Patents
Literature
37 results about "Tert promoter" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
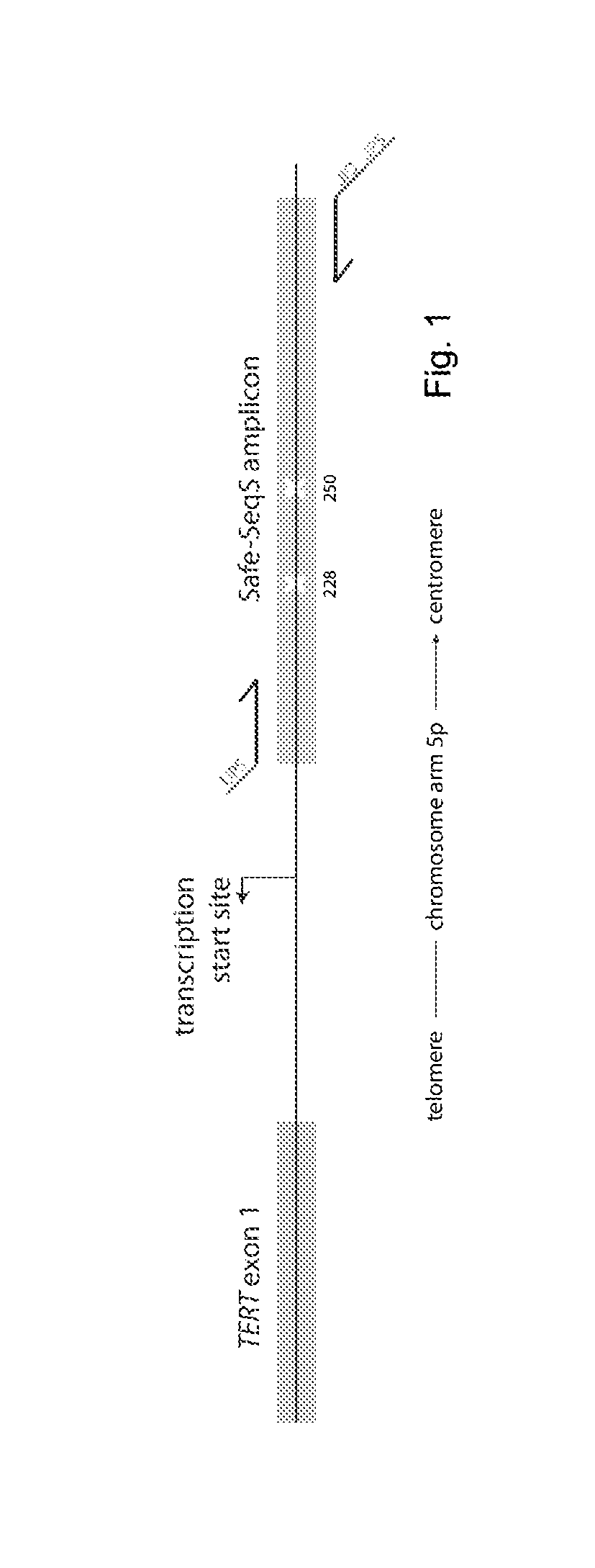
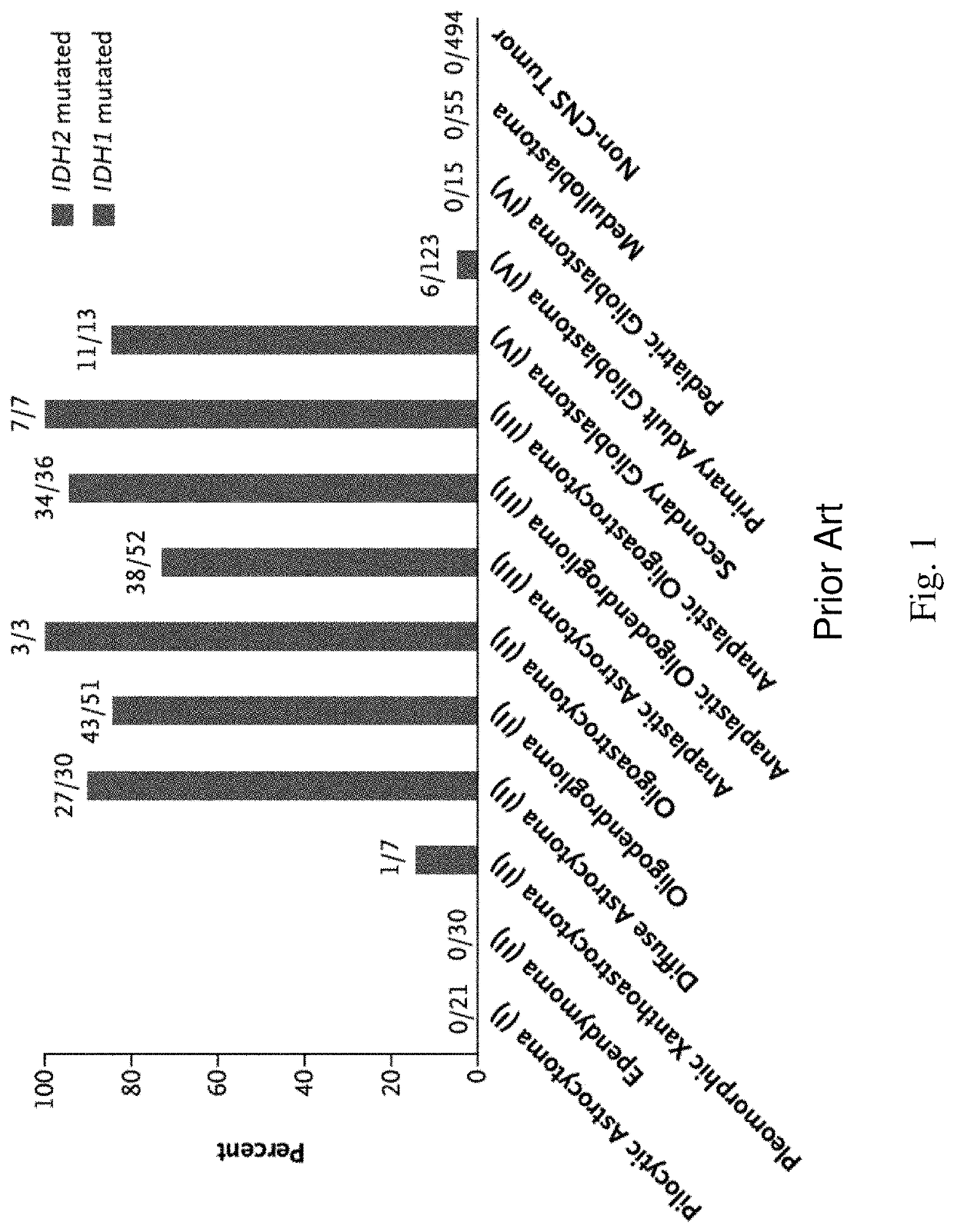
TERT Promoter Mutations in Human Cancers. Reactivation of telomerase reverse transcriptase (TERT) is a fundamental step in the initiation of human cancer. Recently, we have identified the mechanism by which mutations in the TERT promoter lead to telomerase activation.
Selective antibody targeting of undifferentiated stem cells
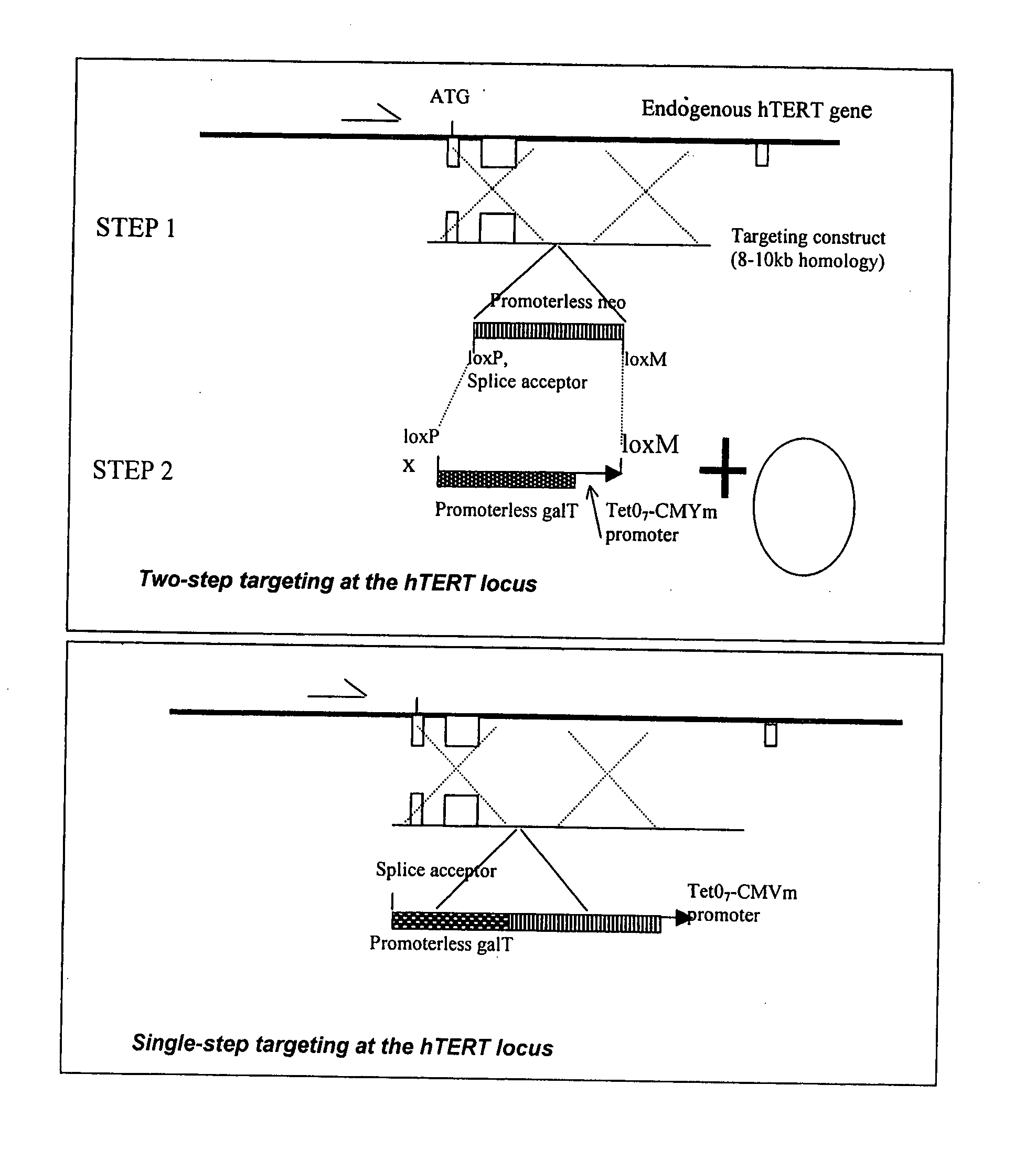
This invention provides a system for producing differentiated cells from a stem cell population for use wherever a relatively homogenous cell population is desirable. The cells contain an effector gene under control of a transcriptional control element (such as the TERT promoter) that causes the gene to be expressed in relatively undifferentiated cells in the population. Expression of the effector gene results in expression of a cell-surface antigen that can be used to deplete the undifferentiated cells. Model effector sequences encode glycosyl transferases that synthesize carbohydrate xenoantigen or alloantigen, which can be used for immunoseparation or as a target for complement-mediated lysis. The differentiated cell populations produced are suitable for use in tissue regeneration and non-therapeutic applications such as drug screening.
Owner:ASTERIAS BIOTHERAPEUTICS INC +1
Differentiated cells suitable for human therapy
InactiveUS20130273651A1Peptide/protein ingredientsMammal material medical ingredientsCell Surface AntigensApoptosis
This invention provides a system for producing differentiated cells from a stem cell population for use wherever a relatively homogenous cell population is desirable. The cells contain an effector gene under control of a transcriptional control element (such as the TERT promoter) that causes the gene to be expressed in relatively undifferentiated cells in the population. Expression of the effector gene results in depletion of undifferentiated cells, or expression of a marker that can be used to remove them later. Suitable effector sequences encode a toxin, a protein that induces apoptosis; a cell-surface antigen, or an enzyme (such as thymidine kinase) that converts a prodrug into a substance that is lethal to the cell. The differentiated cell populations produced according to this disclosure are suitable for use in tissue regeneration, and non-therapeutic applications such as drug screening.
Owner:ASTERIAS BIOTHERAPEUTICS INC
Methods for rapid and sensitive detection of hotspot mutations
Methods that rapidly, sensitively, and specifically detect mutations in IDH1 / 2 and the TERT promoter employ amplification of particular portions of the genes that experience frequent and exquisitely localized mutations. The ability to distinguish between sequences that differ only by one nucleotide and which may be present in very low ratios is essential for such an assay.
Owner:DUKE UNIV
Tert promoter mutations in cancer
ActiveUS20160040250A1Bacterial antigen ingredientsIn-vivo radioactive preparationsTreatment modalityTert promoter
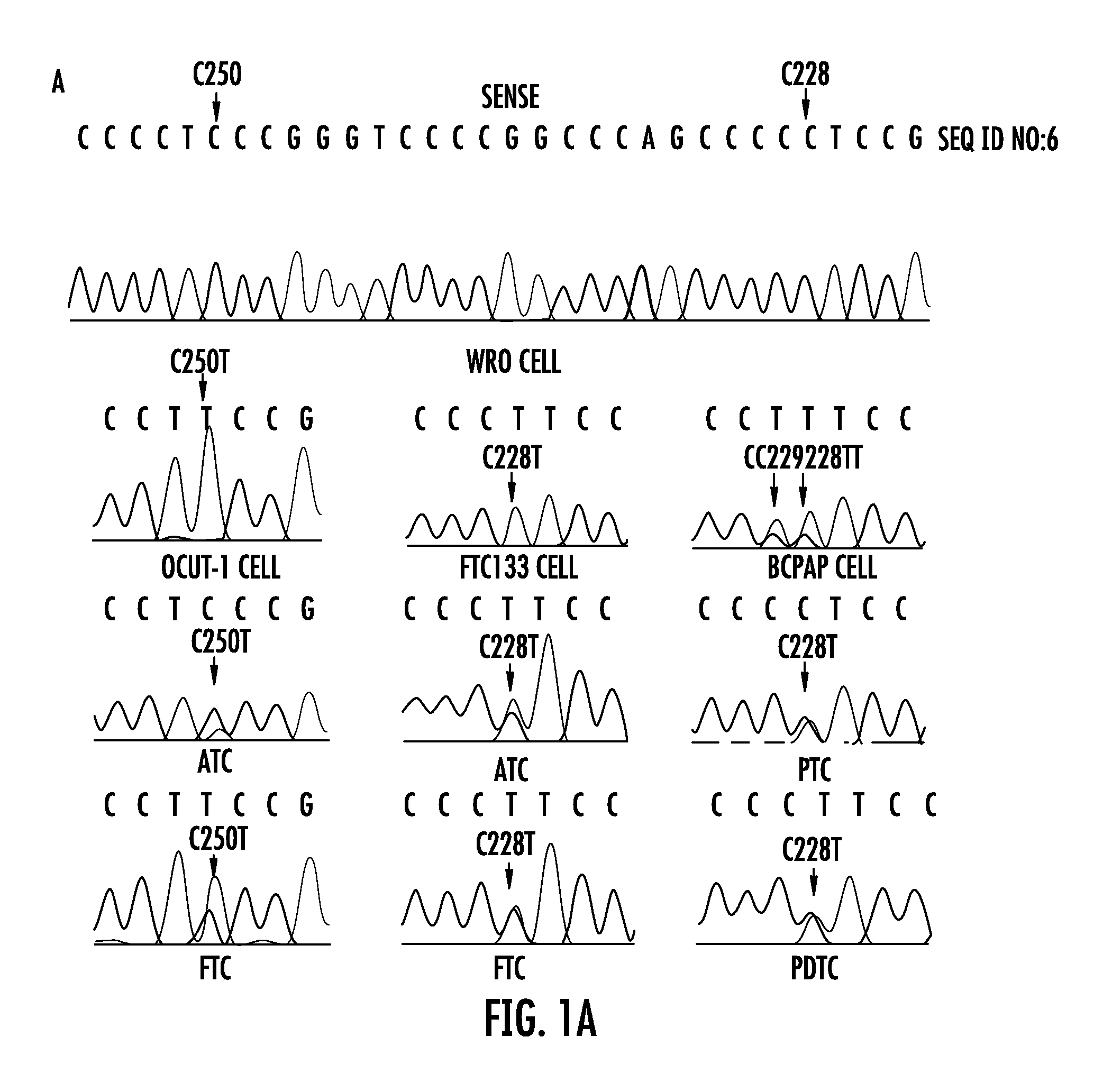
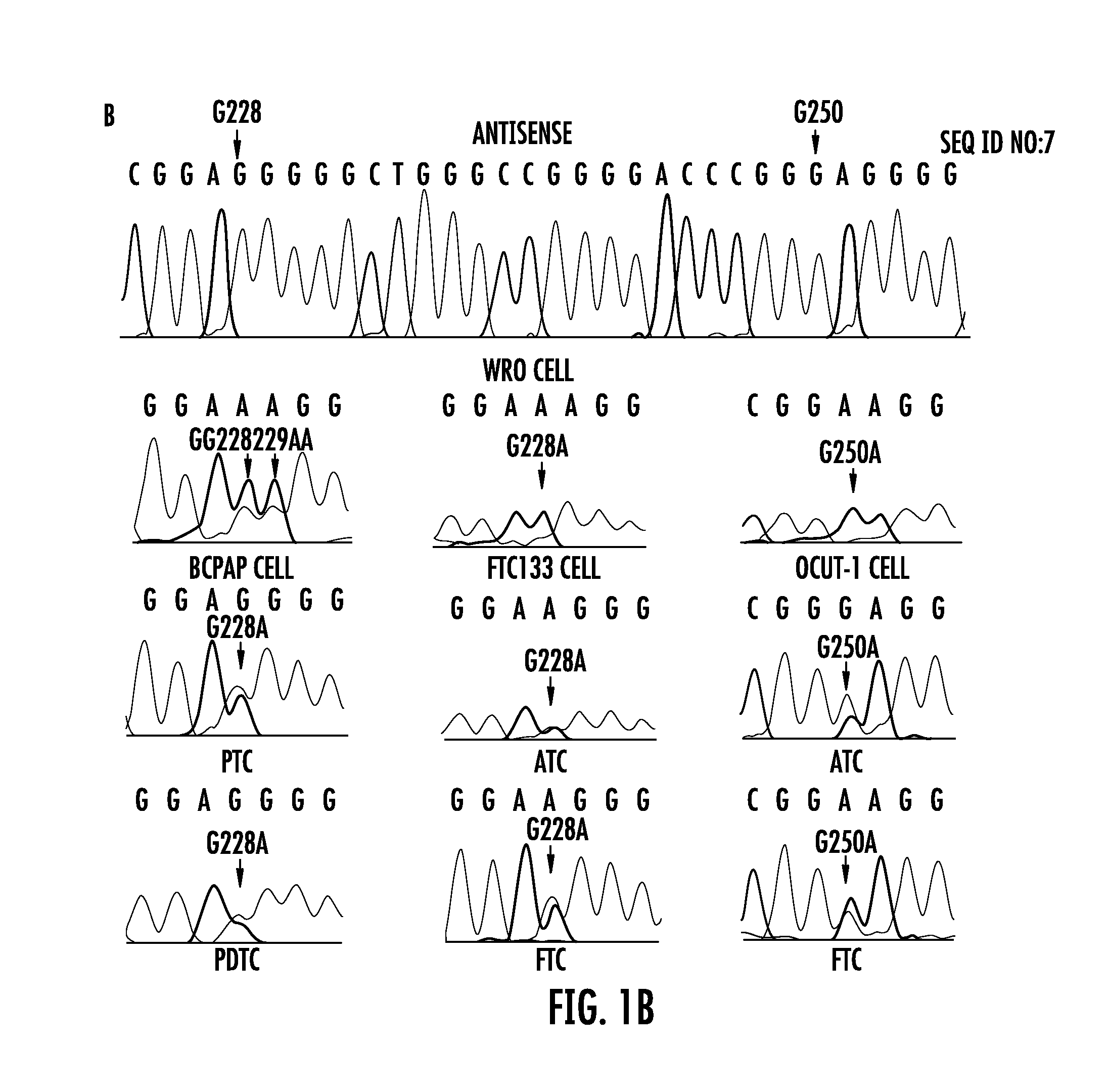
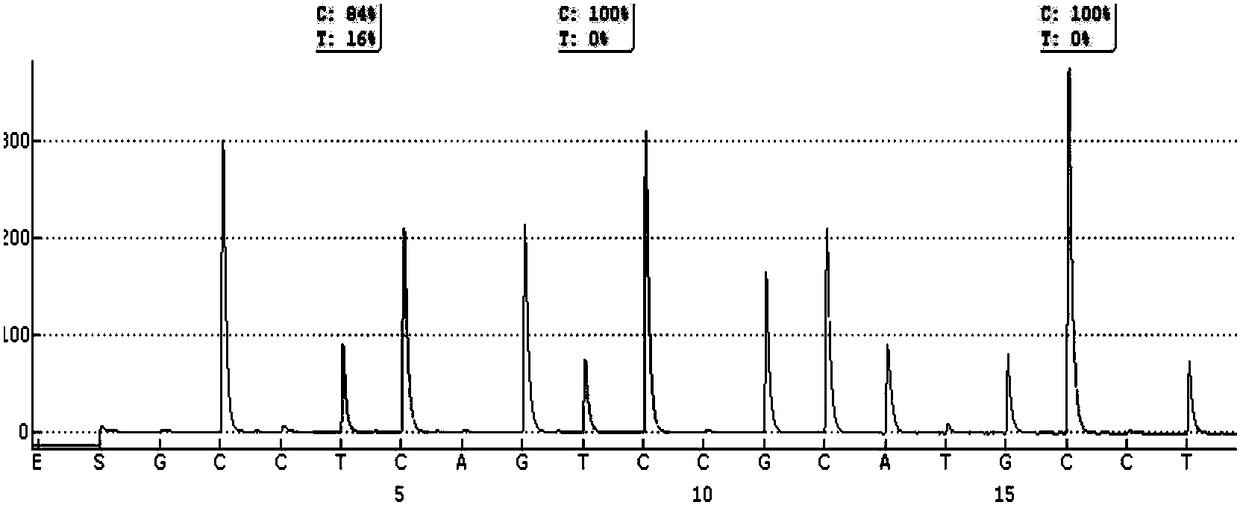
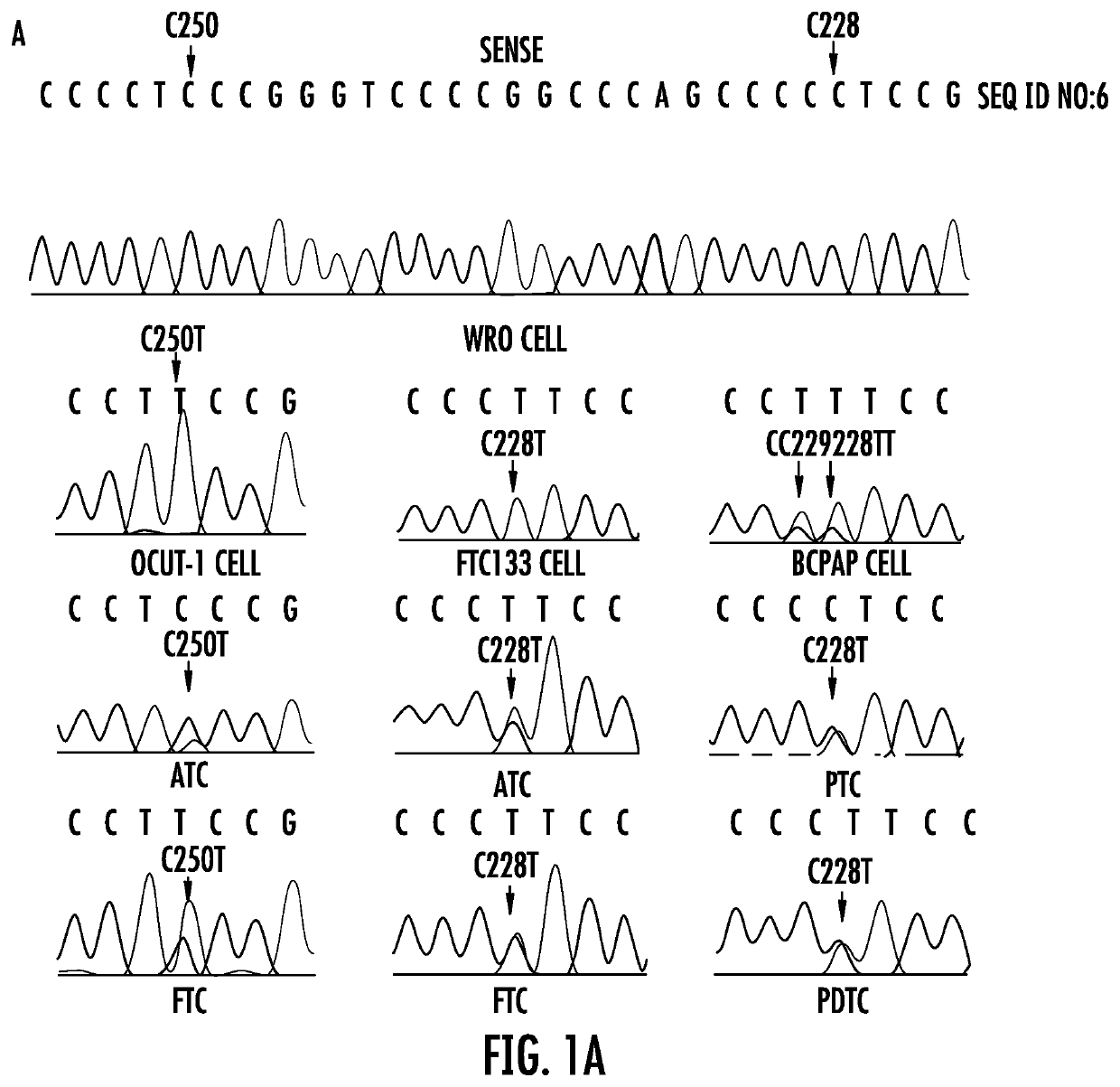
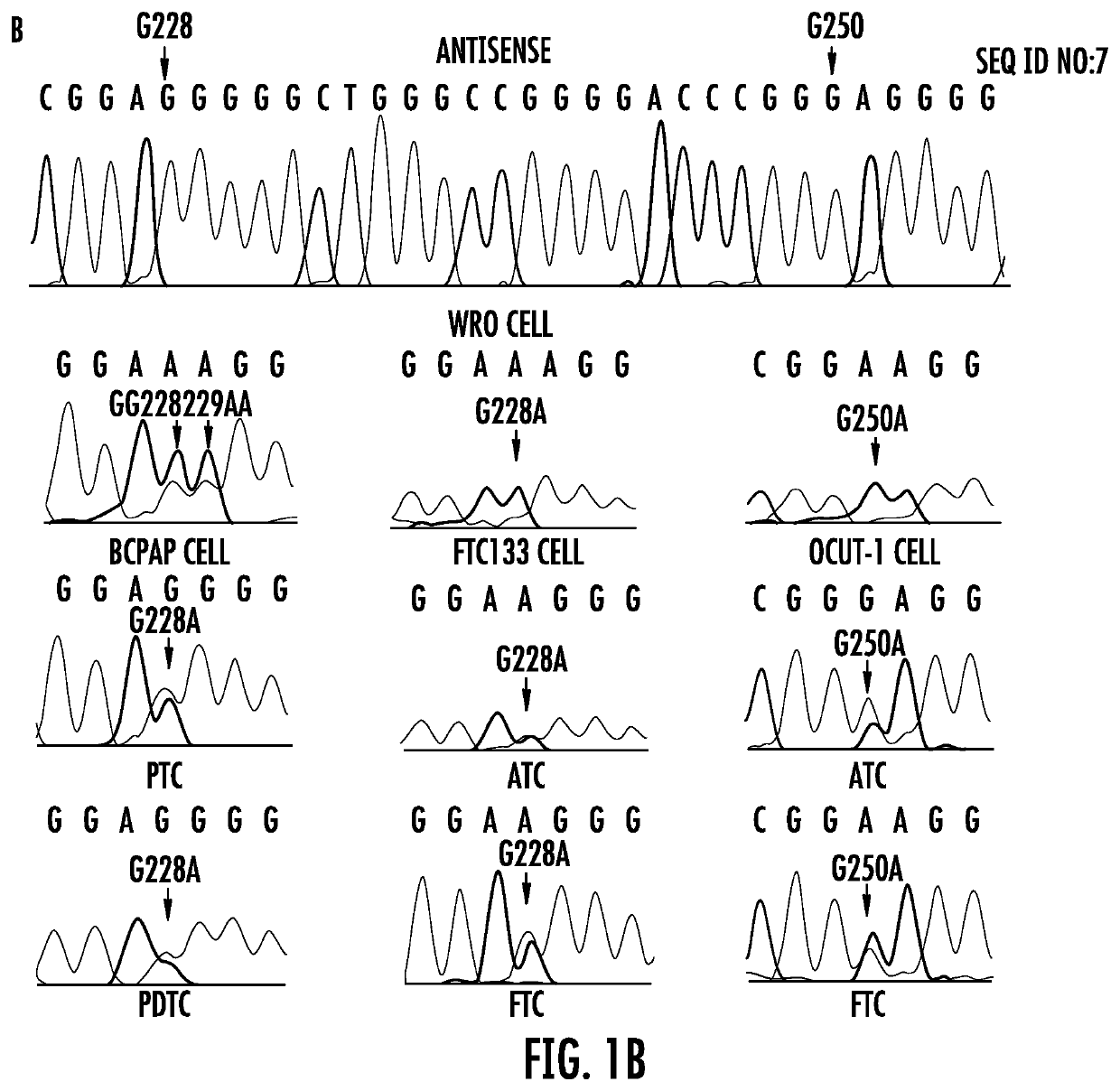
The present invention relates to the field of cancer. More specifically, the present invention provides methods and compositions related to certain promoter mutations in cancer. In one embodiment, a method for treating a subject having thyroid cancer comprises the steps of (a) obtaining a biological sample from the subject; (b) performing an assay on the sample obtained from the subject to identify a mutation at 1 295 228 C>T (C228T) and 1 295 250 C>T (C250T), corresponding to −124 C>T and −146 C>T from the translation start site in the promoter of the telomerase reverse transcriptase (TERT) gene; (c) identifying the subject as having or likely to develop aggressive thyroid cancer if the C228T and / or C250T mutation is identified; and (d) treating the subject with one or more treatment modalities appropriate for a subject having or likely to develop aggressive thyroid cancer.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
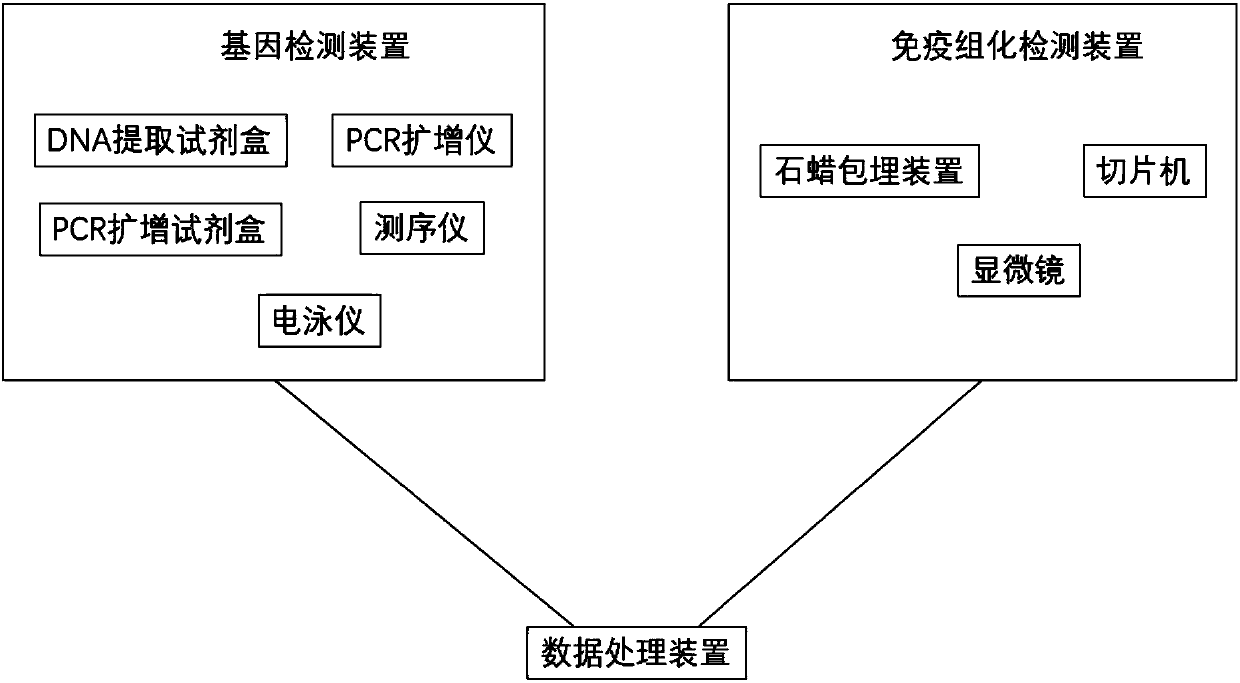
System for predicting chemotherapy sensitivity of patient with bladder cancer
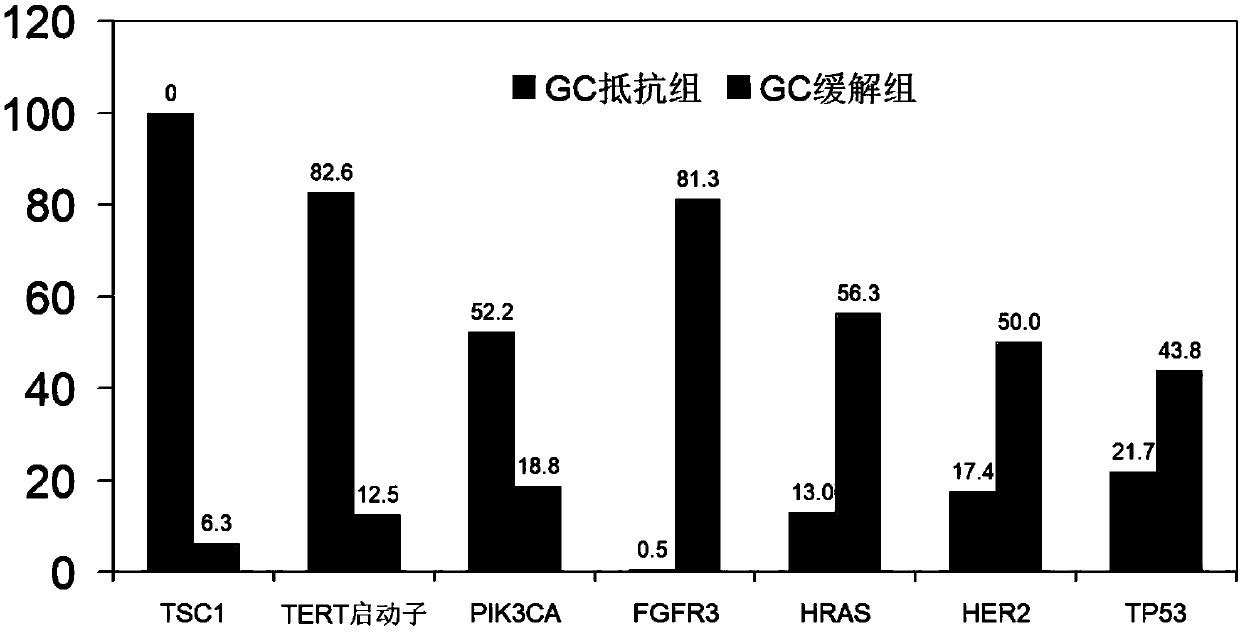
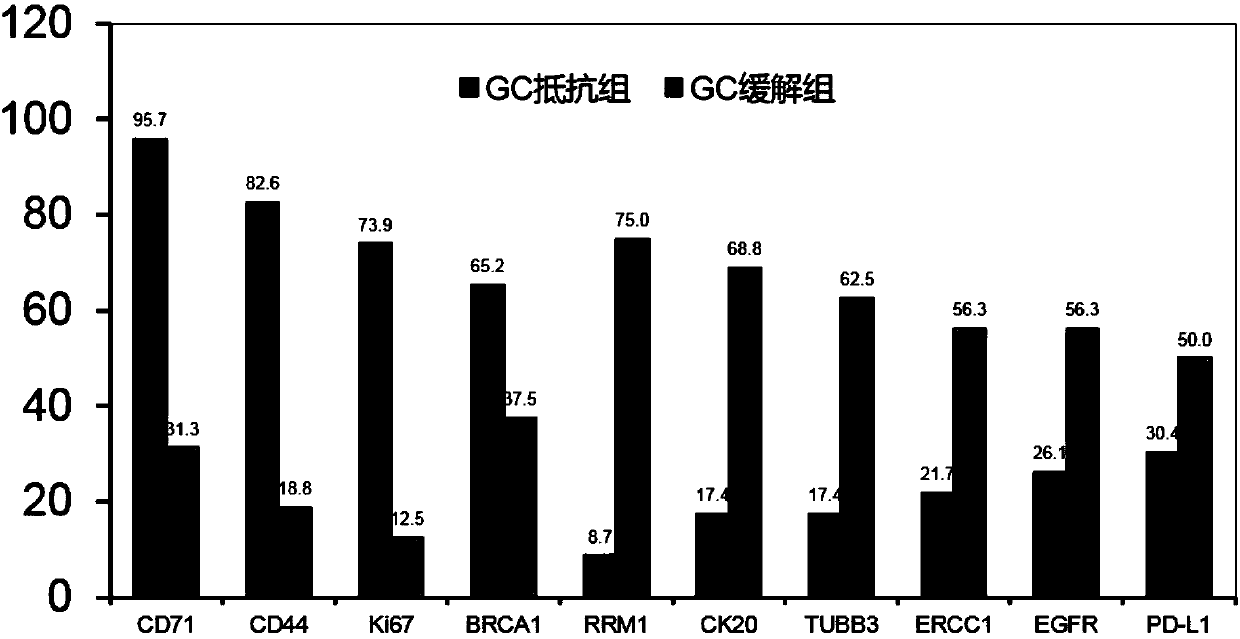
The invention discloses a system for predicting the chemotherapy sensitivity of a patient with bladder cancer. The system comprises a gene detection device, an immunohistochemical detection device anda data processing device, wherein results of the gene detection device and the immunohistochemical detection device are analyzed and processed by virtue of the data processing device; the gene detection device comprises a DNA extraction kit, a primer group, a PCR amplification instrument, a PCR purification kit, a sequencer and an electrophoresis instrument. According to the system, correspondingspecific primers and antibodies are designed and prepared by virtue of bladder cancer mutant genes including FGFR3, HRAS, TERT promoters, TP53, TSC1, PIK3CA and HER2 as well as abnormal expression proteins including EGFR, ERCC1, CK20, BRCA1, CD44, RRM1, PD-L1, TUBB3, Ki67 and CD71, so as to meet the detection requirements of molecular pathological indexes; and the system is simple, convenient, economic and used for guiding the implementation of neoadjuvant chemotherapy, and the prediction accuracy of the chemotherapy sensitivity of the bladder cancer can be greatly improved.
Owner:李翀
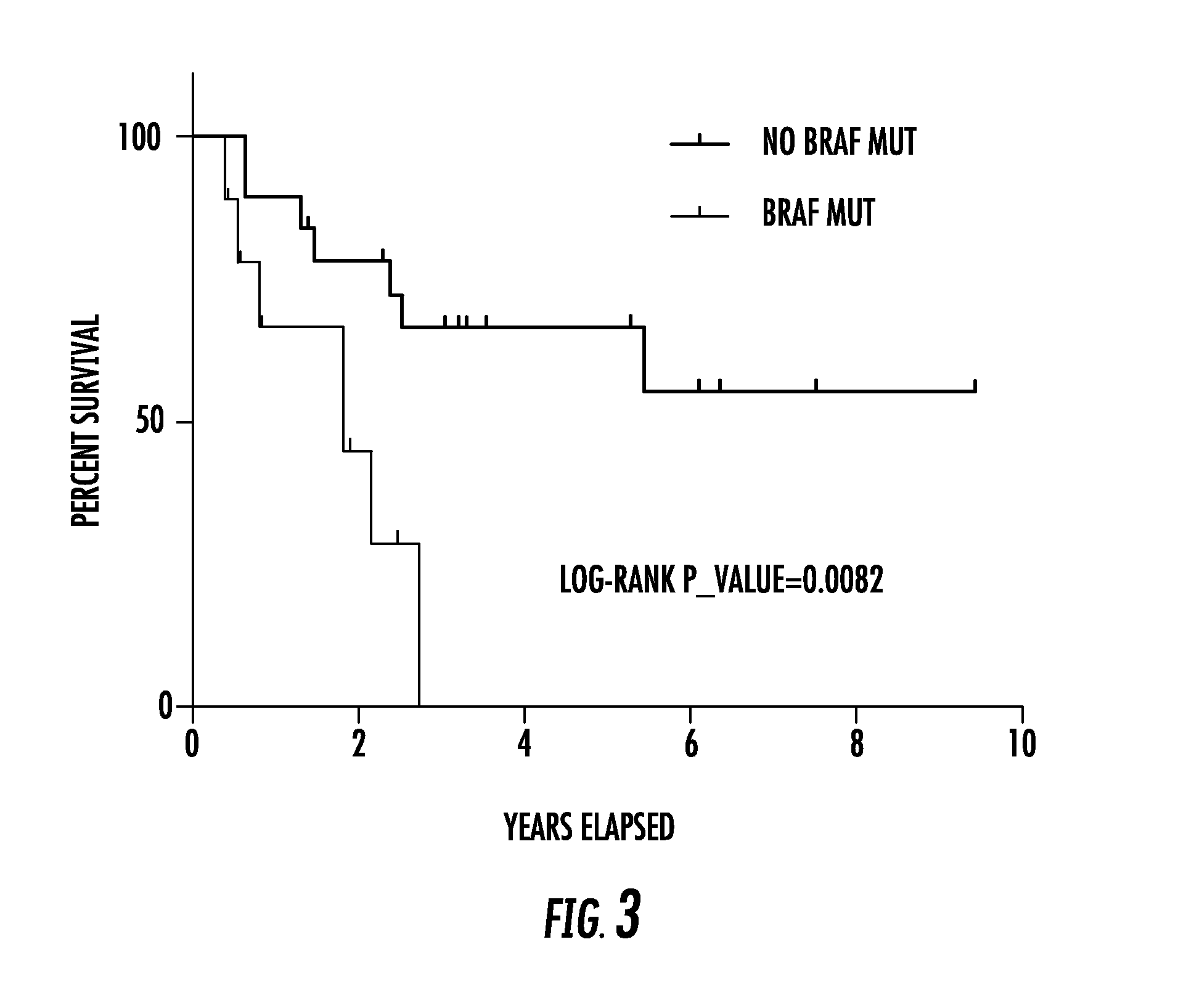
Tert and braf mutations in human cancer
ActiveUS20170022572A1Good risk stratificationEasy to manageMicrobiological testing/measurementReverse transcriptaseTert promoter
The present invention relates to the field of cancer. More specifically, the present invention provides methods and compositions related to certain mutations in cancer. In one embodiment, a method for treating a subject having aggressive thyroid cancer comprises the steps of (a) obtaining a biological sample from the subject; (b) performing an assay on the sample obtained from the subject to identify a mutation at 1 295 228 C>T (C228T), corresponding to −124 C>T from the translation start site in the promoter of the telomerase reverse transcriptase (TERT) gene, and a T1799A mutation in the BRAF gene that results in a V600E amino acid change; (c) identifying the subject as having or likely to develop aggressive thyroid cancer if the C228T and V600E mutations are identified; and (d) treating the subject with one or more treatment modalities appropriate for a subject having or likely to develop aggressive thyroid cancer. Similar approaches are applied to other human cancers harboring both BRAF V600E mutation and TERT promoter mutations.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
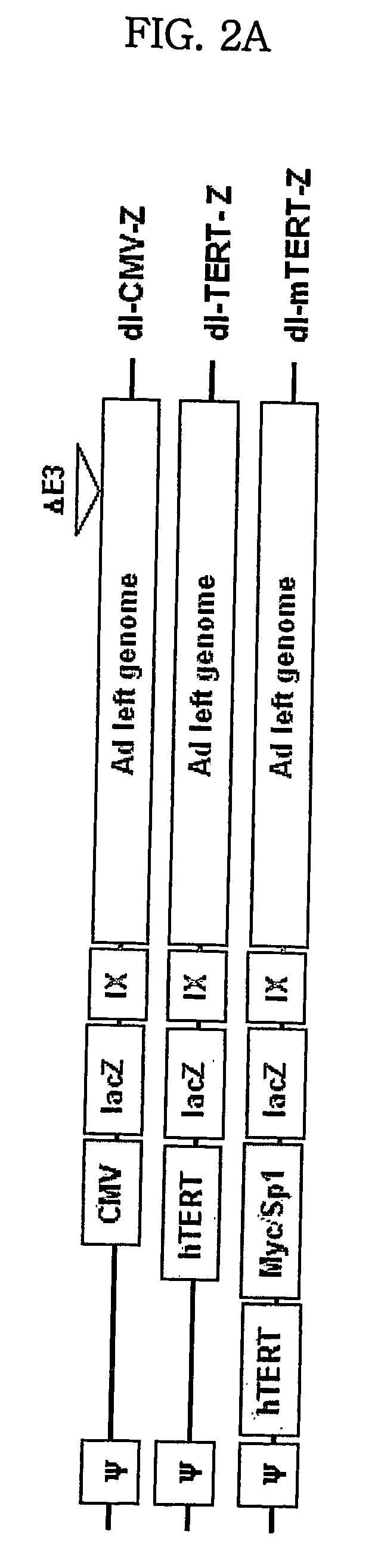
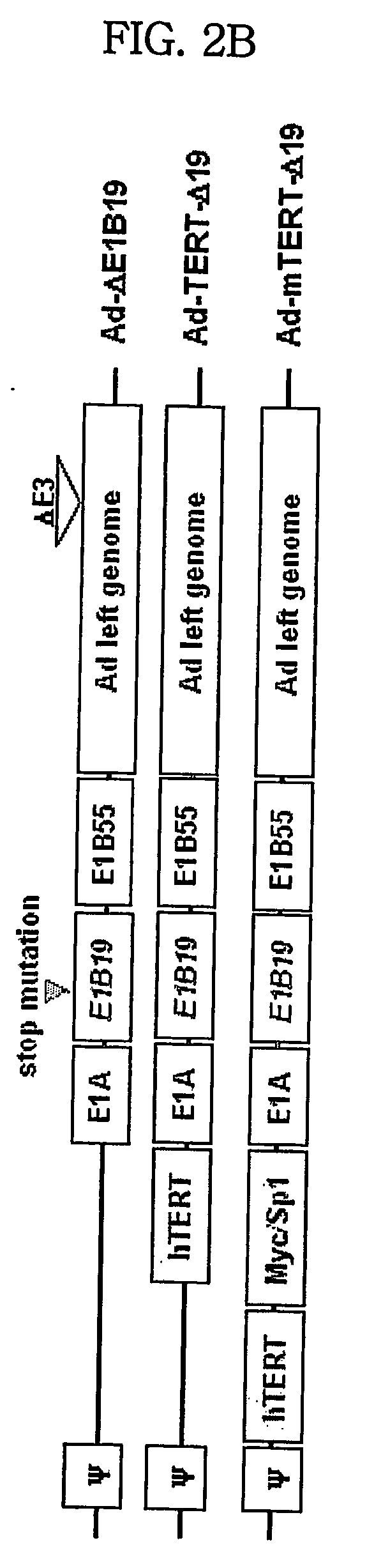
Modified tert promoter with enhanced tumor-specificity and strength and recombinant vector comprising the same
The present invention relates to a transcriptional regulatory sequence with enhanced tumor-specificity and strength and a recombinant vector comprising the transcriptional regulatory sequence. More particularly, the present invention relates to a transcriptional regulatory sequence comprising a human telomere reverse transcriptase (hTERT) promoter linked to a nucleotide sequence that comprises one or more c-Myc binding sites and / or one or more Sp1 binding sites, and a recombinant vector comprising a certain gene that is operably linked to the above transcriptional regulatory sequence.
Owner:GENEMEDICINE CO LTD
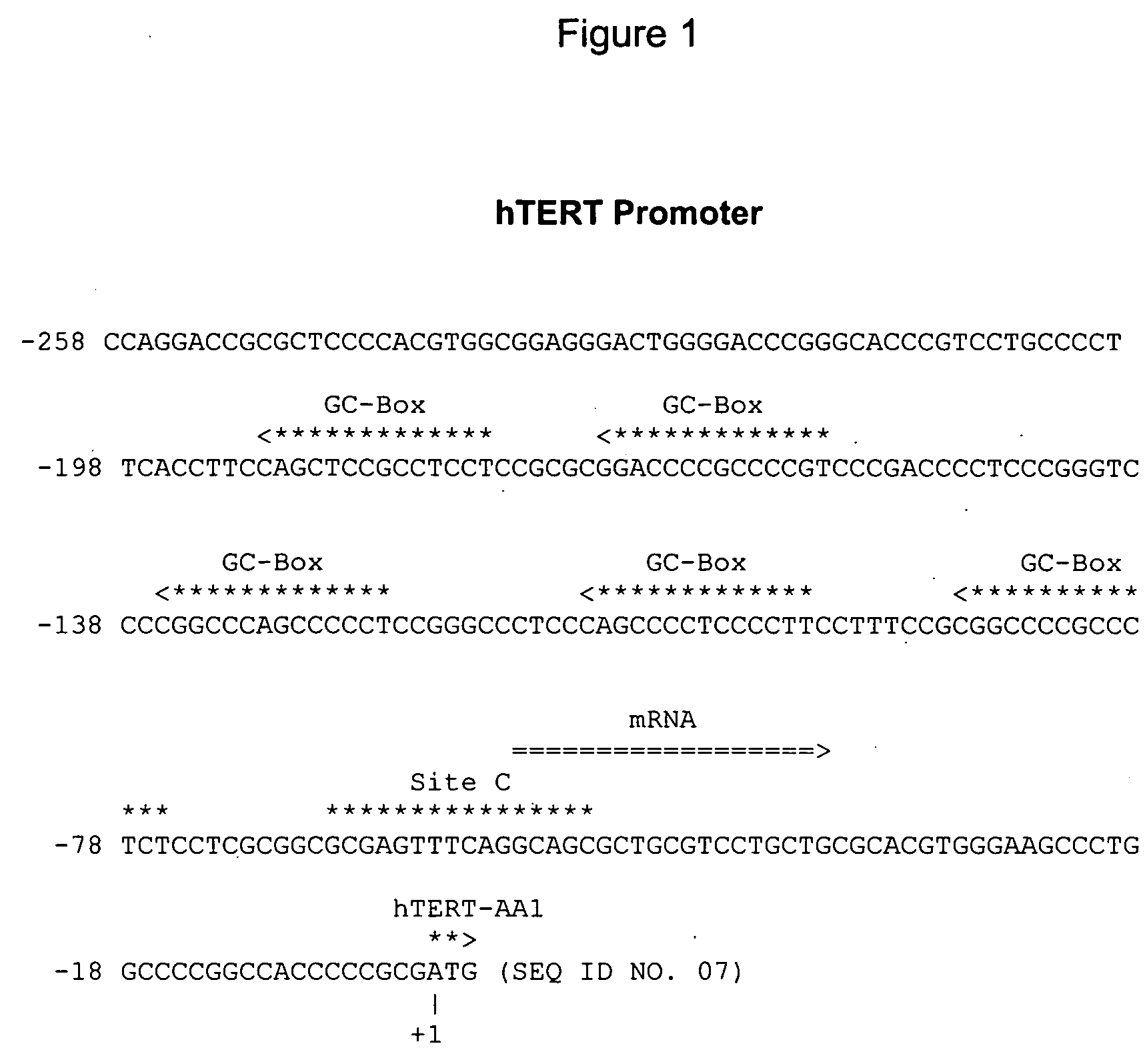
Method of diagnosing cancer comprising detection of the methylation signature in the htert promoter
InactiveUS20150232941A1Effective diagnosisMicrobiological testing/measurementDNA methylationTelomerase
A method of diagnosing cancer in a mammal is provided. The method includes the steps of determining in a nucleic acid-containing sample from the mammal the degree of DNA methylation of a target region within the hTERT promoter from the nucleotide at about position −157 to the nucleotide at about position −580, or a corresponding target region in a TERT promoter in a mammal other than a human, to yield a sample methylation signature, determining the baseline degree of DNA methylation of the target region in a control sample to yield a control methylation signature, comparing the sample methylation signature to the control methylation signature and rendering a diagnosis of cancer when there is at least 1.5 times more methylation in the sample methylation signature as compared to the control methylation signature. Methods of determining tumour grade and progression, predicting survival and determining whether or not a mammal is a candidate for telomerase-targeted or demethylation therapy are also provided.
Owner:HOSPITAL FOR SICK CHILDREN
Methods for rapid and sensitive detection of hotspot mutations
Methods that rapidly, sensitively, and specifically detect mutations in IDH1 / 2 and the TERT promoter employ amplification of particular portions of the genes that experience frequent and exquisitely localized mutations. The ability to distinguish between sequences that differ only by one nucleotide and which may be present in very low ratios is essential for such an assay.
Owner:DUKE UNIV
Assays for TERT promoter modulatory agents using a telomerase structural RNA component
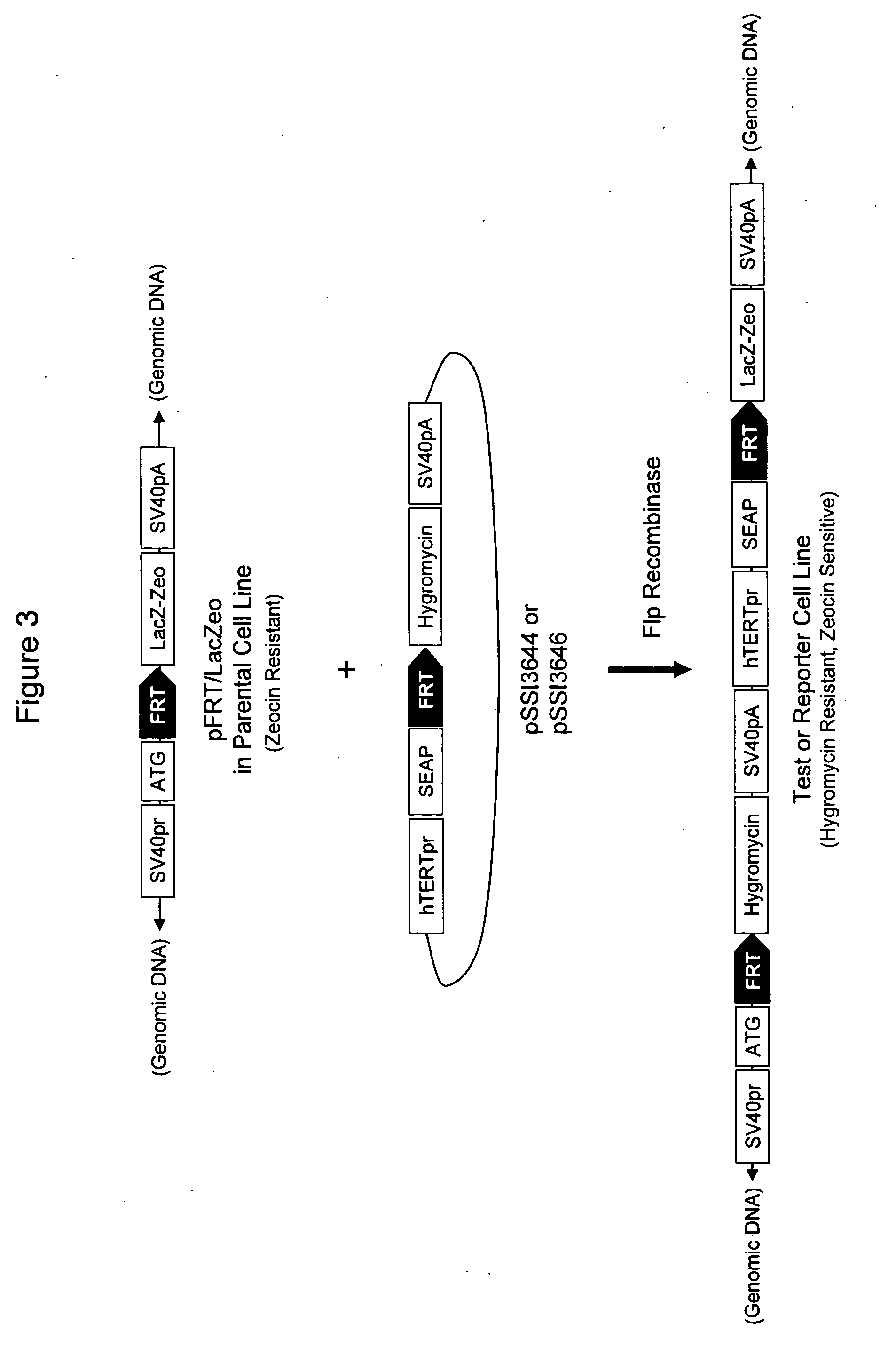
Methods and compositions for assaying an agent for TERT promoter modulatory activity are provided. In the subject methods, an agent is contacted with a cell comprising a mutant telomerase structural RNA component (TR) that results in a detectable phenotype in the presence of telomerase reverse transcriptase (TERT). Also provided are compositions, systems and kits thereof, as well as devices, that find use in practicing the subject methods. The subject invention finds use in assaying agents for TERT promoter modulatory activity, such as in a high throughput format.
Owner:SIERRA SCIENCES
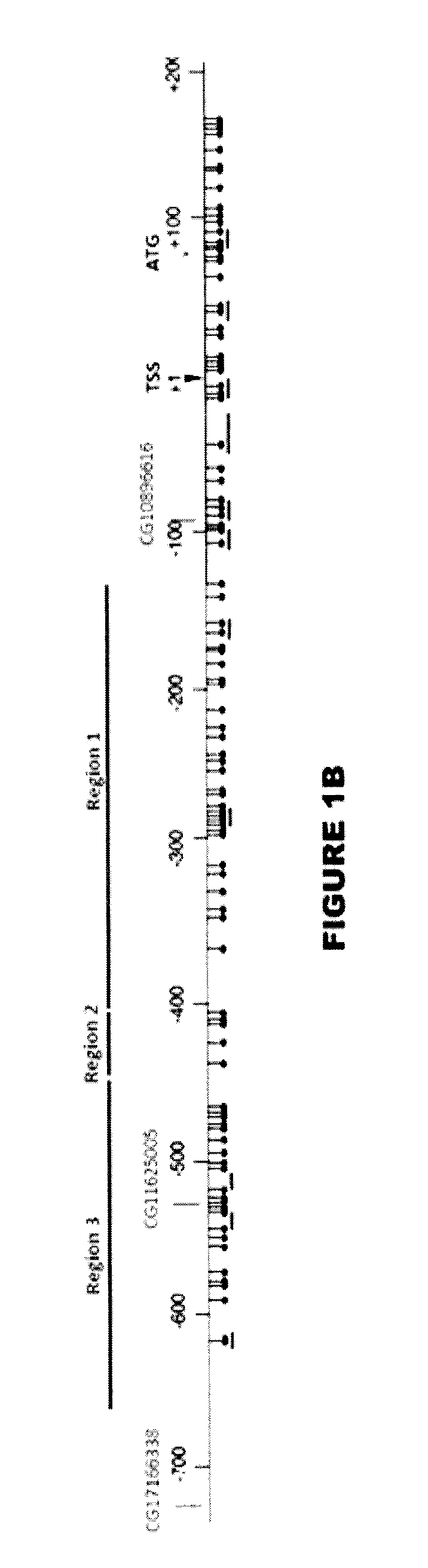
Primer composition for detecting TERT promoter methylation and application thereof
PendingCN112662773ASimple and fast operationHigh feasibilityMicrobiological testing/measurementDNA/RNA fragmentationTert promoterOncology
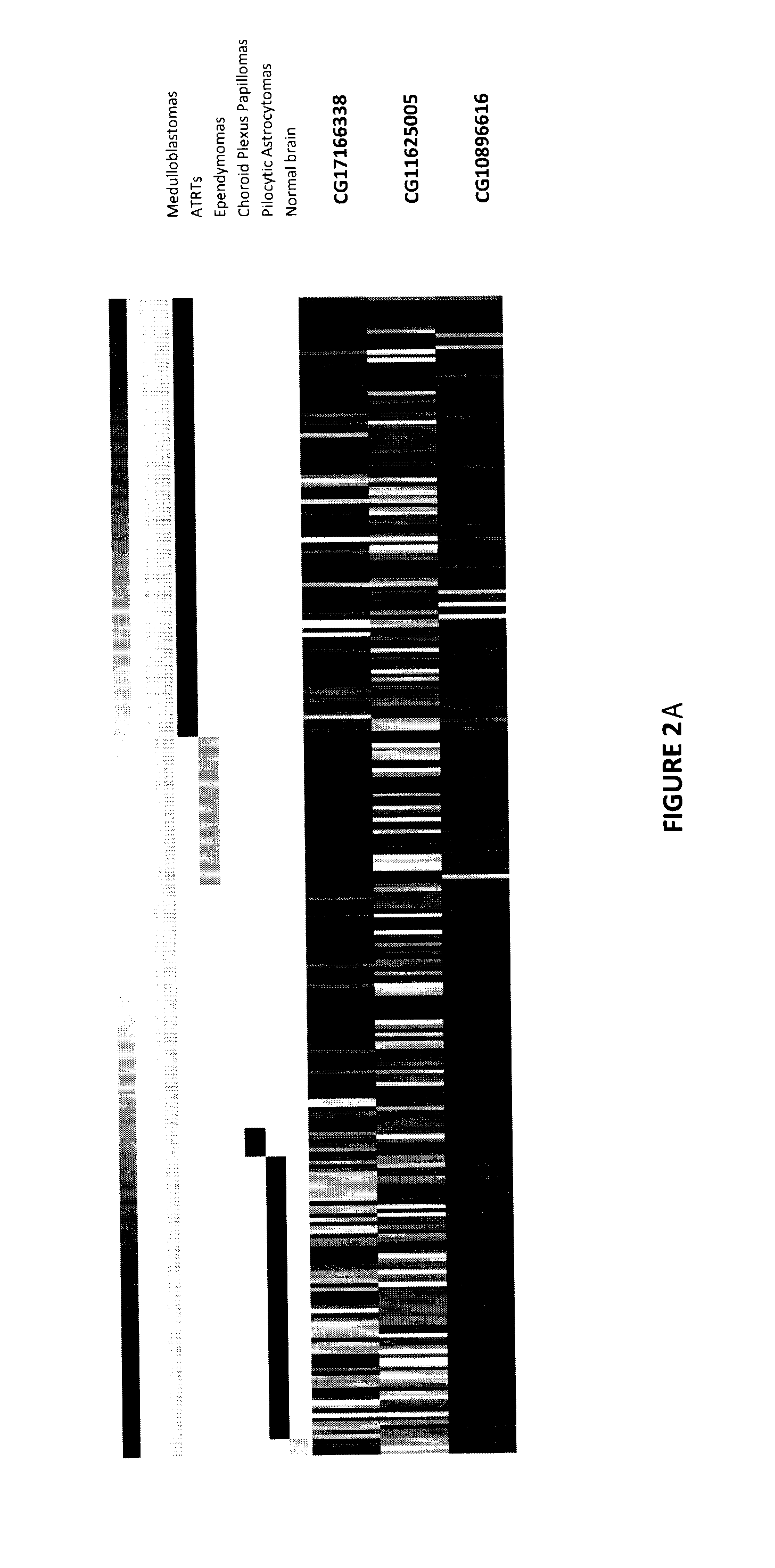
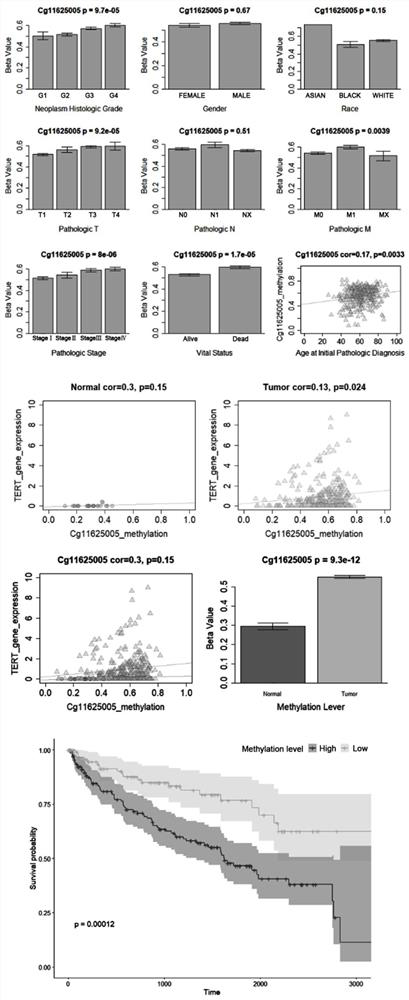
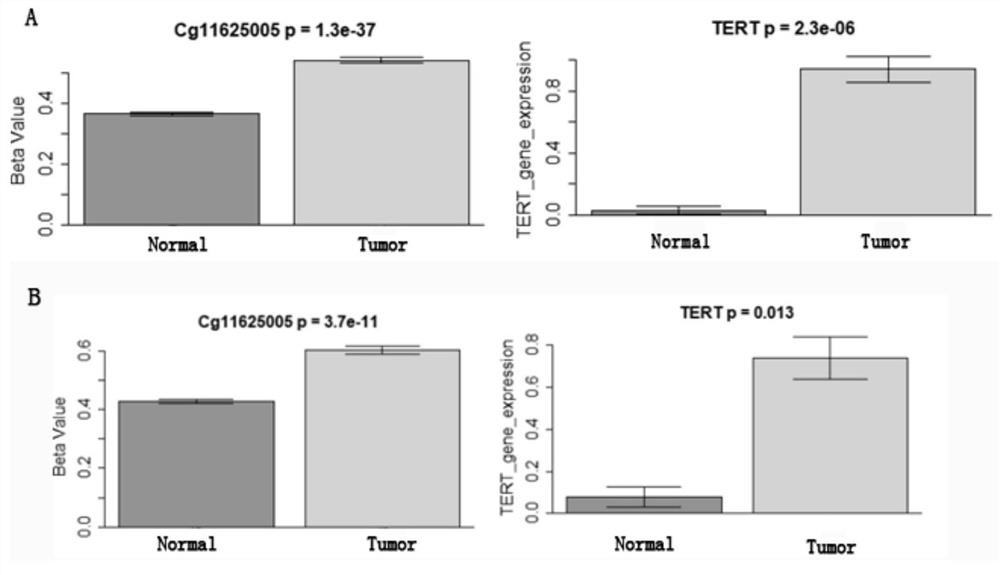
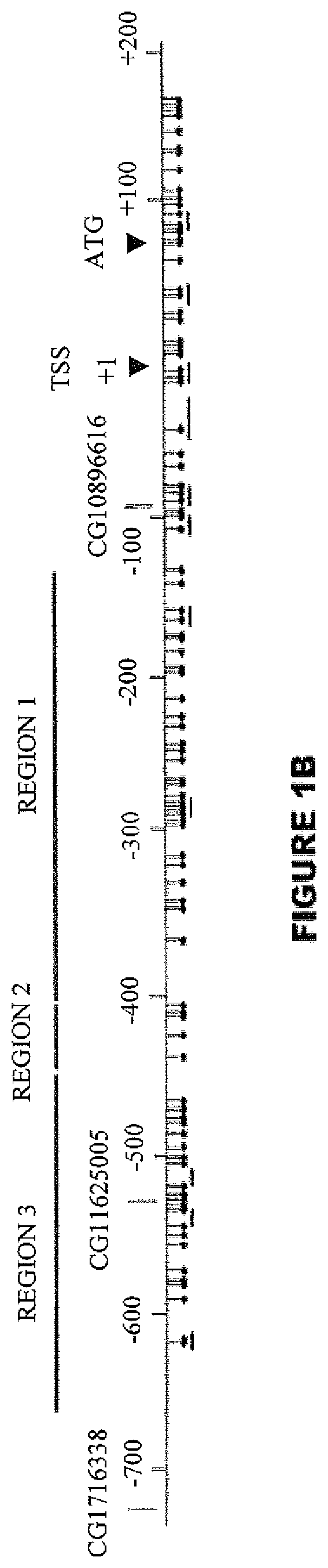
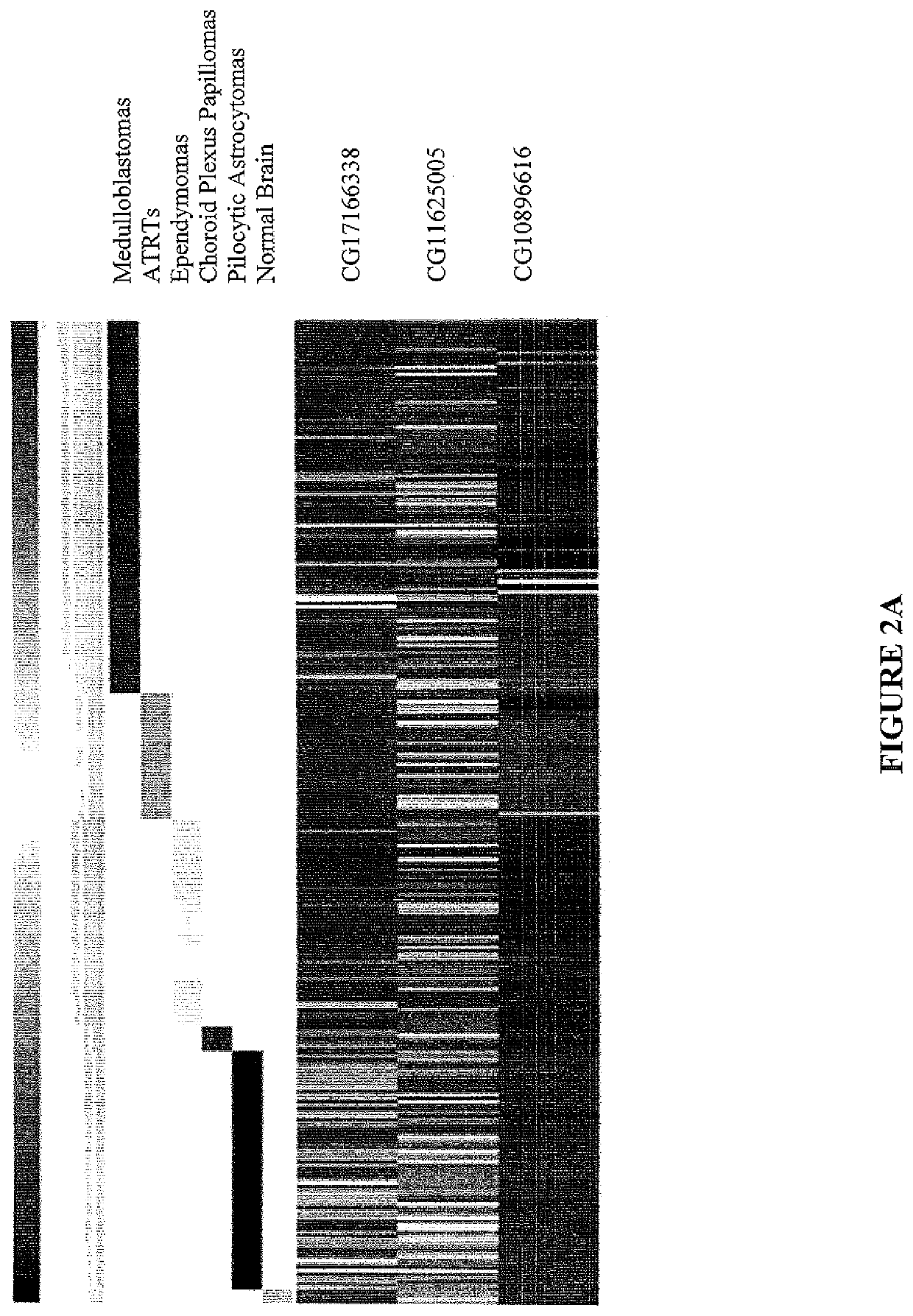
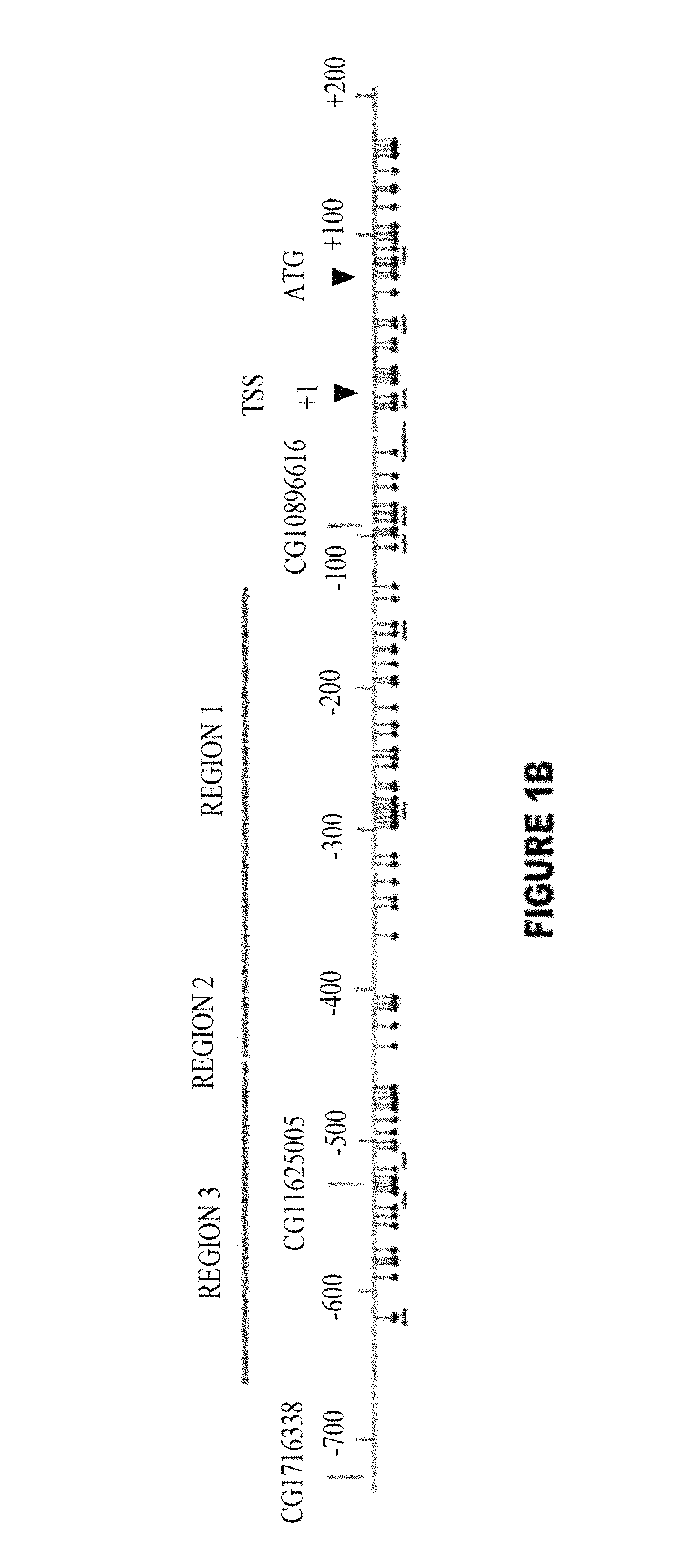
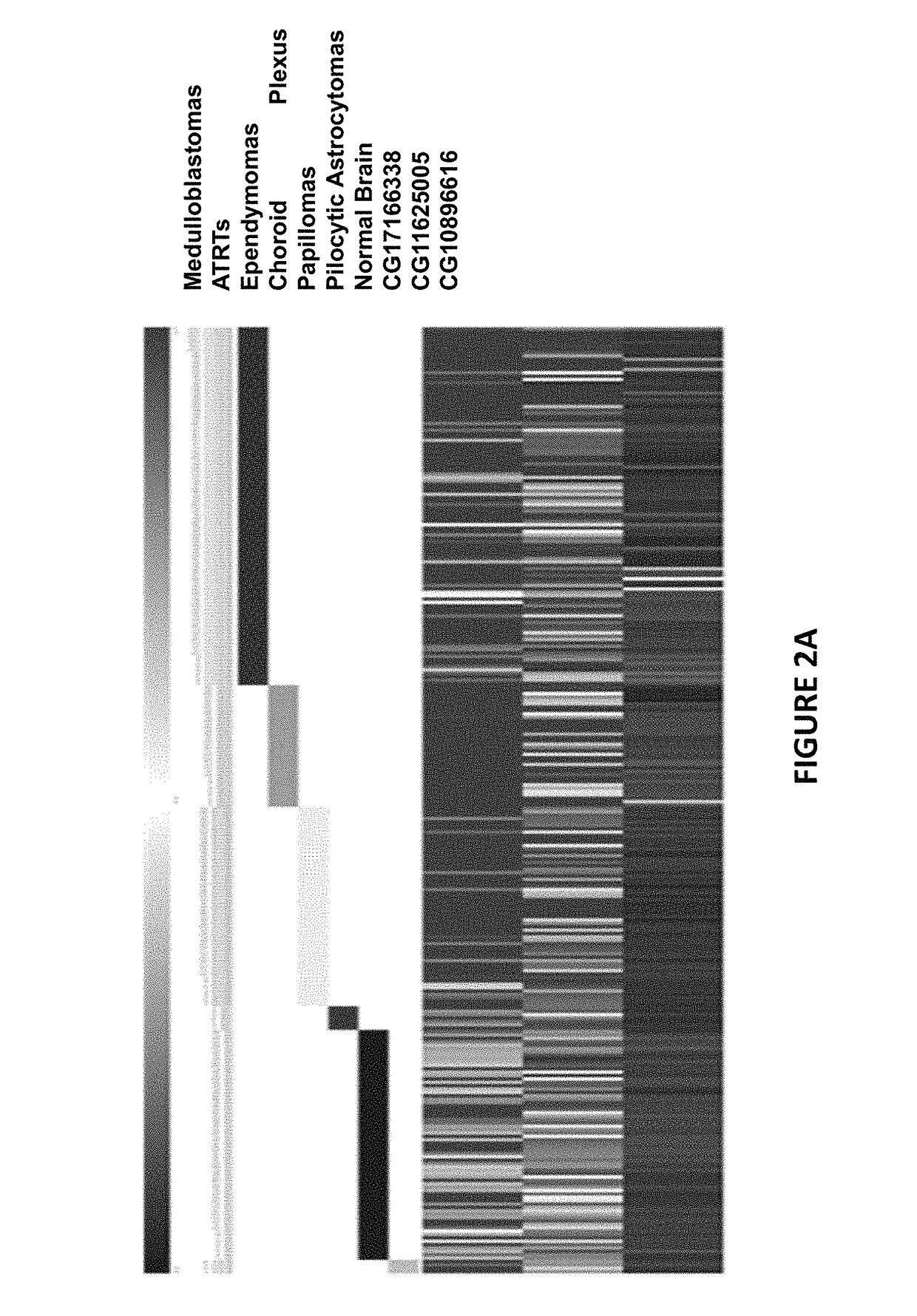
The invention provides a TERT promoter methylation detection primer composition and application of the TERT promoter methylation detection primer composition in kidney cancer detection. According to the primer composition, the methylation level of the cg11625005 site of the TERT promoter can be detected through a pyrosequencing technology, and pathological staging grading is further carried out on the kidney cancer. The TERT promoter provided by the invention is high in methylation level detection sensitivity and accuracy, and has important guiding significance for early detection, prognosis and risk assessment of kidney cancer.
Owner:HUNAN LINGKANG MEDICAL TECH CO LTD
NGS-based brain tumor molecular diagnosis analysis method
InactiveCN112102944AHigh sensitivityStrong specificityMedical automated diagnosisHybridisationRepetitive SequencesMicrosatellite
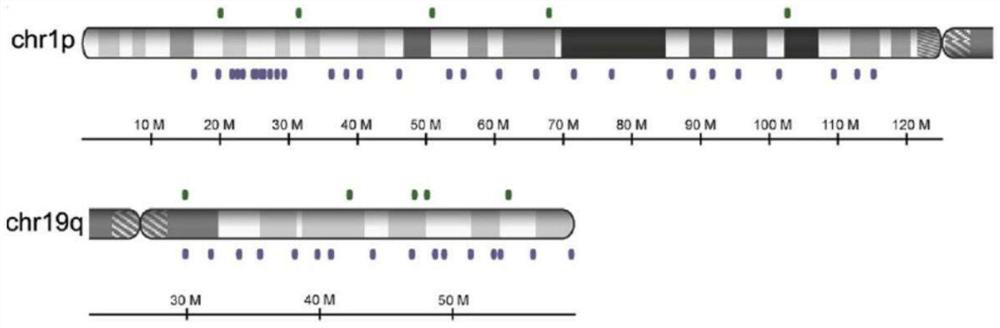
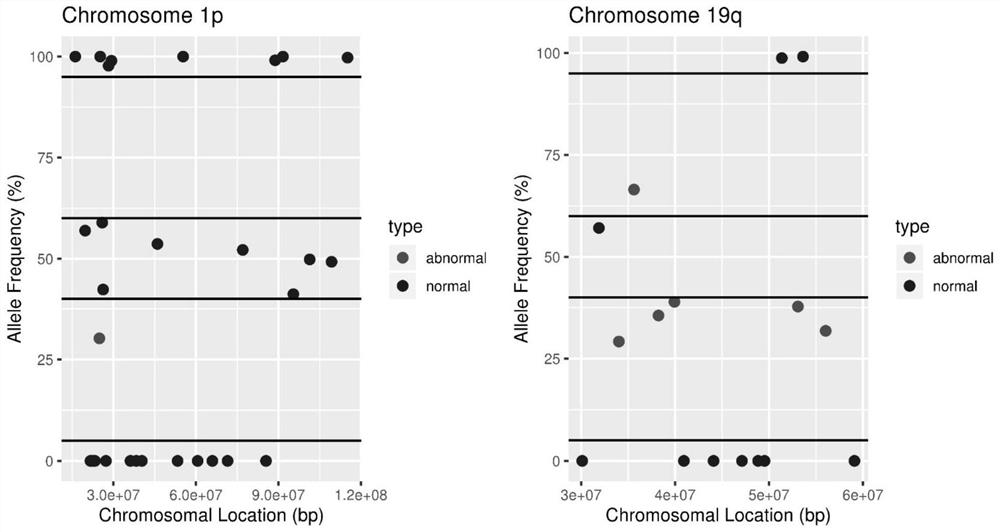
The invention discloses an NGS-based brain tumor molecular diagnosis analysis method. The method comprises the following steps: constructing a cluster based on an Amazon cloud platform; establishing an analysis process of the molecular marker for analyzing the brain tumor on the cluster; a sequence alignment module; removing the repetitive sequence module; a gene fusion analysis module; a call variation module; a cnv analysis structure variation module; the method disclosed by the invention has the advantages of high sensitivity, high specificity, high repeatability, low experimental cost andshort analysis period, and can be used for comprehensively analyzing the hot spot mutation of the TERT promoter, the deletion states of the chromosomes 1p and 19q and other main brain tumor related molecular markers at one time. The NGS-based analytical technique of the present invention enables reliable detection of 1p and / or 19q deletions in the background tumor tissue of 70% normal cells, is more sensitive than microsatellite-based LOH analysis, and requires less DNA.
Owner:阔然生物医药科技(上海)有限公司
Modified tert promoter with enhanced tumor-specificity and strength and recombinant vector comprising the same
The present invention relates to a transcriptional regulatory sequence with enhanced tumor-specificity and strength and a recombinant vector comprising the transcriptional regulatory sequence. More particularly, the present invention relates to a transcriptional regulatory sequence comprising a human telomere reverse transcriptase (hTERT) promoter linked to a nucleotide sequence that comprises one or more c-Myc binding sites and / or one or more Sp1 binding sites, and a recombinant vector comprising a certain gene that is operably linked to the above transcriptional regulatory sequence.
Owner:GENEMEDICINE CO LTD
Method of diagnosing cancer
A method of diagnosing cancer in a mammal is provided. The method includes the steps of determining in a nucleic acid-containing sample from the mammal the degree of DNA methylation of a target region within the hTERT promoter from the nucleotide at about position −157 to the nucleotide at about position −580, or a corresponding target region in a TERT promoter in a mammal other than a human, to yield a sample methylation signature, determining the baseline degree of DNA methylation of the target region in a control sample to yield a control methylation signature, comparing the sample methylation signature to the control methylation signature and rendering a diagnosis of cancer when there is at least 1.5 times more methylation in the sample methylation signature as compared to the control methylation signature. Methods of determining tumour grade and progression, predicting survival and determining whether or not a mammal is a candidate for telomerase-targeted or demethylation therapy are also provided.
Owner:HOSPITAL FOR SICK CHILDREN
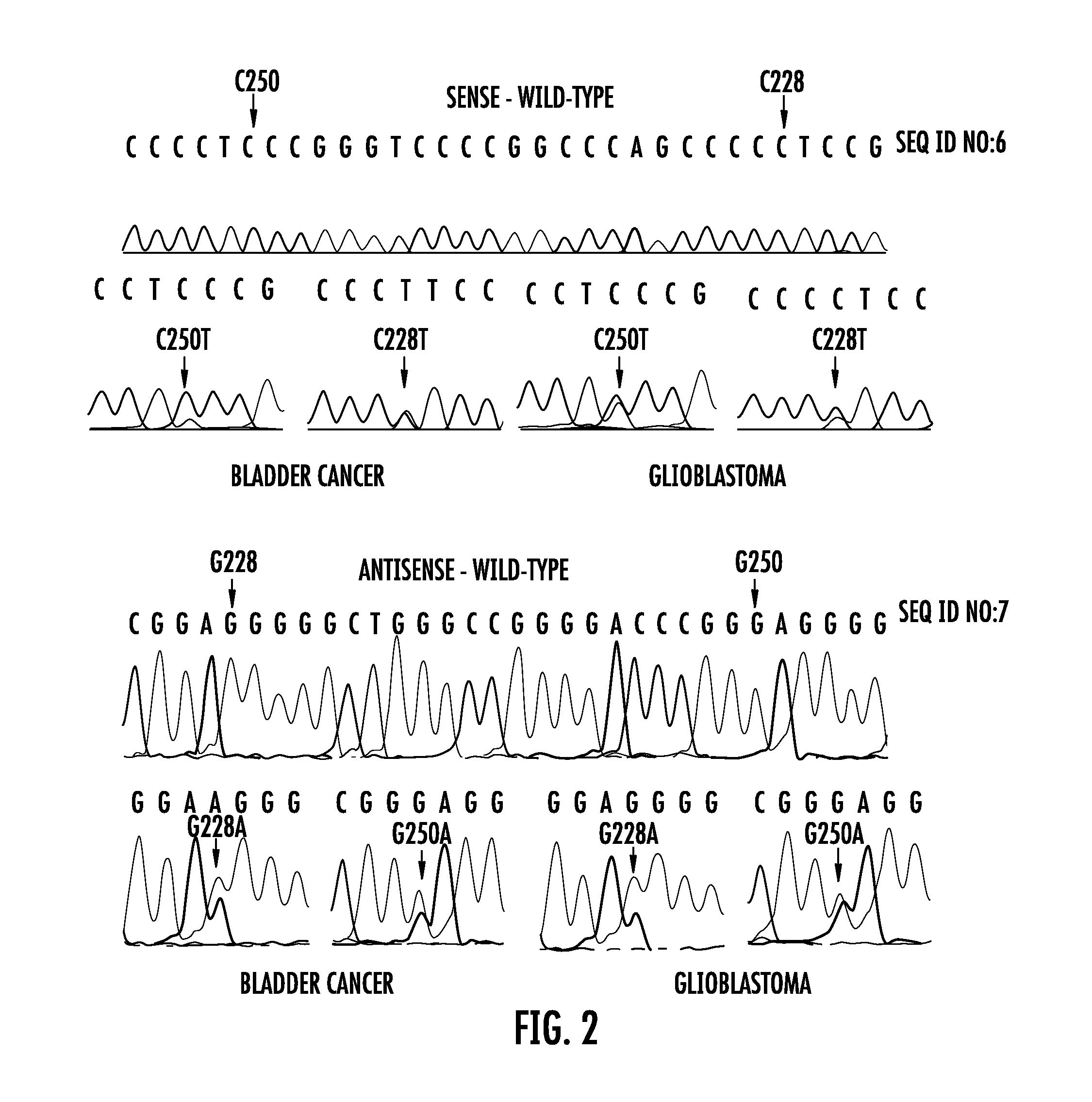
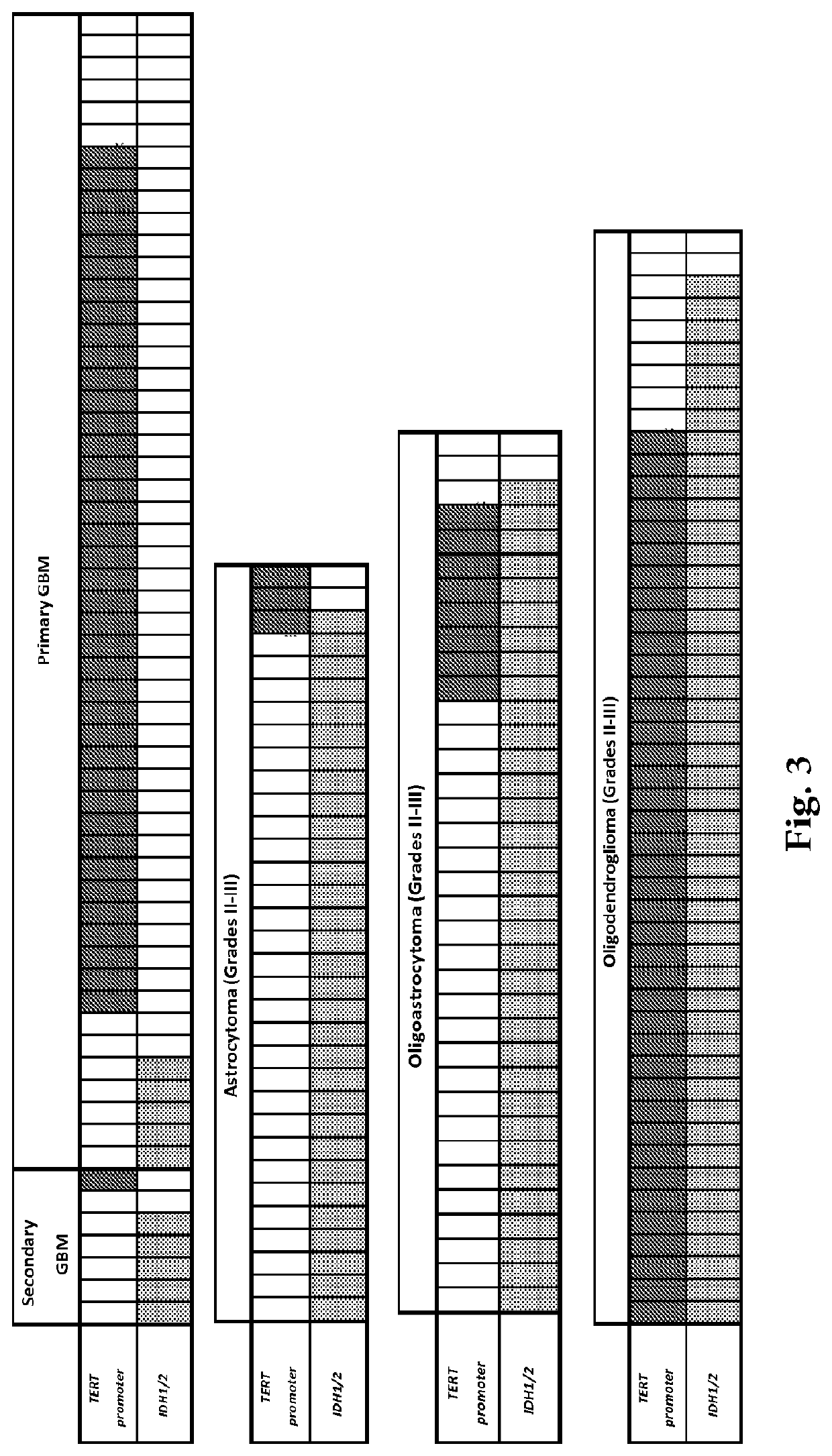
Mutations define clinical subgroups of gliomas
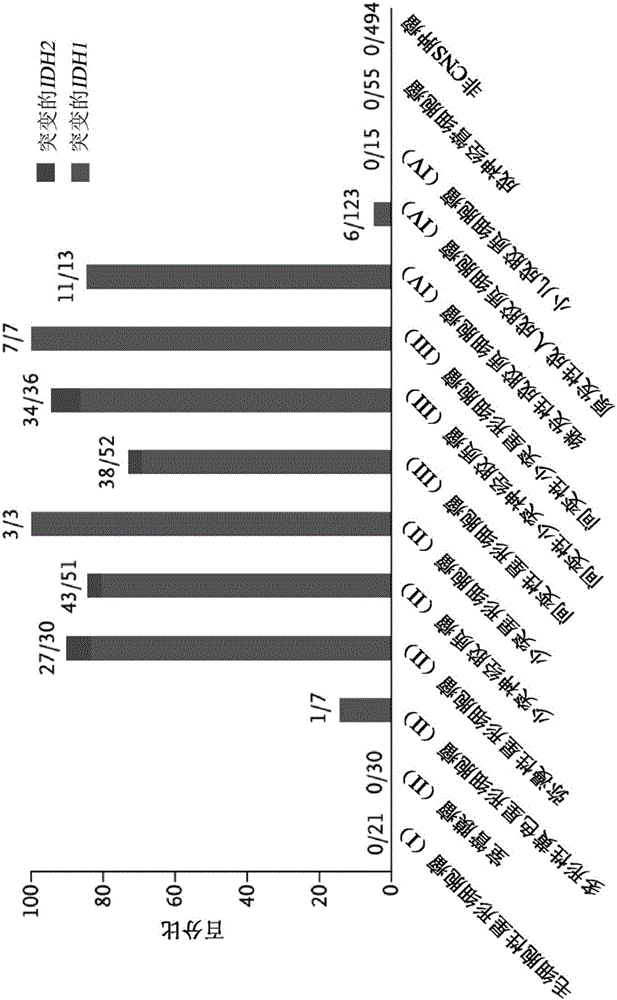
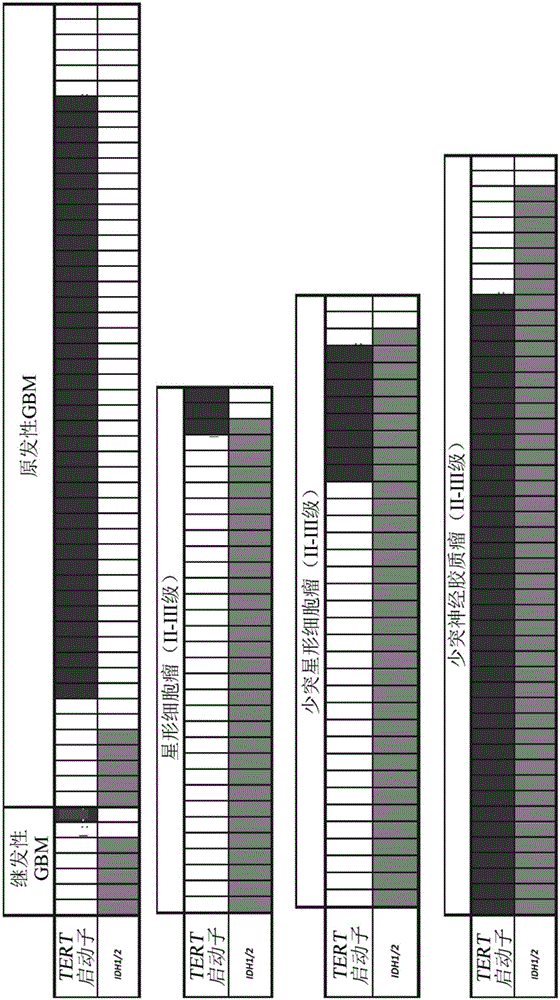
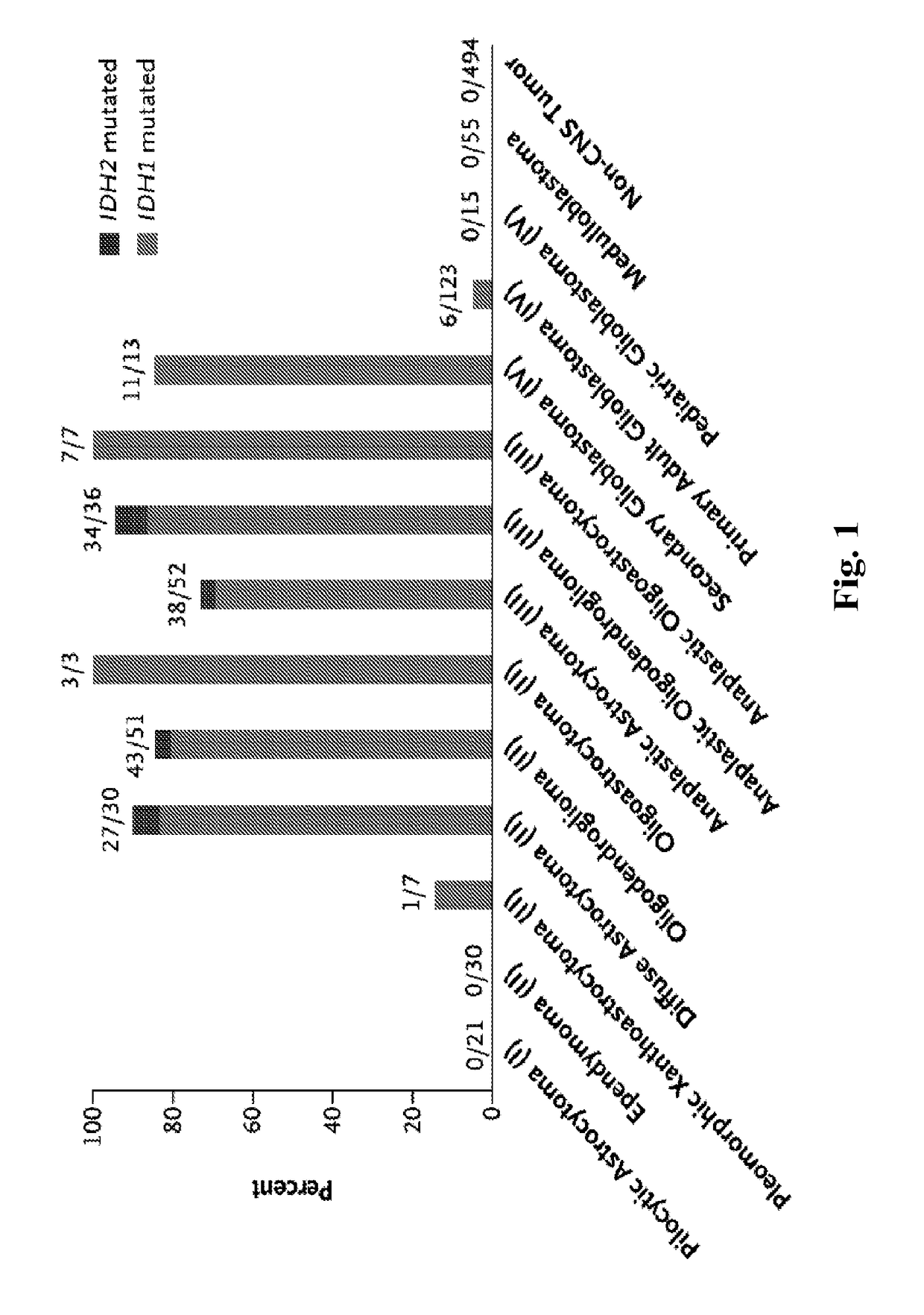
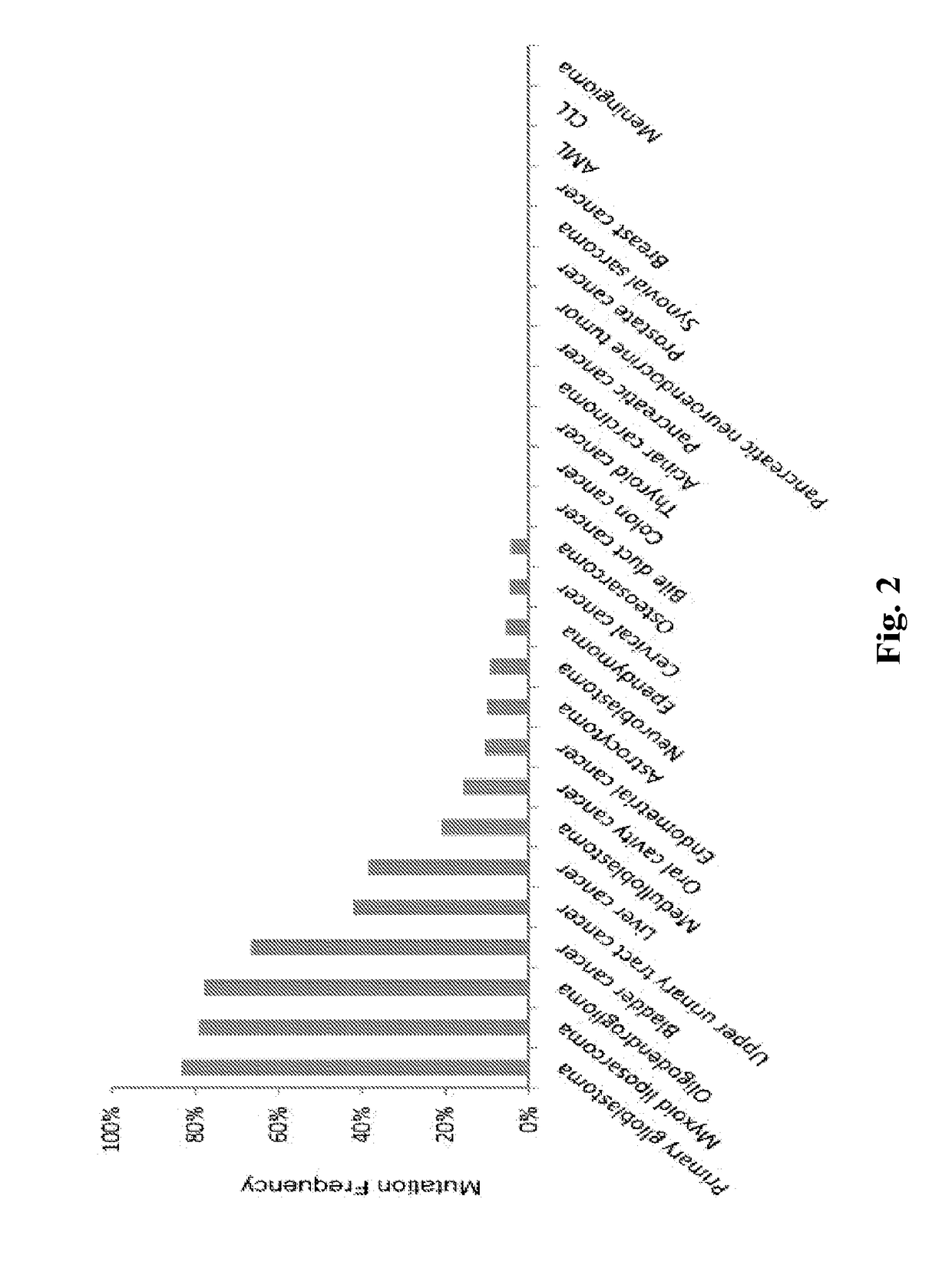
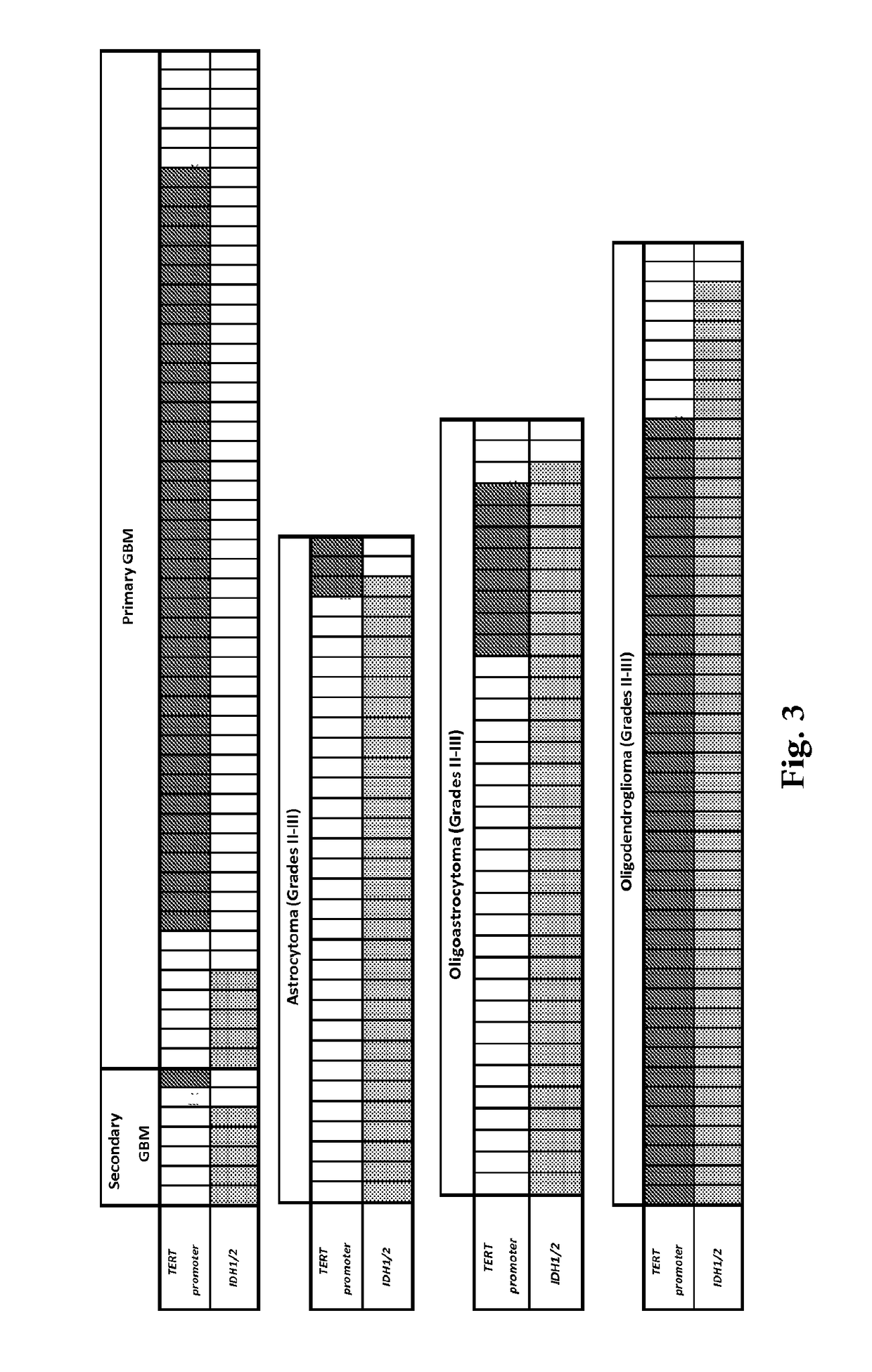
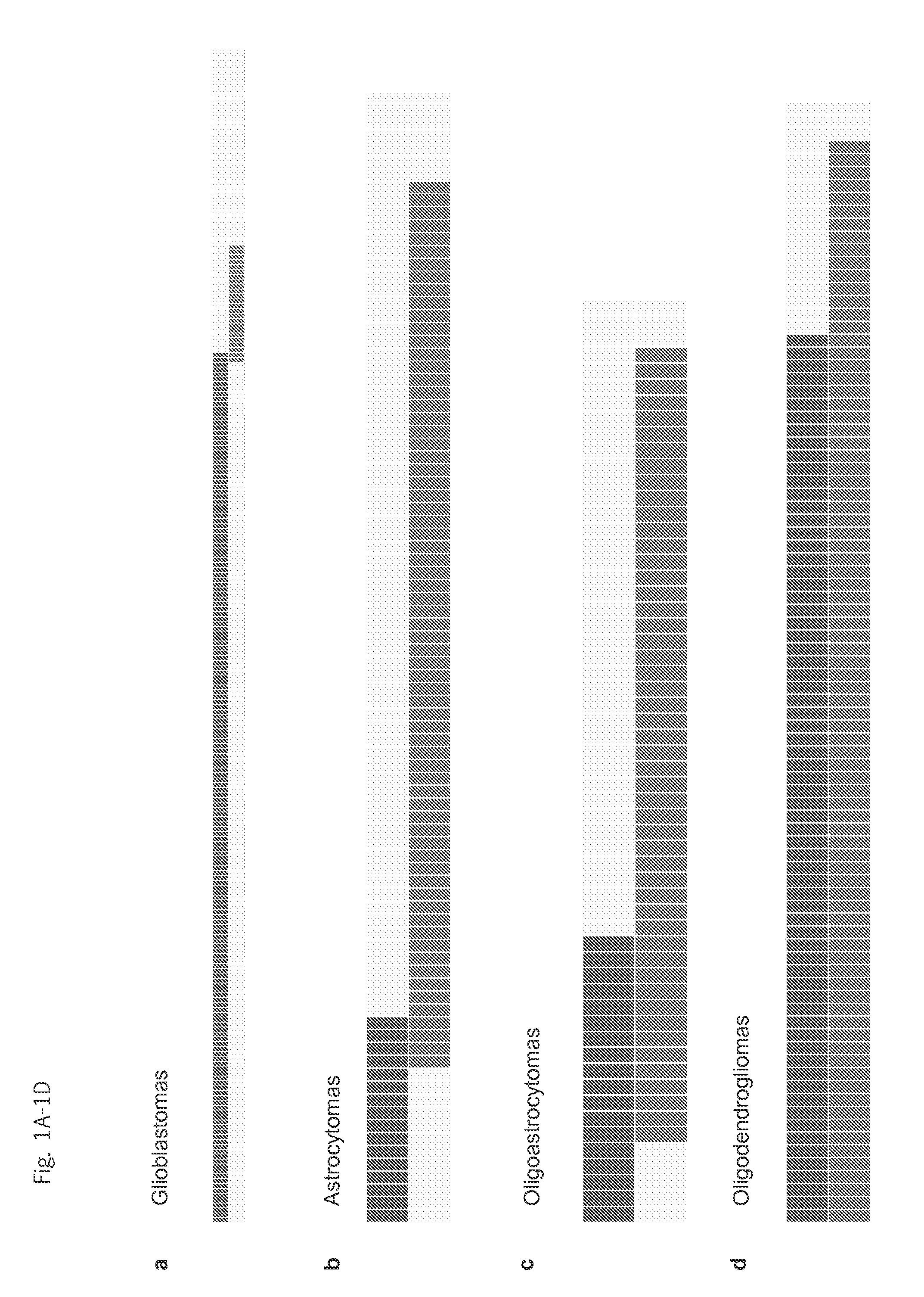
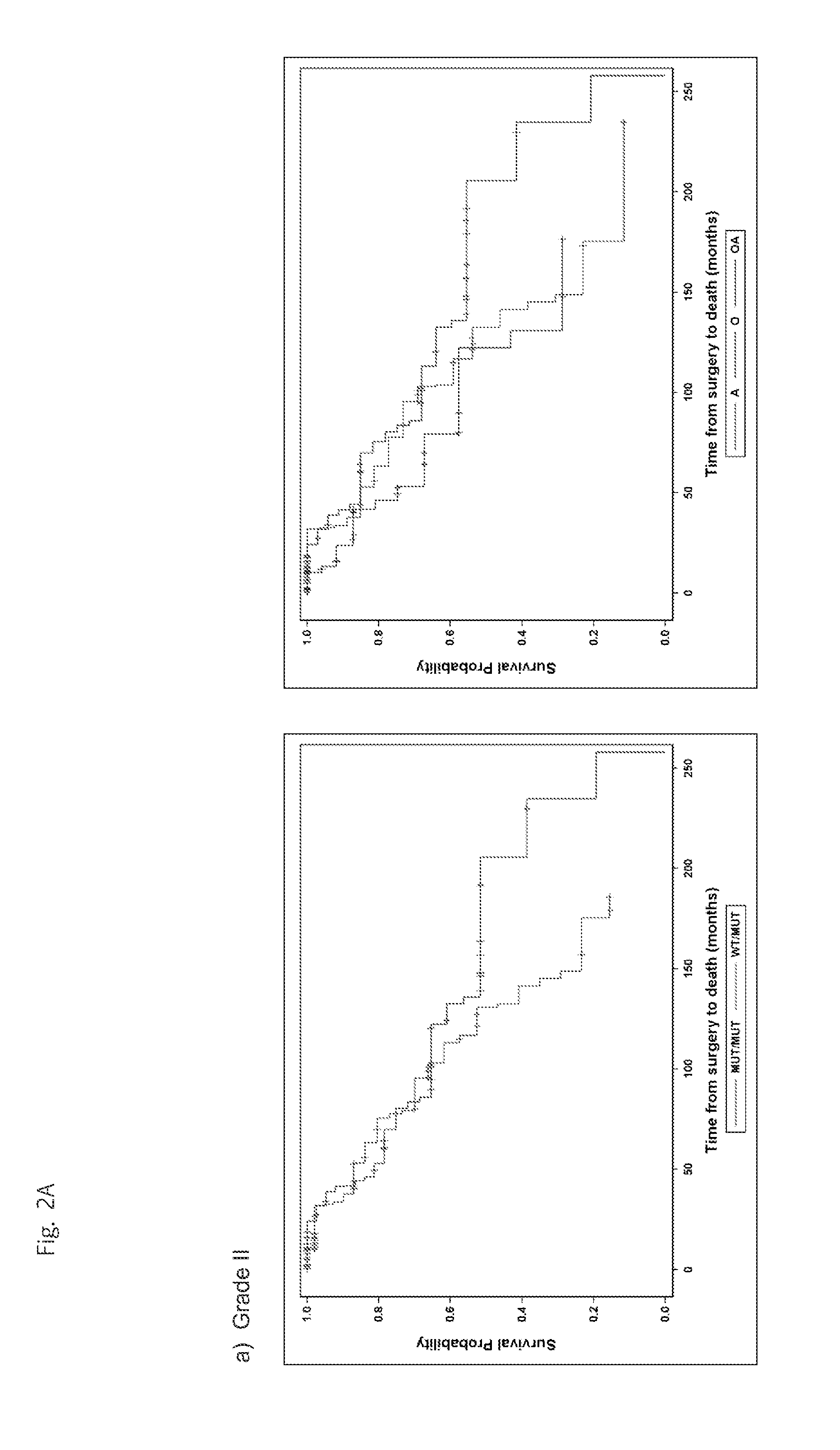
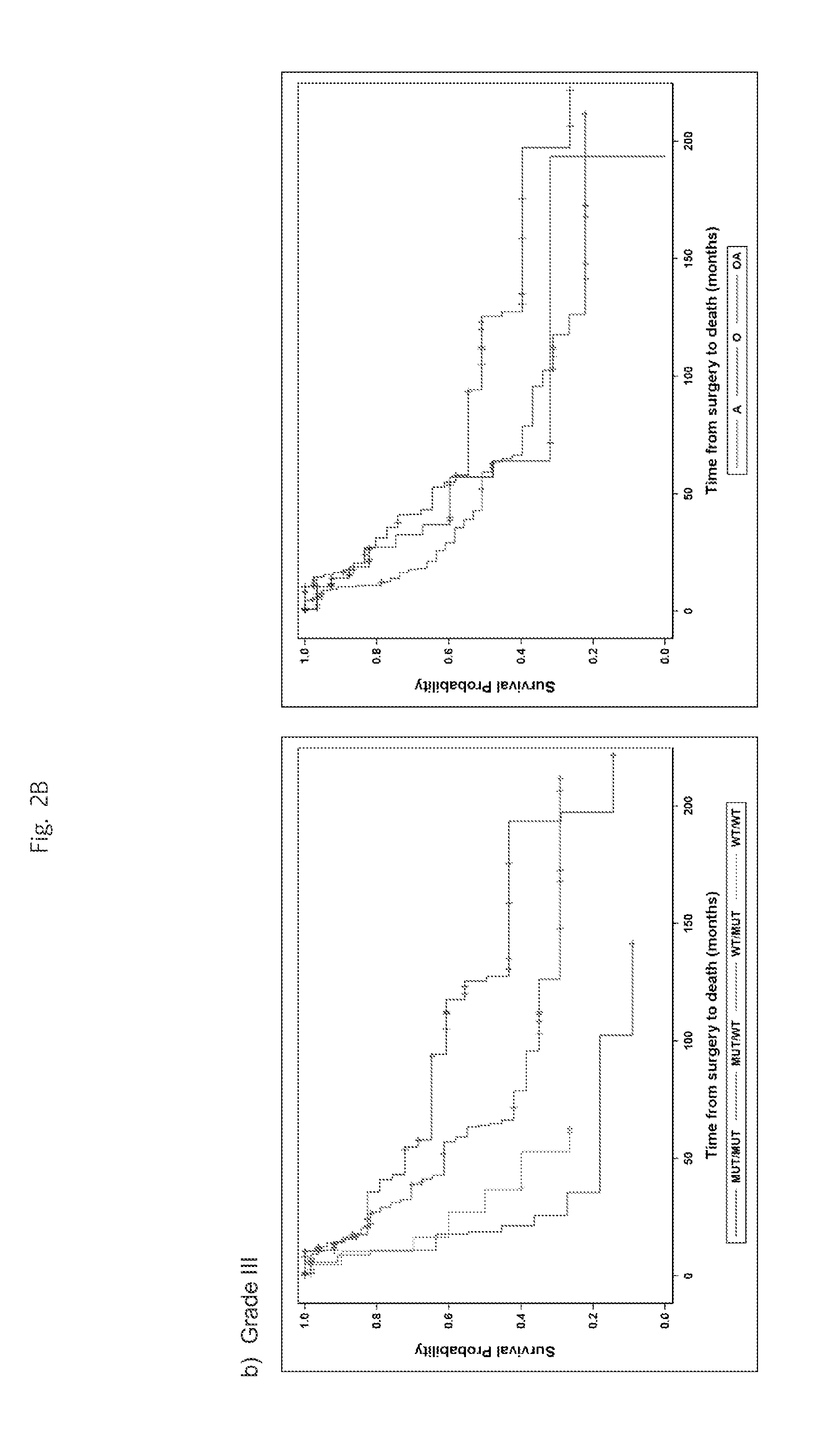
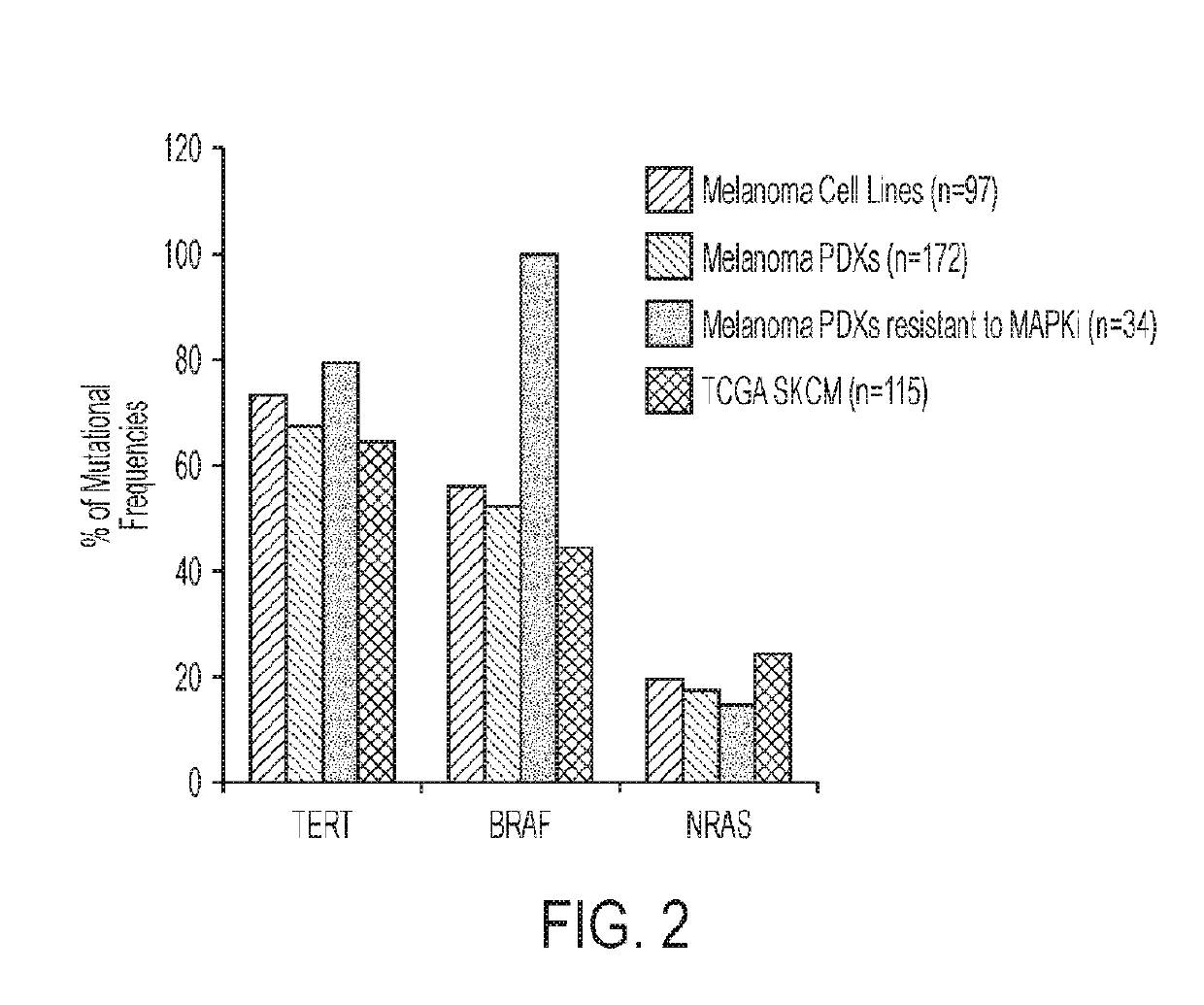
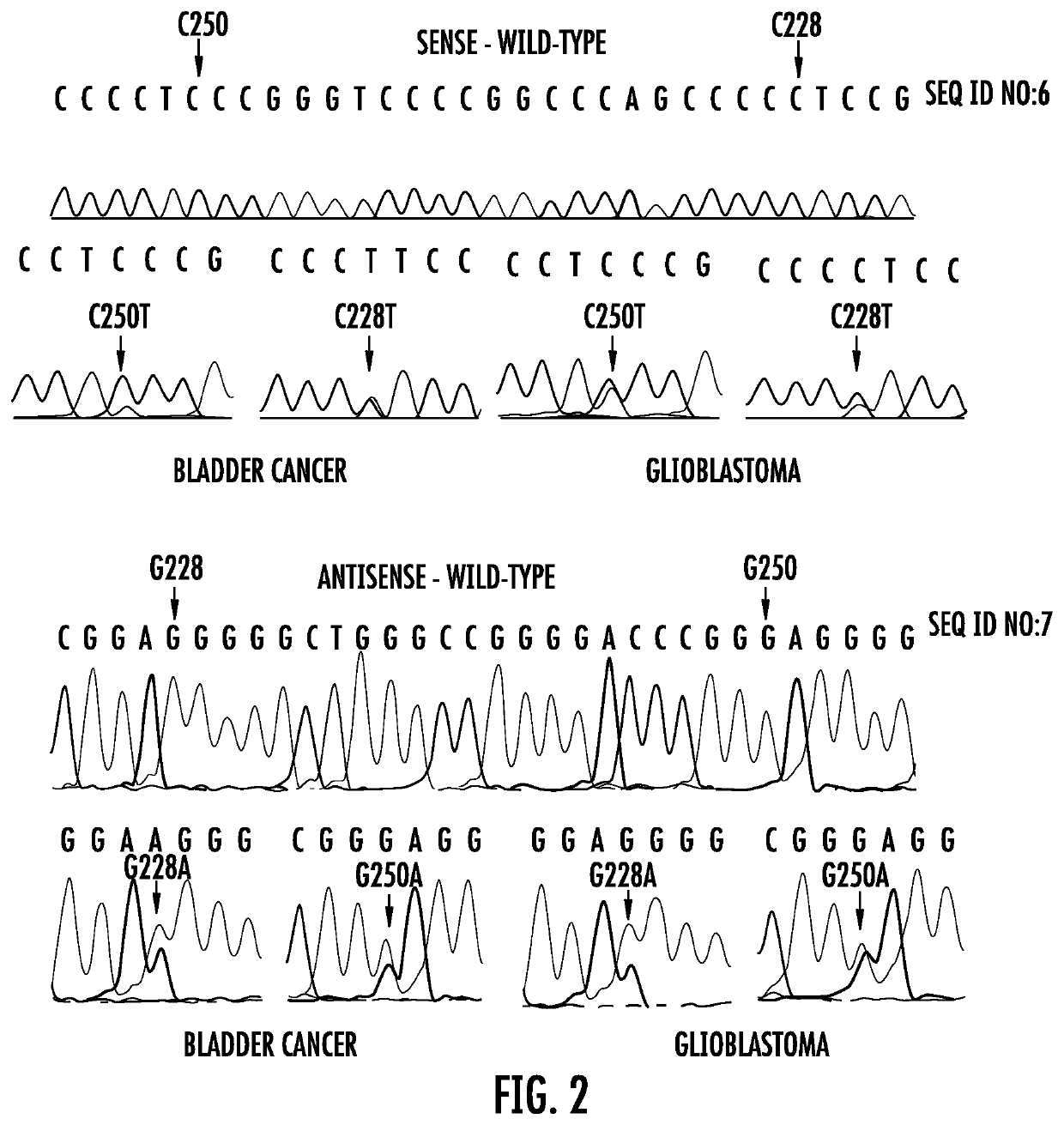
Genetic signatures capable of distinguishing among several types of gliomas provide clinically relevant information that can serve as an adjunct to histopathological diagnosis. For example, mutations in the TERT promoter occurred in 74.2% of glioblastomas (GBM), but occurred in a minority of Grade II-III astrocytomas (18.2%). In contrast, IDH1 / 2 mutations were observed in 78.4% of Grade II-III astrocytomas, but were uncommon in primary GBM. The genetic signatures permit the stratification of the glioma patients into distinct cohorts.
Owner:DUKE UNIV
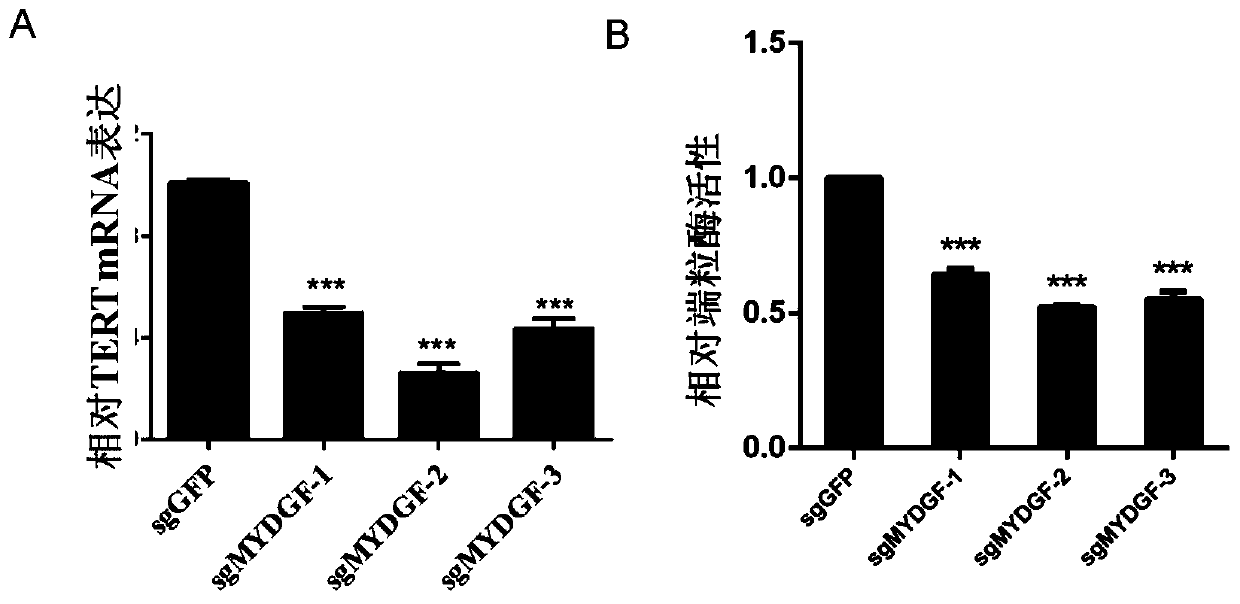
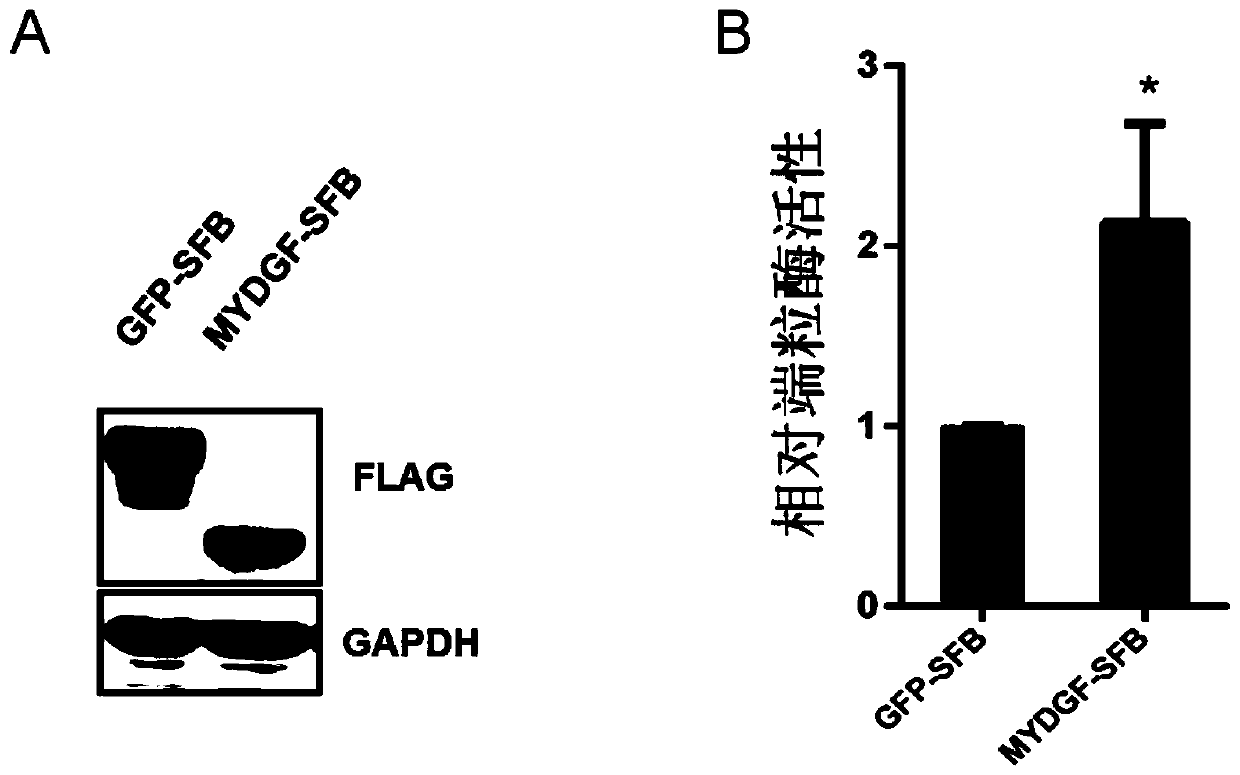
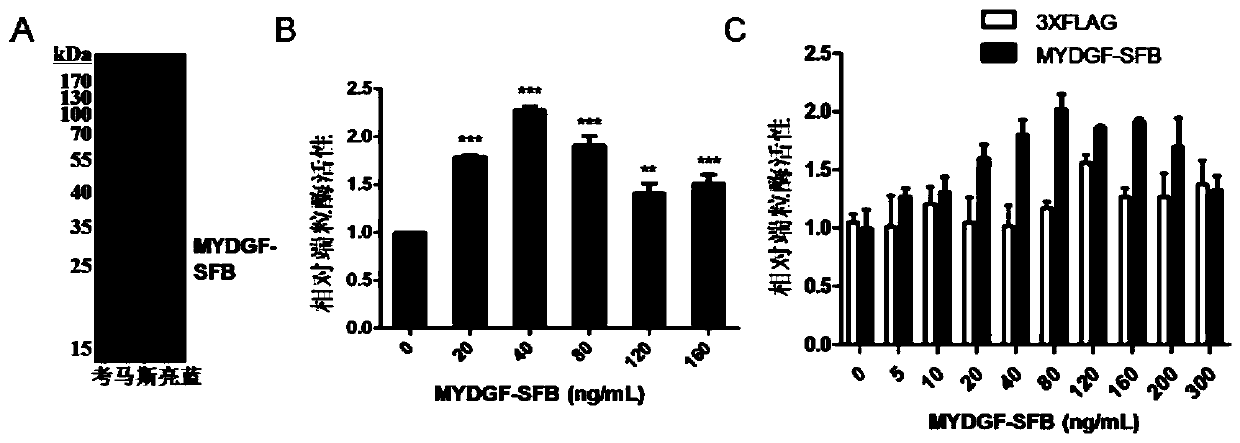
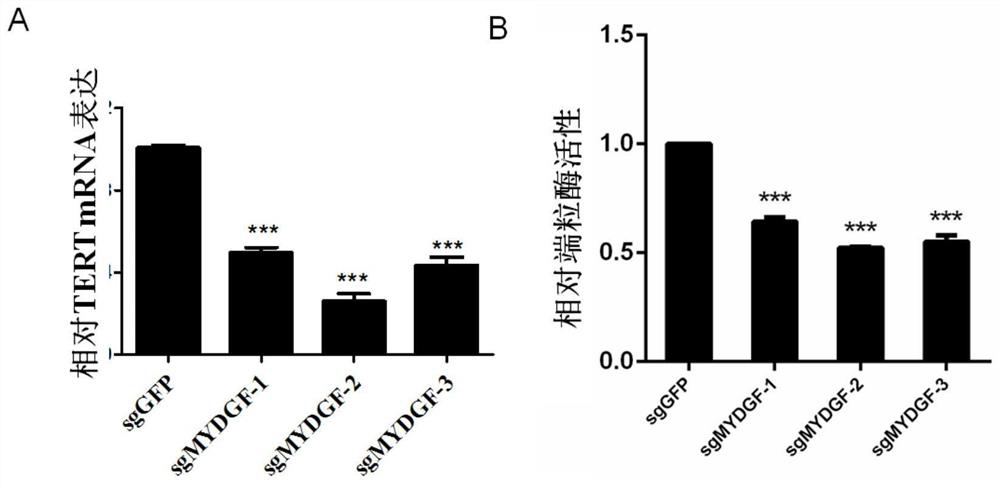
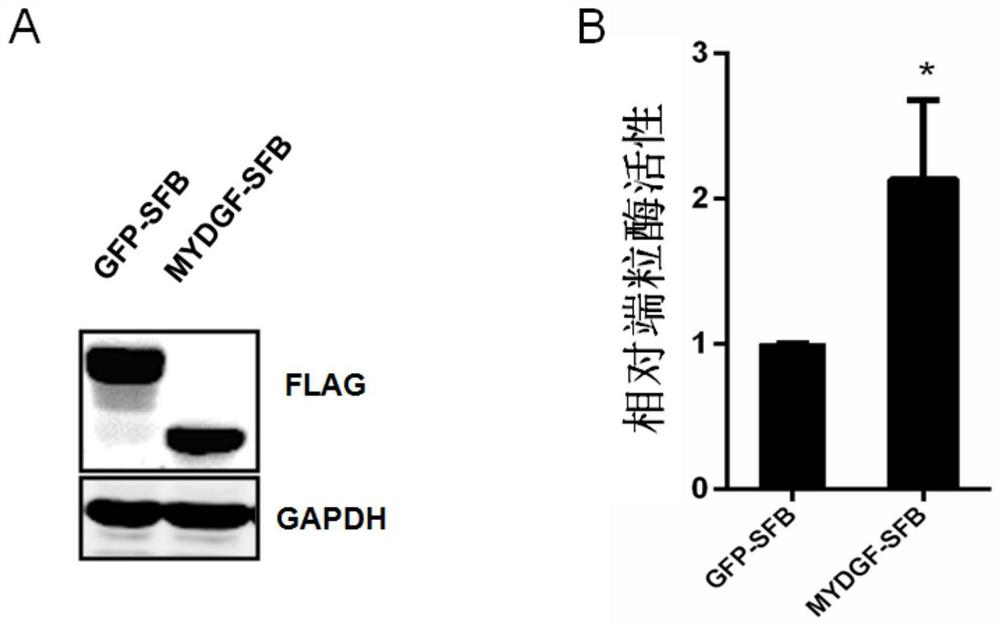
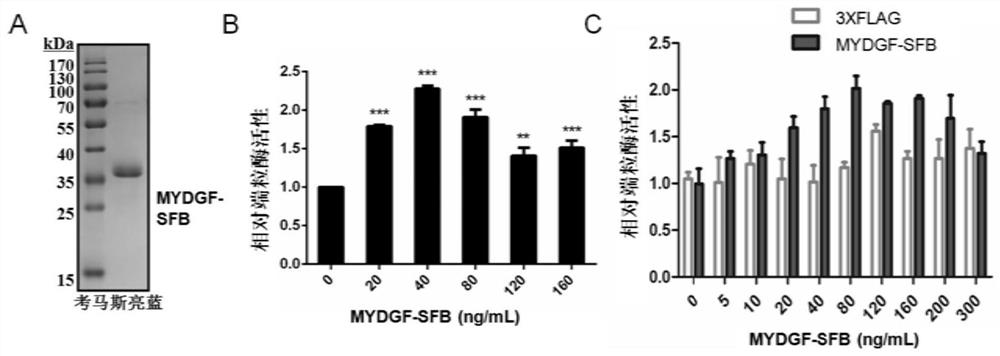
Application of MYDGF protein in preparation of telomerase expression and cell senescence regulating agent
ActiveCN111544572AHigh activityAnti agingPowder deliveryPeptide/protein ingredientsTelomeraseTelomerase Catalytic Subunit
The invention discloses an application of MYDGF protein in preparation of a telomerase expression and cell senescence regulating agent. Research finds that the MYDGF protein acts on a human telomerasecatalytic subunit TERT promoter to activate transcriptional expression of TERT and improve the activity of telomerase. The MYDGF purified protein is added into the human mesenchymal stem cells cultured in vitro, so that telomerase can be activated, reduction of telomerase TERT expression in the replicative aging process is delayed, and cell aging is delayed. Therefore, the protein MYDGF has an important regulation and control function in the telomerase activation and aging process, an effective cell telomerase activation and anti-aging regulation and control agent can be developed for the MYDGF, the cell aging process is delayed, and the protein MYDGF has an important application prospect.
Owner:SUN YAT SEN UNIV
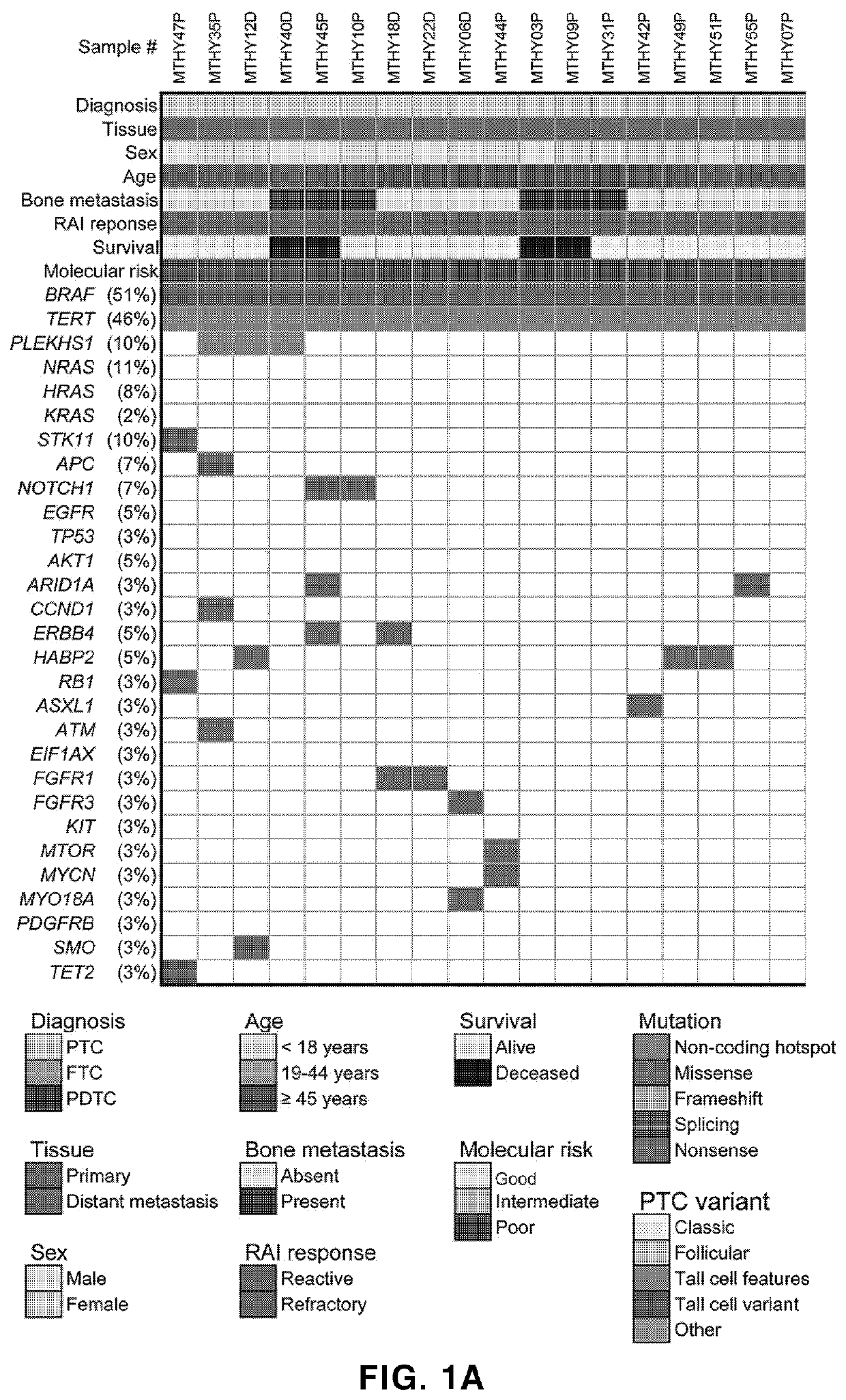
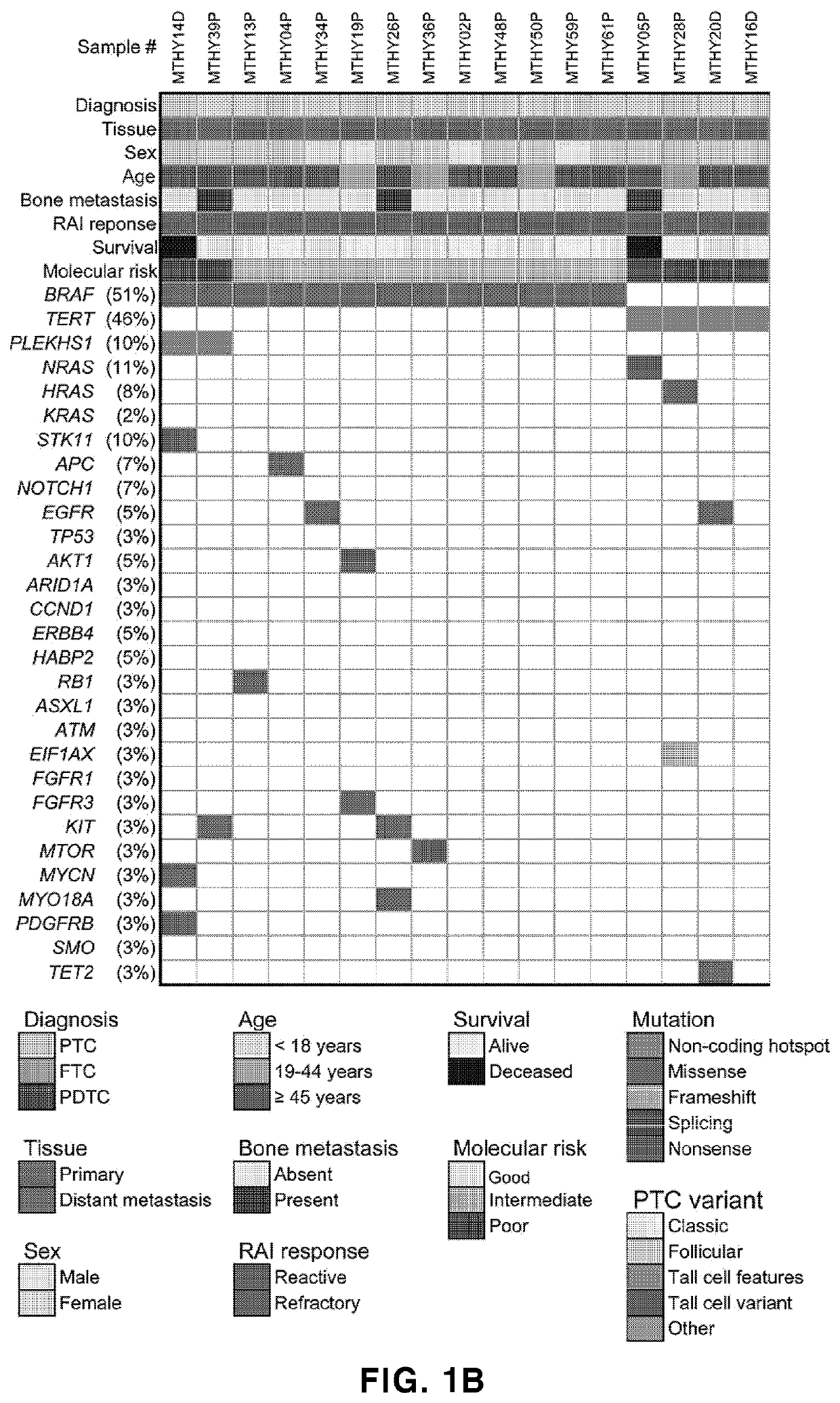
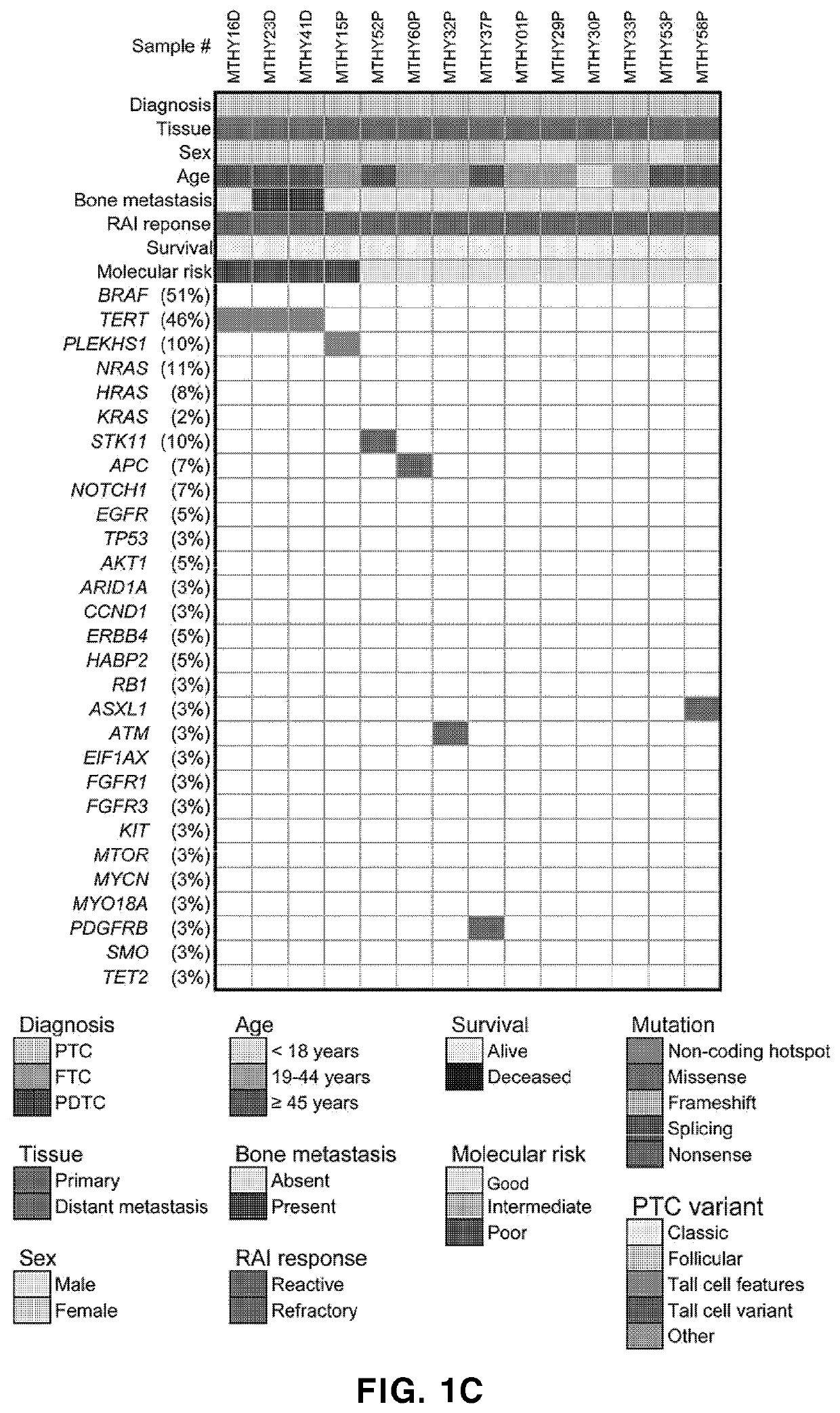
Biomarker composition for diagnosing or predicting prognosis of thyroid cancer, comprising preparation capable of detecting mutation in plekhs1 gene, and use thereof
PendingUS20220356533A1Poor prognosisShorten the lengthMicrobiological testing/measurementRadioactive iodine therapyTert promoter
The present invention relates to: a biomarker composition for diagnosing or predicting the prognosis of thyroid cancer, comprising a preparation capable of detecting a mutation in a PLEKHS1 promoter gene; and a use thereof. The biomarker composition for diagnosing or predicting the prognosis of thyroid cancer of the present invention confirms whether a mutation is present in a PLEKHS1 promoter gene, and thus can provide information needed for diagnosing metastatic (distant metastatic) differentiated thyroid cancer, and also confirms whether a mutation is present in BRAF, TERT promoter, three types of RAS and a TP53 gene in addition to the PLEKHS1 promoter gene, and thus, with respect to radioactive iodine therapy response and survival, can classify the prognosis of a metastatic differentiated thyroid cancer patient into one of three prognosis groups, and predict the same.
Owner:THE CATHOLIC UNIV OF KOREA IND ACADEMIC COOP FOUND
Assays for TERT promoter modulatory agents
InactiveUS20060029962A1High throughput formatMicrobiological testing/measurementHeterologousWild type
Methods and compositions for assaying an agent for TERT promoter modulatory activity are provided. In the subject methods, an agent is contacted with an expression system that includes a TERT promoter nucleic acid operably linked to a heterologous reporter nucleic acid. A feature of the expression system is that it is integrated into a carrier nucleic acid, e.g., a chromosome, in a manner such that it is known to be inactive under wild-type conditions but not positionally silenced. Also provided are compositions, systems and kits thereof, as well as devices, that find use in practicing the subject methods. The subject invention finds use in assaying agents for TERT promoter modulatory activity, such as in a high throughput format.
Owner:SIERRA SCIENCES
Method of diagnosing cancer comprising detection of the methylation signature in the hTERT promoter
A method of diagnosing cancer in a mammal is provided. The method includes the steps of determining in a nucleic acid-containing sample from the mammal the degree of DNA methylation of a target region within the hTERT promoter from the nucleotide at about position −157 to the nucleotide at about position −580, or a corresponding target region in a TERT promoter in a mammal other than a human, to yield a sample methylation signature, determining the baseline degree of DNA methylation of the target region in a control sample to yield a control methylation signature, comparing the sample methylation signature to the control methylation signature and rendering a diagnosis of cancer when there is at least 1.5 times more methylation in the sample methylation signature as compared to the control methylation signature. Methods of determining tumor grade and progression, predicting survival and determining whether or not a mammal is a candidate for telomerase-targeted or demethylation therapy are also provided.
Owner:HOSPITAL FOR SICK CHILDREN
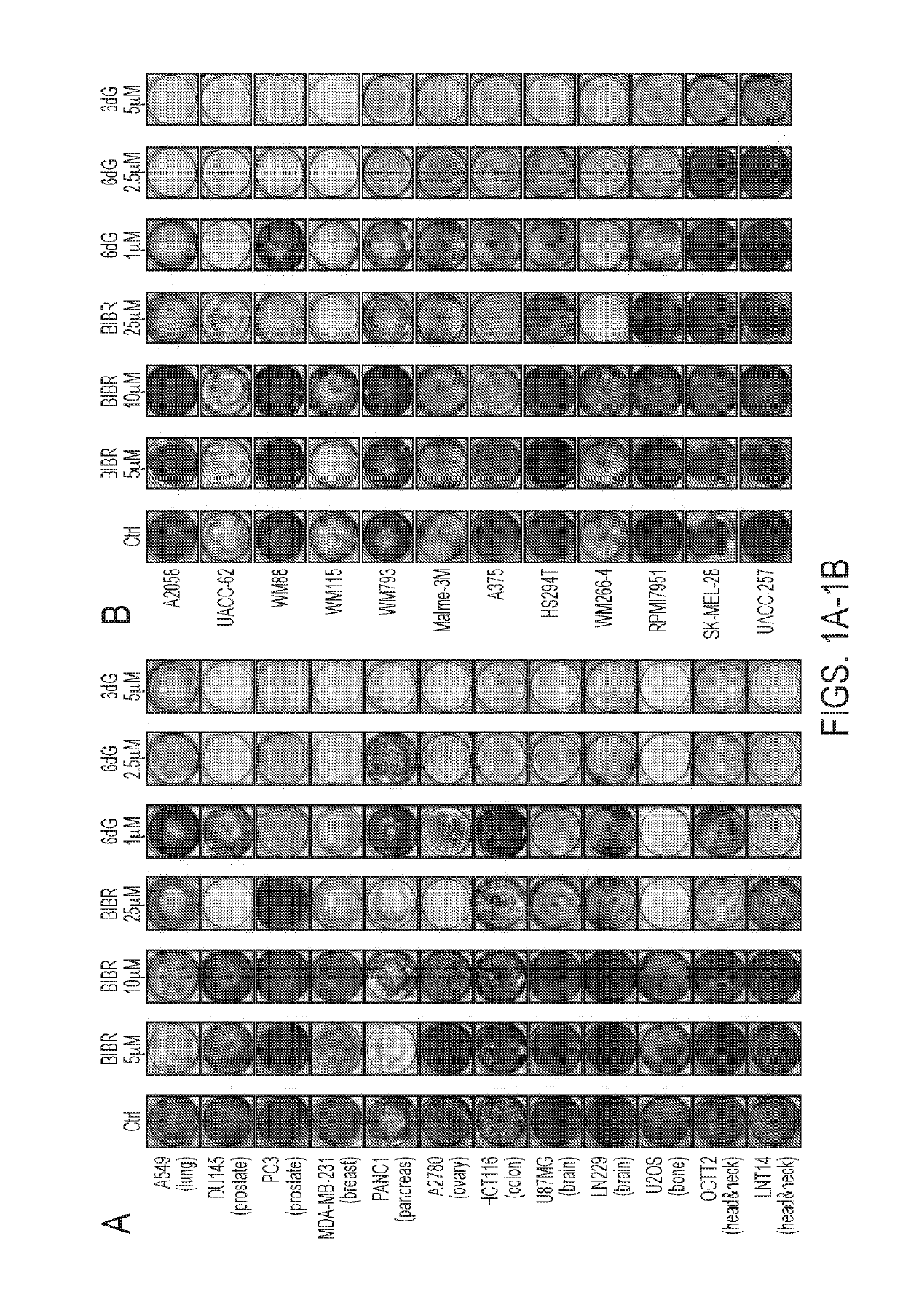
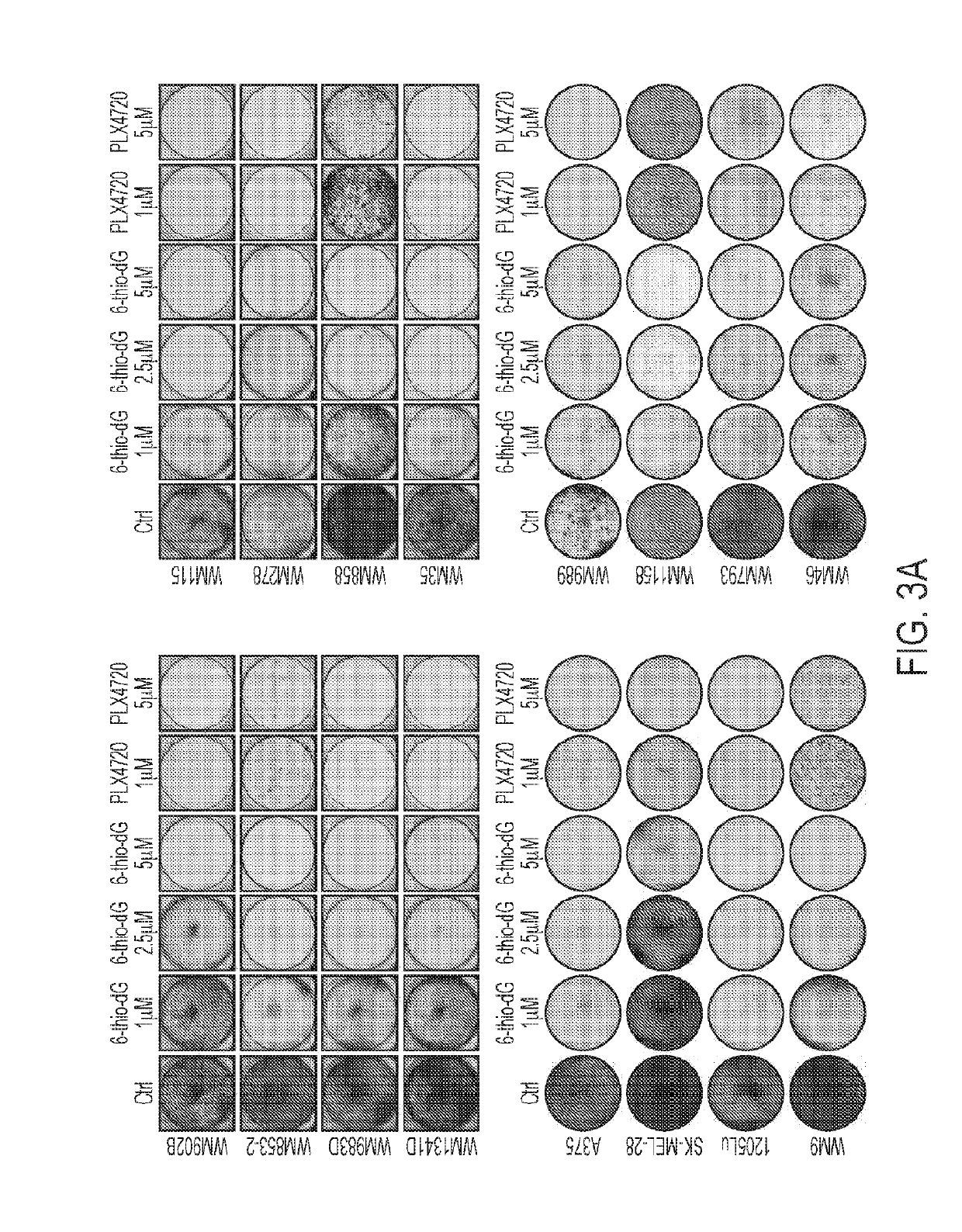
6-thio-2'-deoxyguanosine (6-thio-dg) results in telomerase dependent telomere dysfunction and cell death in various models of therapy-resistant cancer cells
PendingUS20190298751A1Telomeres shortenImprove survivalOrganic active ingredientsAntineoplastic agentsTherapy resistantTelomerase
The present disclosure provide for methods of using 6-thio-2′-deoxyguanosine (6-thio-dG) to treat telomerase-positive cancers that exhibit (a) one or more TERT promoter mutations, and / or (b) enriched telomere transcriptional signature(s). In particular, melanomas, including those who are not sensitive or have become resistant to immune checkpoint inhibition and / or MAPKi therapy are targets for this therapy.
Owner:THE WISTAR INST OF ANATOMY & BIOLOGY +1
Kit and method for detecting mutation of TERT promoter through pyrosequencing method
InactiveCN108285922AAccurate detectionImprove throughputMicrobiological testing/measurementMagnetic beadPolymerase L
The invention discloses a kit and a method for detecting the mutation of a TERT promoter through a pyrosequencing method. The mutation of the TERT promoter specifically refers to mutations of -124bp 1295, 228C>T and -146bp 1259, 250C>T on the upstream of the promoter. The kit comprises a PCR (Polymerase Chain Reaction) buffer solution, a TaqDNA polymerase, a primer, a template DNA, a pyrosequencing kit, a magnetic bead, a combined buffer solution, a washing buffer solution, an annealing buffer solution and a denaturation buffer solution. The kit can accurately efficiently detect the mutation of the TERT promoter at high throughput, so that the evidence is provided for the early diagnosis and screening of the thyroid cancer.
Owner:XIANGYA HOSPITAL CENT SOUTH UNIV
Selective antibody targeting of undifferentiated stem cells
Owner:ASTERIAS BIOTHERAPEUTICS INC
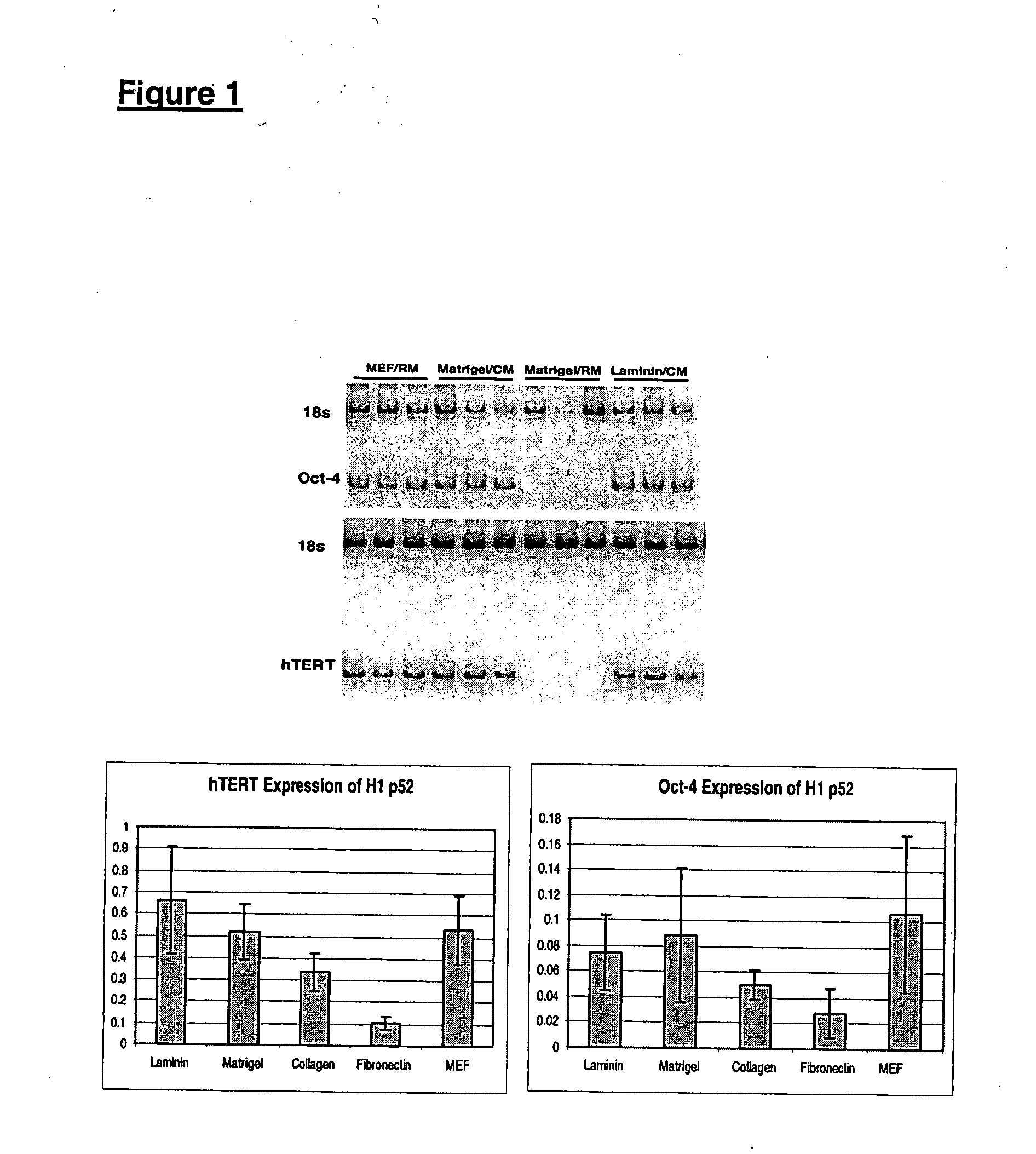
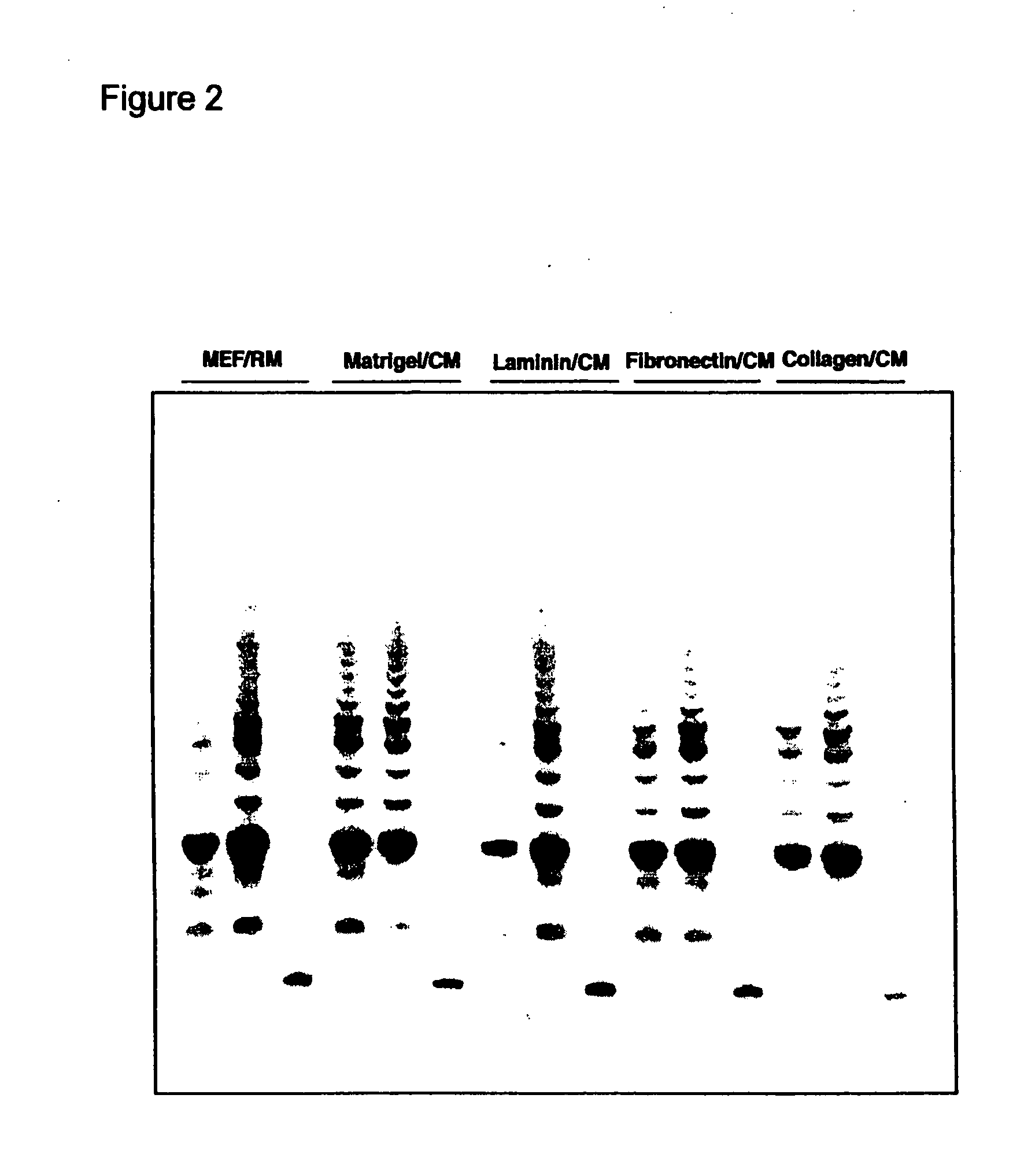
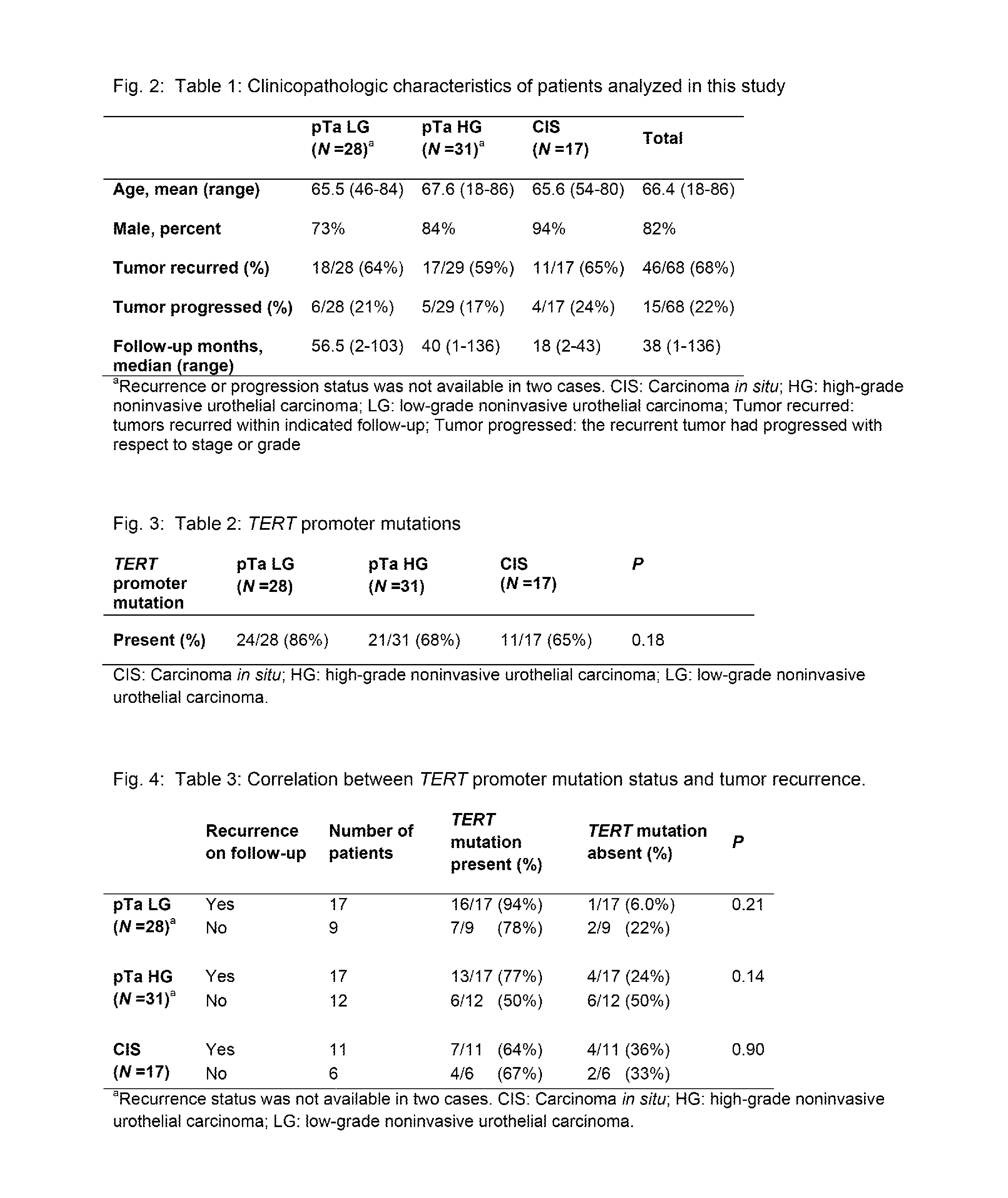
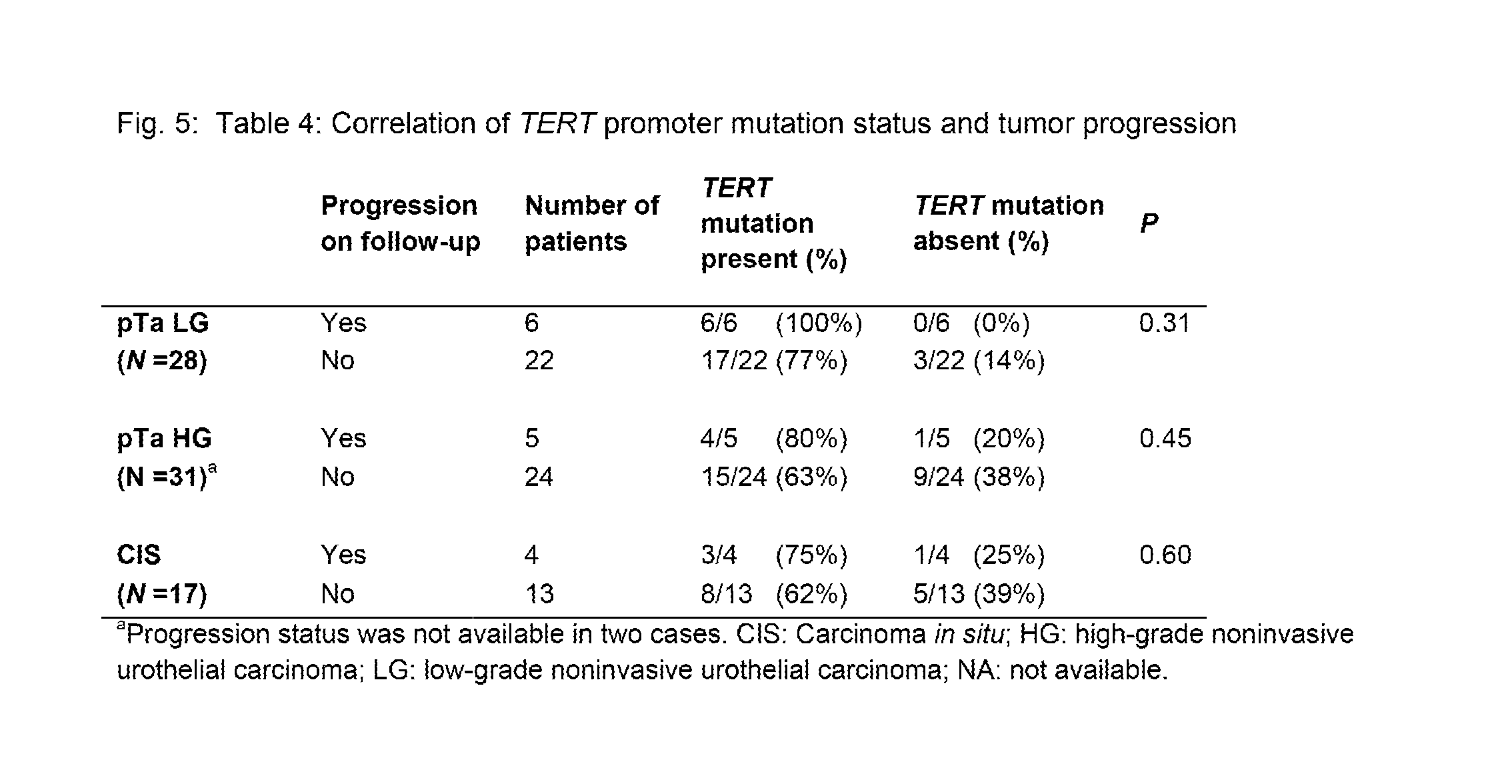
TERT Promoter Mutations in Urothelial Neoplasia
TERT promoter mutations occur in both papillary and flat lesion bladder cancers, are the most frequent genetic alterations identified to date in noninvasive precursor lesions of the bladder, are detectable in urine, and appear to be strongly associated with bladder cancer recurrence. The TERT promoter mutations are useful urinary biomarker for both the early detection and monitoring of bladder neoplasia.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Assays for TERT promoter modulatory agents
Methods and compositions for assaying an agent for TERT promoter modulatory activity are provided. In the subject methods, an agent is contacted with a normal cell under assay conditions that provide for a detectable phenotype, e.g., cell death, upon modulation of TERT promoter transcription control activity. Also provided are compositions, systems and kits thereof, as well as devices, that find use in practicing the subject methods. The subject invention finds use in assaying agents for TERT promoter modulatory activity, such as in a high throughput format.
Owner:SIERRA SCIENCES
Primer composition for tumor methylation site detection and application thereof
ActiveCN113308547ASimple and fast operationHigh feasibilityMicrobiological testing/measurementDNA/RNA fragmentationTert promoterOncology
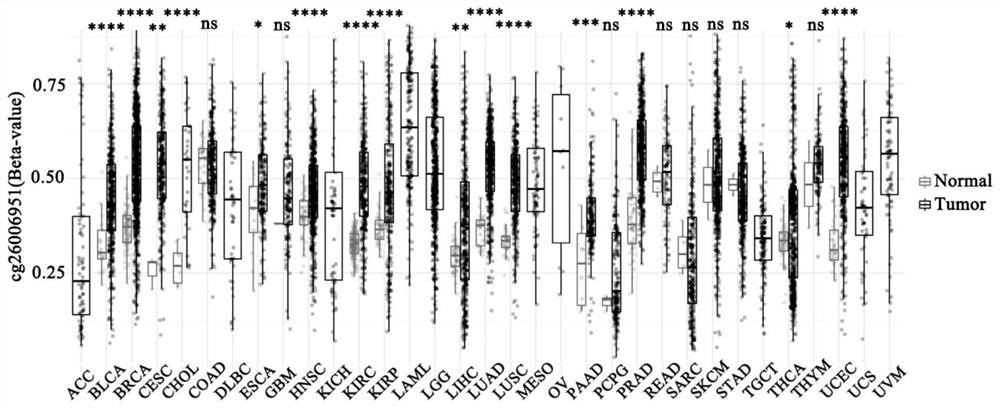
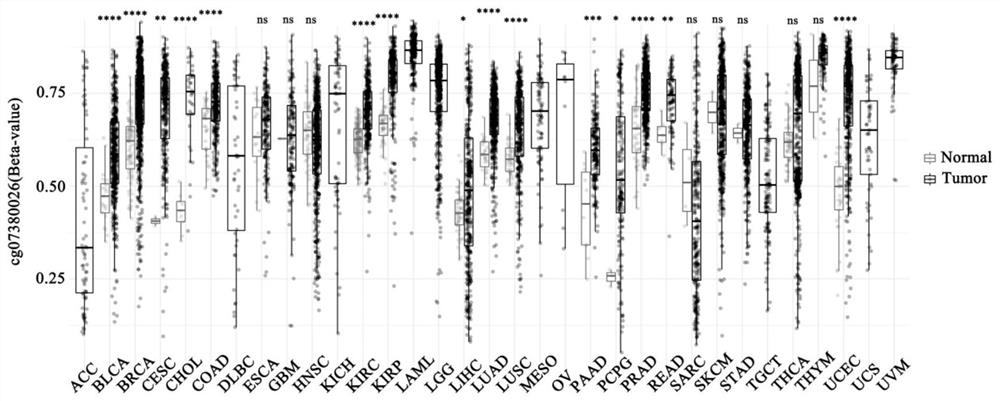
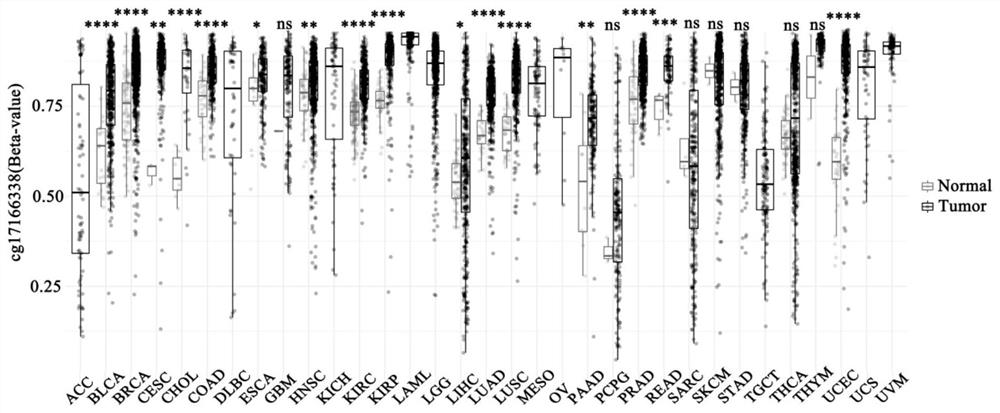
The invention provides a primer composition for detecting the methylation level of a TERT promoter and application thereof in tumor detection. With the application of the primer composition, the methylation levels of the cg26006951 site, the cg17166338 site and the cg07380026 site of the TERT promoter can be detected through the pyrosequencing technology so as to further perform pathological staging grading on the kidney cancer. The TERT promoter methylation level detection sensitivity and accuracy are high, which has important guiding significance for early detection, prognosis and risk assessment of kidney cancer.
Owner:HUNAN LINGKANG MEDICAL TECH CO LTD
Assays for TERT promoter modulatory agents
InactiveUS20060154266A1High throughput assayMicrobiological testing/measurementOther foreign material introduction processesTert promoterBiochemistry
Methods and compositions for assaying an agent for TERT promoter modulatory activity are provided. In the subject methods, an agent is contacted with a cell comprising a mutant telomerase structural RNA component (TR) that results in a detectable phenotype in the presence of telomerase reverse transcriptase (TERT). Also provided are compositions, systems and kits thereof, as well as devices, that find use in practicing the subject methods. The subject invention finds use in assaying agents for TERT promoter modulatory activity, such as in a high throughput format.
Owner:SIERRA SCIENCES
TERT promoter mutations in cancer
The present invention relates to the field of cancer. More specifically, the present invention provides methods and compositions related to certain promoter mutations in cancer. In one embodiment, a method for treating a subject having thyroid cancer comprises the steps of (a) obtaining a biological sample from the subject; (b) performing an assay on the sample obtained from the subject to identify a mutation at 1 295 228 C>T (C228T) and 1 295 250 C>T (C250T), corresponding to −124 C>T and −146 C>T from the translation start site in the promoter of the telomerase reverse transcriptase (TERT) gene; (c) identifying the subject as having or likely to develop aggressive thyroid cancer if the C228T and / or C250T mutation is identified; and (d) treating the subject with one or more treatment modalities appropriate for a subject having or likely to develop aggressive thyroid cancer.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Application of mydgf protein in preparation of regulators for telomerase expression and cell senescence
ActiveCN111544572BHigh activityAnti agingPowder deliveryPeptide/protein ingredientsTelomeraseTelomerase Catalytic Subunit
The invention discloses the application of MYDGF protein in the preparation of telomerase expression and cell senescence regulator. The research of the present invention finds that the MYDGF protein acts on the human telomerase catalytic subunit TERT promoter, activates the transcription and expression of TERT, and improves the activity of telomerase. In human mesenchymal stem cells cultured in vitro, adding MYDGF purified protein can activate telomerase, delay the decline of telomerase TERT expression during replicative aging, and delay cell senescence. Therefore, the protein MYDGF has an important regulatory function in the process of telomerase activation and aging, and effective regulatory agents for cell telomerase activation and anti-aging can be developed for MYDGF to delay the process of cell aging, which has important application prospects.
Owner:SUN YAT SEN UNIV
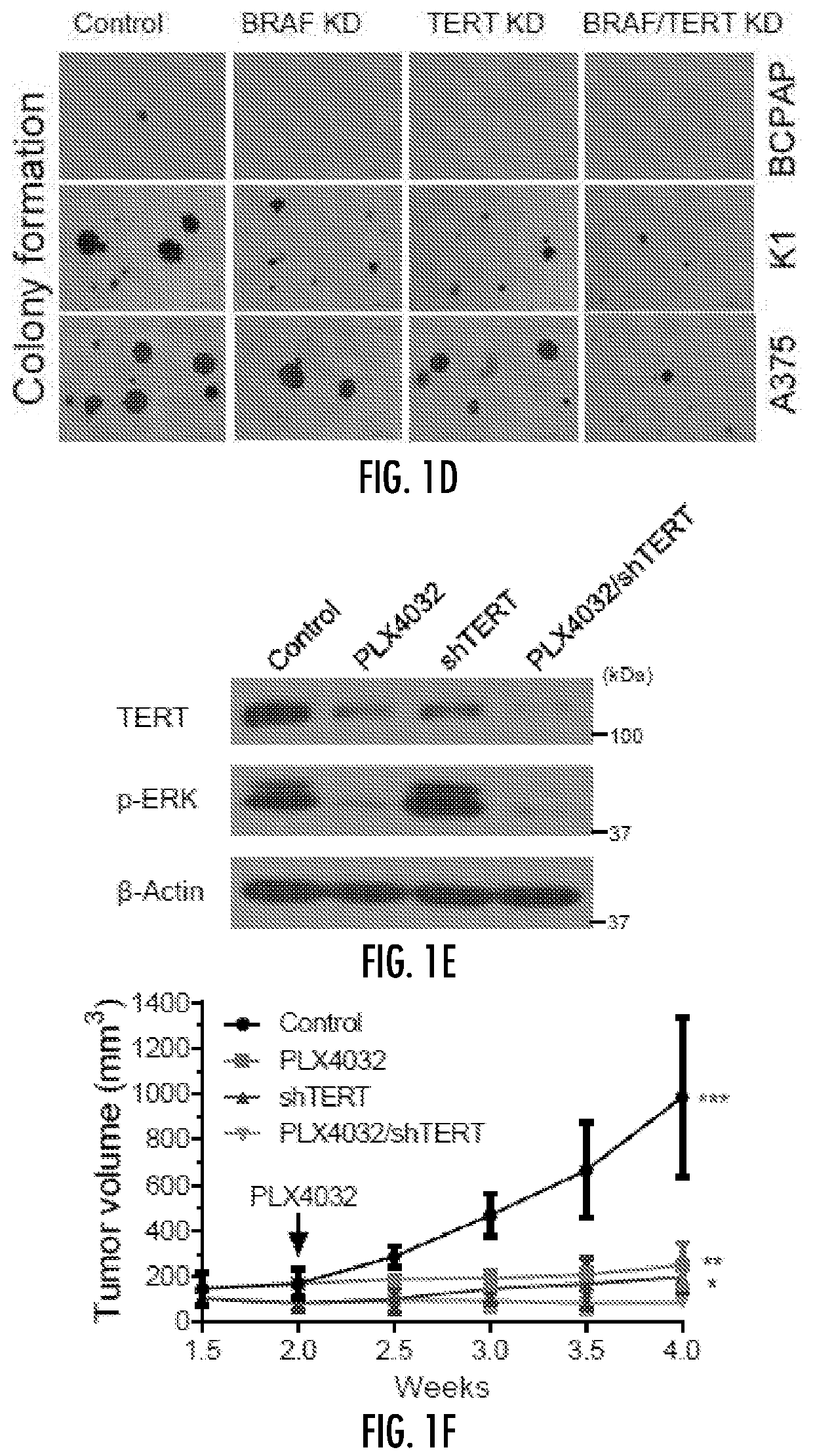
Regulation of mutant tert by braf v600e/map kinase pathway through fos/gabp in human cancer
PendingUS20200323805A1High expressionRobustly upregulating the expression of TERTOrganic active ingredientsAntineoplastic agentsTelomeraseReverse transcriptase
The present invention relates to the field of cancer. More specifically, the present invention provides methods and compositions useful for the treatment of cancer characterized by TERT and BRAF mutations. In a specific embodiment, a method for treating a mutant telomerase reverse trancriptase (TERT) enzyme-associated cancer in a subject comprises the step of administering to the subject an anti-cancer agent that inhibits one or more of FOS, GABPB, the formation of the GABPA-GABPB complex or the binding of the GABPA-GABPB complex to a mutant TERT promoter.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Methods for rapid and sensitive detection of hotspot mutations
Methods that rapidly, sensitively, and specifically detect mutations in IDH1 / 2 and the TERT promoter employ amplification of particular portions of the genes that experience frequent and exquisitely localized mutations. The ability to distinguish between sequences that differ only by one nucleotide and which may be present in very low ratios is essential for such an assay.
Owner:DUKE UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com