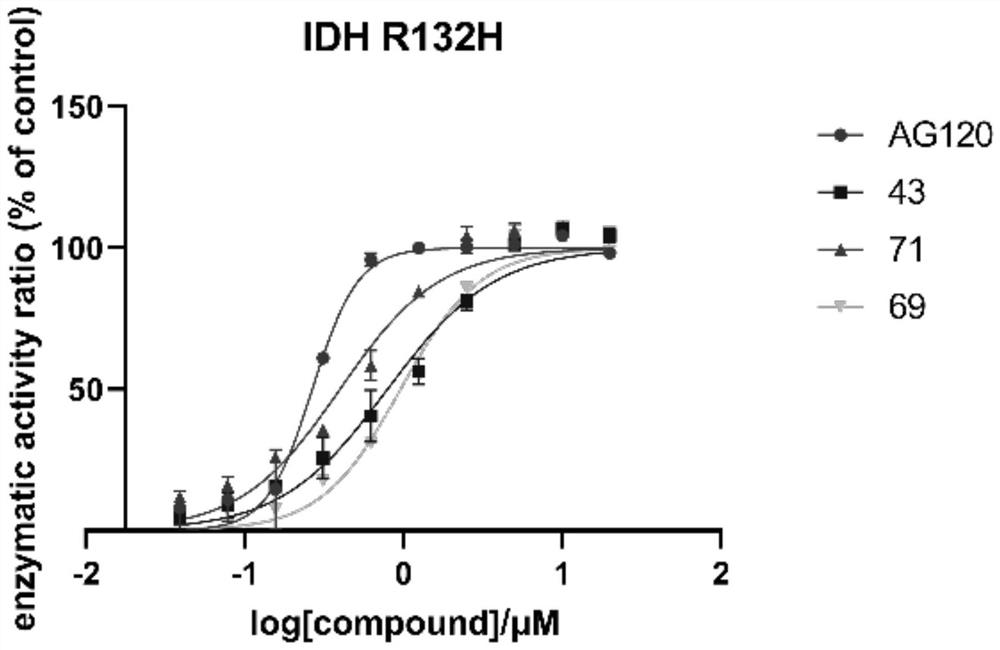
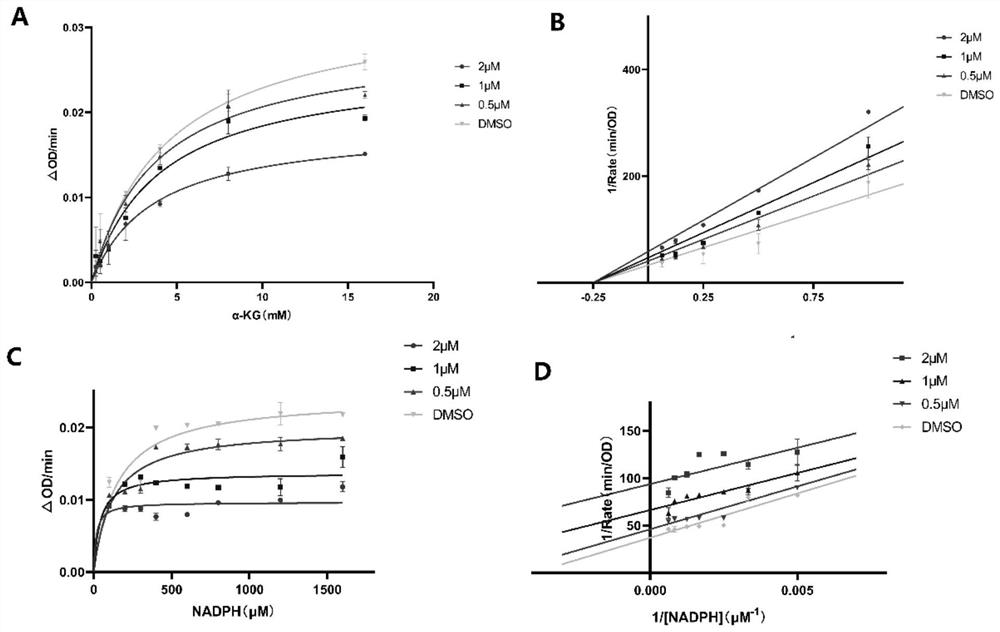
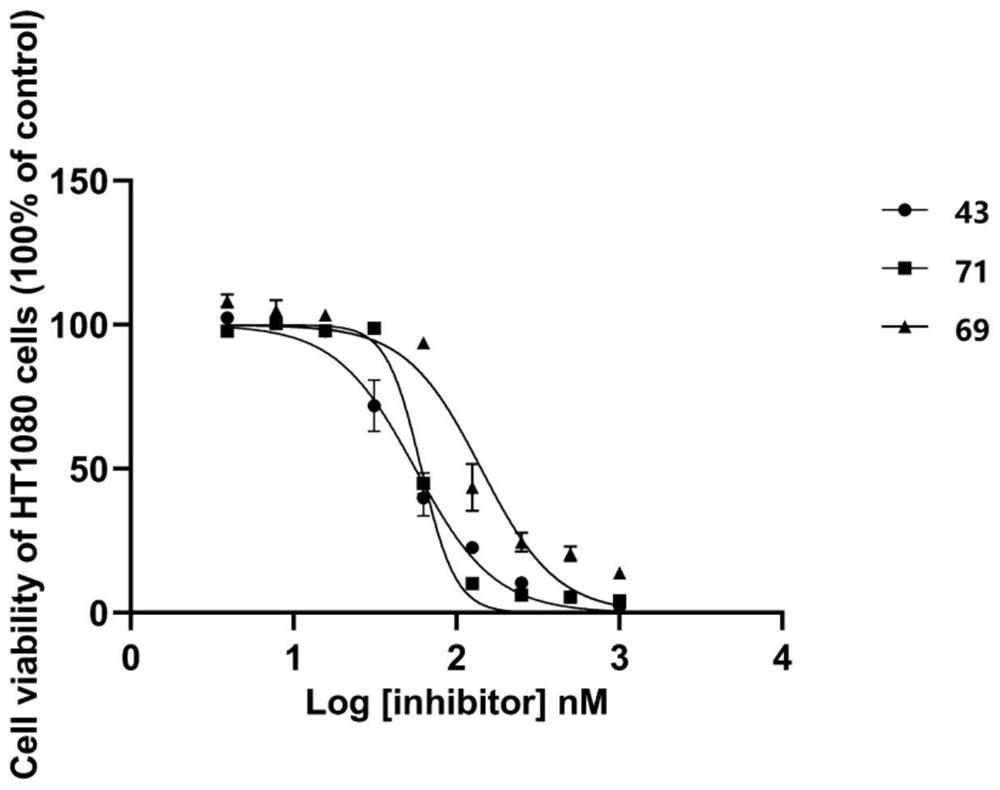
Non-natural peptide IDH1 inhibitor synthesized based on UGI reaction as well as preparation method and application of non-natural peptide IDH1 inhibitor
An IDH1, non-natural technology, applied in the field of chemical biology, can solve the problems of cumbersome preparation methods, high cost, raw materials that cannot be directly purchased from the market, etc., and achieve the effect of improving cytotoxicity and good inhibitory effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-4
[0033] Synthesis of Example 1-4 Linear Unnatural Peptide Analog 1-4
[0034] Embodiment 1 reaction formula:
[0035] Dissolve 2-methylbenzaldehyde (60mg, 0.5mmol) in 2mL of methanol, under stirring conditions, add 1.0 equivalent of 3-fluoroaniline successively and stir at room temperature for 0.5 hours, then add 1.0 equivalent of formic acid and cyclohexyl isocyanide, Continue to stir at room temperature for 2-10 hours, the product precipitates, and is filtered and washed to obtain the pure white solid linear unnatural peptide analog 1 with a yield of 39%. The structure of 1 was confirmed to be correct by NMR detection. HRMS(pos.ESI):m / z[M+Na] + for C 22 h 25 FN 2 NaO 2 calcd: 391.1792, found: 391.1785.
[0036] Embodiment 2 reaction formula:
[0037] Same as Example 1, except that acetic acid was used instead of formic acid to obtain linear non-natural peptide analog 2 with a yield of 49%. Through NMR detection, it was confirmed that the structure of 2 was correc...
Embodiment 3
[0038] Embodiment 3 reaction formula
[0039] Same as Example 1, except that propionic acid was used instead of formic acid to obtain linear non-natural peptide analog 3 with a yield of 22%. Through NMR detection, it was confirmed that the structure of 3 was correct. HRMS(pos.ESI):m / z[M+Na] + for C 24 h 29 FN 2 NaO 2 calcd: 419.2105, found: 419.2108.
[0040] Embodiment 4 reaction formula:
[0041] Same as Example 1, except that butyric acid was used instead of formic acid to obtain linear non-natural peptide analog 4 with a yield of 50%. Through NMR detection, it was confirmed that the structure of 4 was correct. HRMS(pos.ESI):m / z[M+Na] + for C 25 h 31 FN 2 NaO 2 calcd: 433.2262, found: 433.2262.
Embodiment 5
[0042] Synthesis of Example 5 Linear Unnatural Peptide Analog 5
[0043]
[0044] Same as Example 1, except that phenylpropionic acid was used instead of formic acid to obtain linear non-natural peptide analog 5 with a yield of 64%. Through NMR detection, it was confirmed that the structure of 5 was correct. HRMS(pos.ESI):m / z[M+Na] + for C 30 h 33 FN 2 NaO 2 calcd: 495.2418, found: 495.2410.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com