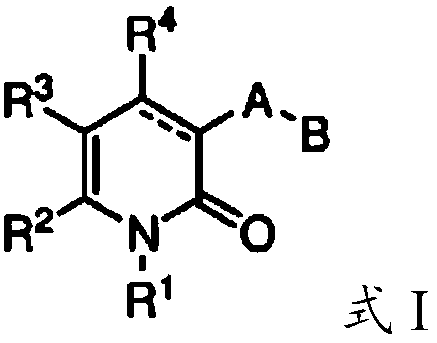
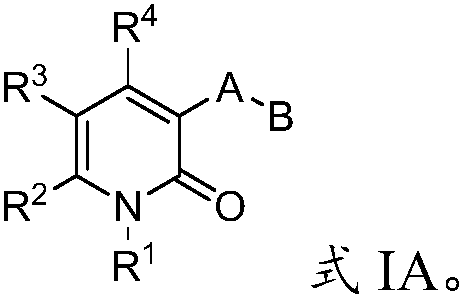
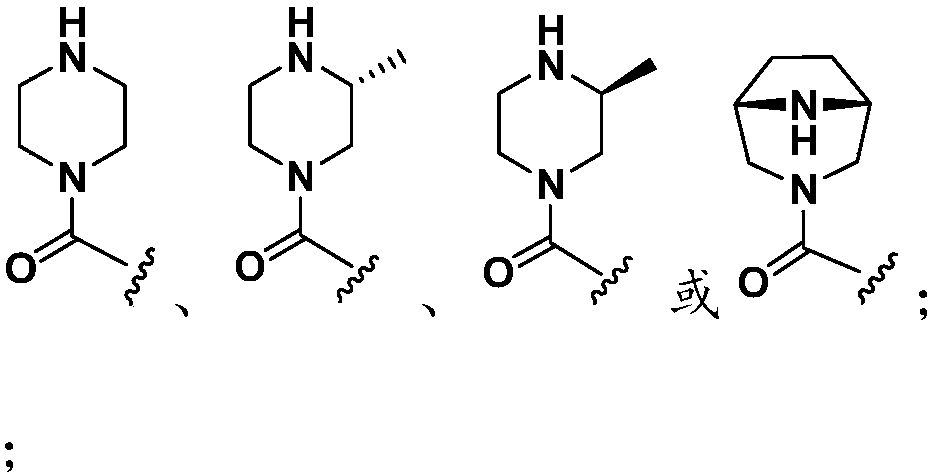
Mutant IDH1 inhibitors useful for treating cancer
A C1-C6, alkyl technology, applied in the field of mutant IDH1 inhibitors that can be used in the treatment of cancer, can solve problems such as increased risk of brain tumors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0209] abbreviation
[0210] AcOH acetic acid
[0211] BOC tert-butoxycarbonyl
[0212] BSA bovine serum albumin
[0213] CBZ benzyloxycarbonyl
[0214] DCM dichloromethane
[0215] DIPEA Diisopropylethylamine
[0216] DMAP 4-(N,N-Dimethylamino)pyridine
[0217] DMF Dimethylformamide
[0218] DMF-DMA dimethylformamide dimethyl acetal
[0219] DMSO Dimethyl Sulfoxide
[0220] EtOAc ethyl acetate
[0221] LCMS liquid chromatography / mass spectrometry
[0222] LiHMDS lithium bis-(trimethylsilyl)amide
[0223] MP SPE Large Pore Solid Phase Extraction
[0224] NADPH reduced nicotinamide adenine dinucleotide phosphate
[0225] NaHMDS sodium bis-(trimethylsilyl)amide
[0226] NBS N-Bromosuccinimide
[0227] NCS N-chlorosuccinimide
[0228] NMR nuclear magnetic resonance
[0229] PEG polyethylene glycol
[0230] RPMI Roseville Parker Memorial Institute Medium (Cell Culture Medium)
[0231] p-TsOH p-toluenesulfonic acid
[0232] THF Tetrahydrofuran
[0233] TFA trifl...
Embodiment 1
[0237] Example 1. Synthesis of selected compounds
[0238]
[0239] Method 1 - Nitrile 1:
[0240] To a solution of 2-bromo-1-(4-chlorophenyl)ethanone (2.33 g, 10 mmol) in ethanol (25 mL) was added 2-cyanothioacetamide (1 g, 10 mmol). The reaction mixture was heated at reflux for 15.5 h. The reaction mixture was cooled to 0 °C. A precipitate formed and was removed by washing with filtration using hexane and then drying under vacuum. The product, 2-(4-(4-chlorophenyl)thiazol-2-yl)acetonitrile (nitrile N1) was a brown powder; LCMS: m / z (M+H) + = 235.0; 1 H NMR (400MHz, CDCl 3 )δ7.88–7.77(m,2H), 7.48(s,1H), 7.44–7.35(m,2H), 4.17(s,2H).
[0241]
[0242] Nitrile 2: synthesized by method 1, substituting 2-bromo-1-phenylethanone as starting material. After reaction, the mixture was concentrated and purified by silica gel chromatography (0% to 30% EtOAc / Hexane). The product was an orange-red solid (1.53 g, 77%); LCMS: m / z (M+H) + = 201.1.
[0243]
[0244] Nitrile ...
Embodiment 2
[0552] Example 2. Enzyme Assay
[0553] Assays were performed in 1536-well black solid bottom plates in a final assay volume of 9 μL. Depletion of the cofactor NADPH is coupled to the second enzyme diaphorase and its corresponding substrate resazurin by mutating the IDH1 enzyme.
[0554] Specifically, for IDH1R132H, 3 μL of enzyme (4 mM β-ME, 0.0005 mg / mL IDH1R132H, 150 mM NaCl, 20 mM Tris pH 7.5, 10 mM MgCl 2 , 0.05% BSA) were added to the plate, followed by the addition of 23 nL of test compound in DMSO. Cover the plate and incubate at room temperature for 30 minutes, at which time 3 μL of substrate (0.016 mM NADPH, 2 mM α-KG, 150 mM NaCl, 20 mM Tris pH 7.5, 10 mM MgCl 2 , 0.05% BSA). The reaction was incubated at room temperature for 60 minutes at which time the assay mix (0.06 mg / mL diaphorase, 0.036 mM resazurin, 150 mM NaCl, 20 mM Tris pH 7.5, 10 mM MgCl 2 , 0.05% BSA). After a 5 min incubation, fluorescence generated by conversion of resazurin to resorufin (excitatio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com