), double-stranded RNA (dsRNA), micro-RNA (miRNA), and short hairpin RNA (shRNA) molecules, that are capable of mediating or that mediate RNA interference (RNAi) against IDH1 or mutant IDH1 gene expression.
Owner:SIRNA THERAPEUTICS INC
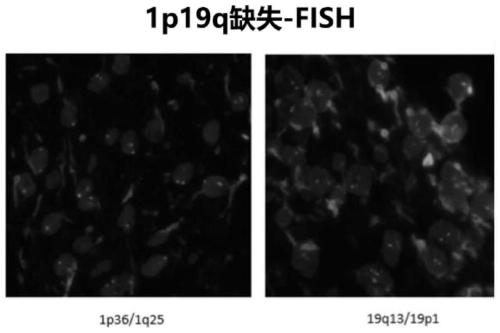
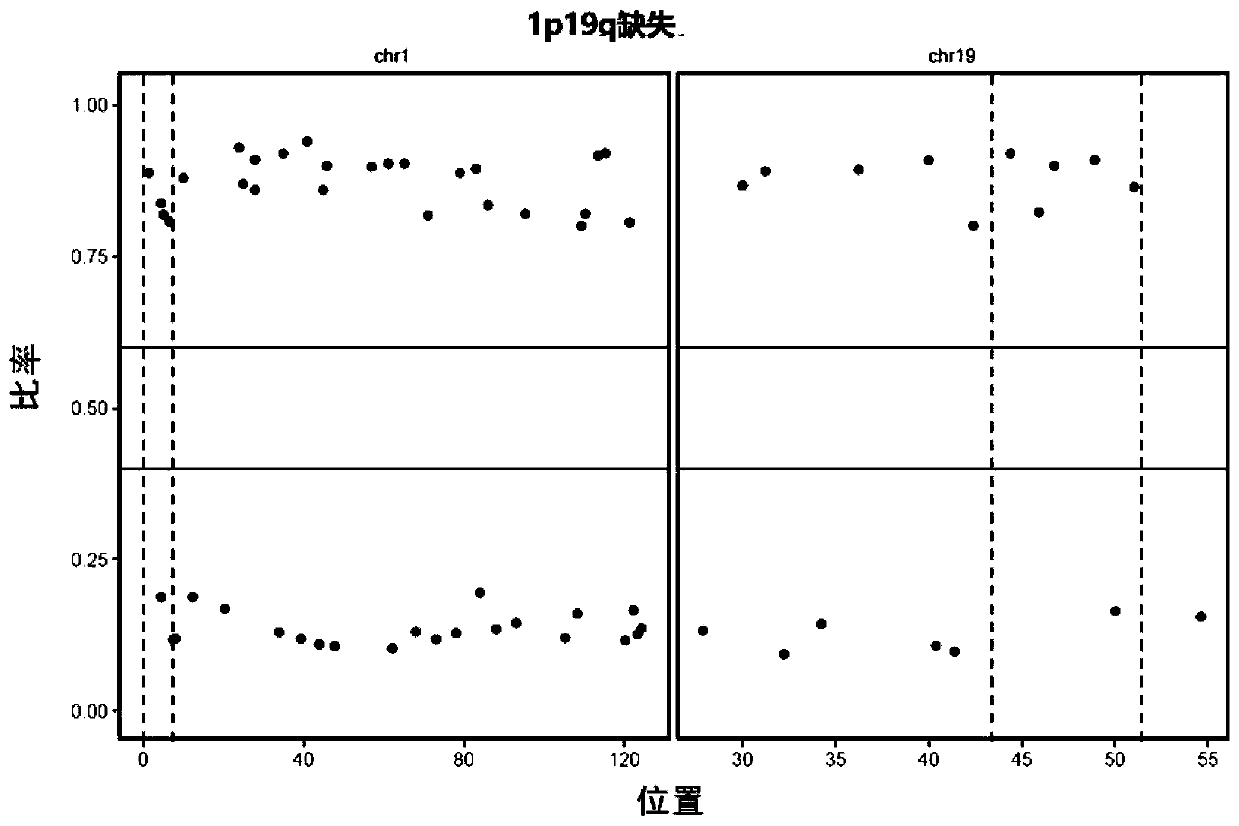
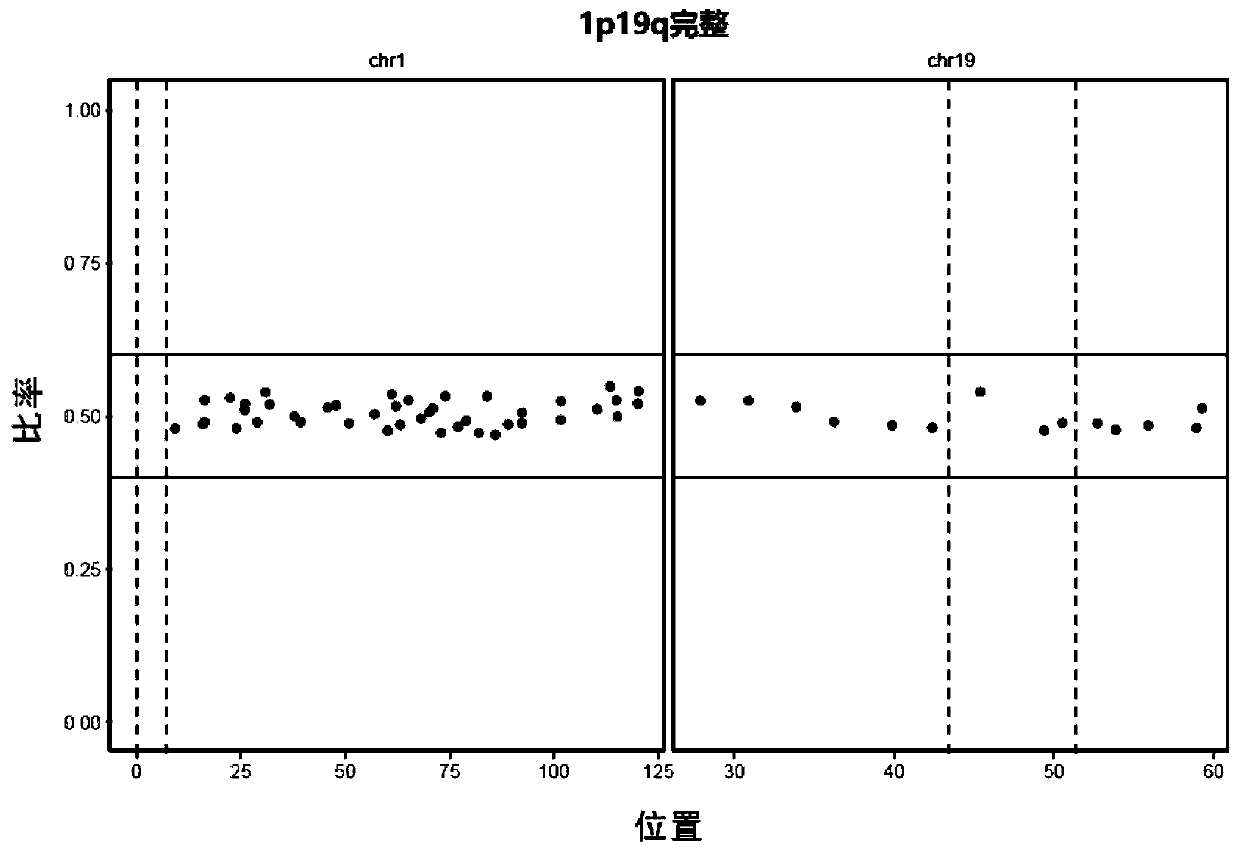
Glioma detection panel based on next-generation sequencing, detection kit and their application
PendingCN110129441APrecision diagnosis and treatment serviceComprehensive medical serviceMicrobiological testing/measurementIDH1Glioma
The invention discloses a glioma detection panel based on next-generation sequencing, a detection kit and their application, wherein the glioma detection panel comprises glioma-related genes and loci;the glioma-related genes and loci include SNP (single-nucleotide polymorphism) locus on No. 1 chromosome, SNP locus on No. 19 chromosome, MGMT, ATRX, H3F3A, ACVR1, CTC, HIST1H3B, MLH1, PLCG1, SMO, AKT1, CTNNB1, HIST1H3C, MSH2, PMS2, TERT, ATRX, DAXX, HRAS, MSH6, PPM1D, TP53, BCOR, DDX3X, IDH1, MYC, PTCH1 and the like. The glioma detection panel herein is suitable for providing a patient with precise comprehensive diagnostic and treatment services just through next-generation sequencing.
Owner:GENECAST WUXI PRECISION MEDICAL DIGNOSTIC LAB
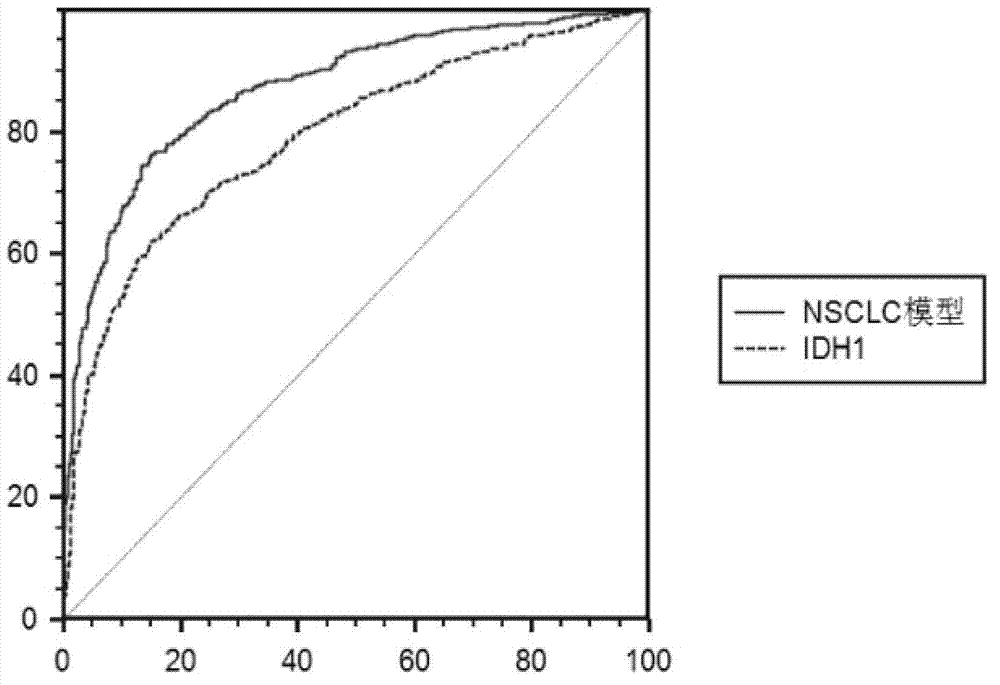
Test kit of auxiliary diagnosis of non-small cell lung cancer patients
ActiveCN103163293AIncreased sensitivityIncrease credibilityBiological testingIDH1Diagnosis standards
The invention discloses a test kit of auxiliary diagnosis of non-small cell lung cancer patients. The test kit comprises a product used for detecting protein landmark isocitrate dehydrogenase 1(IDH1), a product used for detecting protein landmark carbonic anhydrase 125 (CA125), a product used for detecting protein landmark CYFRA21-1 and a carrier on which a functional expression p=1 / (1+(20.186*0.406<x1>*0.923<x2>*0.656<x3>)<-1>) is recorded, wherein x1 represents the concentration of IDH1, x2 represents the concentration of CA125, and x3 represents the concentration of CYFRA21-1. The test kit is adopted and the non-small cell lung cancer patients are diagnosed in an auxiliary mode according to corresponding diagnosis standards, the test kit has the advantages of being high in sensitivity and strong in specificity, reliability of the diagnosis is far higher than that of diagnosis which is carried out with each single protein landmark, and the test kit has great value and application prospects for diagnosis and treatment of non-small cell lung cancer.
Owner:CANCER INST & HOSPITAL CHINESE ACADEMY OF MEDICAL SCI
Microorganism for producing succinic acid
InactiveUS20110300595A1Efficient productionAvoid and reduce yield lossFungiOxidoreductasesMicroorganismIDH1
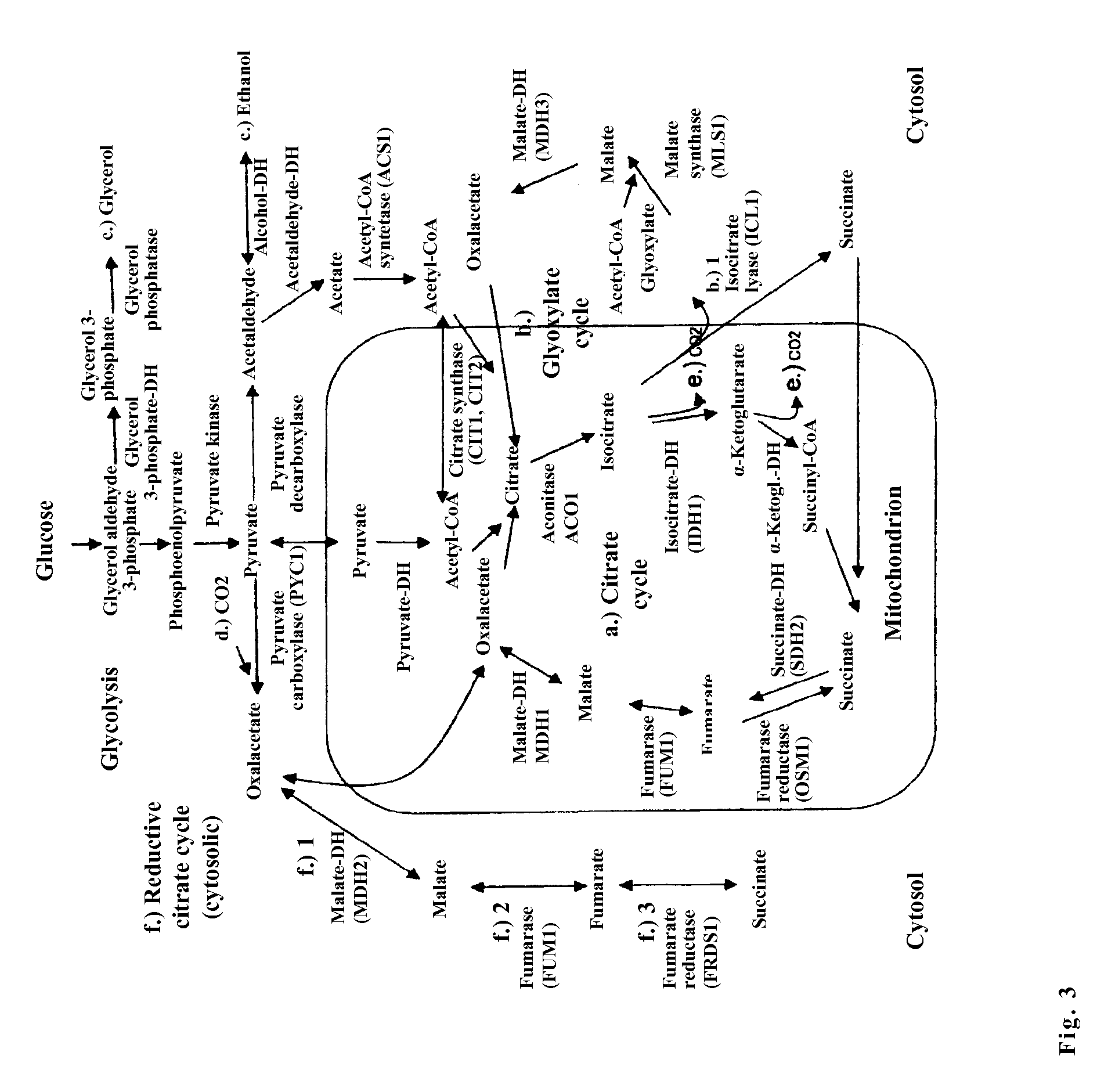
The invention relates to an isolated, genetically modified microorganism, wherein compared to the wild type a) the idh1 and idp1 genes have been deleted or inactivated, and / or b) the sdh2 and sdh1 genes have been deleted or inactivated, and / or c) the PDC2 gene has been deleted or inactivated or is under the control of a promoter which can be suppressed or induced by exposure of the microorganism using an inductor substance, and / or d) one or more genes from the group consisting of ICL1, MLS1, ACS1 and MDH3 has been replaced or supplemented by a corresponding foreign gene or corresponding foreign genes from Crabtree-negative organisms, and to the uses thereof.
Owner:ORGANOBALANCE GMBH
Peripheral blood methylation gene and IDH1 combined detection and diagnosis model for lung cancer
ActiveCN111172279AImprove diagnostic efficiencyWide coverageMicrobiological testing/measurementBiological material analysisIDH1Blood markers
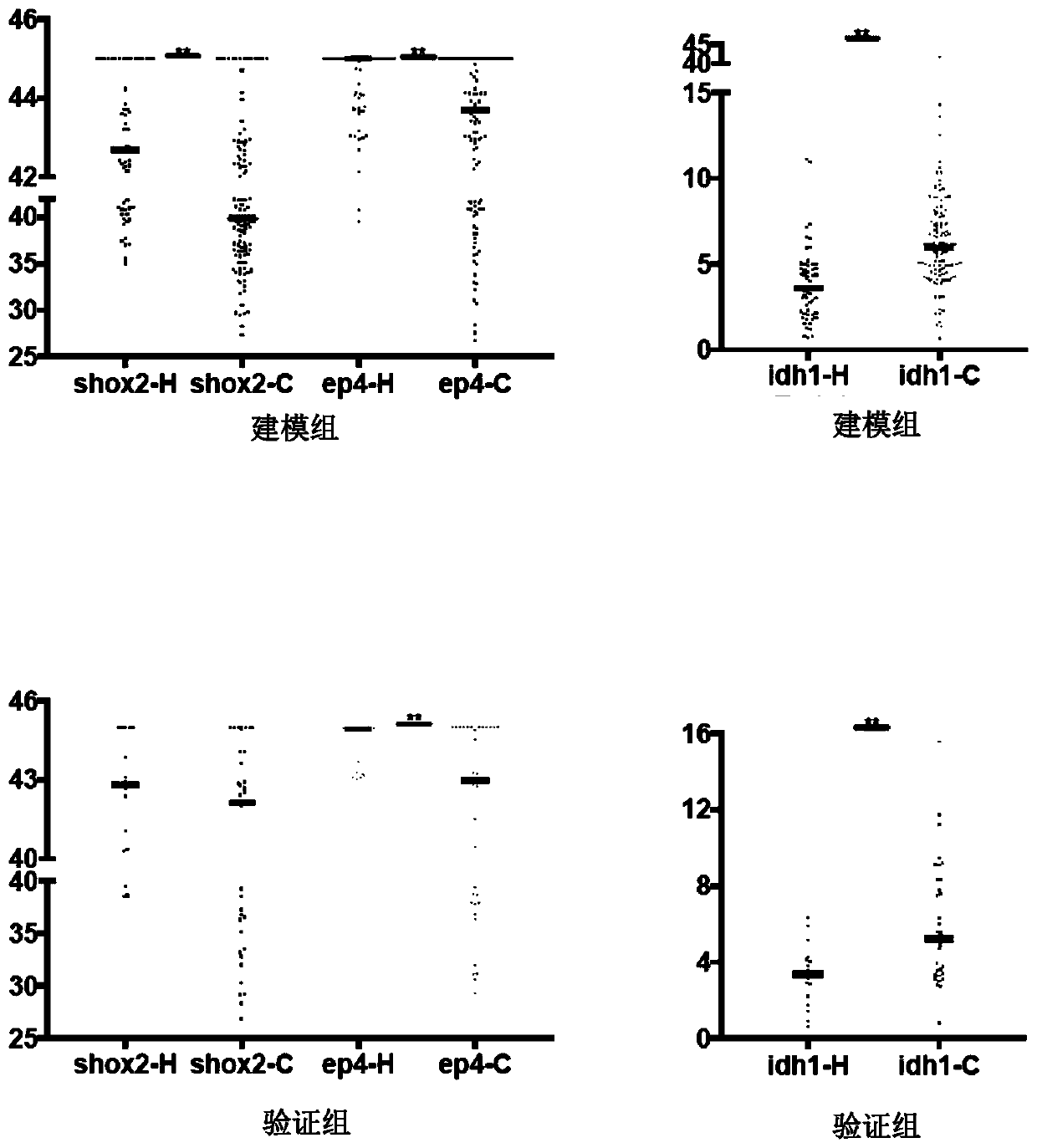
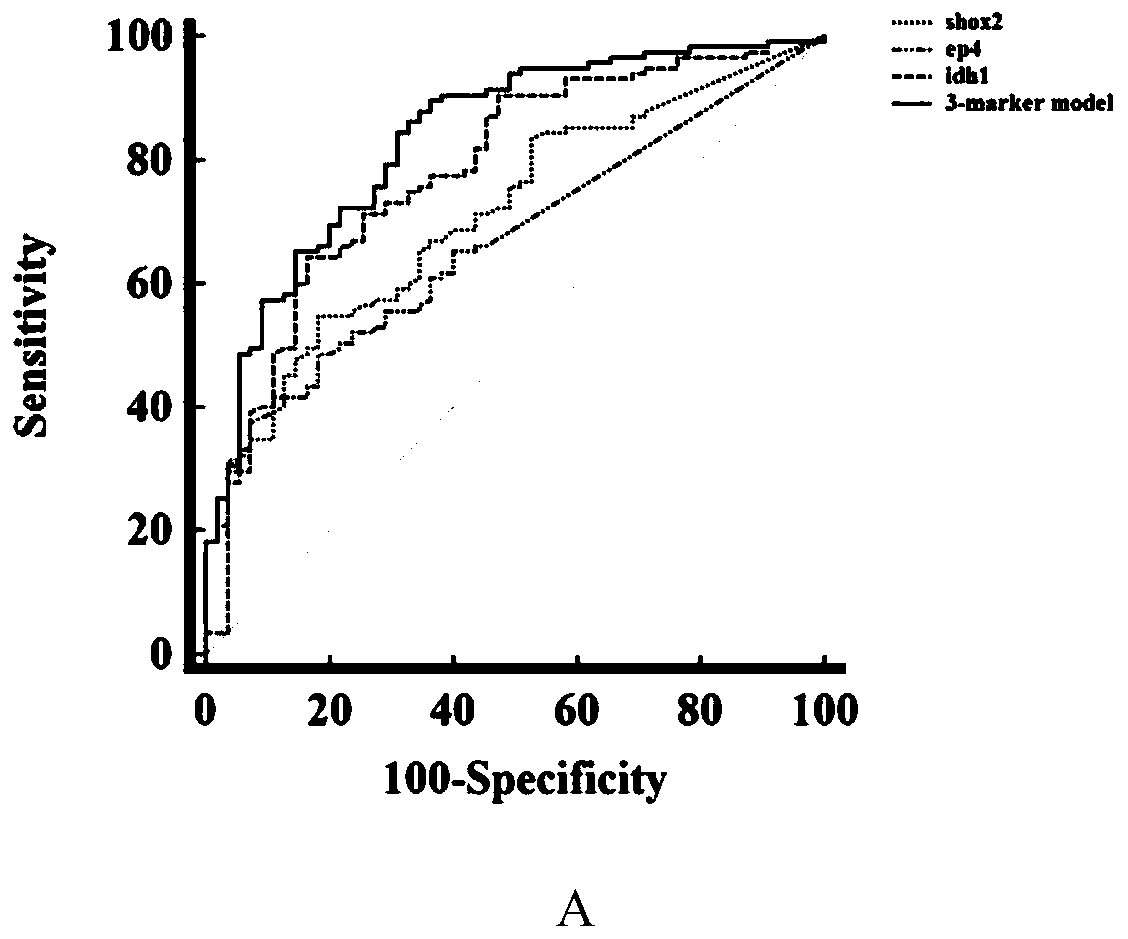
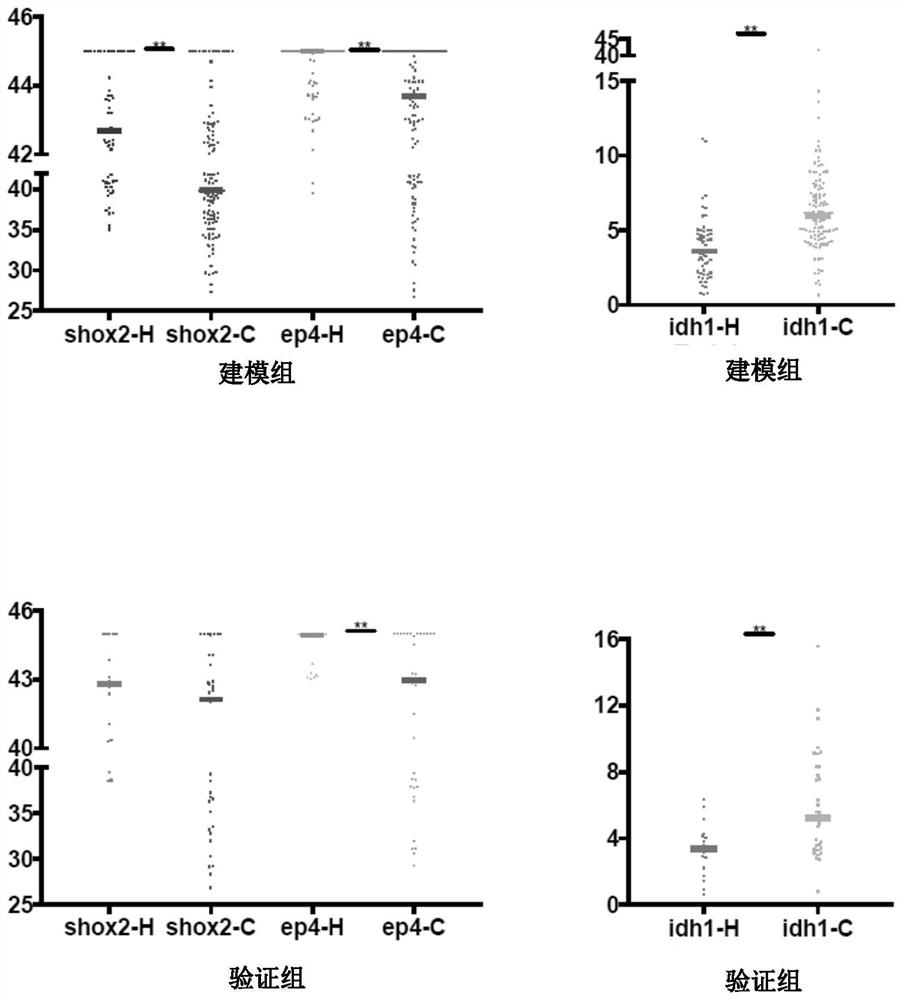
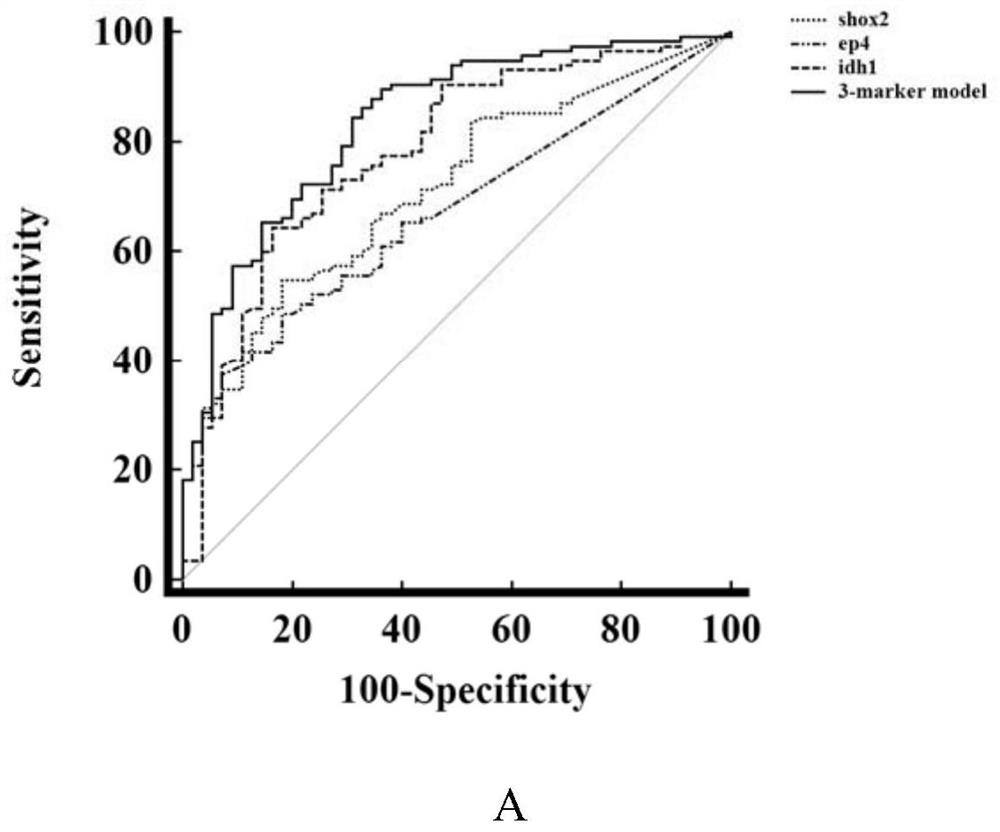
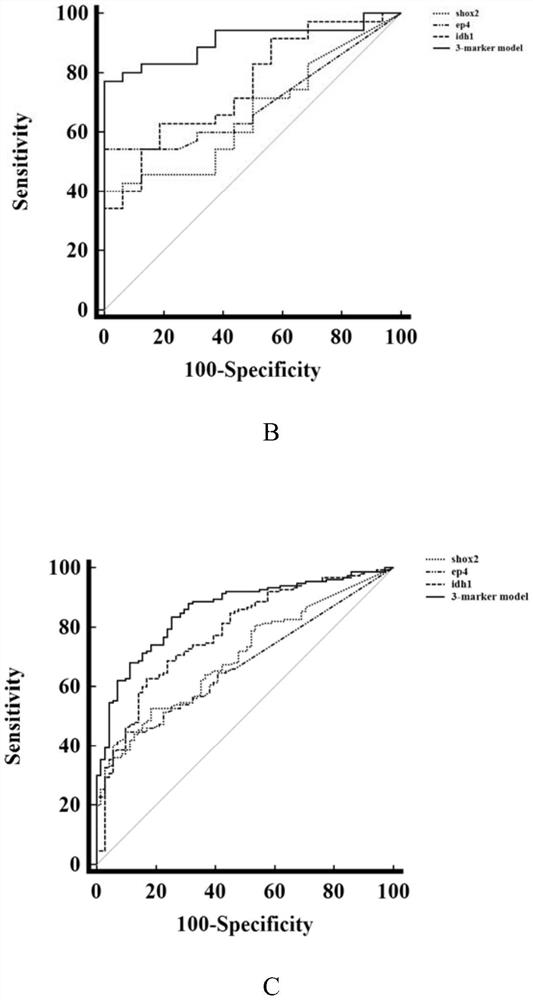
The invention discloses a peripheral blood methylation gene and IDH1 combined detection and diagnosis model for lung cancer. The invention provides application of methylated SHOX2 gene, methylated PTGER4 gene and IDH1 protein as markers in preparation of products for diagnosis or auxiliary diagnosis of lung cancer, and constructs a three-marker combined lung cancer diagnosis model. Experiments prove that: compared with an incorporated single factor, the diagnosis efficiency of the combined diagnosis model is remarkably enhanced. Therefore, the diagnosis efficiency of the lung cancer can be improved by combined detection blood markers.
Owner:CANCER INST & HOSPITAL CHINESE ACADEMY OF MEDICAL SCI
Methods for rapid and sensitive detection of hotspot mutations
Methods that rapidly, sensitively, and specifically detect mutations in IDH1 / 2 and the TERT promoter employ amplification of particular portions of the genes that experience frequent and exquisitely localized mutations. The ability to distinguish between sequences that differ only by one nucleotide and which may be present in very low ratios is essential for such an assay.
Owner:DUKE UNIV
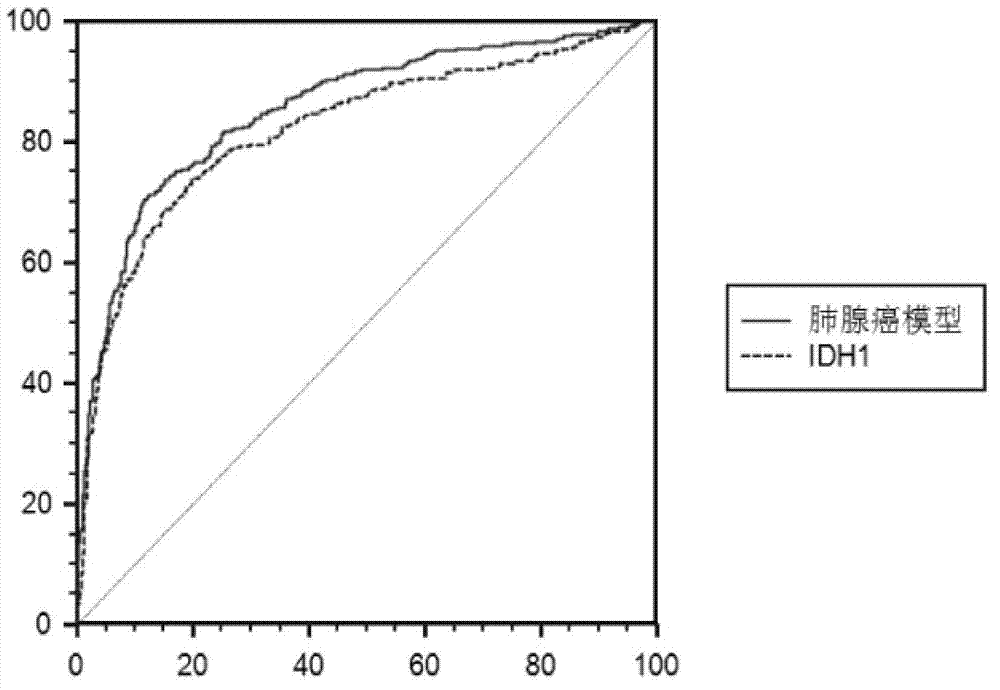
Reagent kit for auxiliary diagnosis of lung adenocarcinoma paitent
ActiveCN103163303AIncreased sensitivityHigh diagnostic confidenceBiological testingProtein markersIDH1
The invention discloses a reagent kit for an auxiliary diagnosis of a lung adenocarcinoma patient. The reagent kit comprises a first product, a second product, a third product and a carrier. The first product is used for detecting protein markers IDH1, the second product is used for detecting protein markers CA125, the third product is used for detecting protein markers CYFRA21-1, and the carrier records the following functional expression: x1 represents the concentration of the IDH1, x2 represents the concentration of the CA125, and x3 represents the concentration of the CYFRA21-1. By means of the reagent kit for the auxiliary diagnosis of the lung adenocarcinoma patient, the lung adenocarcinoma patient is diagnosed in an auxiliary mode according to corresponding diagnostic criteria. Furthermore, the reagent kit for the auxiliary diagnosis of the lung adenocarcinoma patient has the advantages of high sensitivity and strong specificity. In addition, the reliability of the diagnosis is far higher than that of the diagnosis of adopting each individual protein marker, so that the reagent kit for the auxiliary diagnosis of the lung adenocarcinoma patient has a significant value and an application prospect for the diagnosis and treatment of lung adenocarcinoma.
Owner:CANCER INST & HOSPITAL CHINESE ACADEMY OF MEDICAL SCI
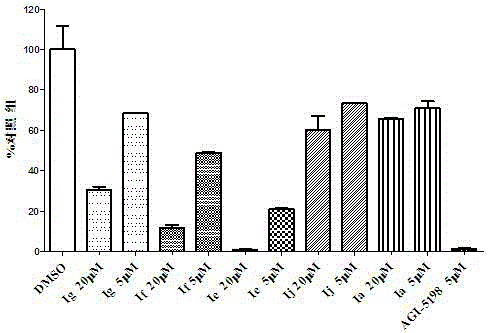
Double-aryl maleimide compound, pharmaceutically acceptable salt thereof, method for preparing double-aryl maleimide compound and pharmaceutically acceptable salt and application of double-aryl maleimide compound and pharmaceutically acceptable salt
The invention discloses a double-aryl maleimide compound, pharmaceutically acceptable salt thereof, a method for preparing the double-aryl maleimide compound and the pharmaceutically acceptable salt and application of the double-aryl maleimide compound and the pharmaceutically acceptable salt. The double-aryl maleimide compound and the pharmaceutically acceptable salt can be particularly used as isocitrate dehydrogenase 1 (IDH1) mutant inhibitors and can be applied to treating malignant tumor such as glioma and acute myeloid leukemia. The double-aryl maleimide compound, the pharmaceutically acceptable salt, the method and the application have the advantages that the double-aryl maleimide compound has efficiently selective IDH1 mutant inhibitory activity, and accordingly the double-aryl maleimide compound and the pharmaceutically acceptable salt can be used for treating the IDH1 mutant mediated malignant tumor such as the glioma and the acute myeloid leukemia; the double-aryl maleimide compound, the pharmaceutically acceptable salt and the method are reasonable in design, and the method is simple and practical.
Owner:ZHEJIANG UNIV OF TECH +1
Kit for auxiliary diagnosis of squamous cell lung carcinoma patient
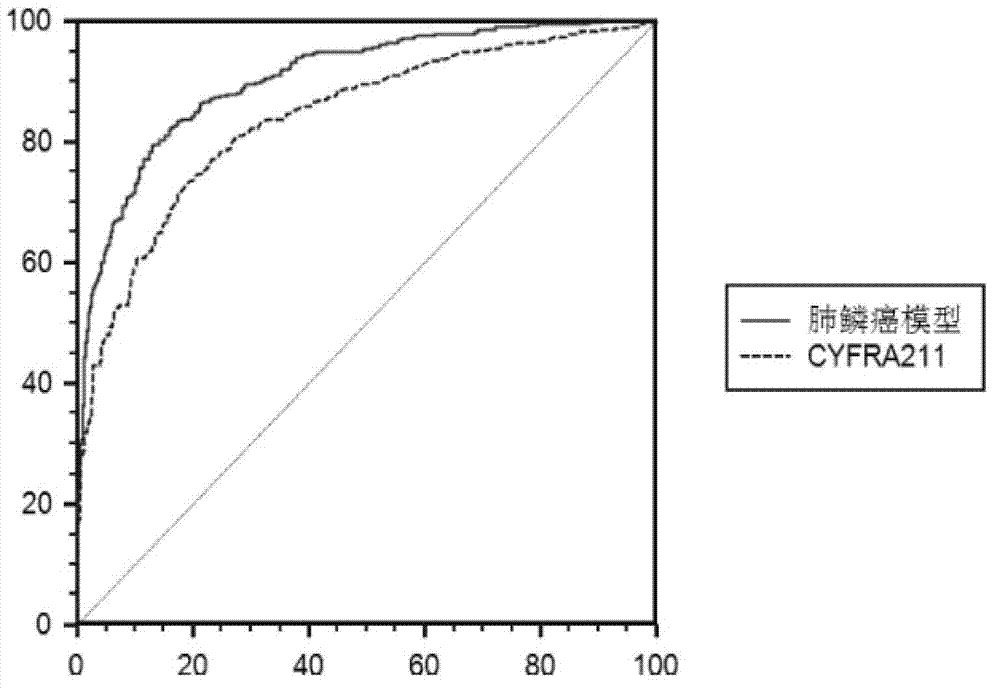
ActiveCN103175969AIncreased sensitivityHigh diagnostic confidenceBiological testingLung squamous cell carcinomaIDH1
The invention discloses a kit for auxiliary diagnosis of squamous cell lung carcinoma patient. The kit provided by the invention comprises a product for detecting a protein marker IDH1, a product for detecting a protein marker CA125, a product for detecting a protein marker CYFRA21-1 and a carrier recorded with the following functional expressions (as shown in the description): x1 stands for the concentration of the IDH1, x2 stands for the concentration of the CA125, and x3 stands for the concentration of the CYFRA21-1. The kit provided by the invention is used for the auxiliary diagnosis of squamous cell lung carcinoma patient according to the corresponding diagnosis standard, and has the characteristics of high flexibility and strong specificity, the diagnosis result reliability is far better than the diagnosis by adopting the single protein marker, and has important value and application prospect for the diagnosis and treatment of the squamous cell lung carcinoma.
Owner:CANCER INST & HOSPITAL CHINESE ACADEMY OF MEDICAL SCI
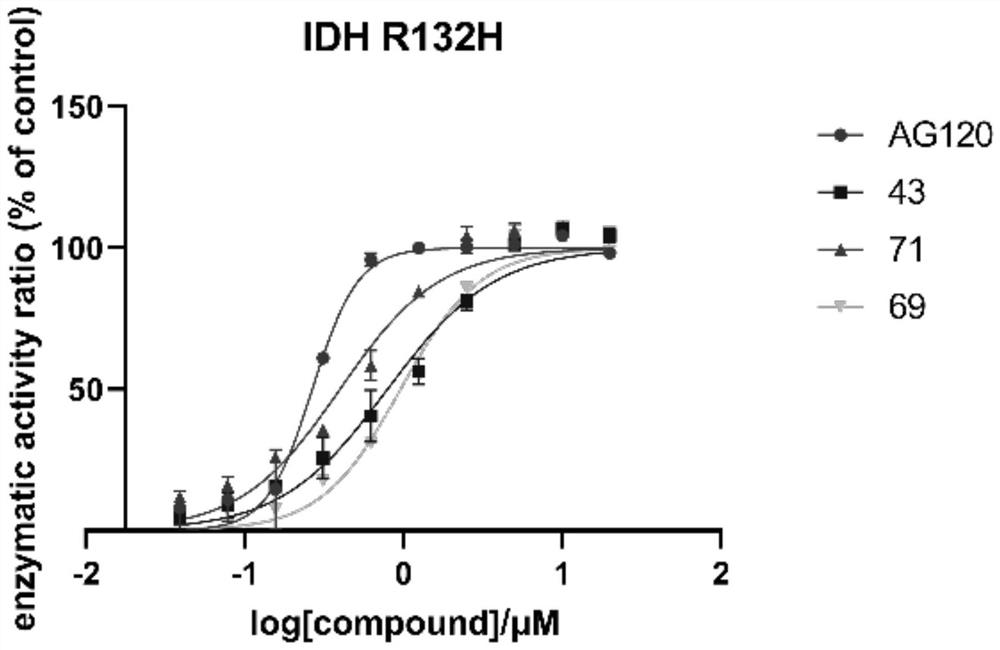
Mutant IDH1 inhibitors useful for treating cancer
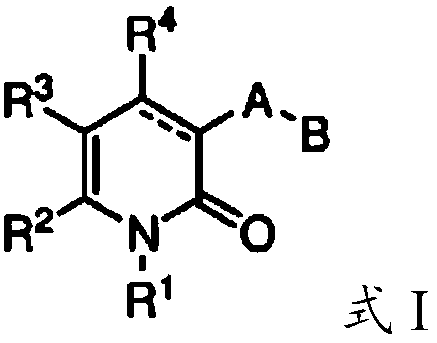
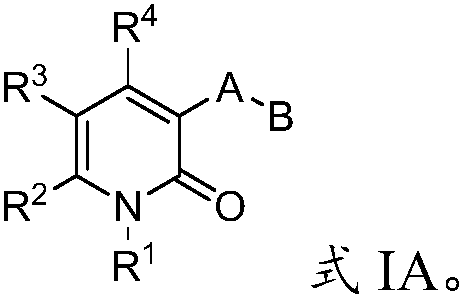
Compounds of Formula I and Formula II and the pharmaceutically acceptable salts thereof are disclosed. The variables A, B, Y, Z, X1, X2, R1-4 and R13-18 are disclosed herein. The compounds are useful for treating cancer disorders, especially those involving mutant IDH1 enzymes. Pharmaceutical compositions containing the compounds of the Formula I or Formula II and methods of treatment comprising administering the compounds of Formula I and Formula II are also disclosed.
Owner:UNITED STATES OF AMERICA +1
Steroidal compounds as well as extraction method and application thereof
ActiveCN108440471AEnhanced inhibitory effectOrganic active ingredientsSteroidsIsocitrate dehydrogenasePhotochemistry
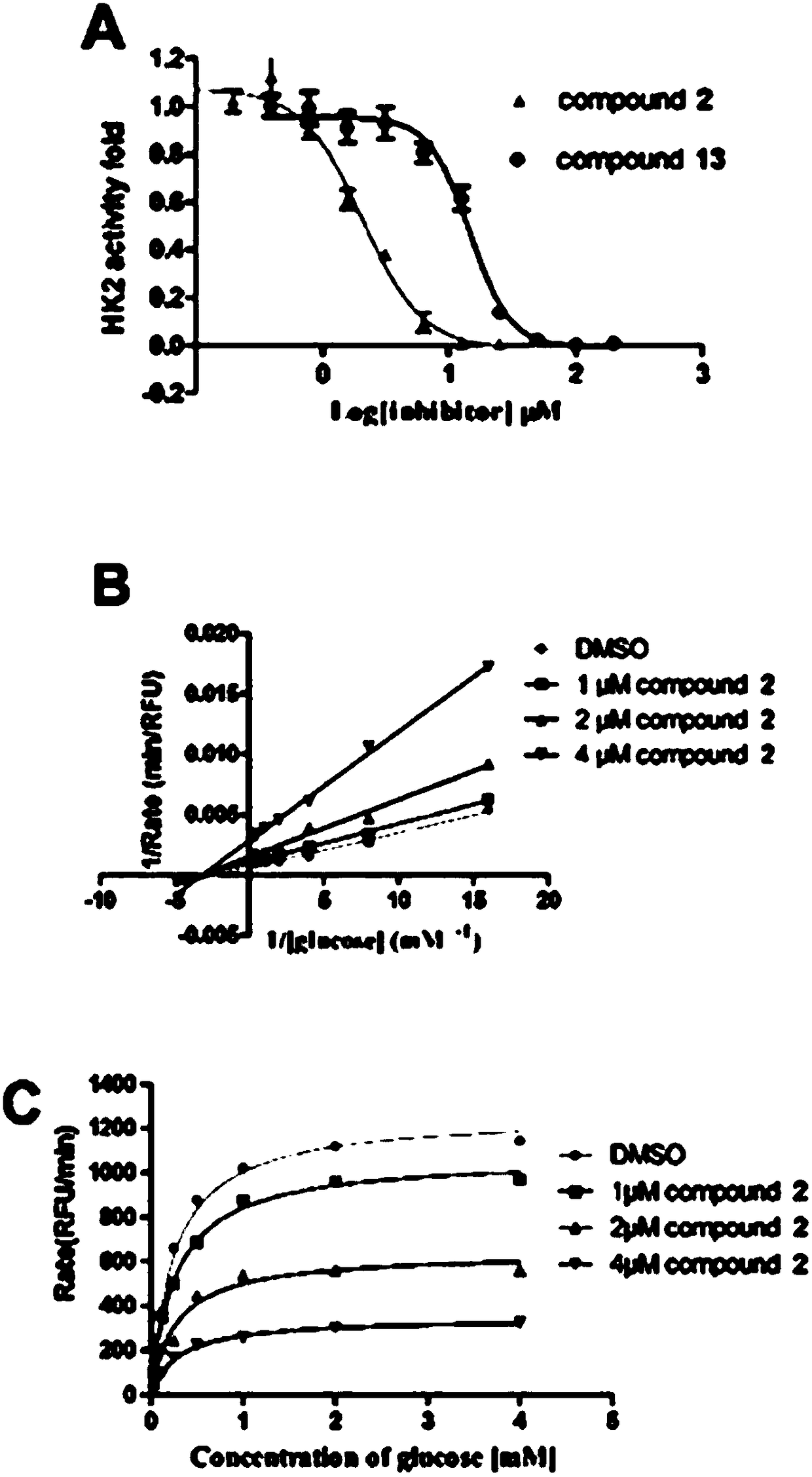
The invention belongs to the technical field of medicines, and discloses an extraction method for steroidal components in glossy ganoderma and an application of the steroidal components. The compoundshave structures represented by a formula (I) and a formula (II) shown in the description. The compounds and compositions of the compounds disclosed by the invention have an inhibitory effect on glycolysis first rate-limiting enzyme HK-2 or isocitrate dehydrogenase (IDH1), thereby having a significant anti-tumor effect; and the compounds have good research and development prospects.
Owner:SHENYANG PHARMA UNIVERSITY
Methods for rapid and sensitive detection of hotspot mutations
Methods that rapidly, sensitively, and specifically detect mutations in IDH1 / 2 and the TERT promoter employ amplification of particular portions of the genes that experience frequent and exquisitely localized mutations. The ability to distinguish between sequences that differ only by one nucleotide and which may be present in very low ratios is essential for such an assay.
Owner:DUKE UNIV
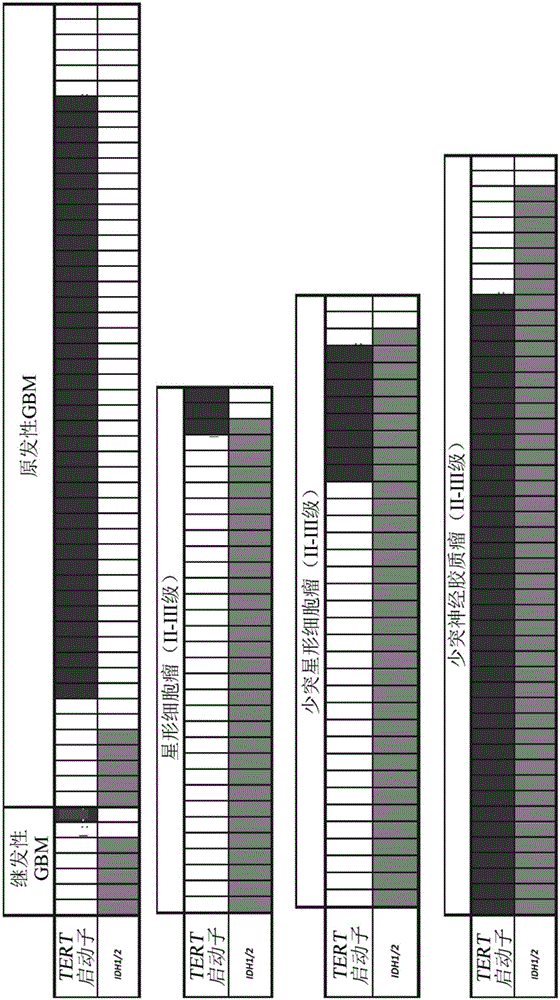
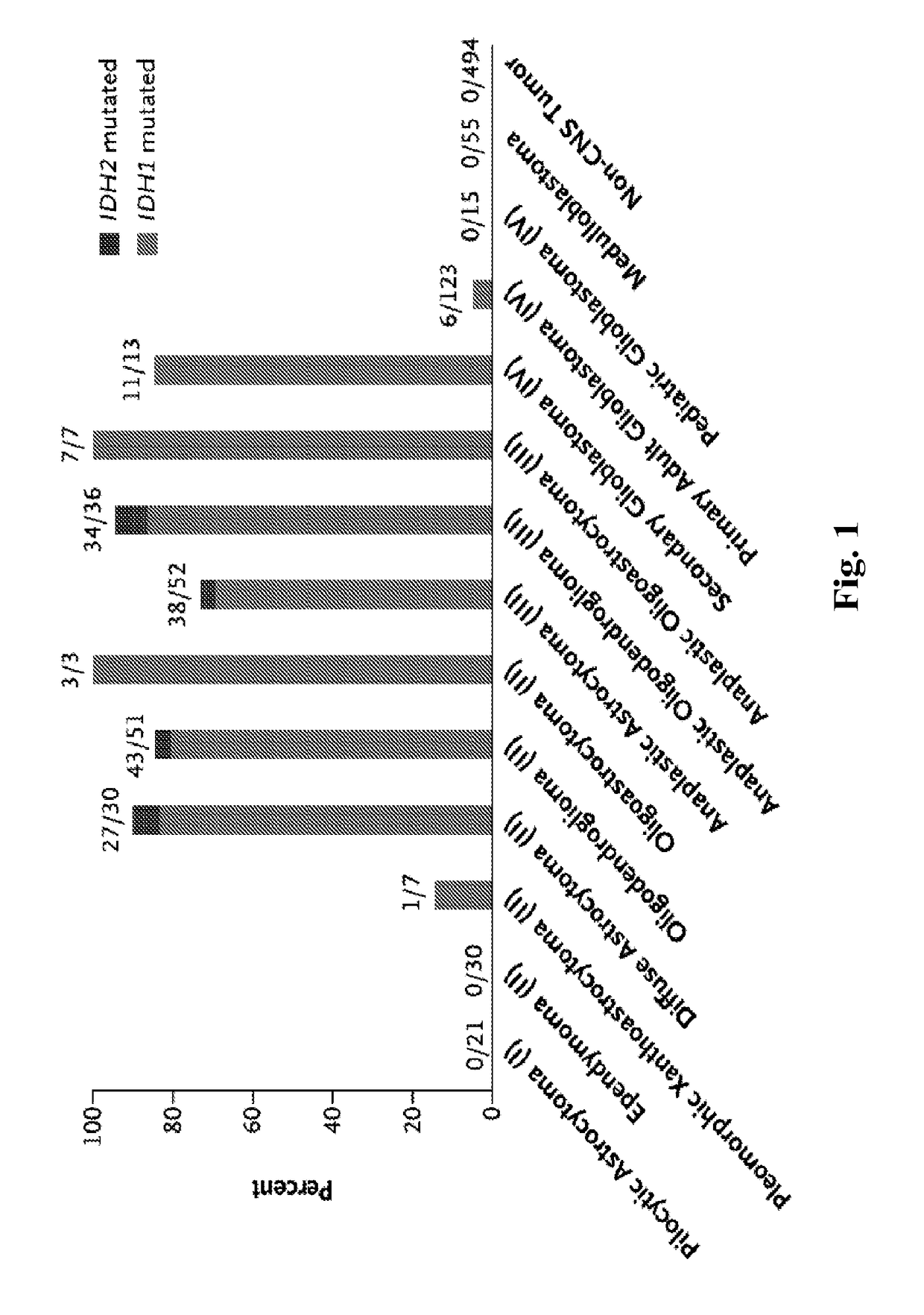
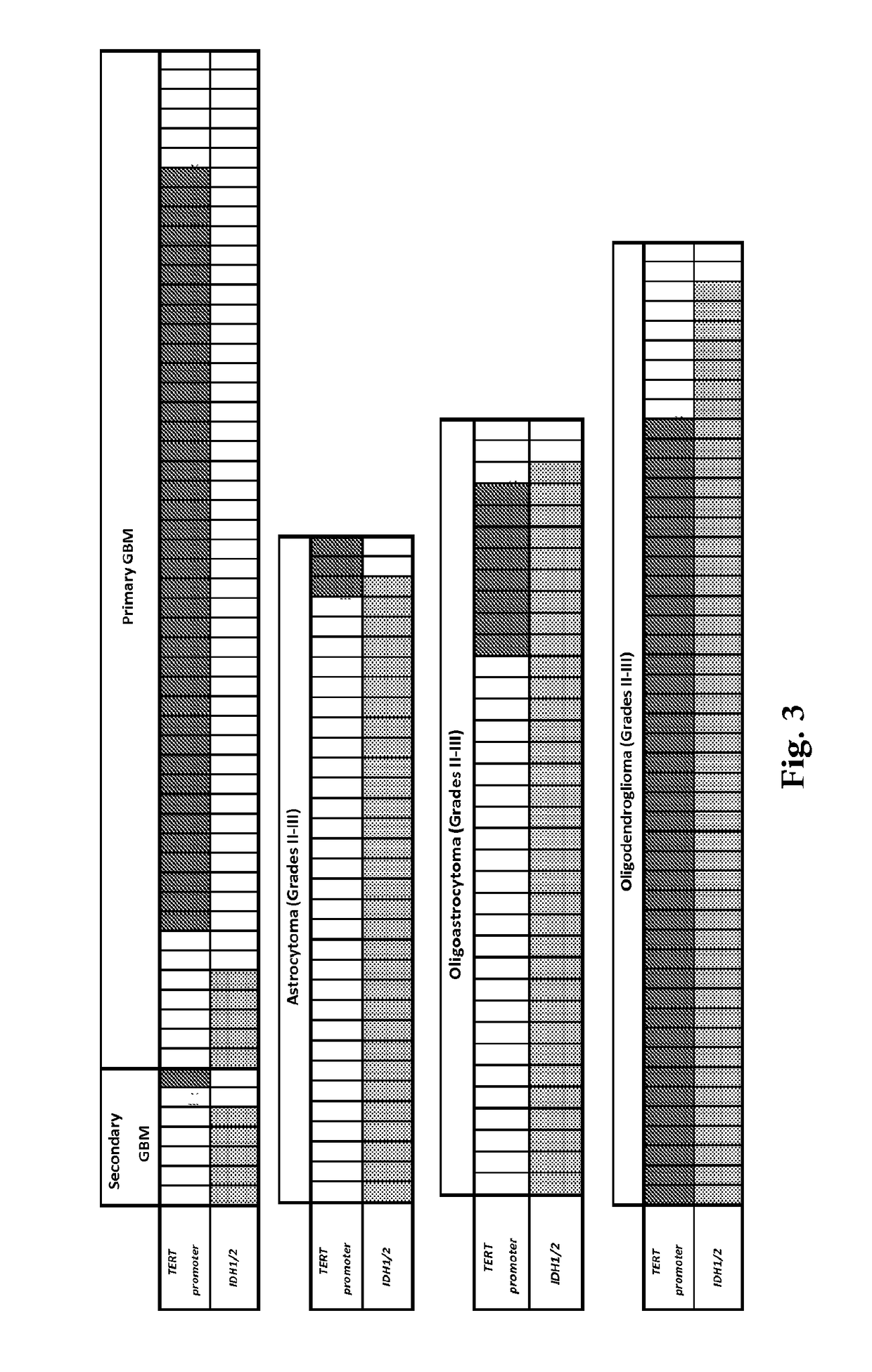
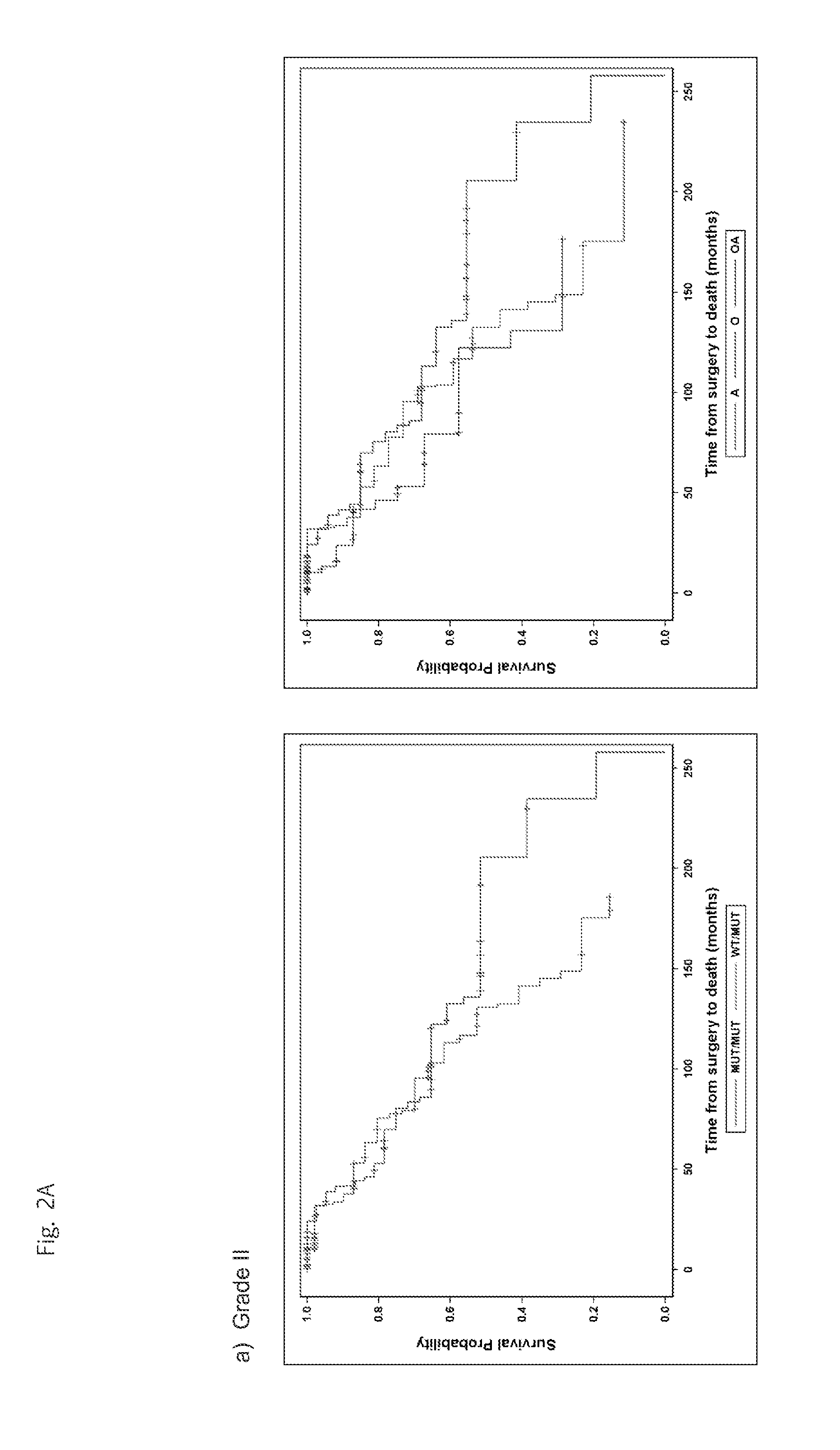
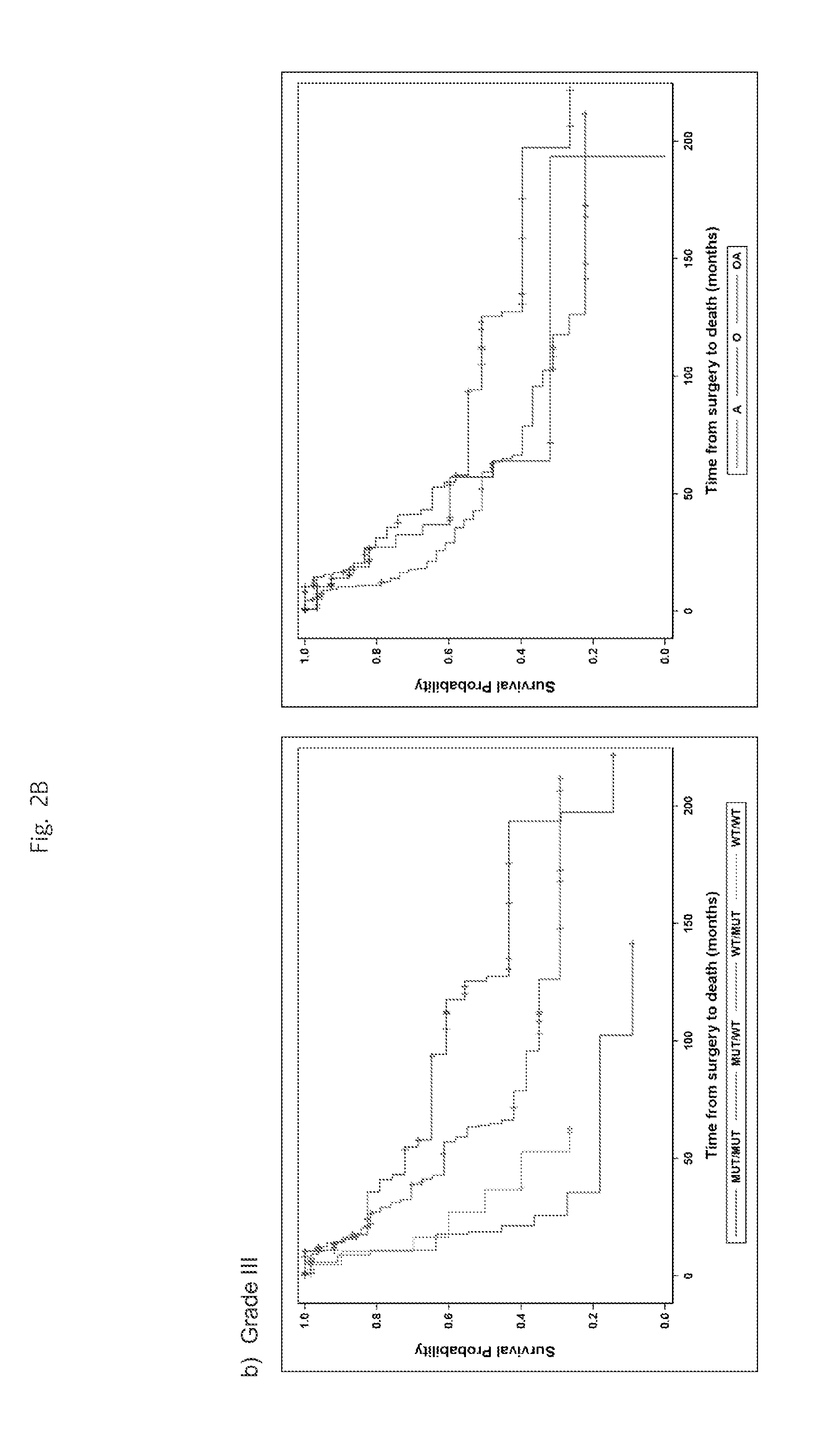
Mutations define clinical subgroups of gliomas
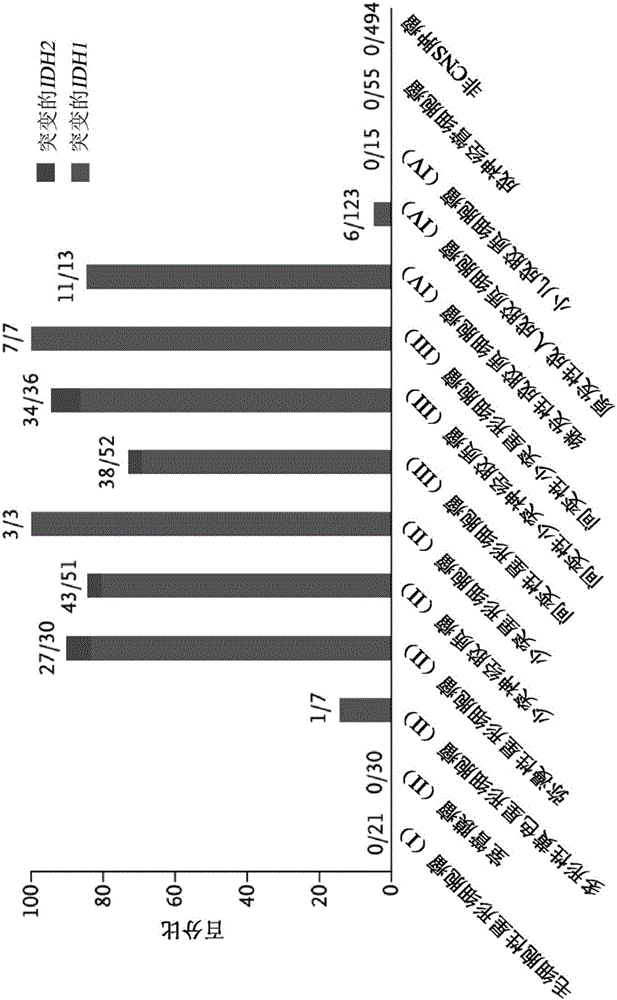
Genetic signatures capable of distinguishing among several types of gliomas provide clinically relevant information that can serve as an adjunct to histopathological diagnosis. For example, mutations in the TERT promoter occurred in 74.2% of glioblastomas (GBM), but occurred in a minority of Grade II-III astrocytomas (18.2%). In contrast, IDH1 / 2 mutations were observed in 78.4% of Grade II-III astrocytomas, but were uncommon in primary GBM. The genetic signatures permit the stratification of the glioma patients into distinct cohorts.
Owner:DUKE UNIV
Non-natural peptide IDH1 inhibitor synthesized based on UGI reaction as well as preparation method and application of non-natural peptide IDH1 inhibitor
PendingCN113845440AReduce concentrationPromote growthOrganic compound preparationCarboxylic acid amides preparationIDH1Disease
The invention discloses a non-natural peptide IDH1 inhibitor synthesized based on UGI reaction as well as a preparation method and application thereof, and belongs to the technical field of chemical biology. The non-natural peptide IDH1 inhibitor synthesized based on the UGI reaction, shown in a general formula I, or pharmaceutically acceptable salt, hydrate or prodrug of the non-natural peptide IDH1 inhibitor are provided. The invention also discloses a pharmaceutical composition containing the non-natural peptide IDH1 inhibitor synthesized based on the UGI reaction. The non-natural peptide IDH1 inhibitor synthesized based on the UGI reaction can be combined with IDH1 mutant protein, inhibit the activity of IDH1 mutant enzyme and reduce the generation of 2-HG in cells, and is used for treating tumors and / or other diseases. R1, R2, R3 and R4 in the general formula I are described in the claims and the specifications.
Owner:SHENYANG PHARMA UNIVERSITY
Primers, reagent kit and method for detecting IDH1 (isocitrate dehydrogenase 1) R132H gene variation by aid of ddPCR (droplet digital polymerase chain reaction) technologies
InactiveCN108384857AImproved prognosisStrong specificityMicrobiological testing/measurementDNA/RNA fragmentationIDH1Small fragment
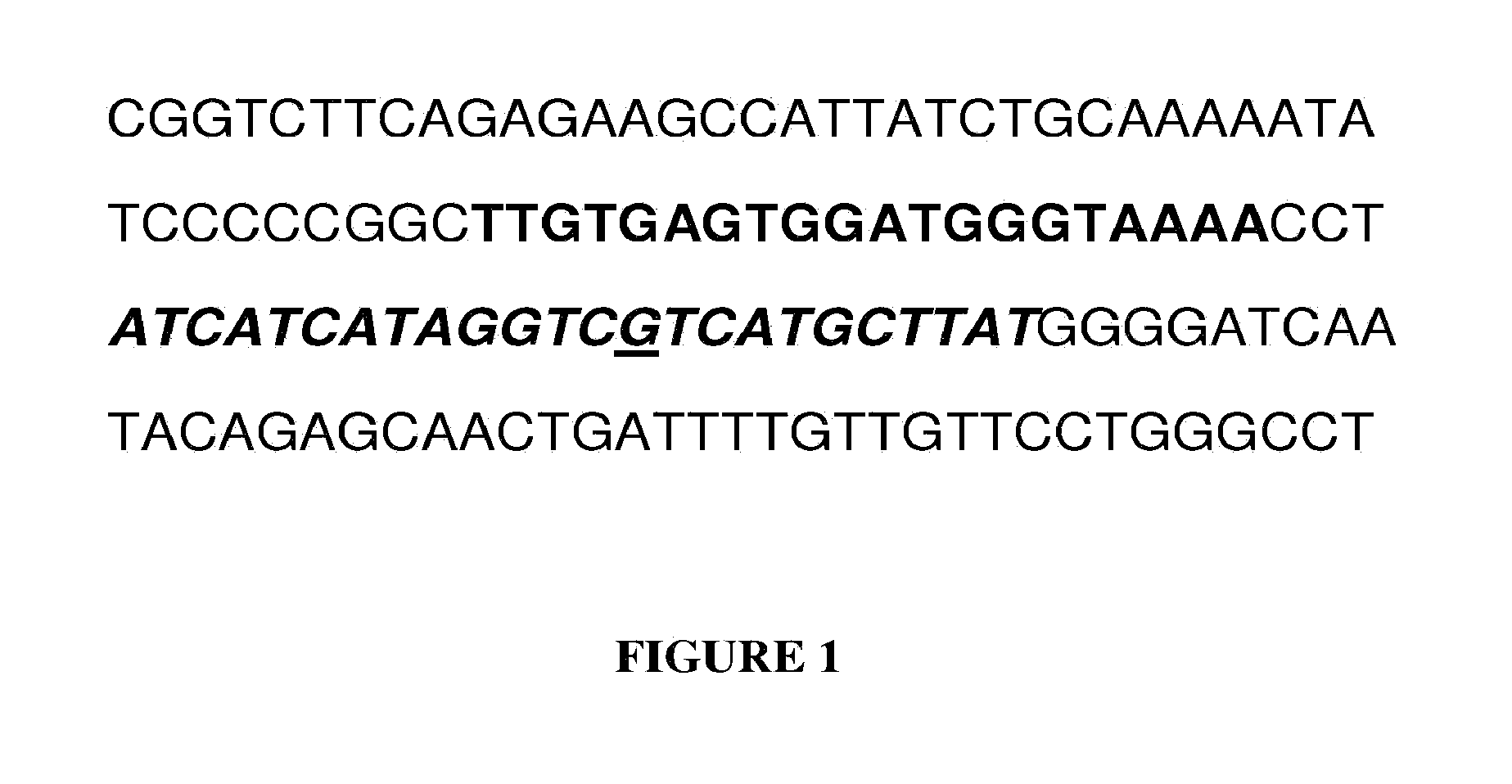
The invention discloses primers, a reagent kit and a method for detecting IDH1 (isocitrate dehydrogenase 1) R132H gene variation by the aid of ddPCR (droplet digital polymerase chain reaction) technologies. The method includes extracting cfDNA (cell-free deoxyribonucleic acid) in peripheral blood; designing two probes and a pair of primers for IDH1R132H mutant types and wild types; carrying out ddPCR amplification by the aid of the cfDNA used as a template; carrying out data analysis on obtained amplification products to obtain absolute quantities and proportions of IDH1R132H mutant genes. Thereagent kit for detecting the IDH1R132H gene variation is based on the method. The primers, the reagent kit and the method have the advantages that provided primer amplification fragments 83bp are particularly applicable to amplifying small-fragment DNA samples such as plasma free DNA and can be combined with the detection probes, accordingly, the IDH1R132H gene variation with extremely low abundance can be detected from the cfDNA, the primers, the reagent kit and the method are good in specificity and high in sensitivity, human IDH1R132H gene variation can be absolutely quantitatively detected by the aid of the primers, the reagent kit and the method, and important effects can be realized by the primers, the reagent kit and the method for guiding clinical treatment and improving patientprognosis; the IDH1R132H gene variation can be detected only by the aid of the peripheral blood without tissue specimen collection, and accordingly the method is particularly suitable for patients intolerant to tissue sampling.
Owner:PRIMBIO GENES BIOTECH WUHAN CO LTD
Microvesicle-based assays
Methods are disclosed herein for assaying a biological sample or a bodily fluid obtained from a subject by isolating, obtaining or using a microvesicle fraction from the biological sample or bodily fluid and detecting in the microvesicle fraction the presence or absence of a genetic aberration in an IDH1, TDH2, TP53, PTEN, CDKN2A, NF1, EGFR, RB1, PIK3CA, or BRAF gene. The methods may be used for aiding the diagnosis, prognosis, monitoring, or therapy selection in relation to a disease or other medical condition (e.g., a glioma) in a subject.
Owner:THE GENERAL HOSPITAL CORP
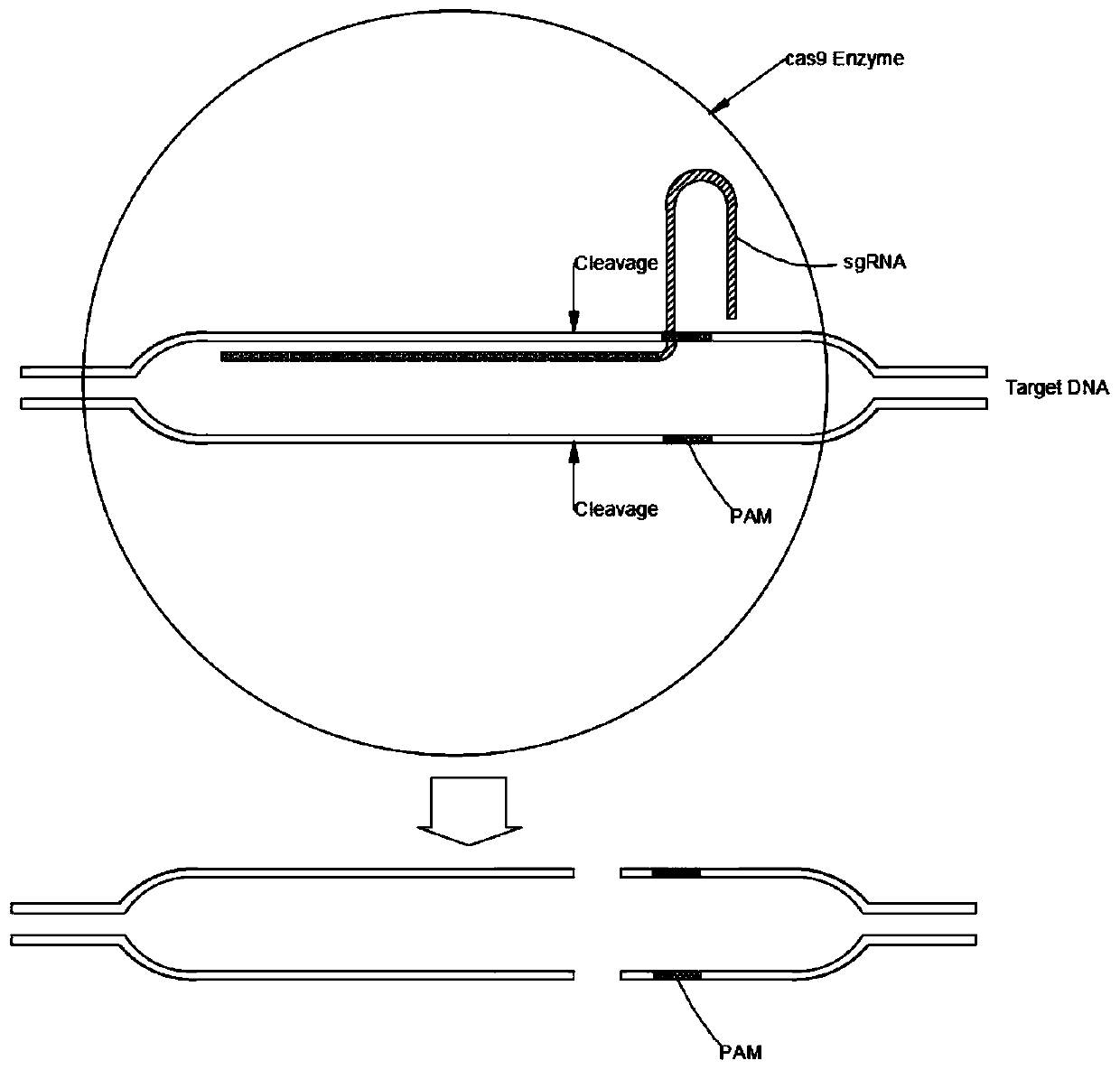
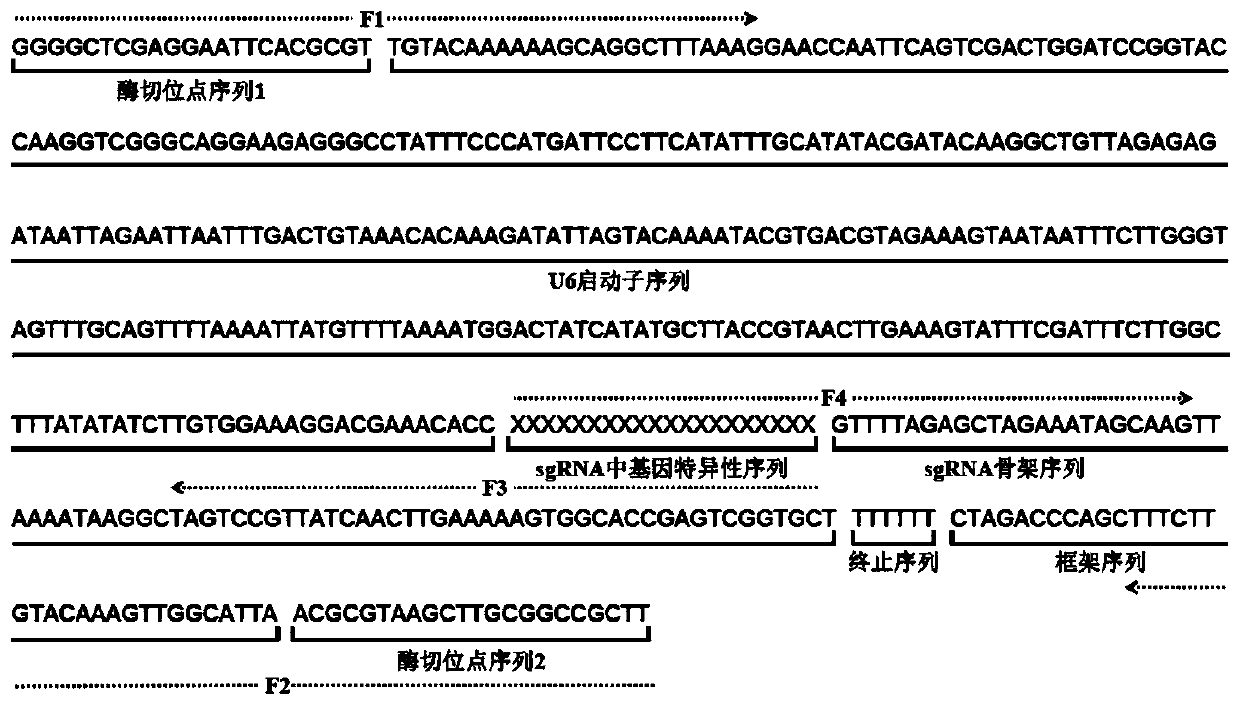
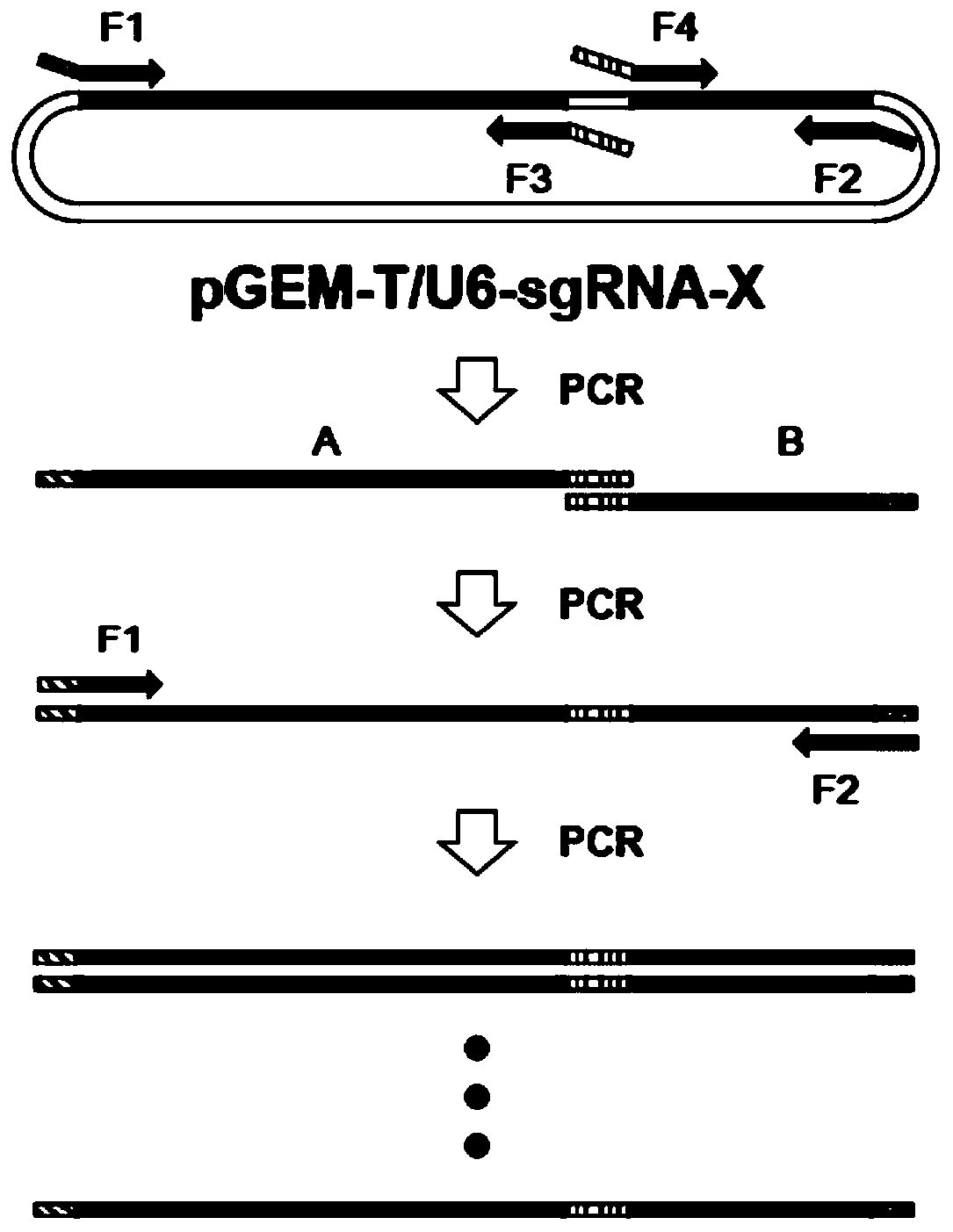
Establishing method of sgRNA expression component driven by gene editing U6 promoter
InactiveCN109777829AGuaranteed success rateEfficient expressionStable introduction of DNAVector-based foreign material introductionHuman DNA sequencingComplete sequence
The invention discloses an establishing method of a sgRNA expression component driven by a gene editing U6 promoter, and belongs to biogenetic engineering. By using an IDH1 gene of a human genome as atarget gene for CRISPR / Cas9 gene editing and designing the specific sequence of the gene in sgRNA, the complete sequence of the sgRNA expression component with the U6 promoter is obtained, the U6-sgRNA expression component is amplified, a PCR primer of the U6-sgRNA expression component is synthesized, PAGE purification is conducted, and finally the step of overlapping PCR to obtain a complete double-chain DNA fragment of the U6-sgRNA expression component is conducted for completing establishment; the whole establishing method is high in gene editing success rate, convenient to use and low incost.
Owner:ANHUI NORMAL UNIV
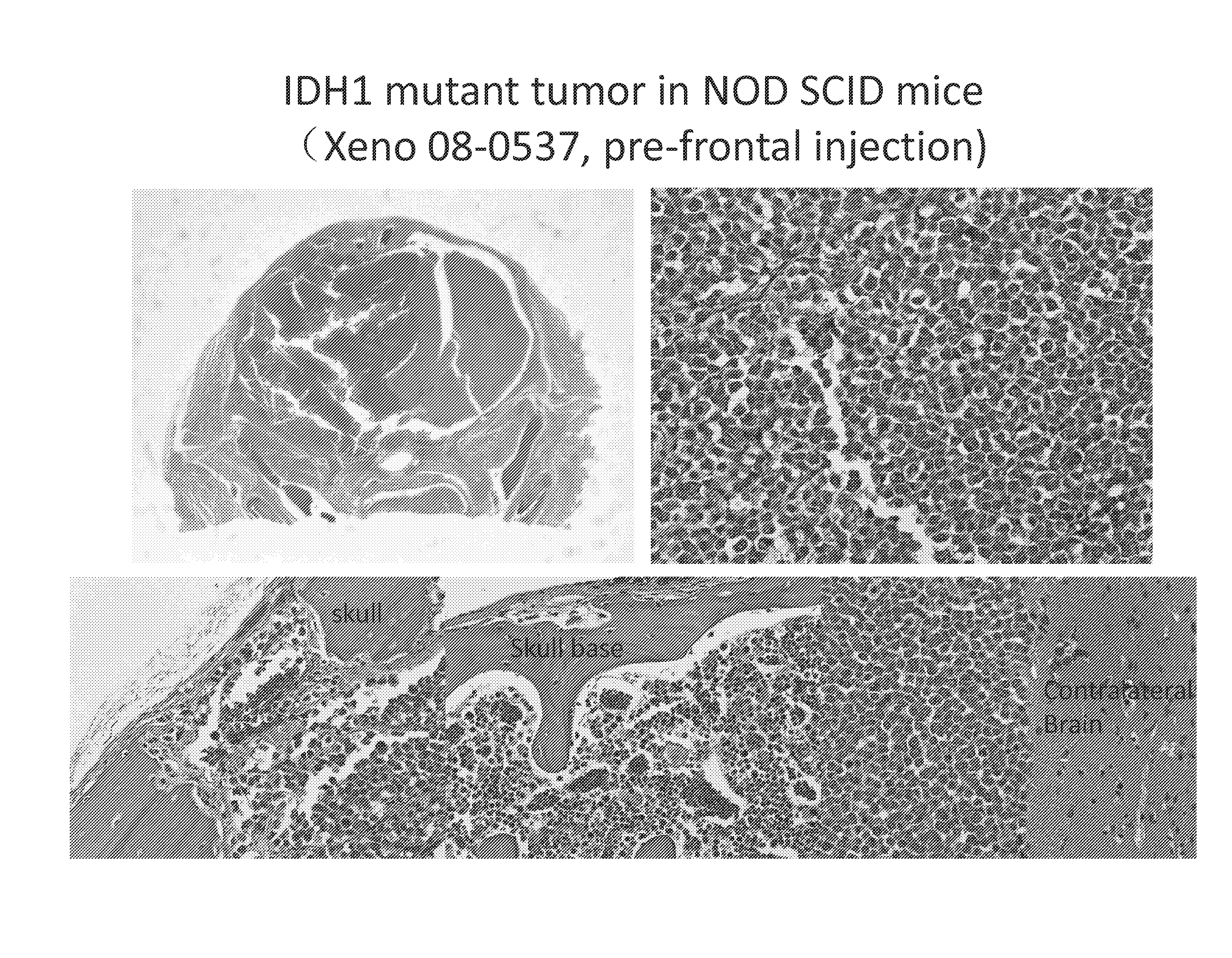
IDH1-Mutated Human Glioblastoma Cell Lines and Xenografts
InactiveUS20140356291A1Reduces cancerous morphologyWell formedCompounds screening/testingMicrobiological testing/measurementIDH1Glioblastoma cell line
IDH1-mutant cell lines and xenografts (e.g., IDH1R132H heterozygous and IDH1R132H homozygous) are derived from human glioblastoma multiforme (GBM) samples. Methods use said cells and xenografts as tools for determining the impact of IDH1R132H on cancer properties including cellular morphology, tumorigenesis, DNA, apoptosis, and metabolic profiles. Methods also use these cell lines for the screening and identification of candidate therapeutic targets.
Owner:DUKE UNIV
Isoxazole derivative as mutated isocitrate dehydrogenase 1 inhibitor
ActiveCN106795146AStrong activity inhibitionInhibitionOrganic active ingredientsOrganic chemistryIDH1Limonoate dehydrogenase
It was discovered that a compound of general formula (I) that has an isoxazole skeleton has an excellent inhibitory activity on a mutated IDH1 protein, inhibits 2-HG production by the aforesaid protein and can effectively inhibit the proliferation of various tumors expressing the aforesaid protein. In general formula (I), R1, R2, R3, Y and Z are each as defined in claim 1.
Owner:DAIICHI SANKYO CO LTD +1
Microorganism for the production of succinic acid
The invention relates to an isolated genetically modified microorganism in which the gene IDH1 and at least one of the genes SDH2 and DIC1 are under the control of a first promoter that is repressed to a growth culture medium by means of a cultivation additive and is active in the absence of the cultivation additive. The genes that are part of the group comprising “PYC1, ACS1, CIT1, ACO1, ICL1, MSL1, and CIT2, optionally also MDH3” are constitutively active. The invention further relates to uses of such a microorganism, especially for producing succinic acid.
Owner:NOVOZYMES AS
Detection system of IDH1/2 gene mutation and kit thereof
PendingCN107841541ASolve highResolving Mutation EnrichmentMicrobiological testing/measurementDNA/RNA fragmentationMutation detectionTrue positive rate
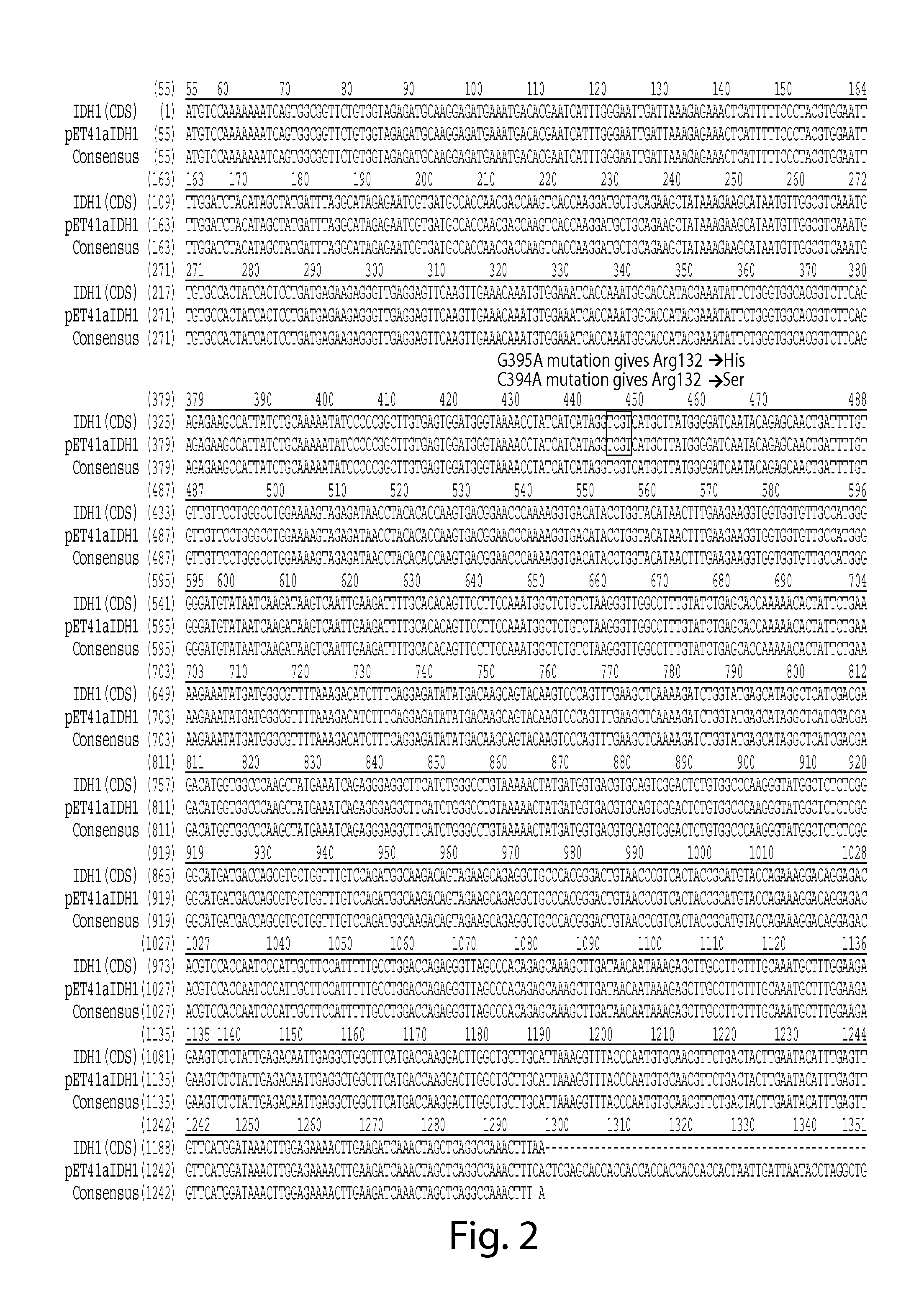
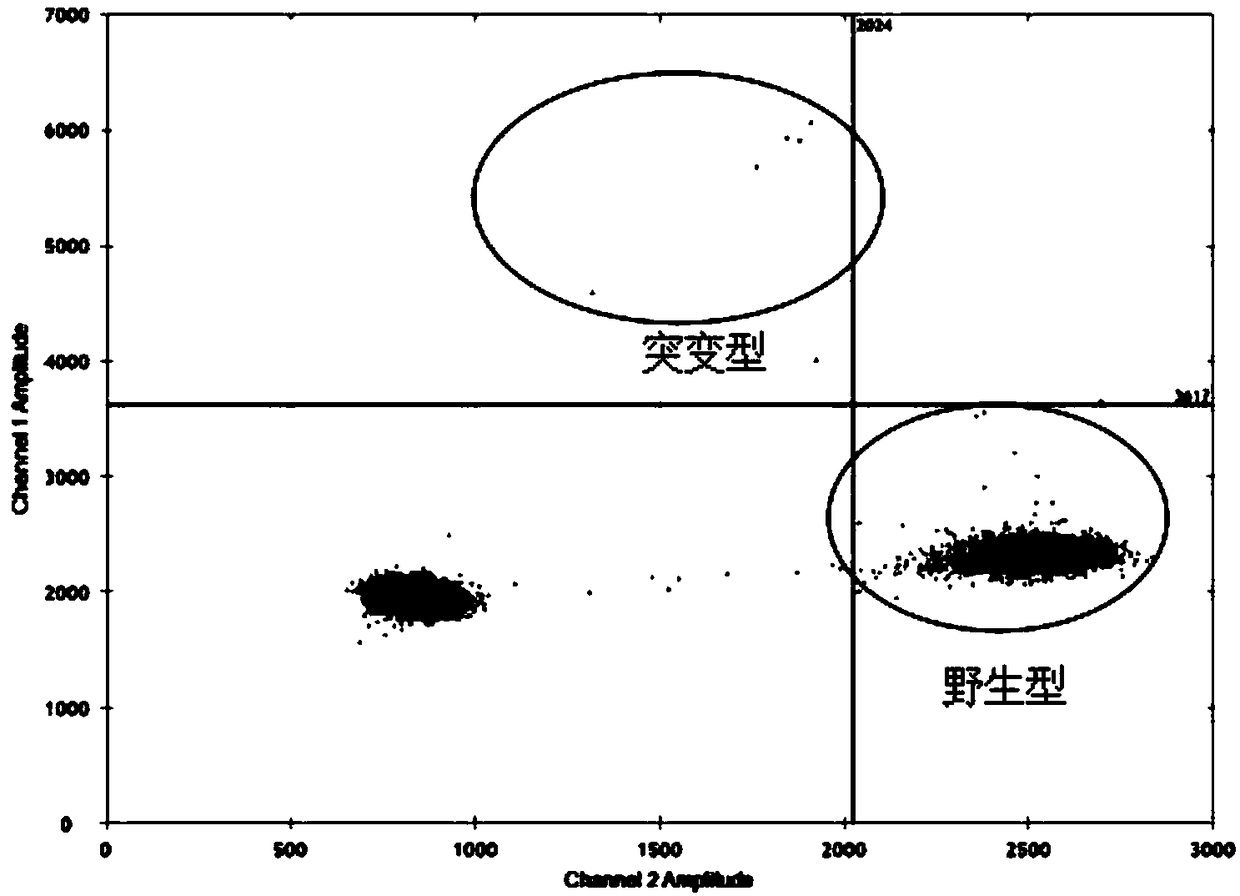
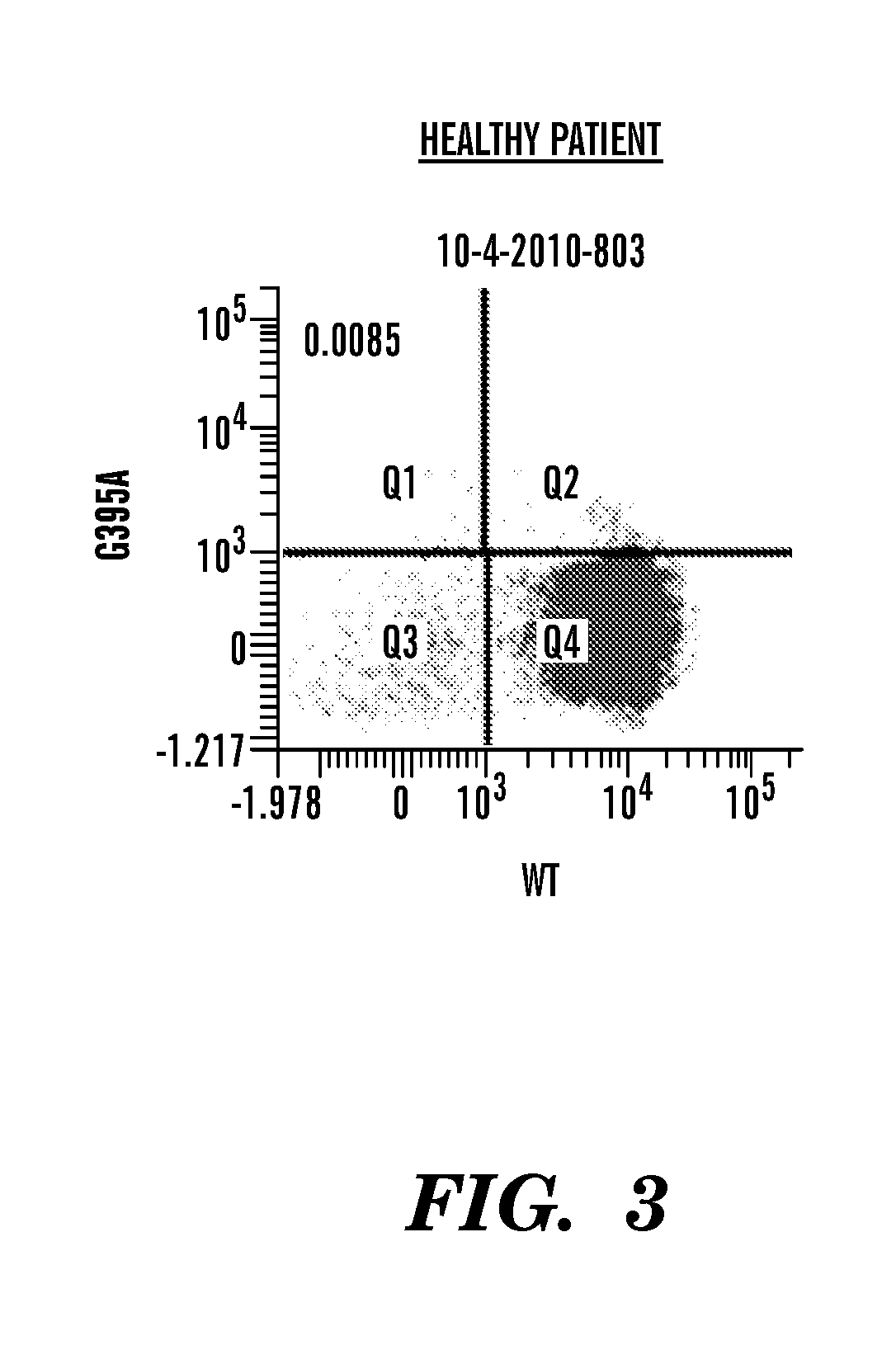
TThe invention relates to a detection system of IDH1 / 2 gene mutation and a kit thereof. The kit comprises PCR detection liquid and SYBR GreenI mixed liquor; the PCR detection liquid A comprises a forward primer A and a reverse primer A and peptide nucleic acid A which are used for detecting a 394th codon C394T site and a 395th codon G395A site of IDH1 / 2 gene, the PCR detection liquid B comprises aforward primer B and a reverse primer B and peptide nucleic acid B which are used for detecting a 419th codon G419A site of IDH1 / 2 gene, and the PCR detection liquid C comprises a forward primer C and a reverse primer C and peptide nucleic acid C which are used for detecting a 515th codon G515A site of IDH1 / 2 gene. The IDH 1 / 2 gene mutation detection system and the kit thereof have high sensitivity and specificity, sample demand is low, and the system and the kit can be used for rapidly, conveniently and accurately carrying out mutation detection of IDH1 / 2 gene.
Owner:上海赛安生物医药科技股份有限公司
Isoxazole derivatives as mutant isocitrate dehydrogenase 1 inhibitors
ActiveCN106795146BInhibitionStrong activity inhibitionOrganic active ingredientsOrganic chemistryIDH1Isocitrate Dehydrogenase (NAD+)
Owner:DAIICHI SANKYO CO LTD +1
Homozygous and heterozygous IDH1 gene-defective cell lines derived from human colorectal cells
IDH1 gene-defective cell lines (e.g., IDH1R132H heterozygous) have been made from a robust cell line, HCT116. The IDH1 gene-defective cell lines can be used to determine the effect of IDH1R132H on cell biology, tumorigenesis, and cellular metabolic profiles. These cell lines can be used to test potential therapeutic targets and to screen potential therapeutic agents. Kits and xenografts are also contemplated.
Owner:DUKE UNIV
Combined detection of peripheral blood methylation gene and idh1 in the diagnosis of lung cancer model
ActiveCN111172279BImprove diagnostic efficiencyWide coverageMicrobiological testing/measurementBiological material analysisIDH1Blood markers
Owner:CANCER INST & HOSPITAL CHINESE ACADEMY OF MEDICAL SCI
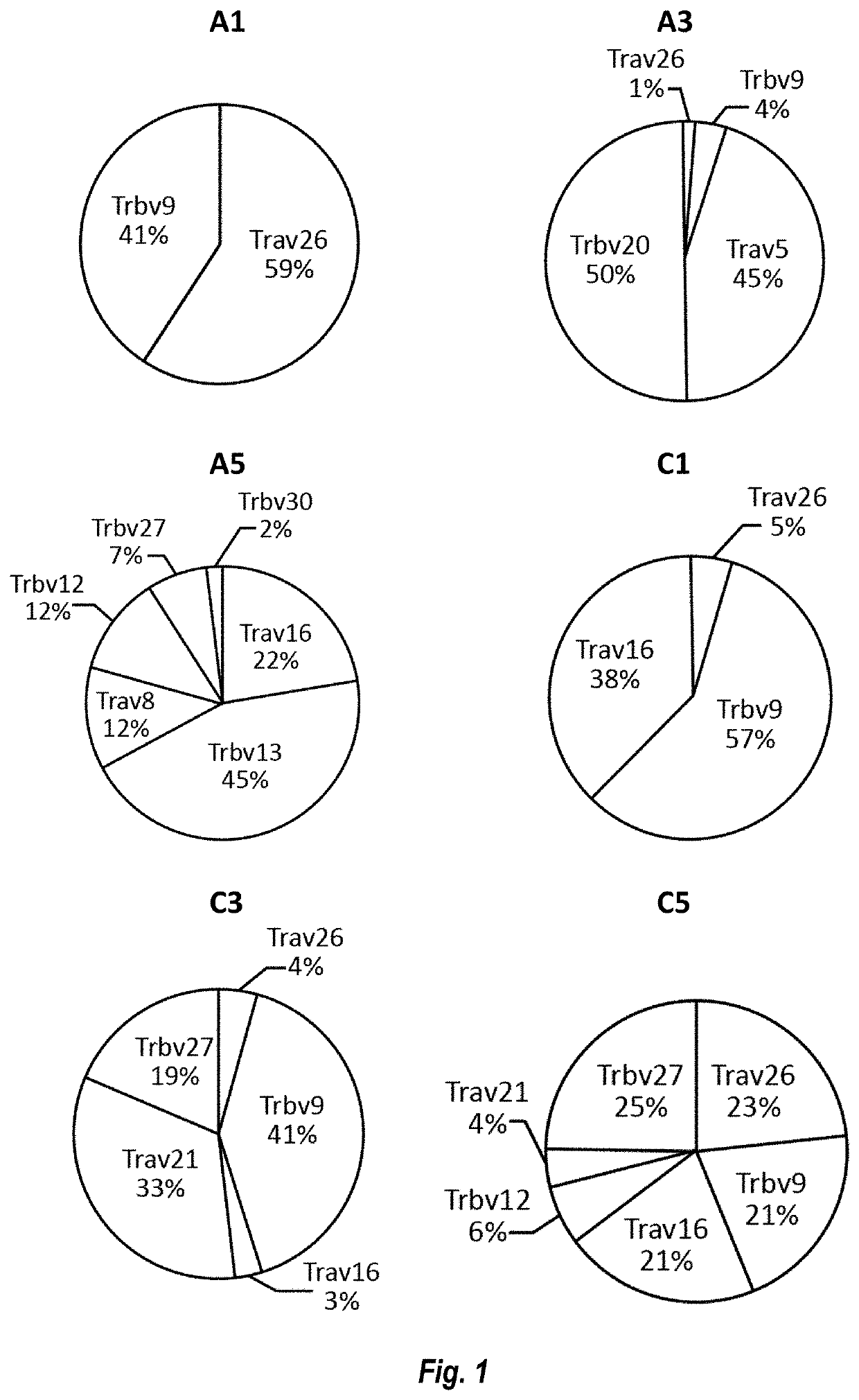
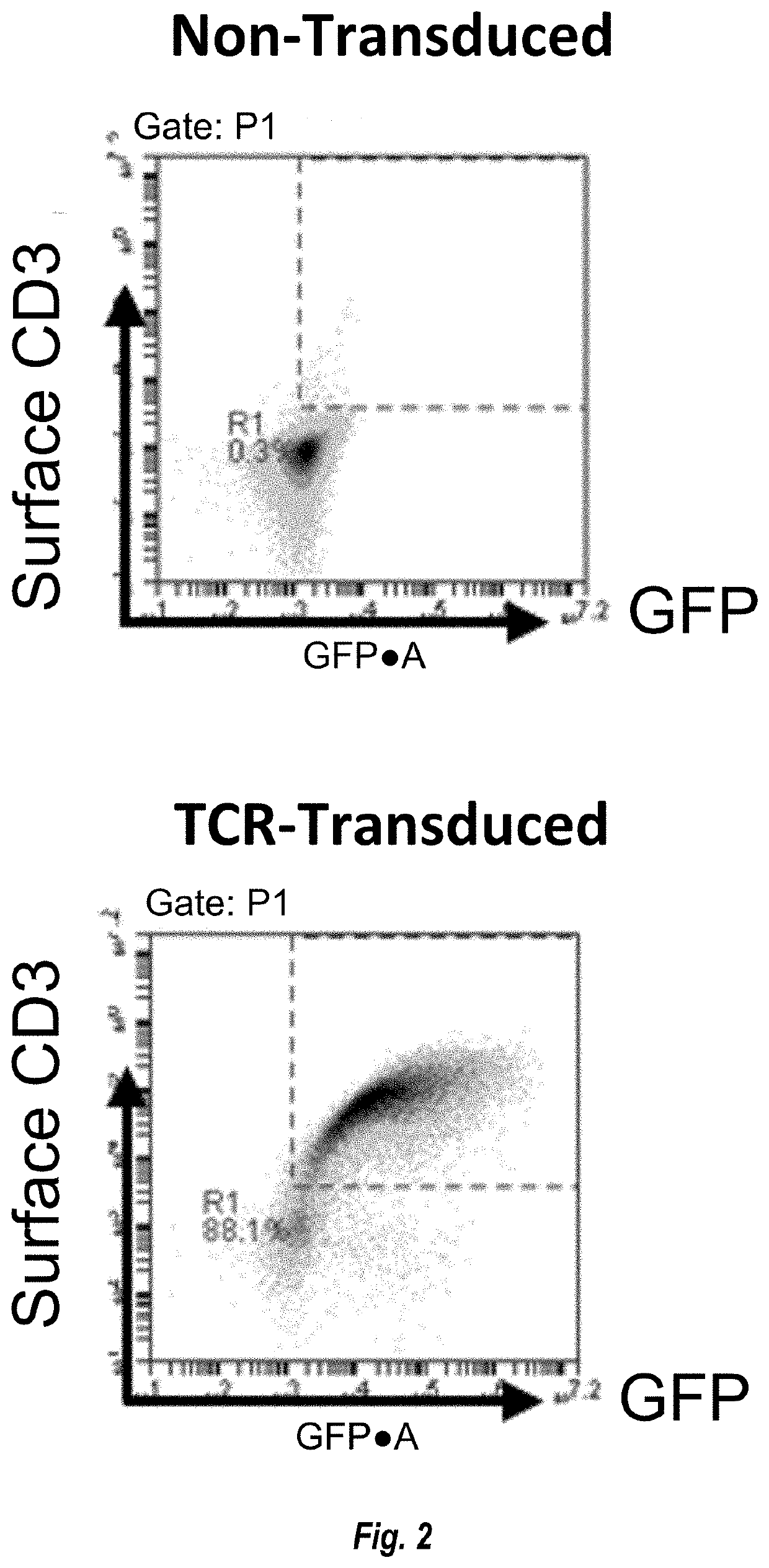
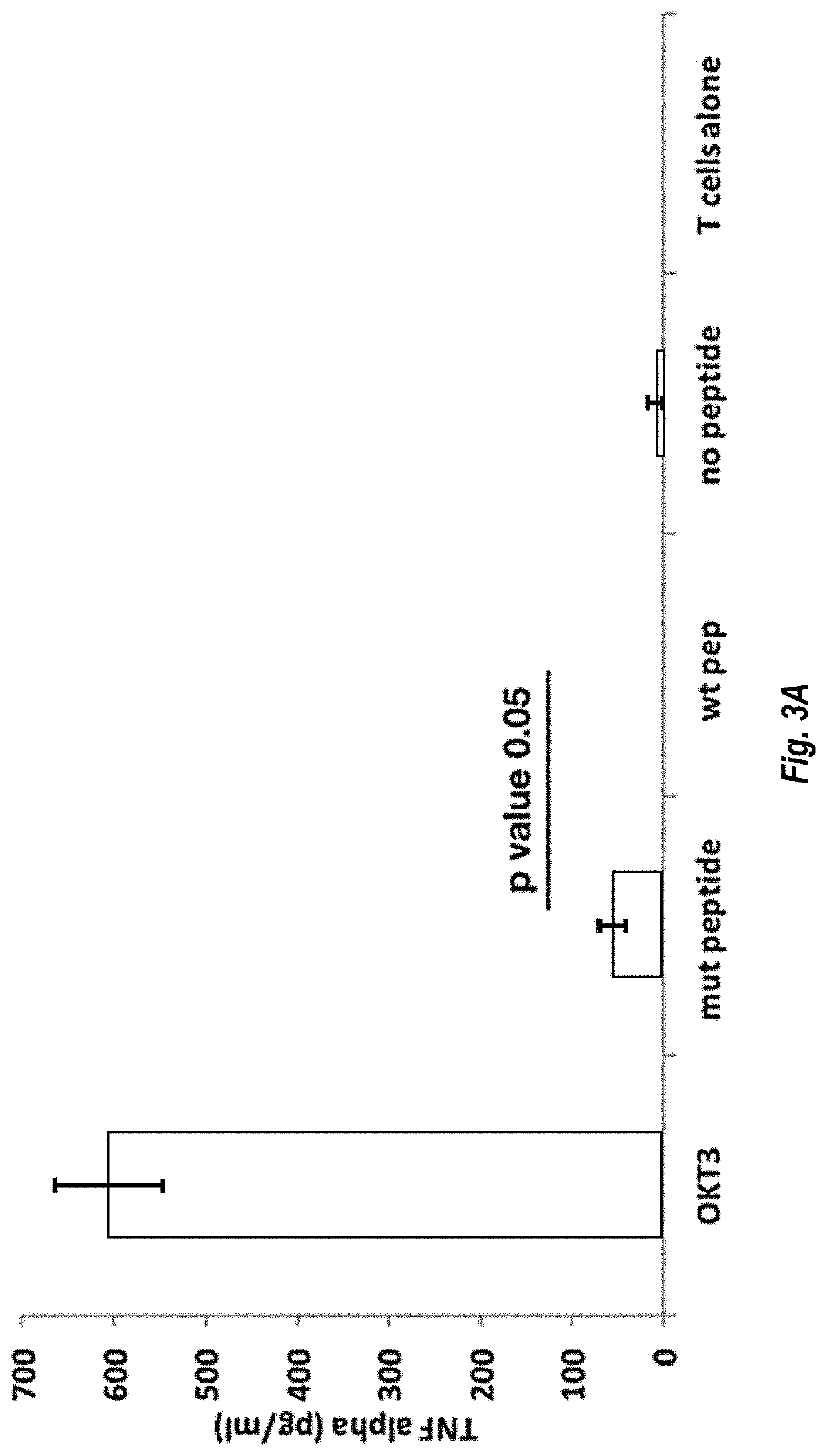
Mutant IDH1 Specific T Cell Receptor
PendingUS20210087252A1Limit scopeImmunoglobulin superfamilyPeptide/protein ingredientsIDH1Biochemistry
Owner:BERKELEY LIGHTS +1
Homozygous and heterozygous IDH1 gene-defective human astrocytoma cell lines
We provide IDH1 gene-defective cell lines (e.g., IDH1R132H heterozygous and IDH1R132H homozygous) derived from dissociated human astrocytoma samples. The cells can be used alone or in combination with each other or other cell types as a tool for determining the impact of IDH1R132H on cellular biology, tumorigenesis, and metabolic profiles. The cell lines may be used to test and identify therapeutic targets and to screen for molecular therapeutic agents.
Owner:DUKE UNIV
Probe method for detecting human IDH1 gene R132H mutation and kit thereof
PendingCN113913520AThe detection process is fastImprove efficiencyMicrobiological testing/measurementDNA/RNA fragmentationHuman DNA sequencingForward primer
The invention discloses a probe method for detecting human IDH1 gene R132H mutation and a kit thereof, and relates to the technical field of biological medical treatment. The probe method for detecting the human IDH1 gene R132H mutation comprises the following steps of: (1) adding human genome DNA into an IDH1R132HPCR reaction mixed solution to form an R132H reaction system, and performing fluorescent quantitative PCR amplification; (2) result judgment: performing analysis according to a mutation Ct value of PCR amplification, wherein the IDH1R132H reaction mixed solution comprises an IDH1R132H forward primer with a sequence shown in SEQ ID No. 3 in a sequence table, an IDH1R132H reverse primer with a sequence shown in SEQ ID No. 4 in the sequence table and a PCR premix solution containing a fluorescent dye. The probe method and the kit have the beneficial effects that the probe method and the kit provide high-sensitivity detection of the IDH1 gene R132H mutation for clinical application, and the IDH1 gene R132H mutation detection is high in speed, high in efficiency and high in specificity.
Owner:武汉承启医学科技有限公司
Homozygous and heterozygous IDH1 gene-defective human astrocytoma cell lines
We provide IDH1 gene-defective cell lines (e.g., IDH1R132H heterozygous and IDH1R132H homozygous) derived from dissociated human astrocytoma samples. The cells can be used alone or in combination with each other or other cell types as a tool for determining the impact of IDH1R132H on cellular biology, tumorigenesis, and metabolic profiles. The cell lines may be used to test and identify therapeutic targets and to screen for molecular therapeutic agents.
Owner:DUKE UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com