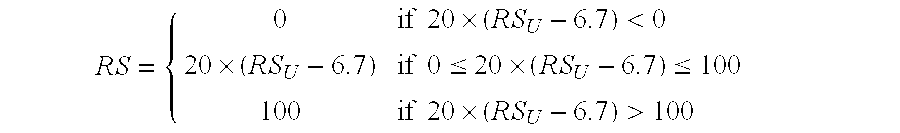Patents
Literature
91 results about "Therapy response" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
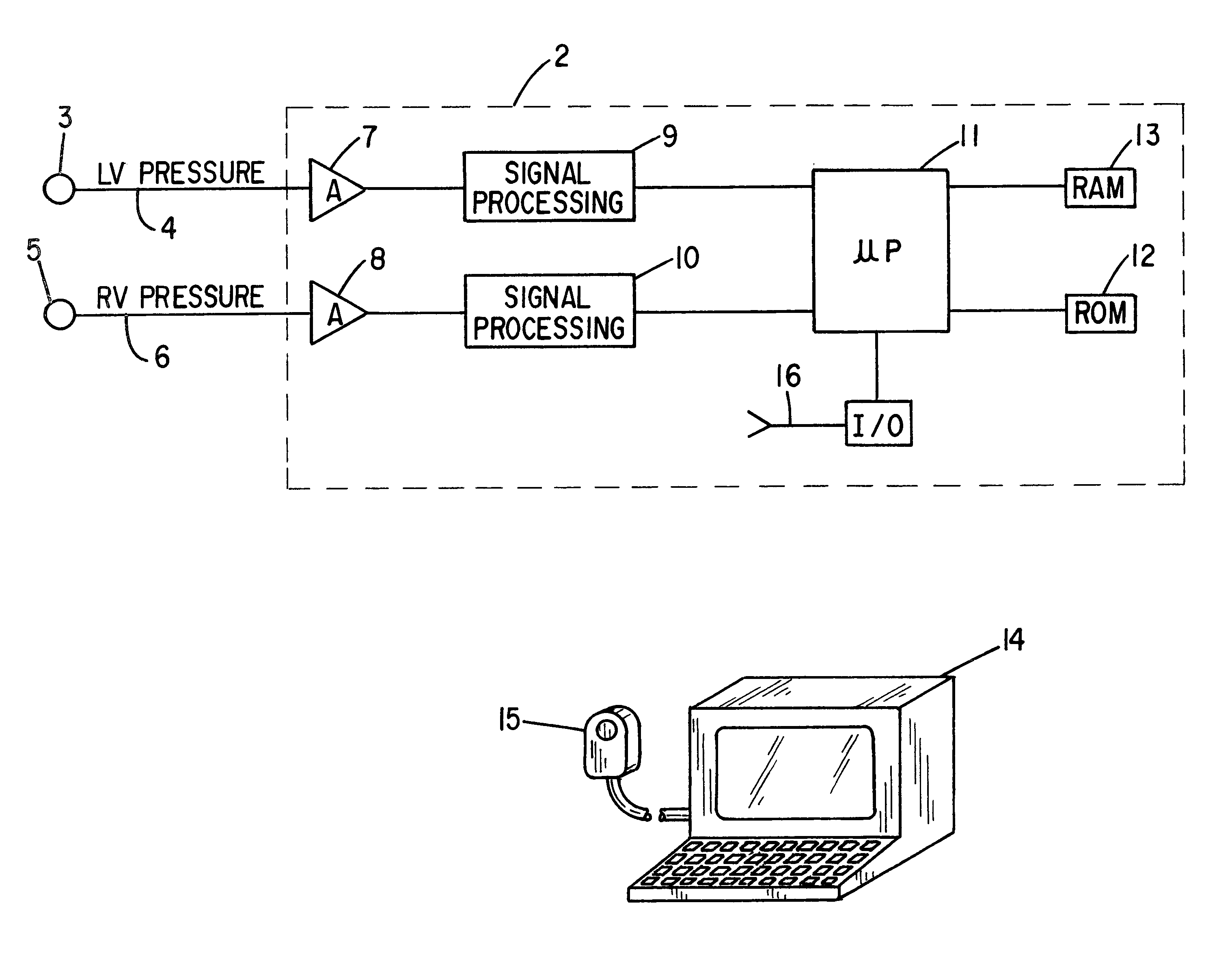
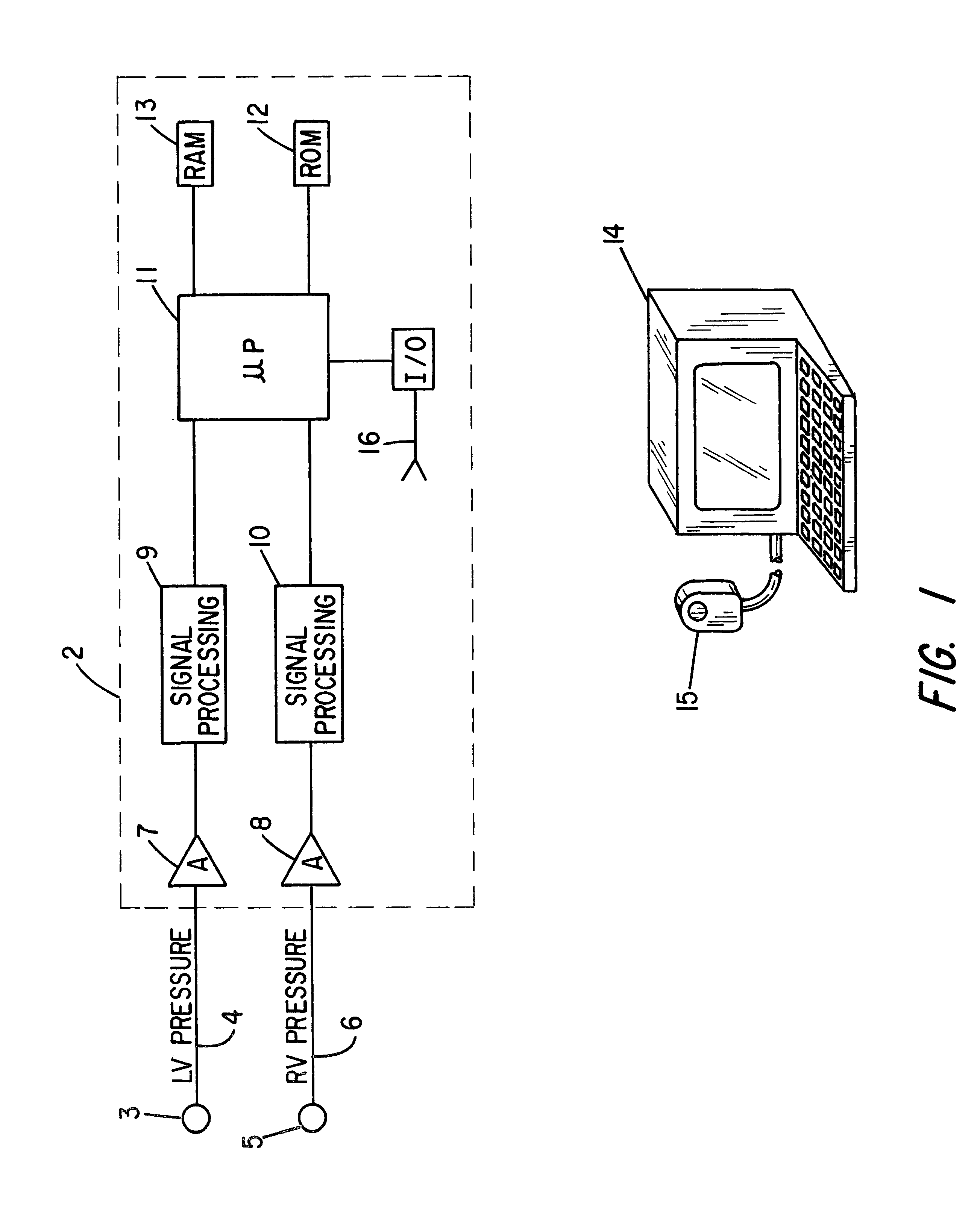
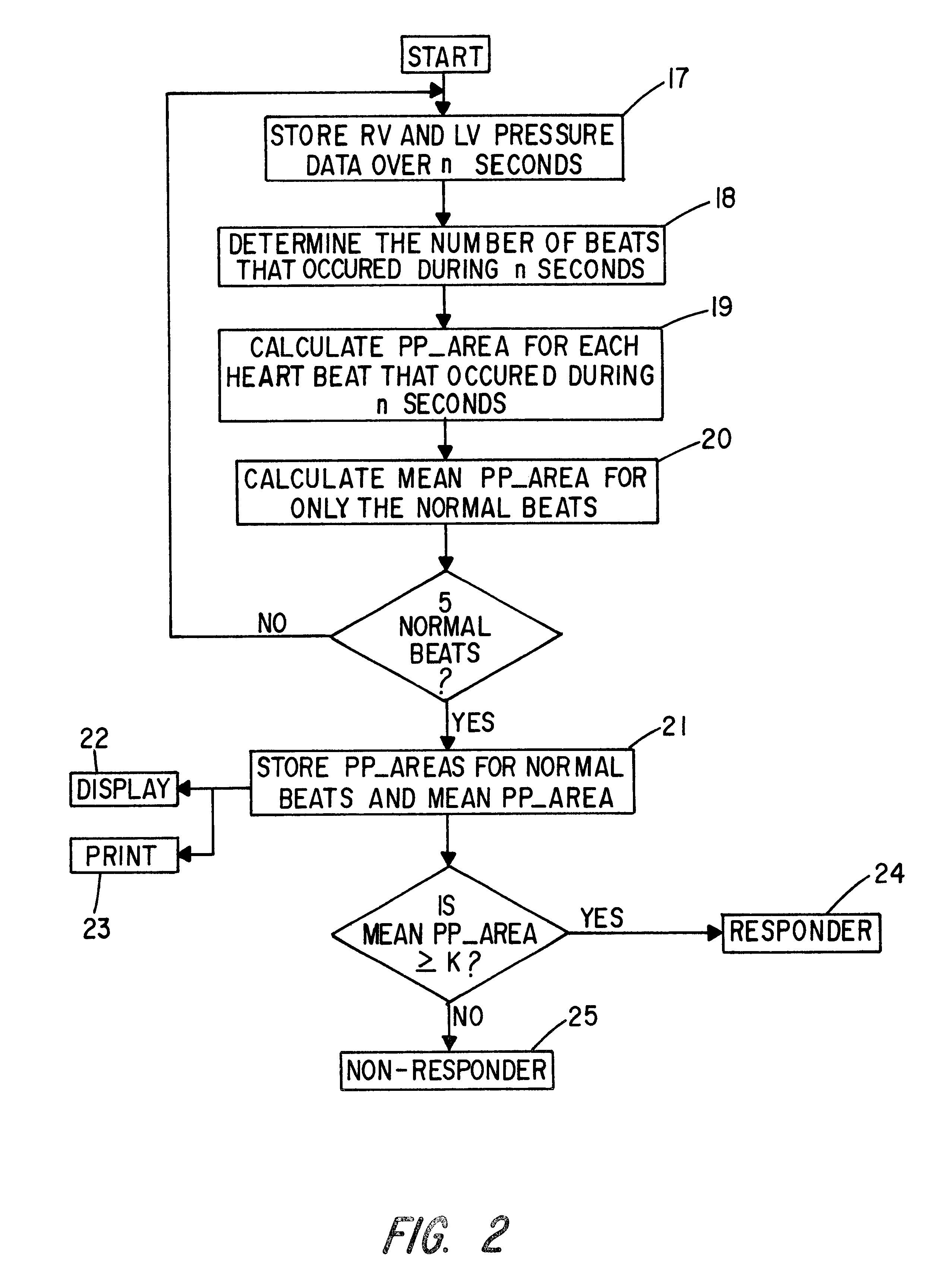
Patient identification for the pacing therapy using LV-RV pressure loop
InactiveUS6280389B1Evaluation of blood vesselsCatheterLeft ventricular sizeCongestive heart failure chf
A method and apparatus for determining whether a patient with congestive heart failure (CHF) will benefit from pacing therapy through the use of an implantable cardiac rhythm management device. A patient's right ventricular and left ventricular pressures are measured, and the patient's PP_Area is calculated for each normal heartbeat that occurs during the testing period. Depending upon the value of the patient's mean PP_Area, it can be determined whether the patient will or will not respond well acutely to pacing therapy. A mean PP_Area value of greater than or equal to a predetermined threshold, which is about 0.3, indicates that the patient is a responder to pacing therapy, while a value of less than the predetermined threshold of about 0.3 indicates that the patient is a non-responder.
Owner:CARDIAC PACEMAKERS INC
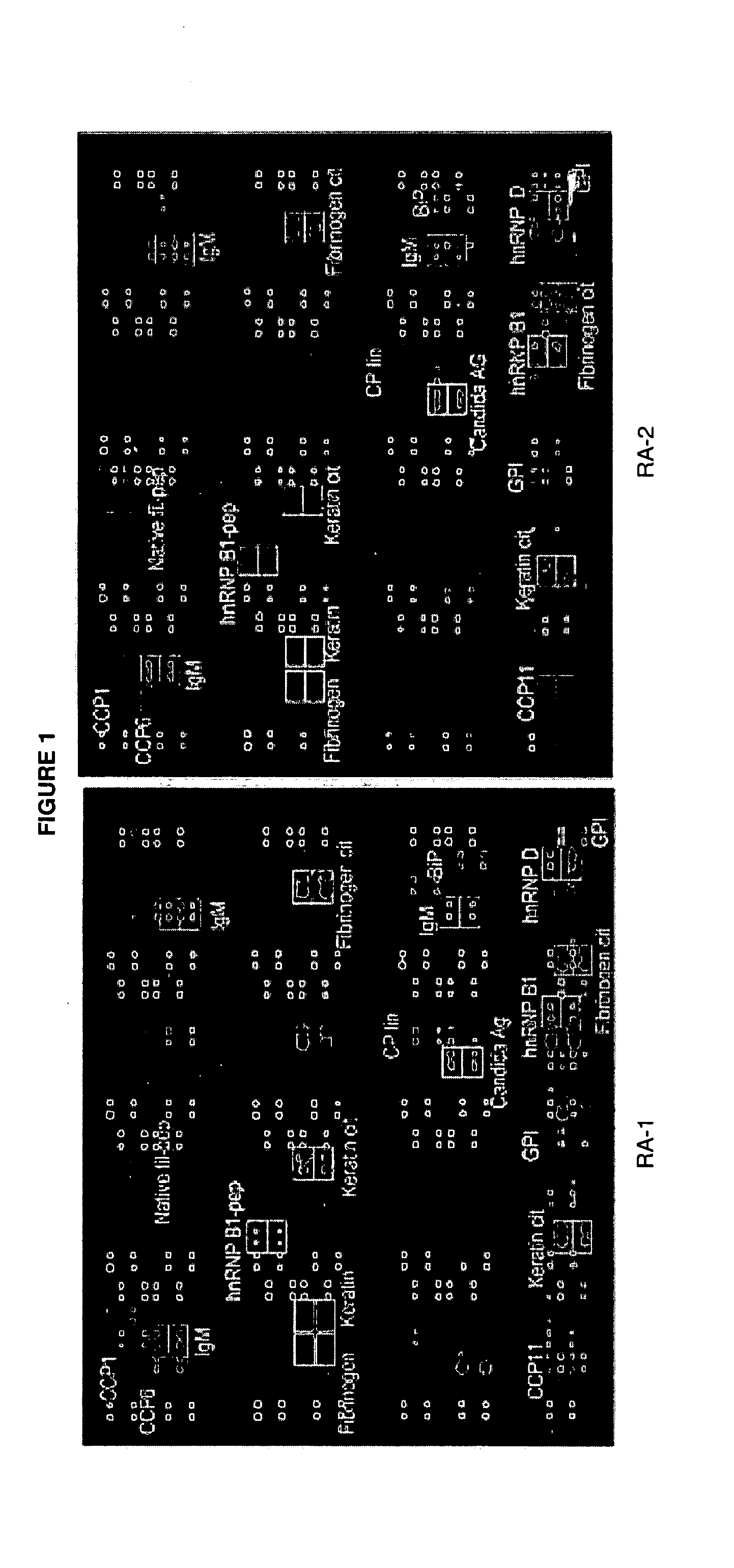
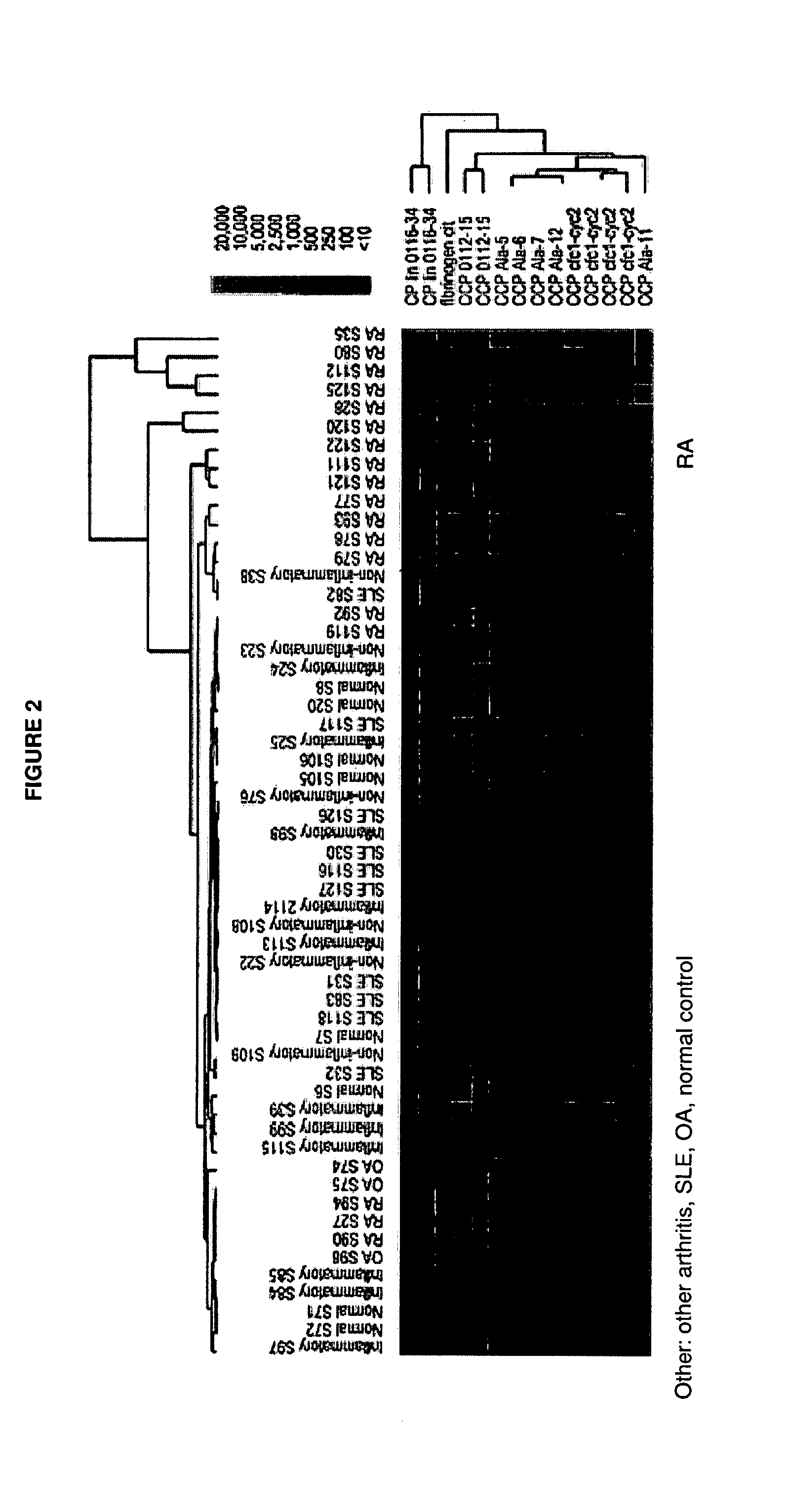
Antibody profiling for determination of patient responsiveness
InactiveUS20080026485A1Increase probabilityConvenient careDisease diagnosisDiseaseAutoimmune disease
Compositions and methods are provided for prognostic classification of autoimmune disease patients into subtypes, which subtypes are informative of the patient's need for therapy and responsiveness to a therapy of interest. The patterns of circulating blood levels of serum autoantibodies and / or cytokines provides for a signature pattern that can identify patients likely to benefit from therapeutic intervention as well as discriminate patients that have a high probability of responsiveness to a therapy from those that have a low probability of responsiveness. Additionally, serum autoantibody and / or cytokine signature patterns can be utilized to monitor responses to therapy. Assessment of this signature pattern of autoantibodies and / or cytokines in a patient thus allows improved methods of care. In one embodiment of the invention, the autoimmune disease is rheumatoid arthritis.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
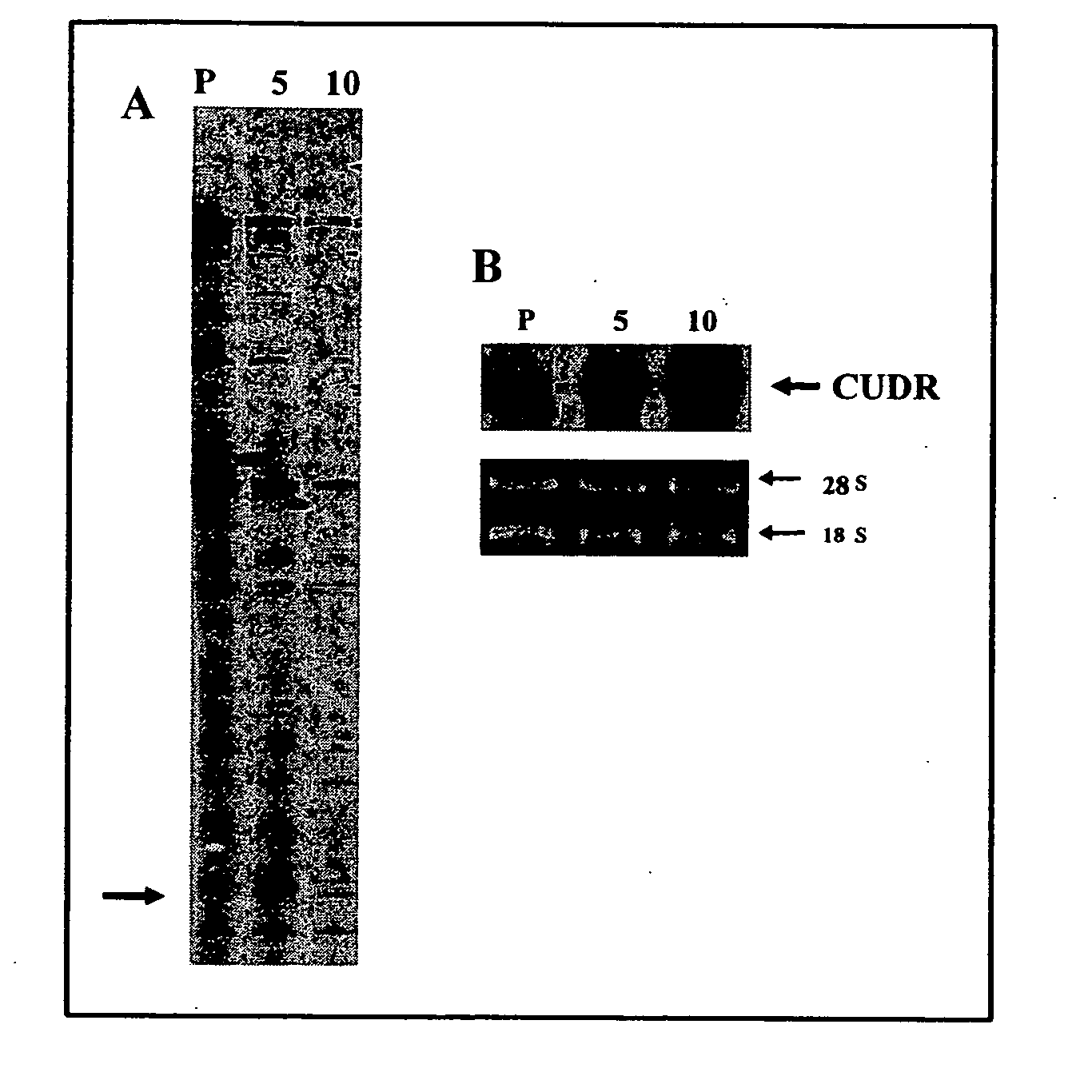
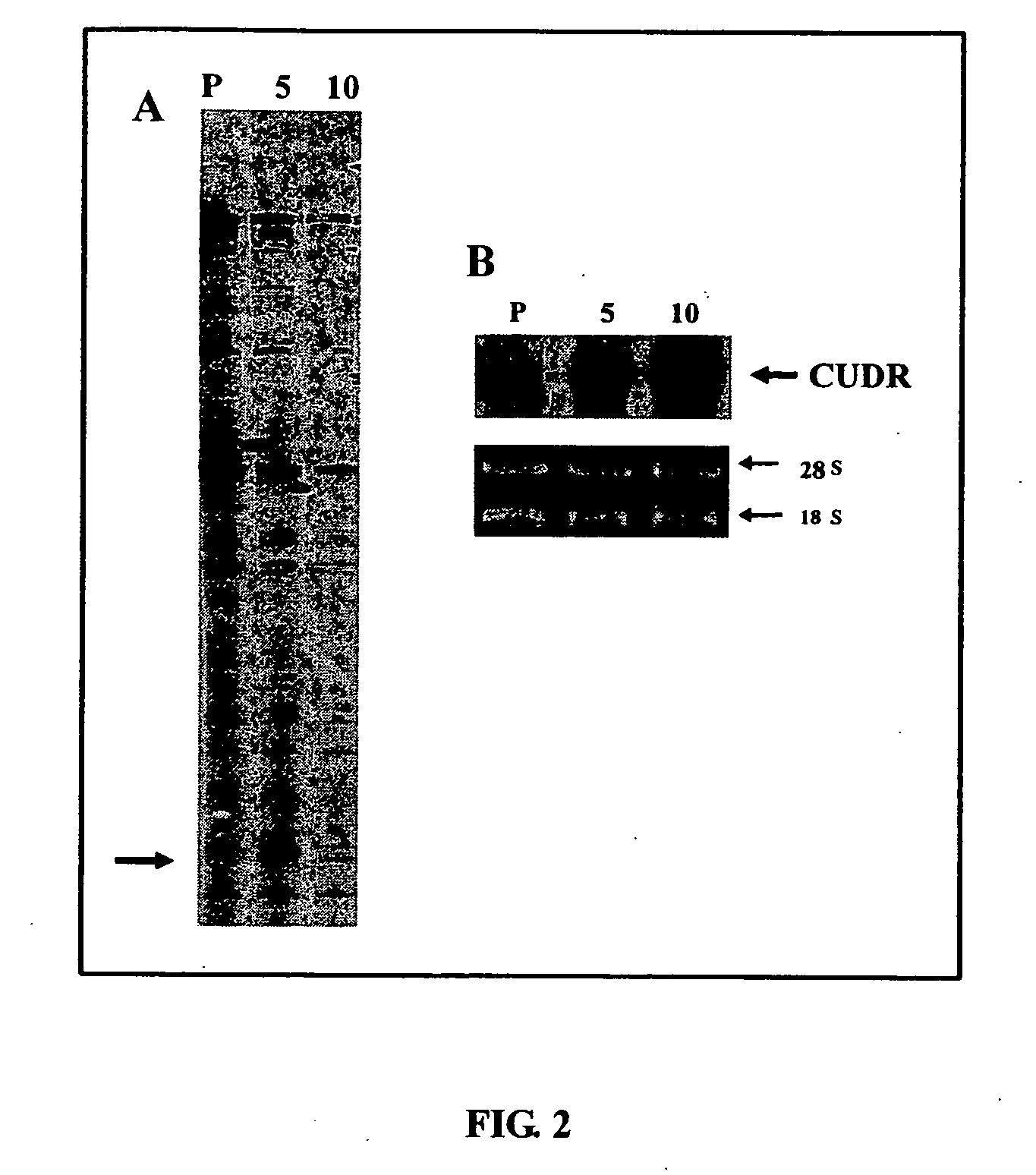
CUDR as biomarker for cancer progression and therapeutic response
InactiveUS20080044828A1Sugar derivativesMicrobiological testing/measurementHuman cancerCancer therapy
Owner:THE CHINESE UNIVERSITY OF HONG KONG
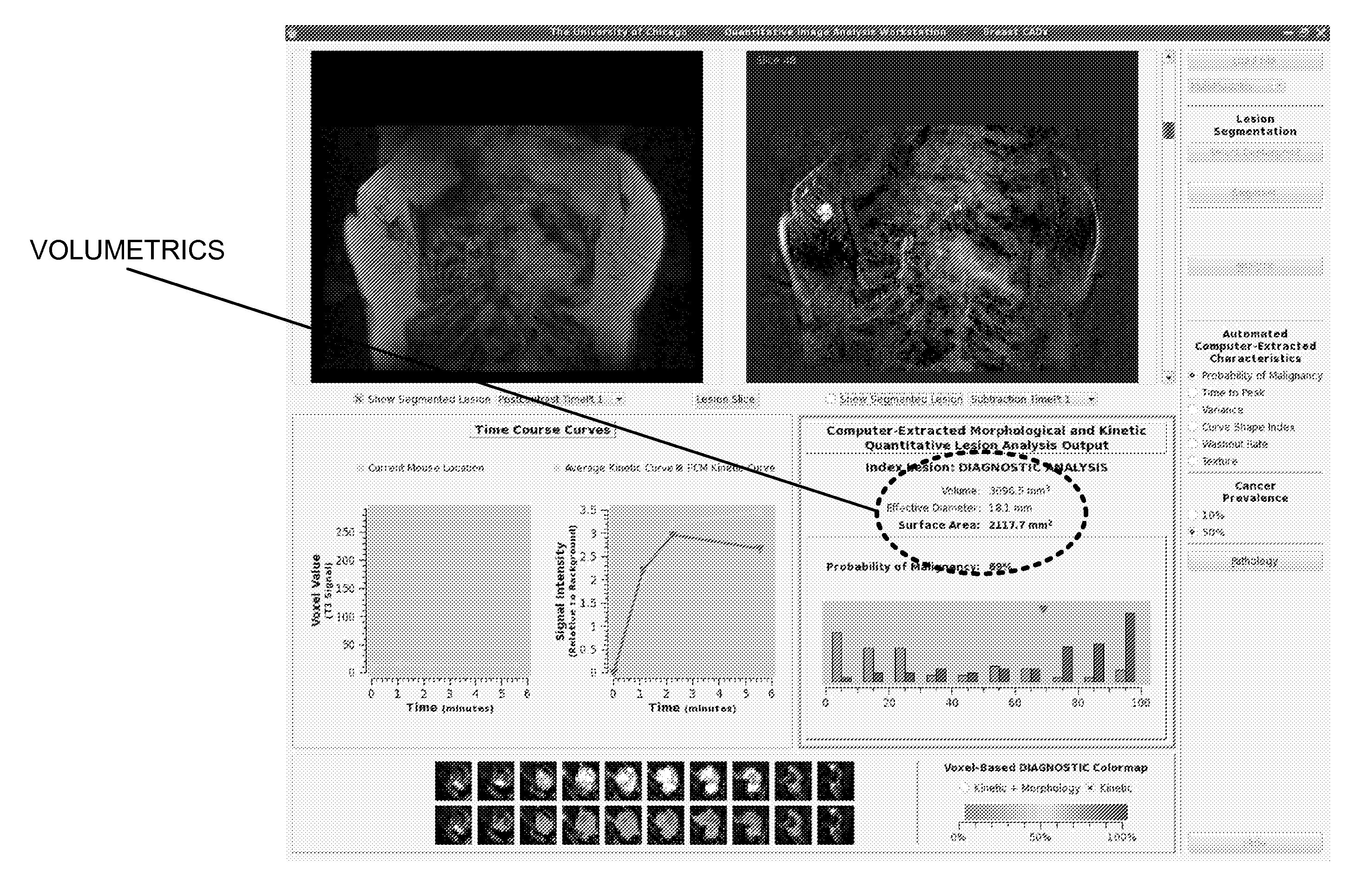
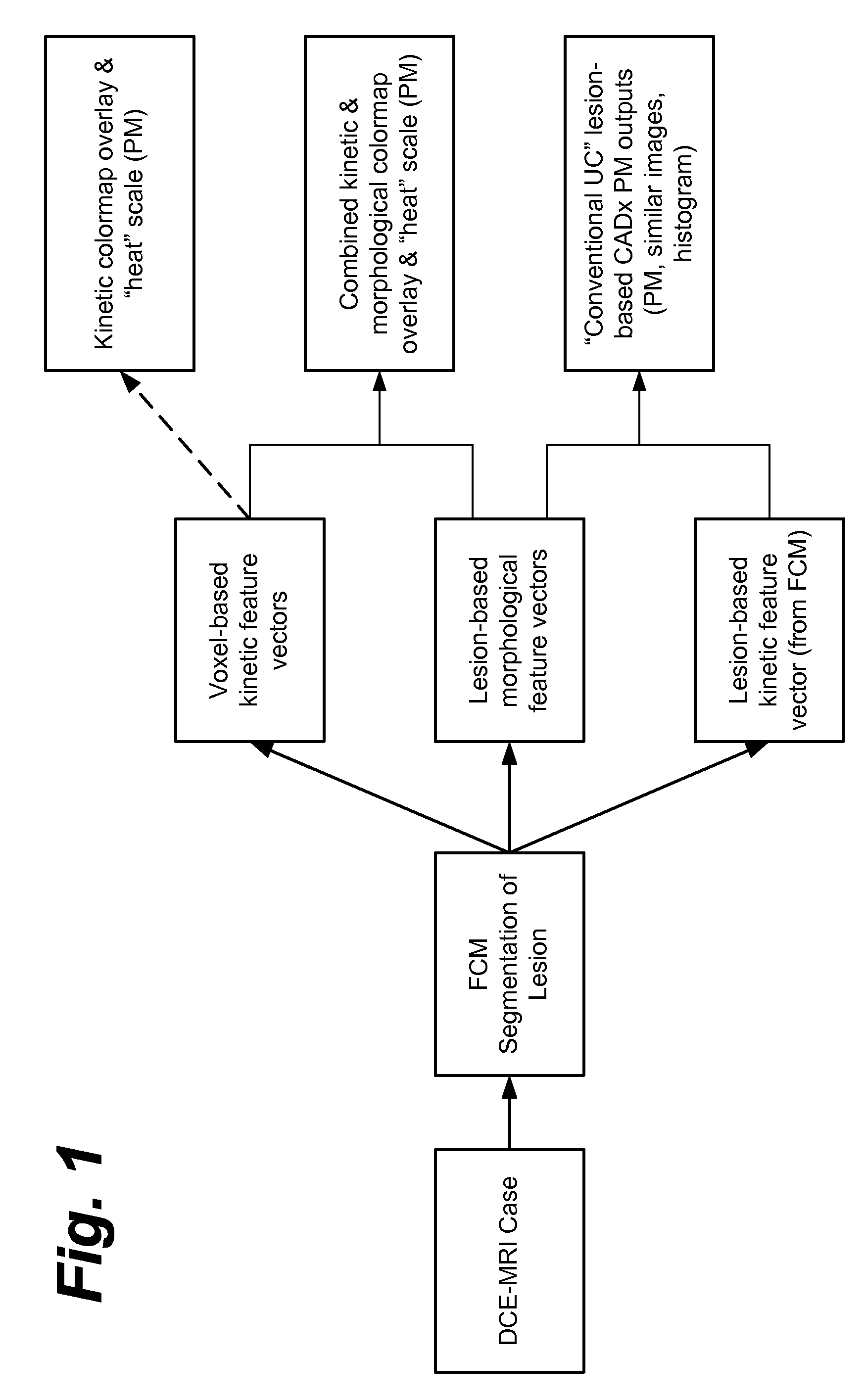
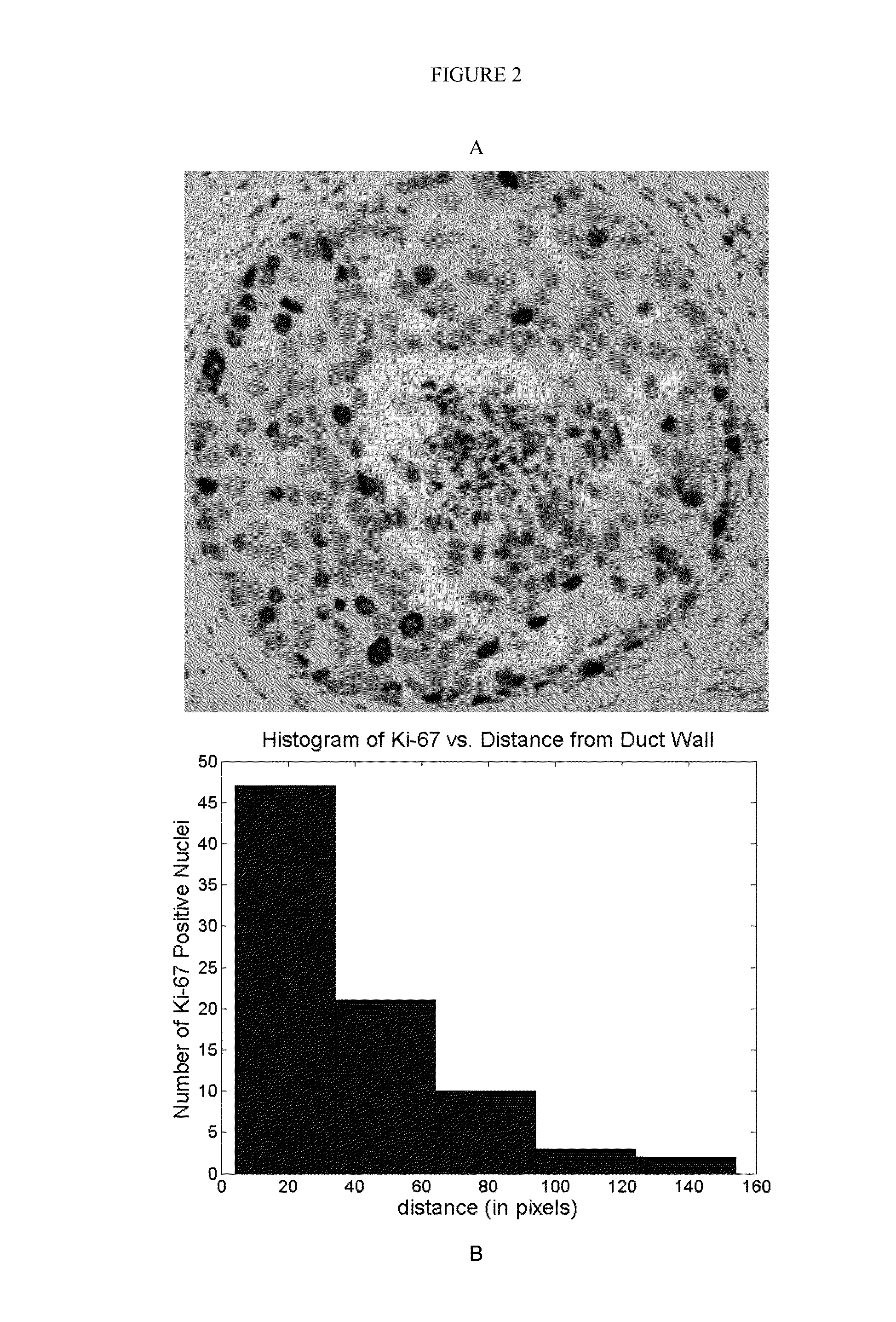
Method, system, software and medium for advanced intelligent image analysis and display of medical images and information
ActiveUS20120189176A1Obtain dataReduced dimensionImage enhancementReconstruction from projectionVoxelLesion
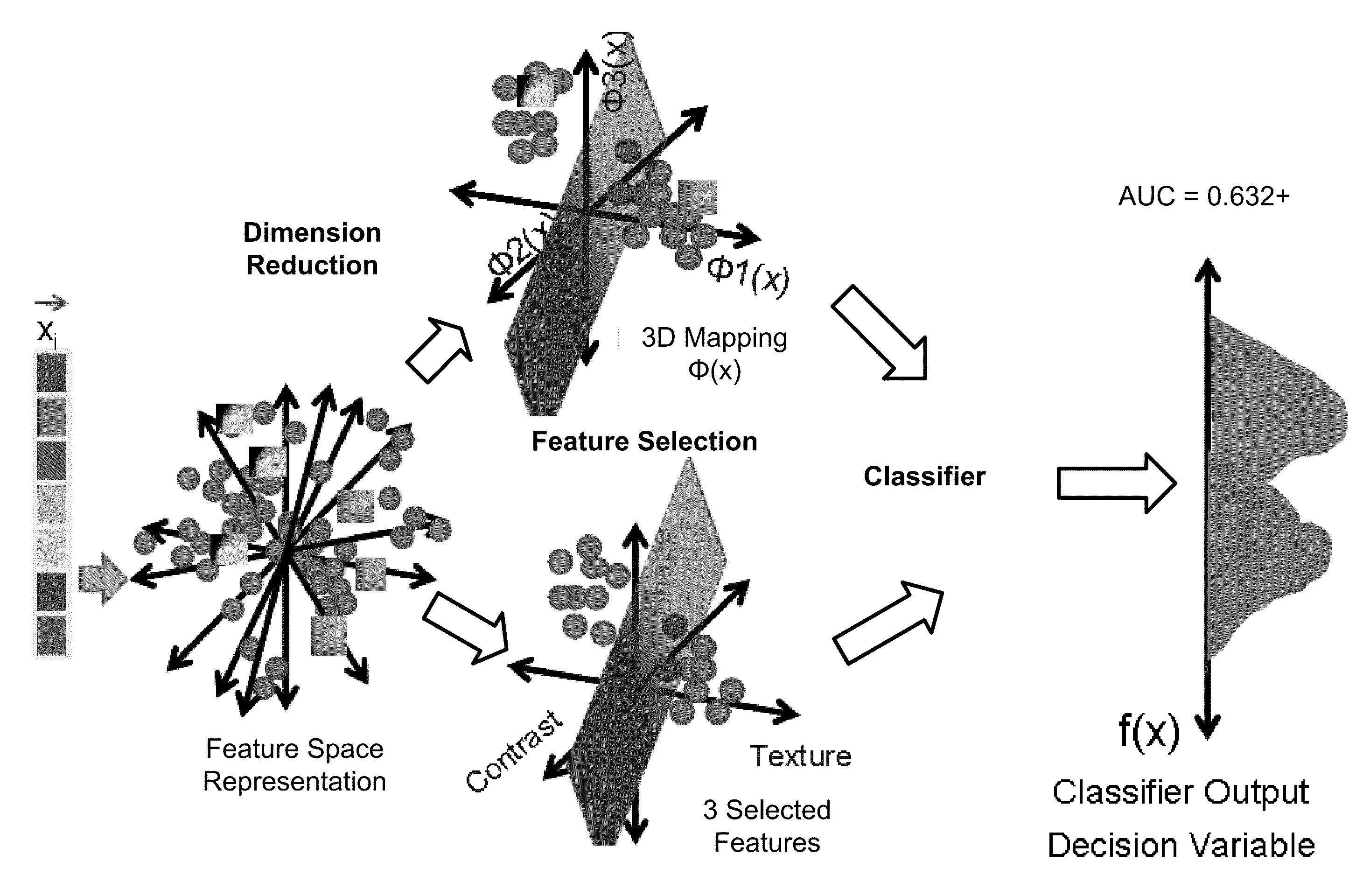
Computerized interpretation of medical images for quantitative analysis of multi-modality breast images including analysis of FFDM, 2D / 3D ultrasound, MRI, or other breast imaging methods. Real-time characterization of tumors and background tissue, and calculation of image-based biomarkers is provided for breast cancer detection, diagnosis, prognosis, risk assessment, and therapy response. Analysis includes lesion segmentation, and extraction of relevant characteristics (textural / morphological / kinetic features) from lesion-based or voxel-based analyses. Combinations of characteristics in several classification tasks using artificial intelligence is provided. Output in terms of 1D, 2D or 3D distributions in which an unknown case is identified relative to calculations on known or unlabeled cases, which can go through a dimension-reduction technique. Output to 3D shows relationships of the unknown case to a cloud of known or unlabeled cases, in which the cloud demonstrates the structure of the population of patients with and without the disease.
Owner:QLARITY IMAGING LLC
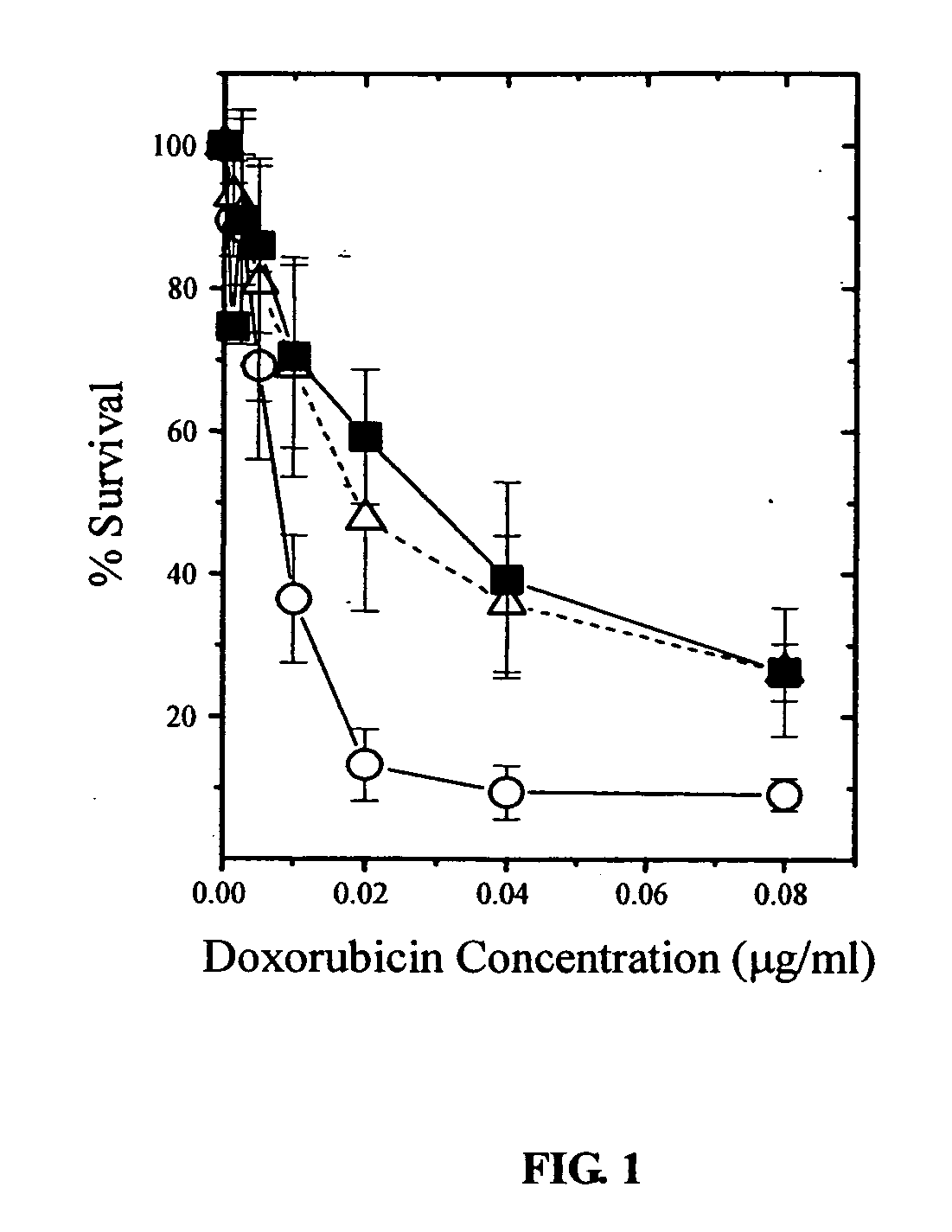
Combination anti-cancer therapy
InactiveUS20090263397A1Improve the level ofHeavy metal active ingredientsBiocideLymphatic SpreadAnticarcinogen
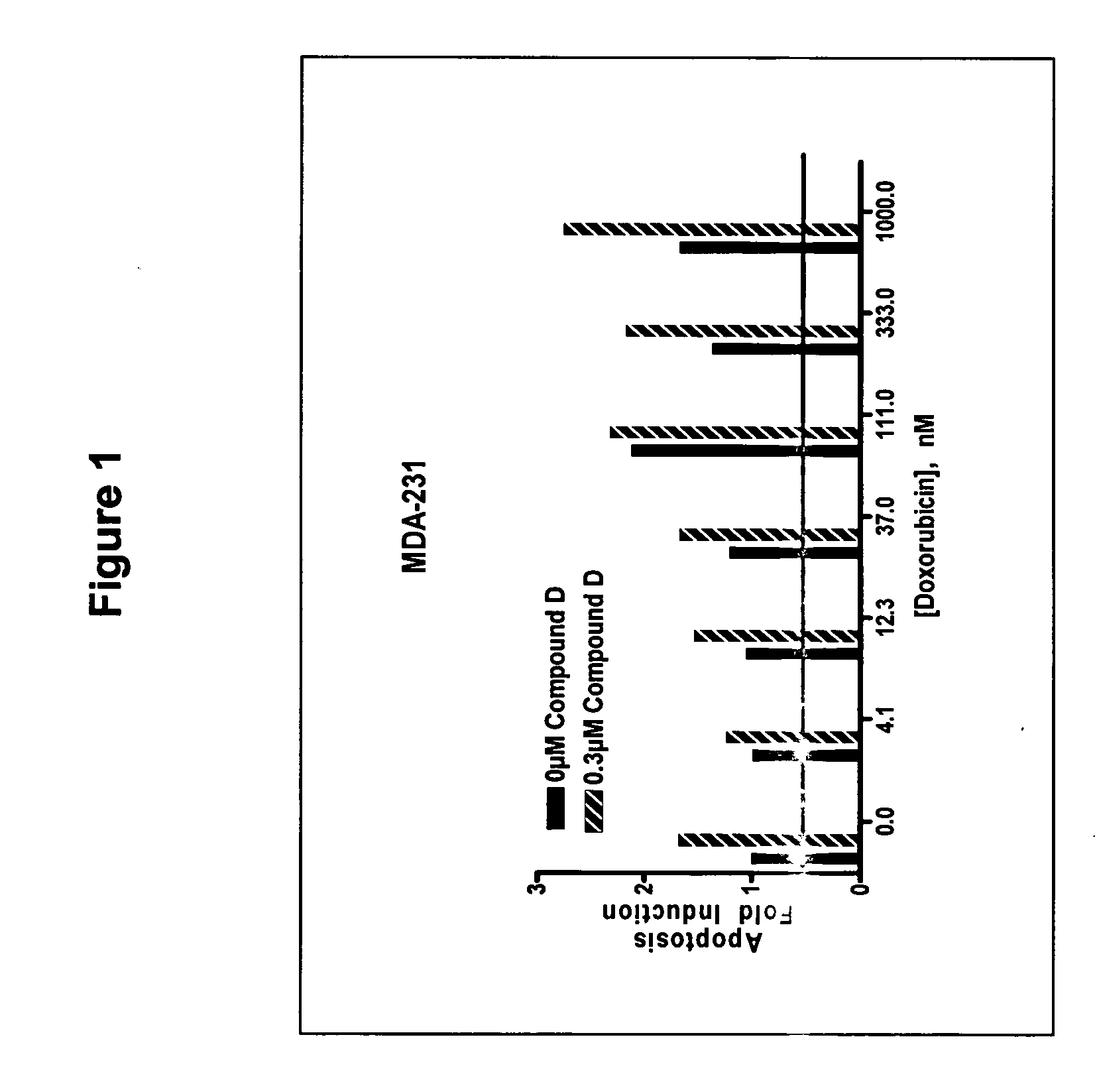
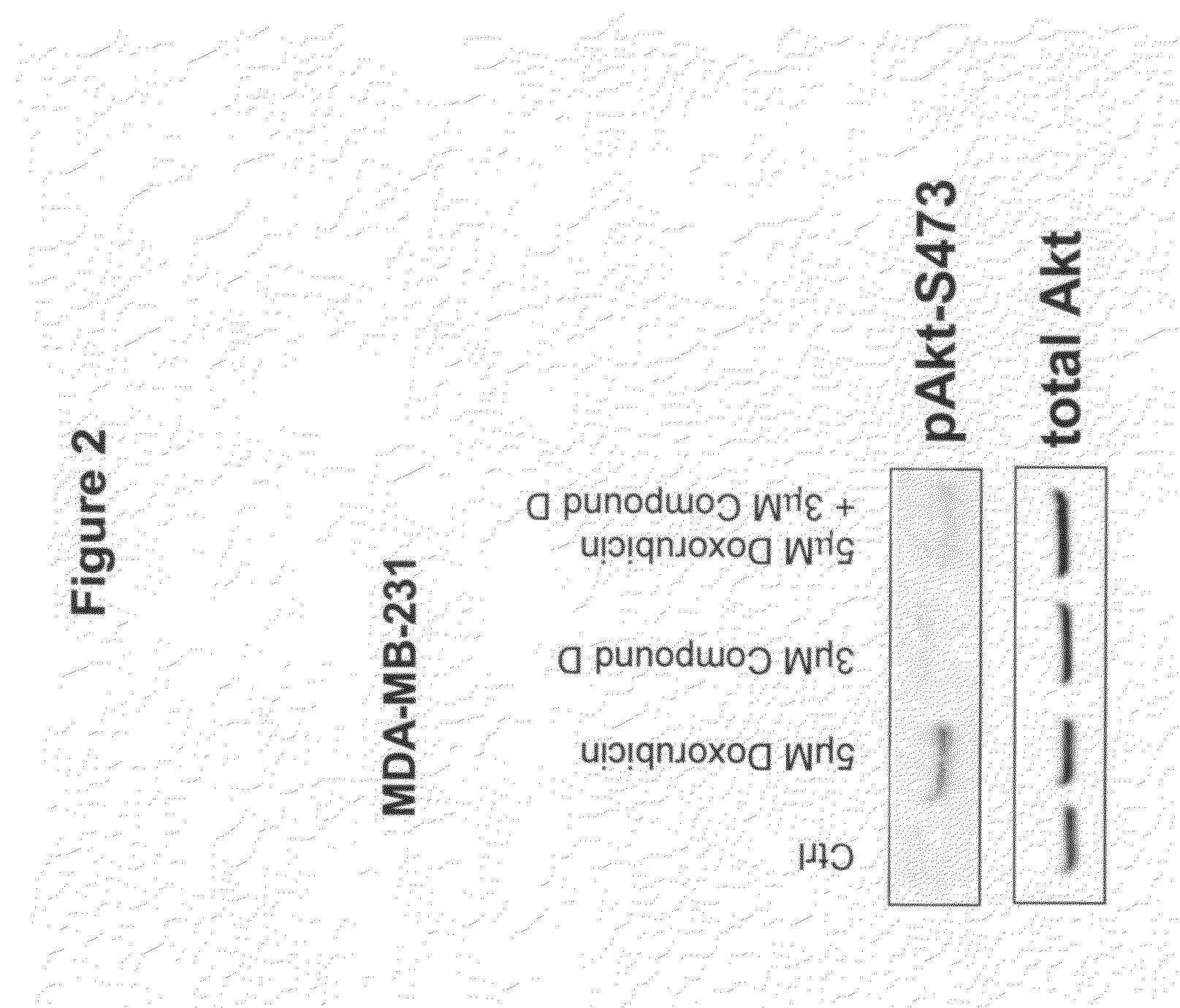
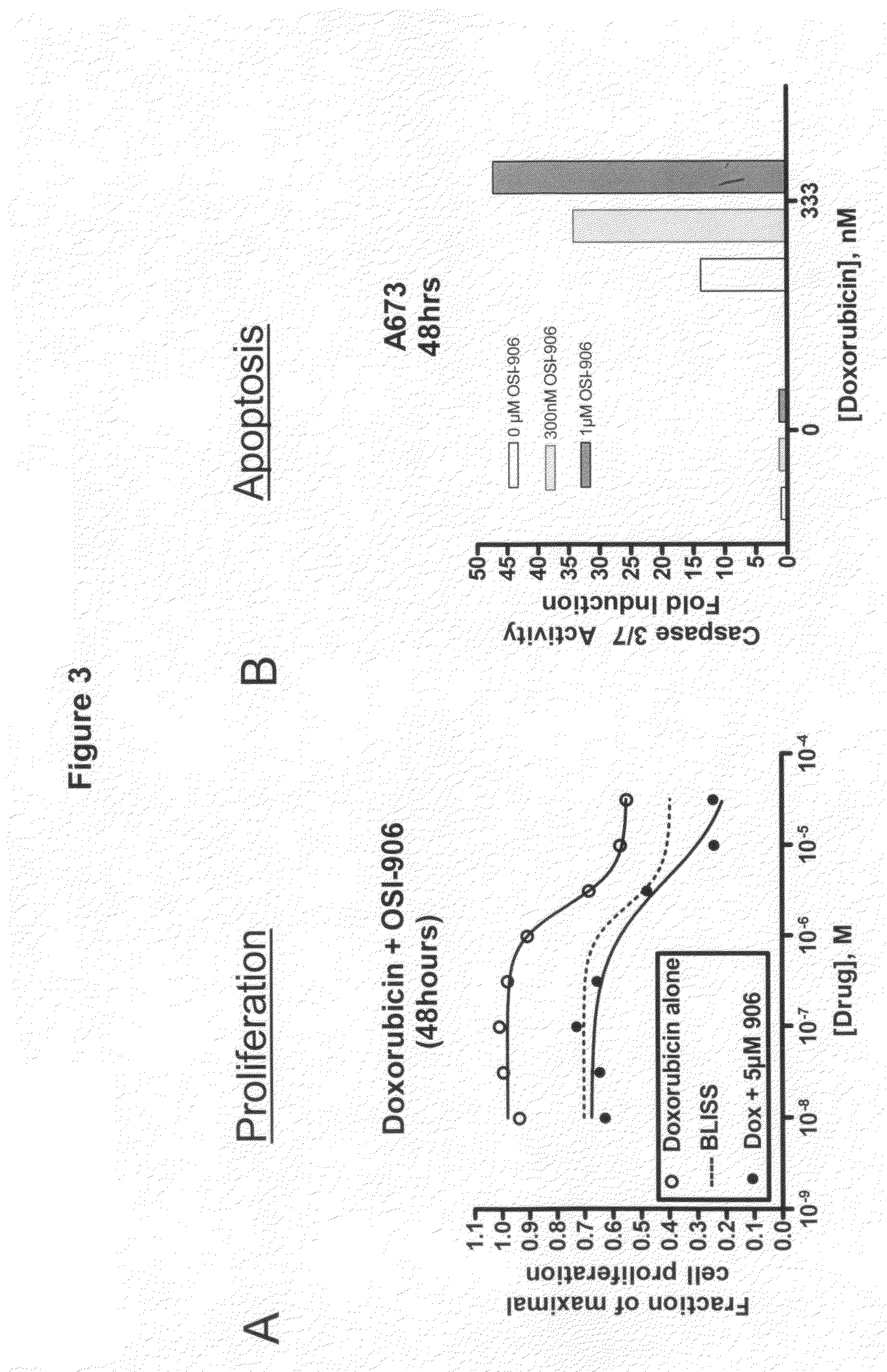
The present invention provides a method for treating tumors or tumor metastases in a patient, comprising administering to said patient simultaneously or sequentially a therapeutically effective amount of a combination of an anti-cancer agent or treatment that elevates pAkt levels in tumor cells and an IGF-1R kinase inhibitor of Formula (I) (e.g. OSI-906). Examples of such anti-cancer agents or treatments include doxorubicin, cisplatin, and ionizing radiation. The present invention also provides a pharmaceutical composition comprising an anti-cancer agent that elevates pAkt levels in tumor cells and an IGF-1R kinase inhibitor of Formula (I), in a pharmaceutically acceptable carrier. The present invention also provides a method of identifying tumor cells that will respond most favorably to treatment with a combination of an anti-cancer agent or treatment that elevates pAkt levels in tumor cells and an IGF-1R kinase inhibitor.
Owner:OSI PHARMA INC
Vaccination by topical application of recombinant vectors
InactiveUS20030045492A1Improve vaccination schemeEfficient methodSsRNA viruses negative-senseGenetic material ingredientsGene deliveryVaccination
The present invention relates to techniques of skin-targeted non-invasive gene delivery to elicit immune responses and uses thereof. The invention further relates to methods of non-invasive genetic immunization in an animal and / or methods of inducing a systemic immune or therapeutic response in an animal following topical application of vectors, products therefrom and uses for the methods and products therefrom. The methods can include contacting skin of the animal with a vector in an amount effective to induce the systemic immune or therapeutic response in the animal as well as such a method further including disposing the vector in and / or on the delivery device. The vector can be gram negative bacteria, preferably Salmonella and most preferably Salmonella typhimurium.
Owner:UAB RES FOUND
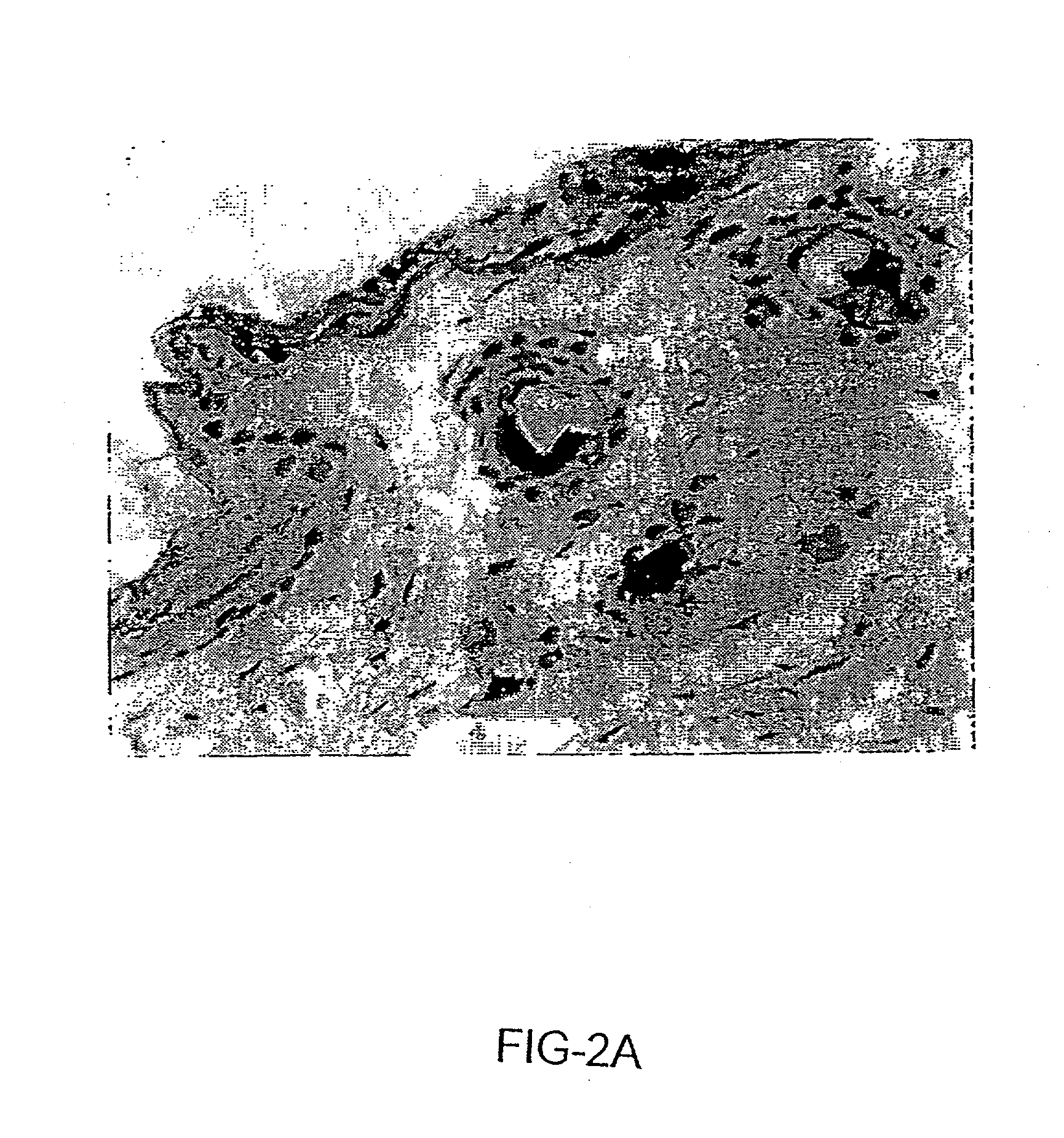
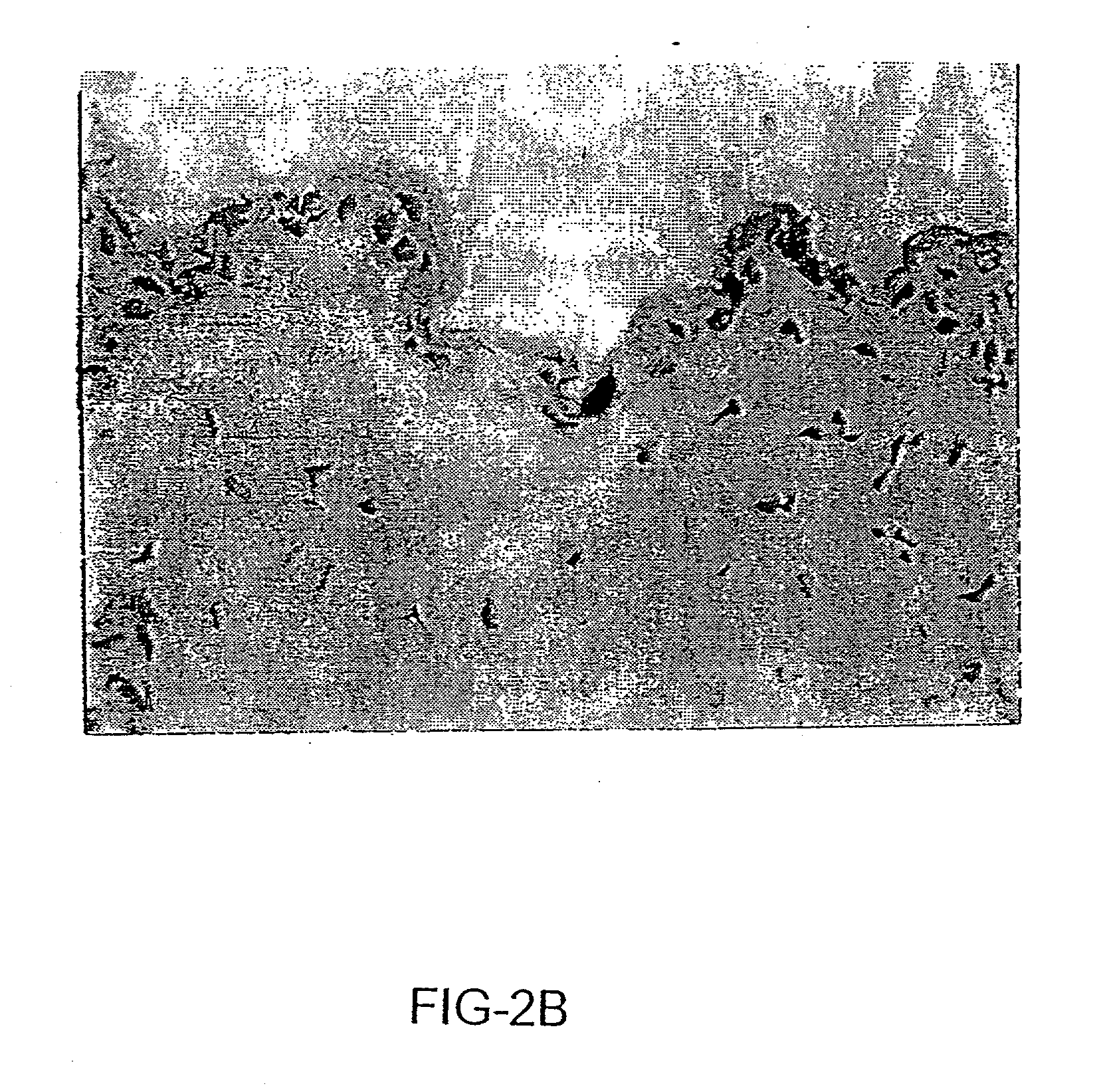
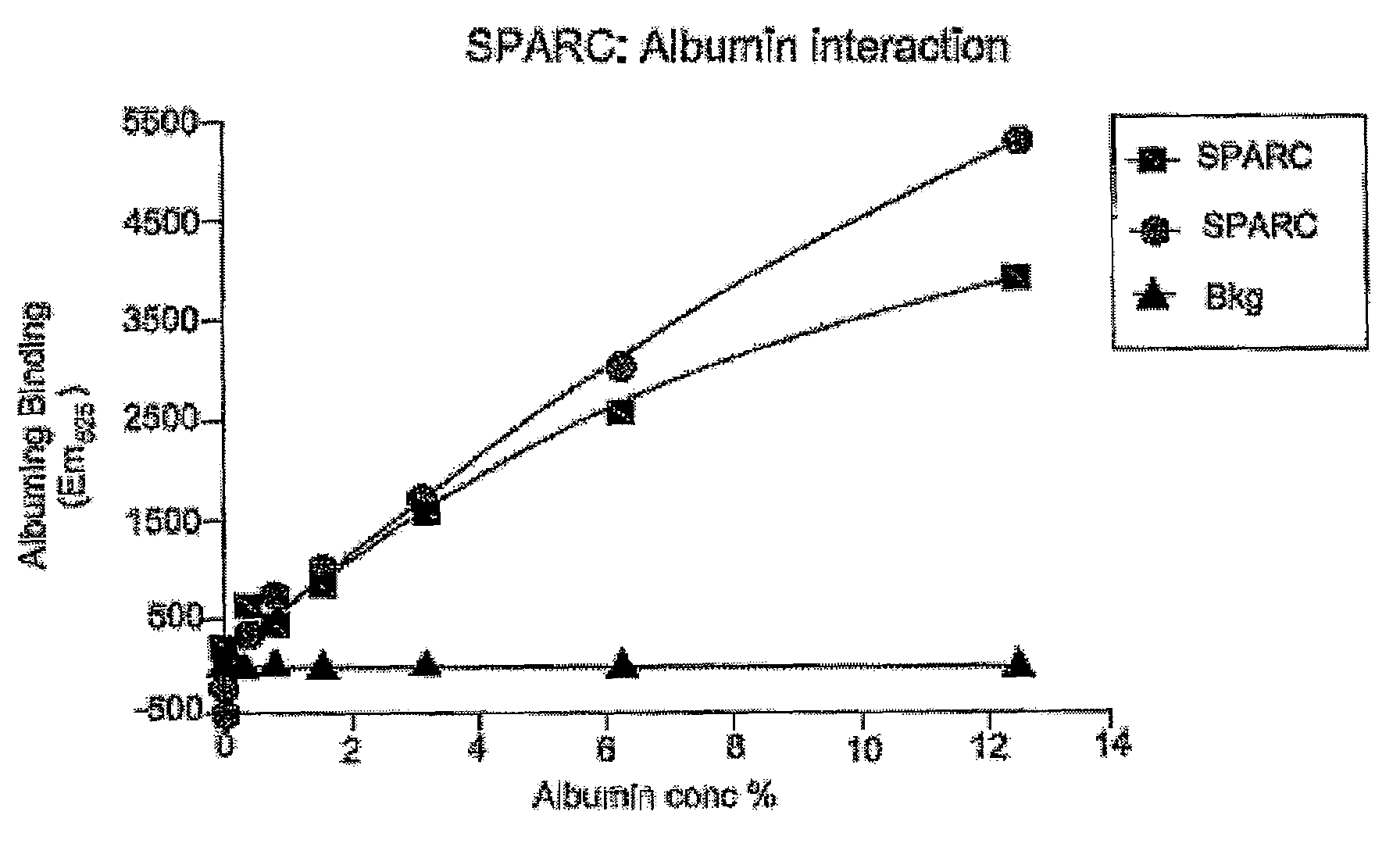
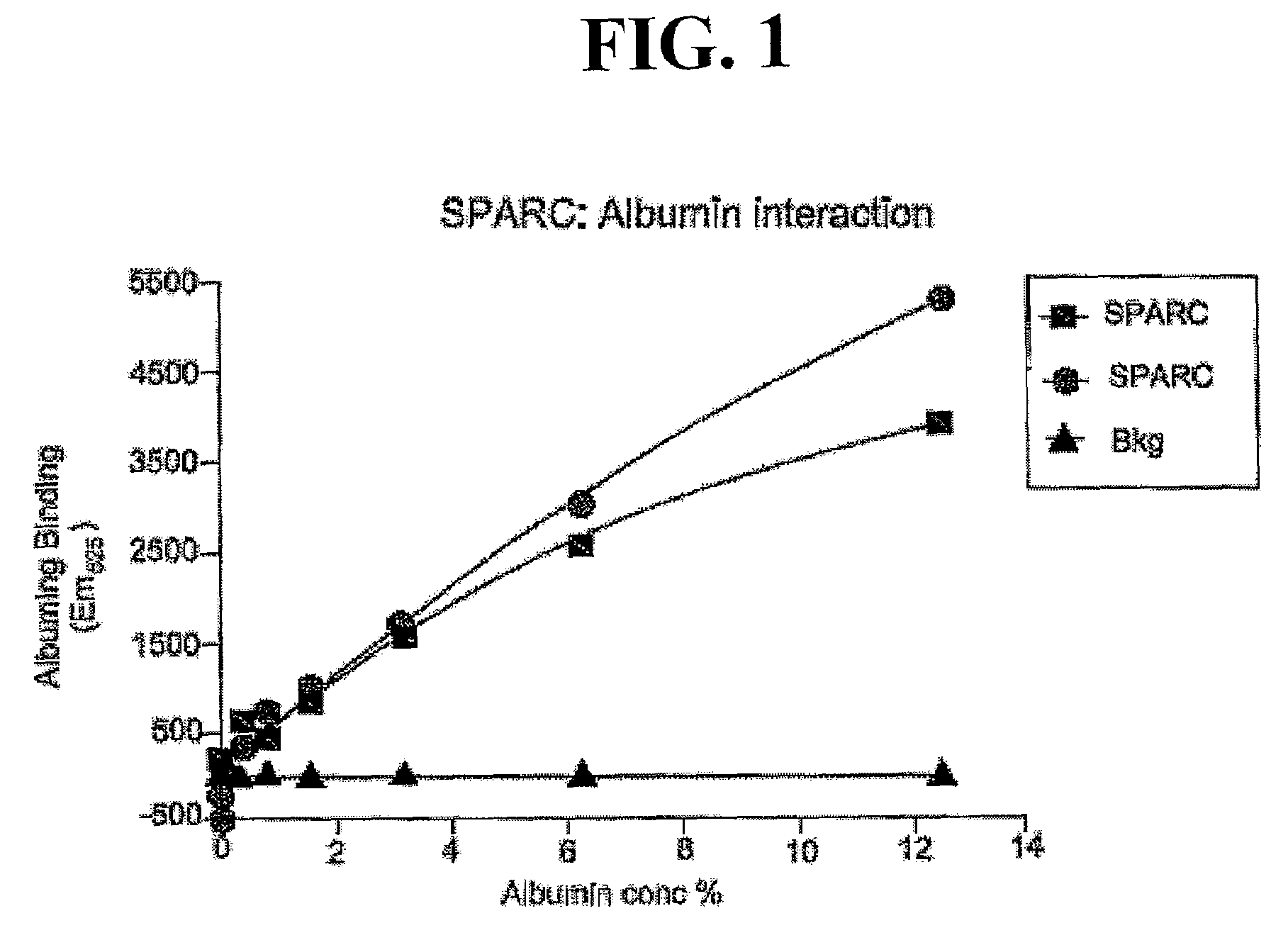
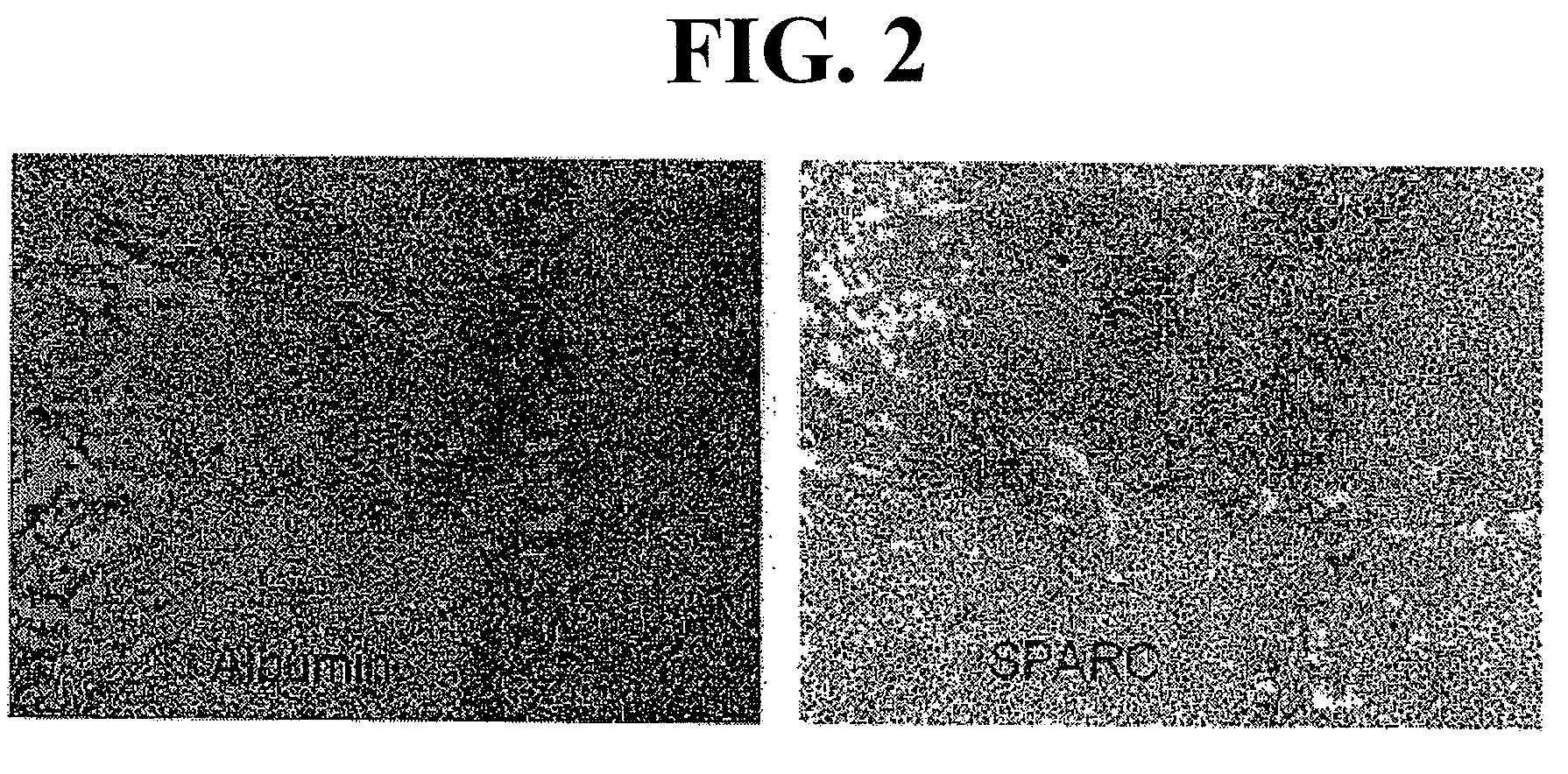
Sparc and methods of use thereof
InactiveUS20080255035A1Organic active ingredientsPeptide/protein ingredientsOncologyCombination therapy
The invention provides methods of treating a mammalian tumors comprising combination therapy with SPARC polypeptides, an angiogenesis inhibitor and paclitaxel. The invention provides also methods of treating a mammalian tumors comprising combination therapy with SPARC polypeptides and paclitaxel. Further, the invention produces kits and methods to predict therapy responses.
Owner:ABRAXIS BIOSCI LLC
Tape stripping methods for analysis of skin disease and pathological skin state
ActiveUS20050221334A1Less traumaticEfficient methodBioreactor/fermenter combinationsBiological substance pretreatmentsAdhesiveNon invasive
The present invention provides non-invasive methods for detecting, monitoring, and diagnosing skin disease and pathological skin states such as irritated skin and psoriasis. The methods include using tape stripping to analyze expression in epidermal samples, of one or more skin markers. In illustrative examples, the tape stripping is performed using pliable tape that has a rubber adhesive. Furthermore, the present invention provides methods for predicting and monitoring response to therapy for a skin disease, such as psoriasis or dermatitis. Finally, the methods can include the use of a micro array.
Owner:DERMTECH INT
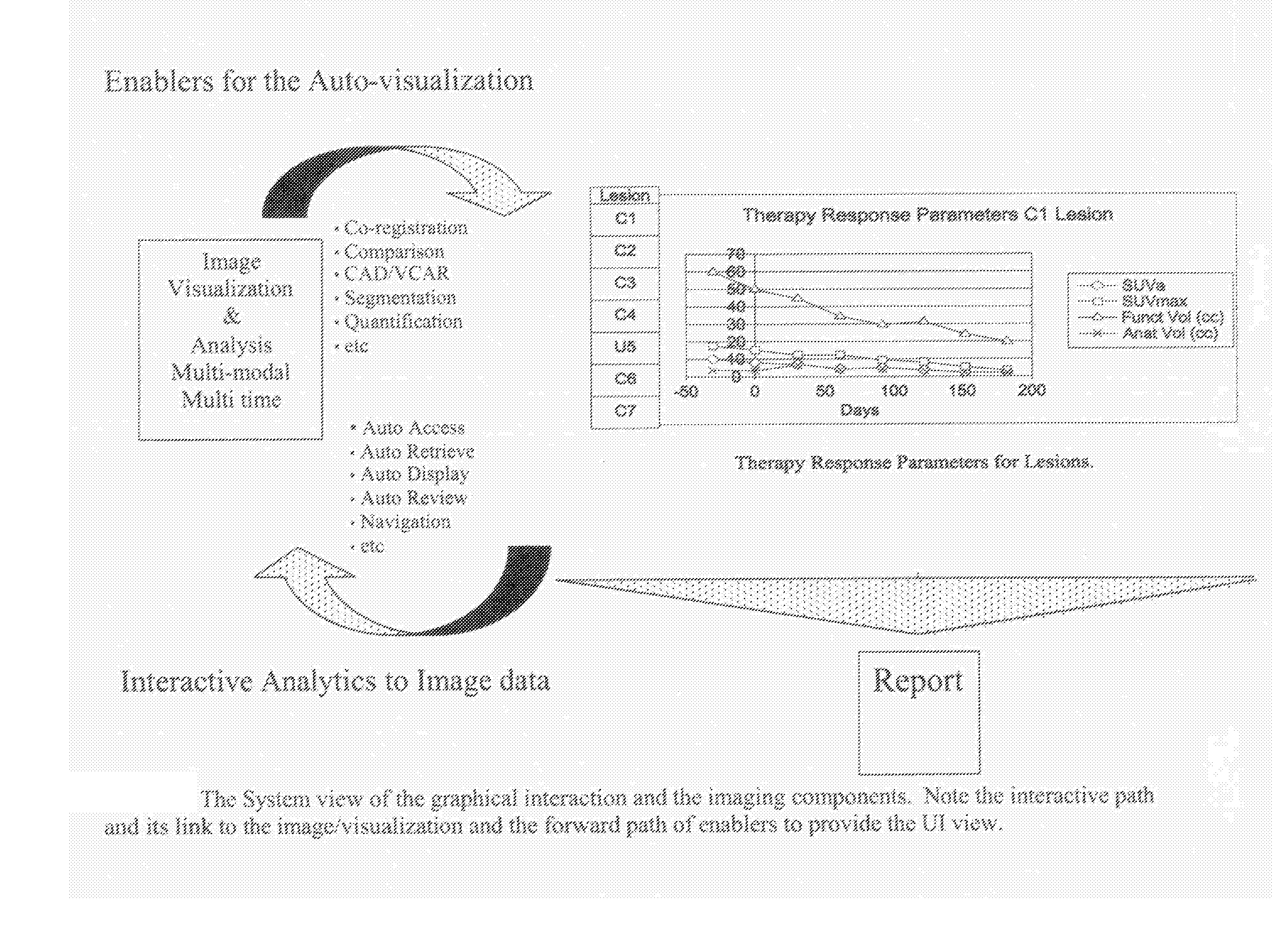
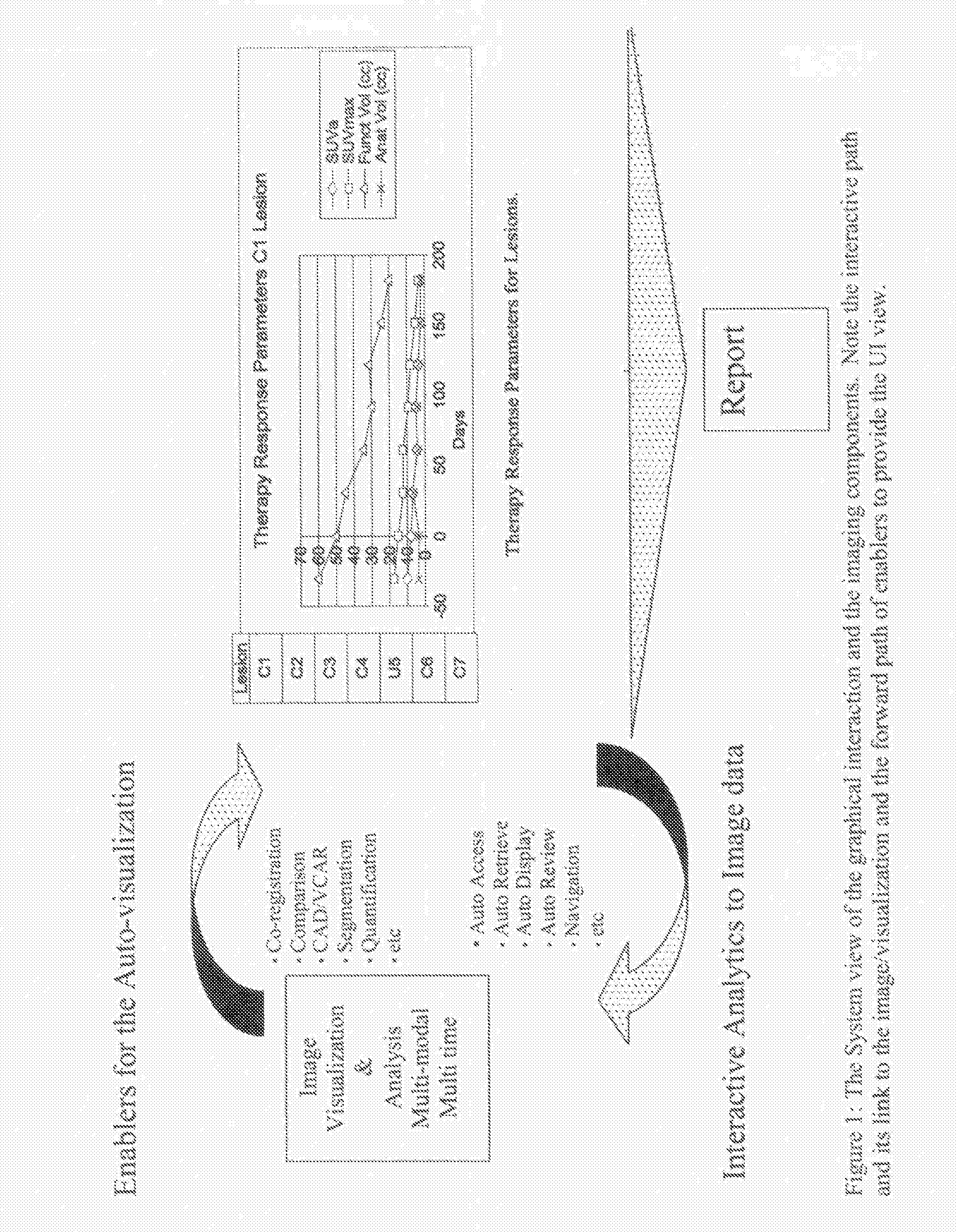
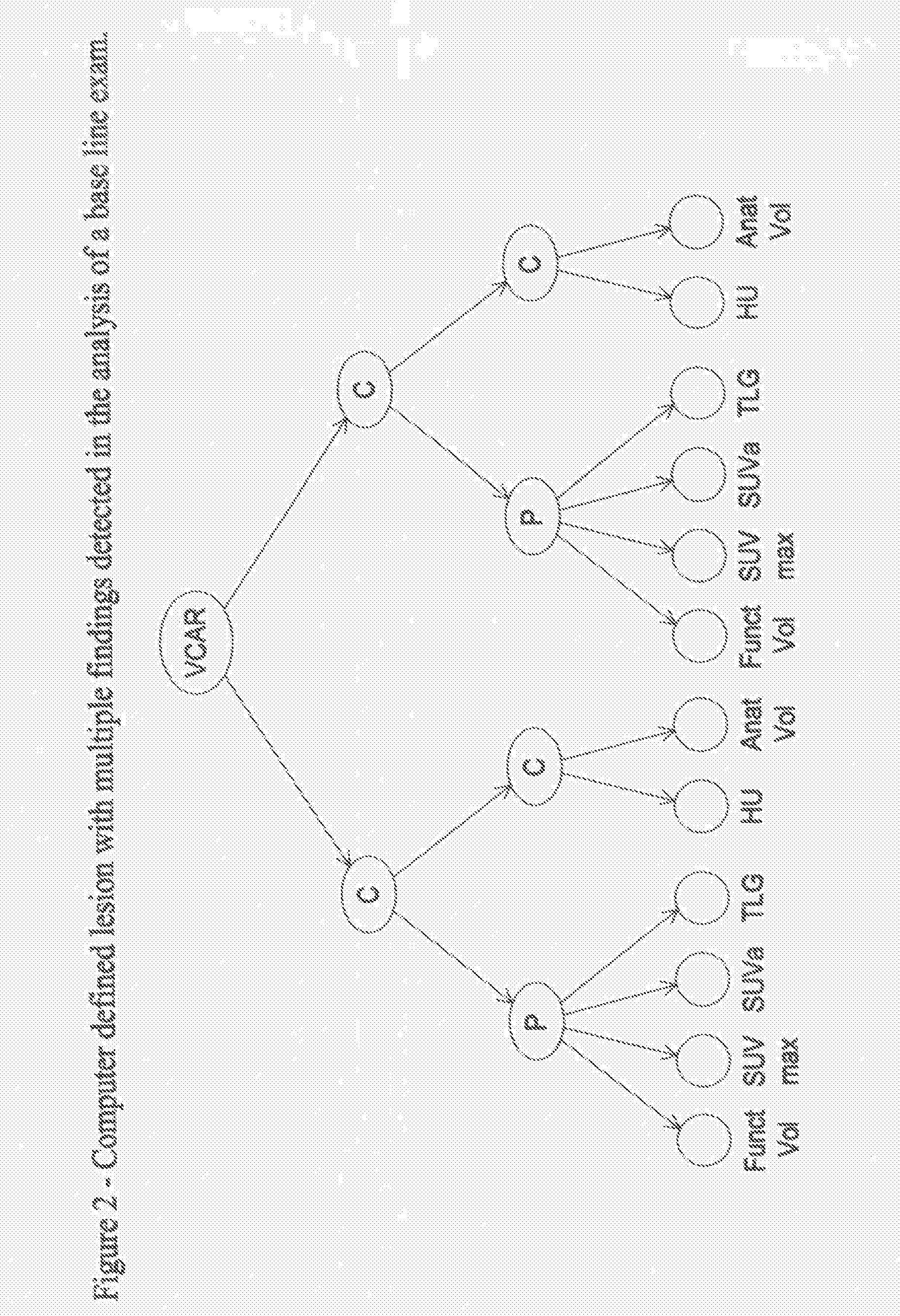
Methods and Apparatus for Volume Computer Assisted Reading Management and Review
InactiveUS20080021301A1Efficient analysisImage enhancementImage analysisComputer-aidedComputer science
A method includes providing an auto visualization display based on at least one quantitative analysis of at least one object of interest's progress over time regarding therapy response parameters over time.
Owner:GENERAL ELECTRIC CO
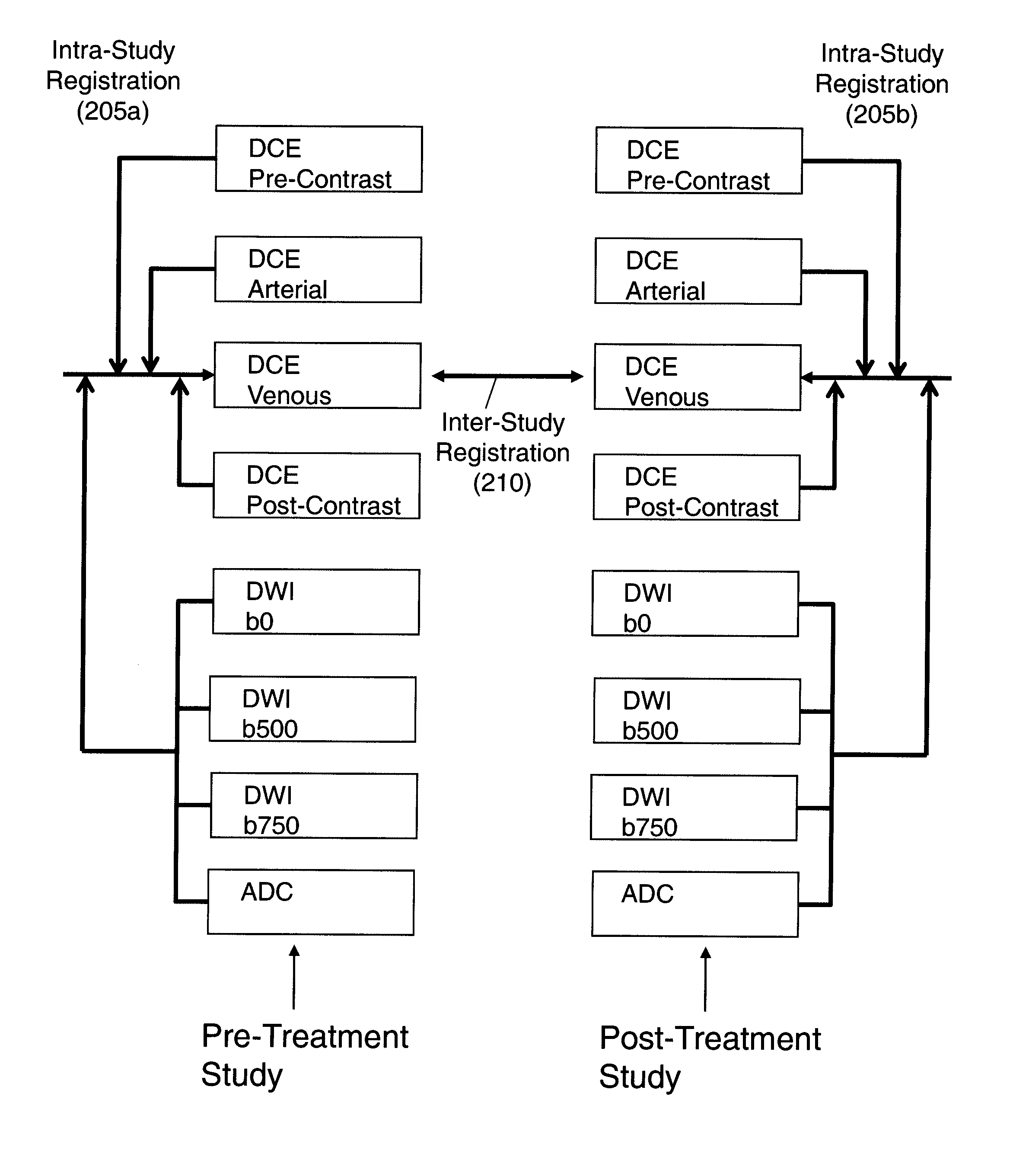
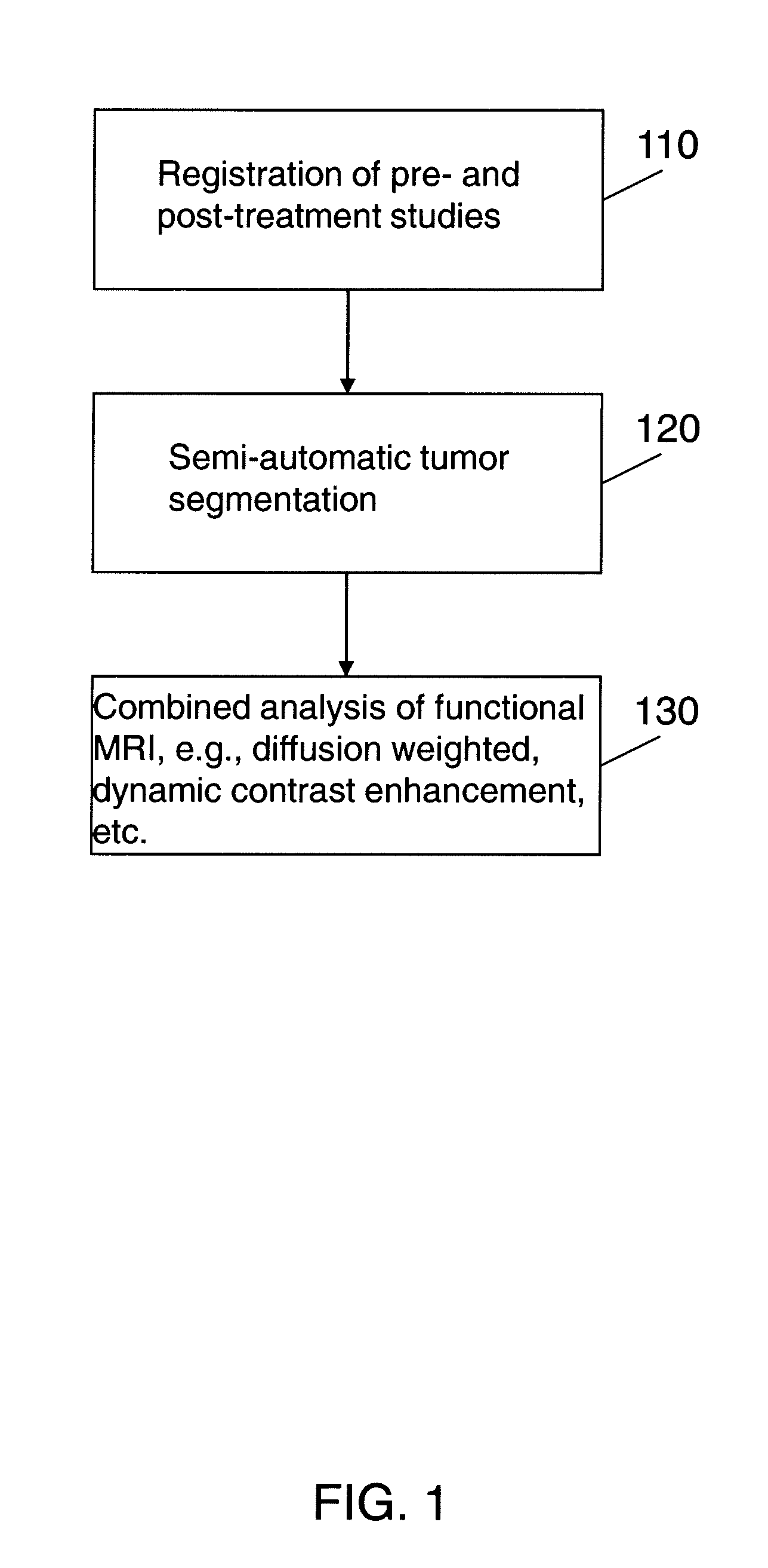
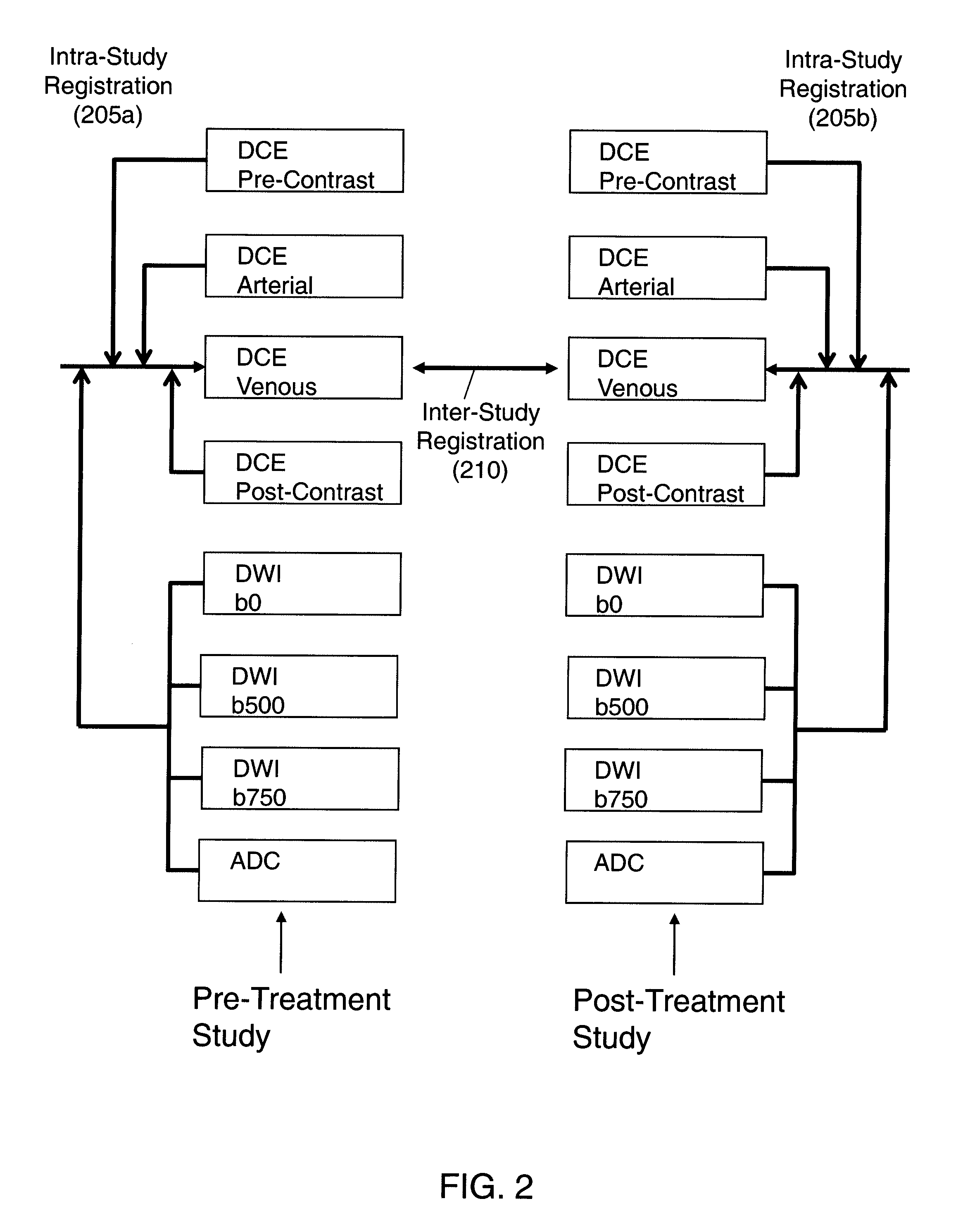
Automated method for assessment of tumor response to therapy with multi-parametric MRI
A method for assessing a tumor's response to therapy, includes providing images of a first study of a patient and images of a second study of the patient, the second study occurring after the first study and after the patient undergoes therapy to treat a tumor, each study comprising first and second types of functional magnetic resonance (fMR) images, performing a first registration in which the images within each study are registered, performing a second registration in which reference images from both studies are co-registered, segmenting the tumor in an image of each of the second registered studies; and determining that first and second fMR measure differences exist between the segmented tumor's of the first and second studies, the first fMR measure difference being obtained from the first type of fMR images, the second fMR measure difference being obtained from the second type of fMR images.
Owner:SIEMENS AG
Multi-scale complex systems transdisciplinary analysis of response to therapy
Described herein are methods and systems to measure dynamics of disease progression, including cancer growth and response, at multiple scales by multiple techniques on the same biologic system. Methods and systems according to the invention permit personalized virtual disease models. Moreover, the invention allows for the integration of previously unconnected data points into an in silico disease model, providing for the prediction of disease progression with and without therapeutic intervention.
Owner:UNIV OF SOUTHERN CALIFORNIA +1
Method for diagnosis and monitoring of disease activity and response to treatment in systemic lupus erythematosus (SLE) and other autoimmune diseases
InactiveUS20100233752A1Sensitive methodMicrobiological testing/measurementDisease diagnosisAutoimmune thyroid diseaseWhite blood cell
The present invention provides methods of diagnosing and monitoring systemic lupus erythematosus and drug-induced lupus erythematosus by measuring cell-based complement activation products in a subject's blood. In particular, the invention describes a diagnostic method employing the measurement of multiple complement activation products, such as C3d and C4d, on the surfaces of red blood cells, white blood cells, and platelets. Kits and automated systems for performing the methods of the invention are also disclosed.
Owner:EXAGEN DIAGNOSTICS +1
Method of providing customized drug delivery systems
InactiveUS20060078621A1Promote absorptionMaximum therapeutic effectivenessPowder deliveryPill deliveryAdditive ingredientBlood plasma
A novel method of correlating the disposition of a specific drug in an individual patient to a controlled and modulated delivery system for optimizing therapeutic response of orally ingested dosage forms is provided. Such a method broadly encompasses a first determination of an individual's metabolic rate in terms of absorption of pharmaceutical materials from within the gastrointestinal tract measured as blood plasma concentration over a specific period of time after ingestion or by other commercially available methods and subsequent determination: 1) predicting a proper pharmaceutical compositions, in terms of amount of active available for absorption by the target patient; and 2) amount of such active pharmaceutical ingredient (API) to be formulated within a drug-delivery device that will take into account the unique metabolic profile of the drug (or drugs) in a specific patient. As a result, the API may be formulated as beads, pellets, minitablets, powders, granules, suspensions, and / or emulsions present within the drug-delivery source. As one potentially preferred embodiment, such beads and / or pellets, which may be coated with different polymers and differing levels of coatings, are selected in response to the initial determination of the patient's metabolic profile in order to ensure the specific targeted patient receives the most efficient dosage of the active drug at a rate unique to that individual.
Owner:J M HUBER CORP
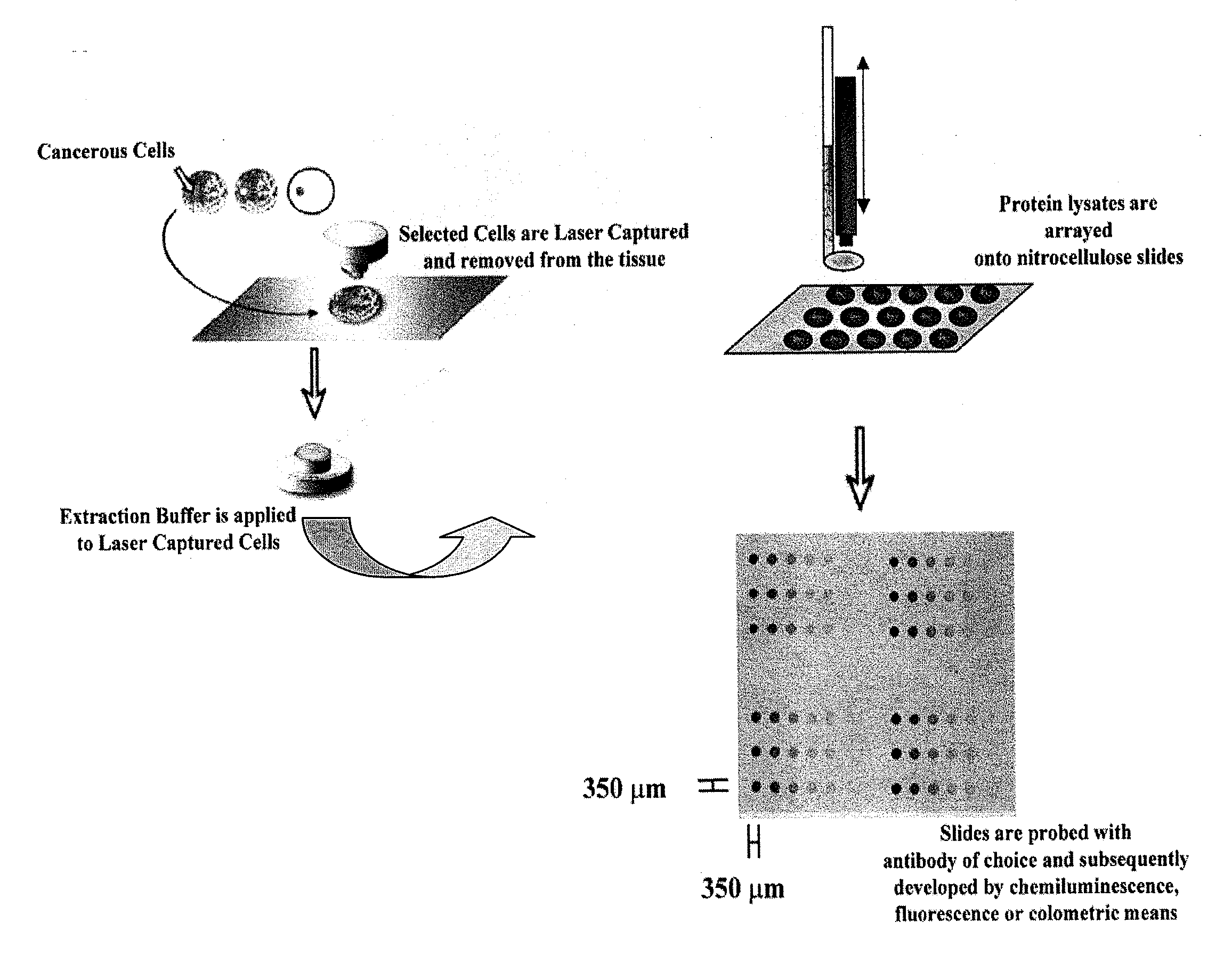
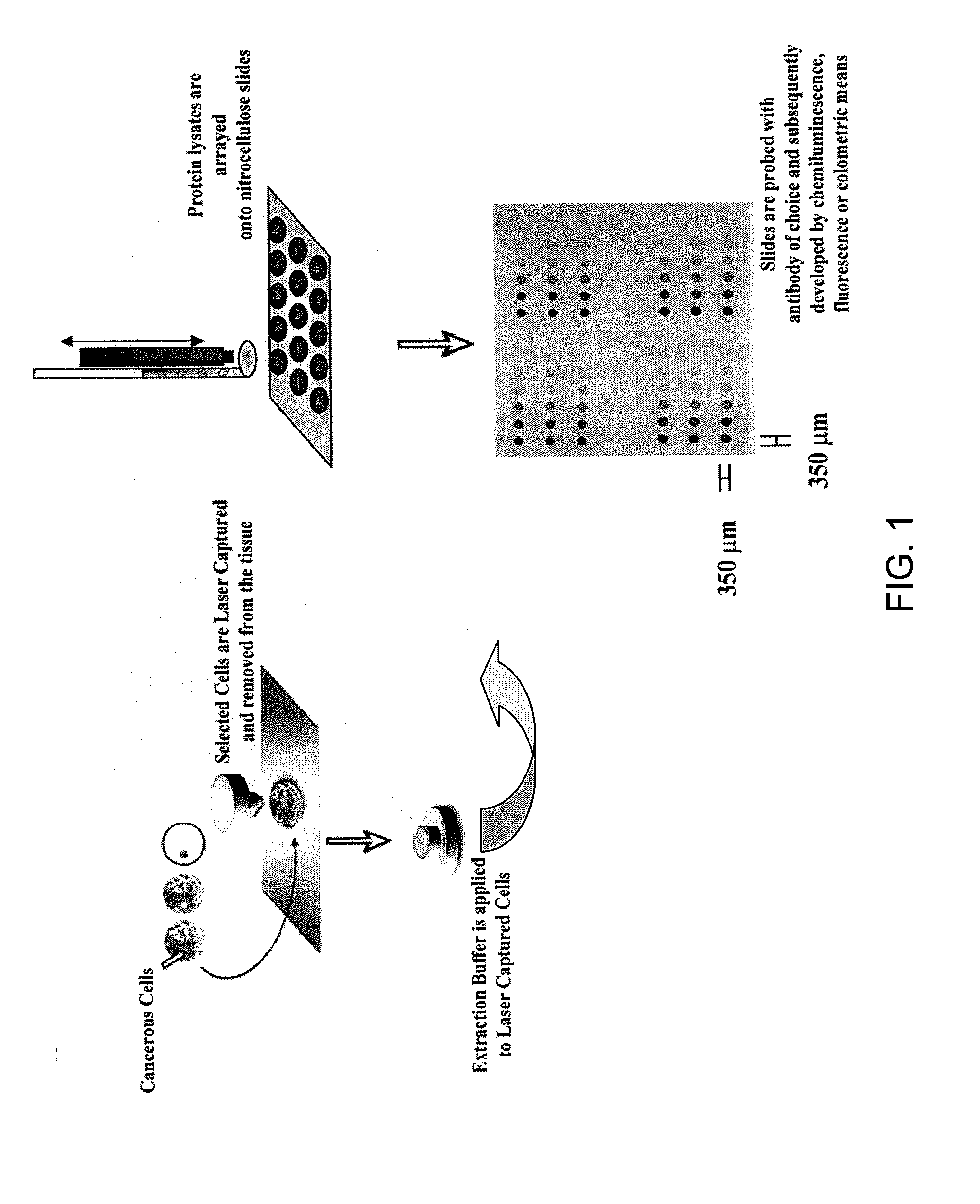
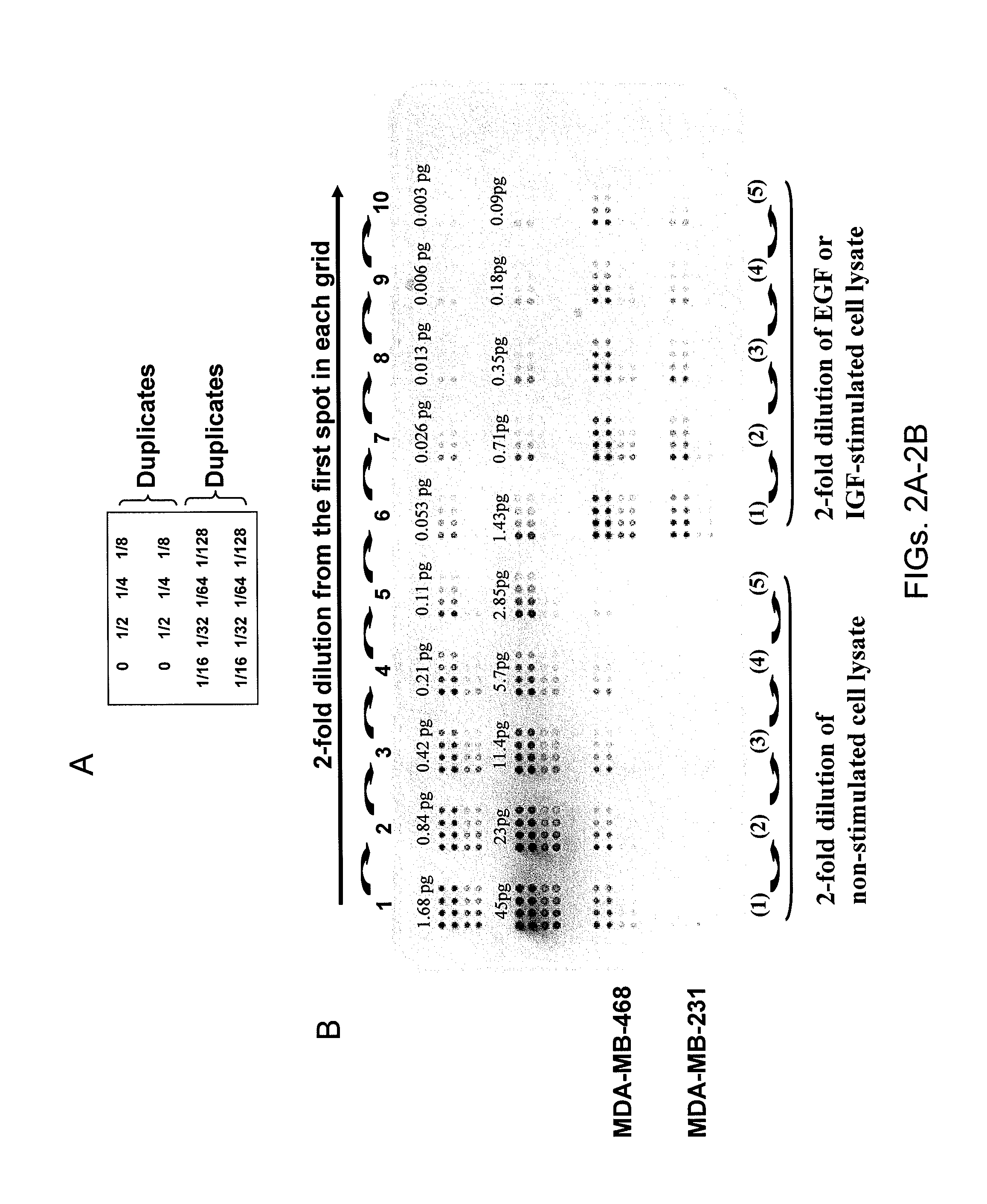
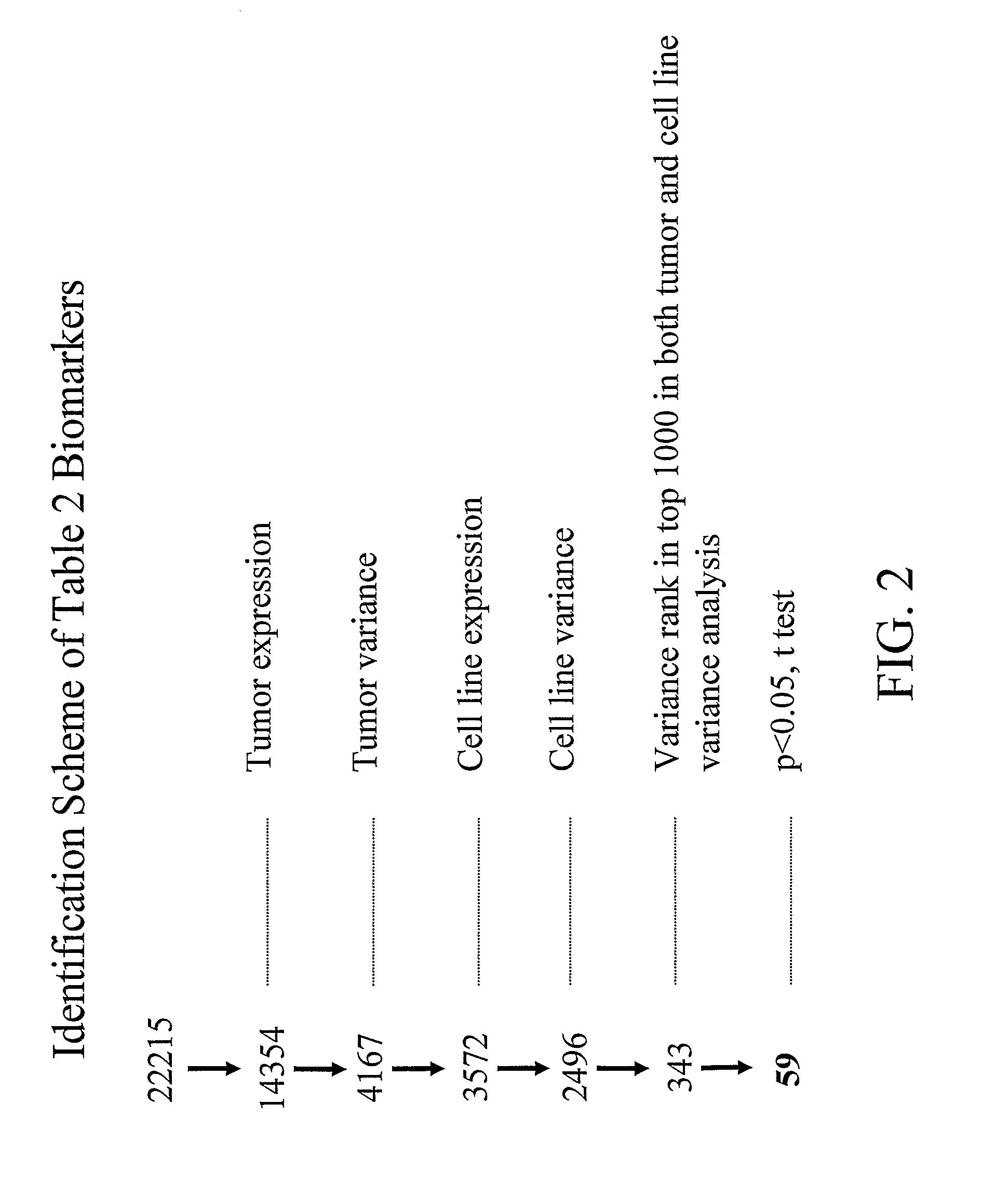
Proteomic Patterns of Cancer Prognostic and Predictive Signatures
The invention provides method for predicting whether a cancer patient will respond to a therapy. Methods of the invention may involve examining protein from a cell of the cancer patient by determining the binding of a panel of antibodies to the protein. Methods of the invention may be used to generate both expression and activation profiles for cells from a cancer patient. Profiles from a cancer patient may then be compared to known profiles for therapy responders and non-responders to predict the individual response of the patient. For example, methods of the invention may be used to determine whether an ovarian or breast cancer patient will respond to a therapeutic protocol.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
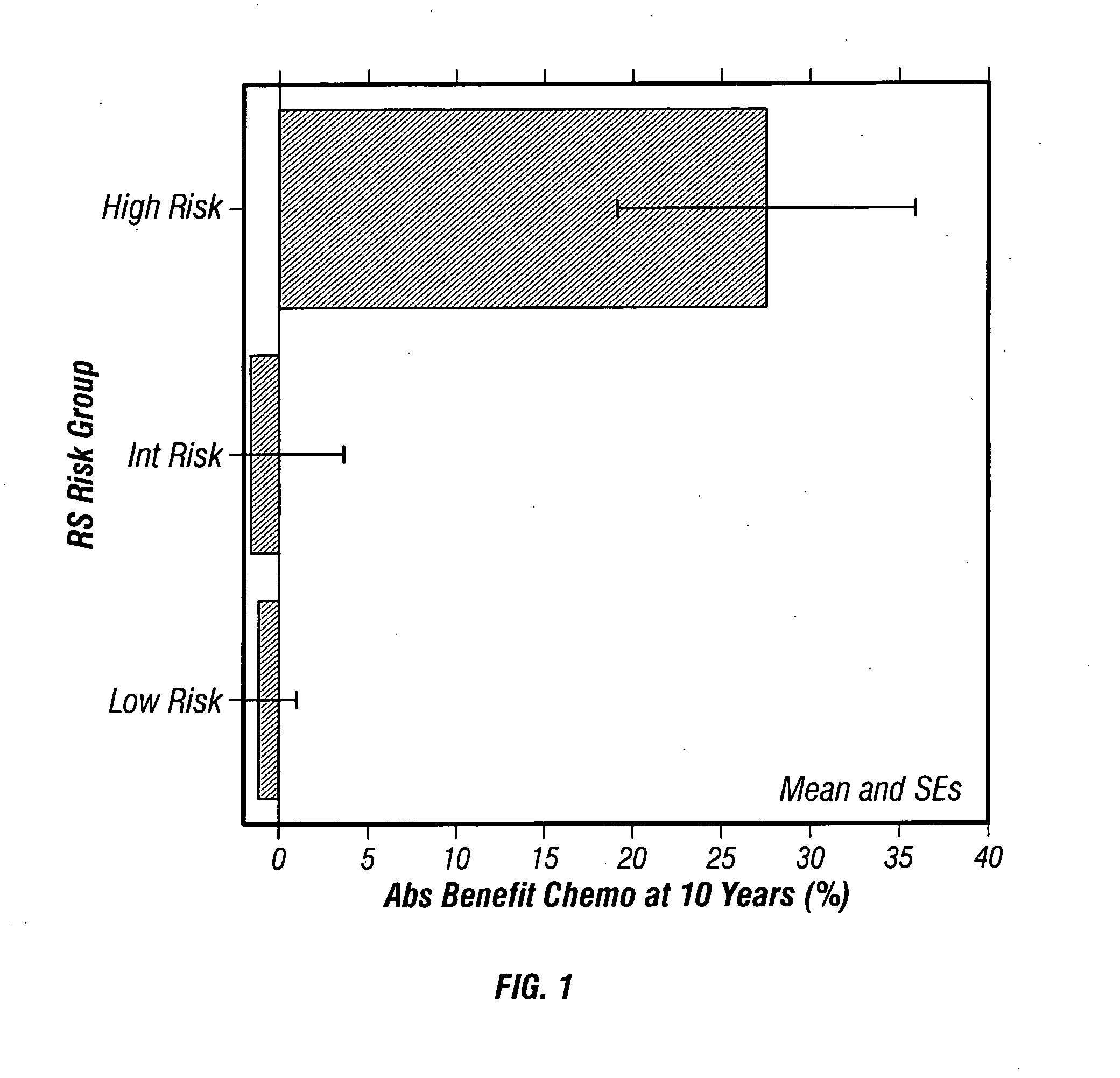
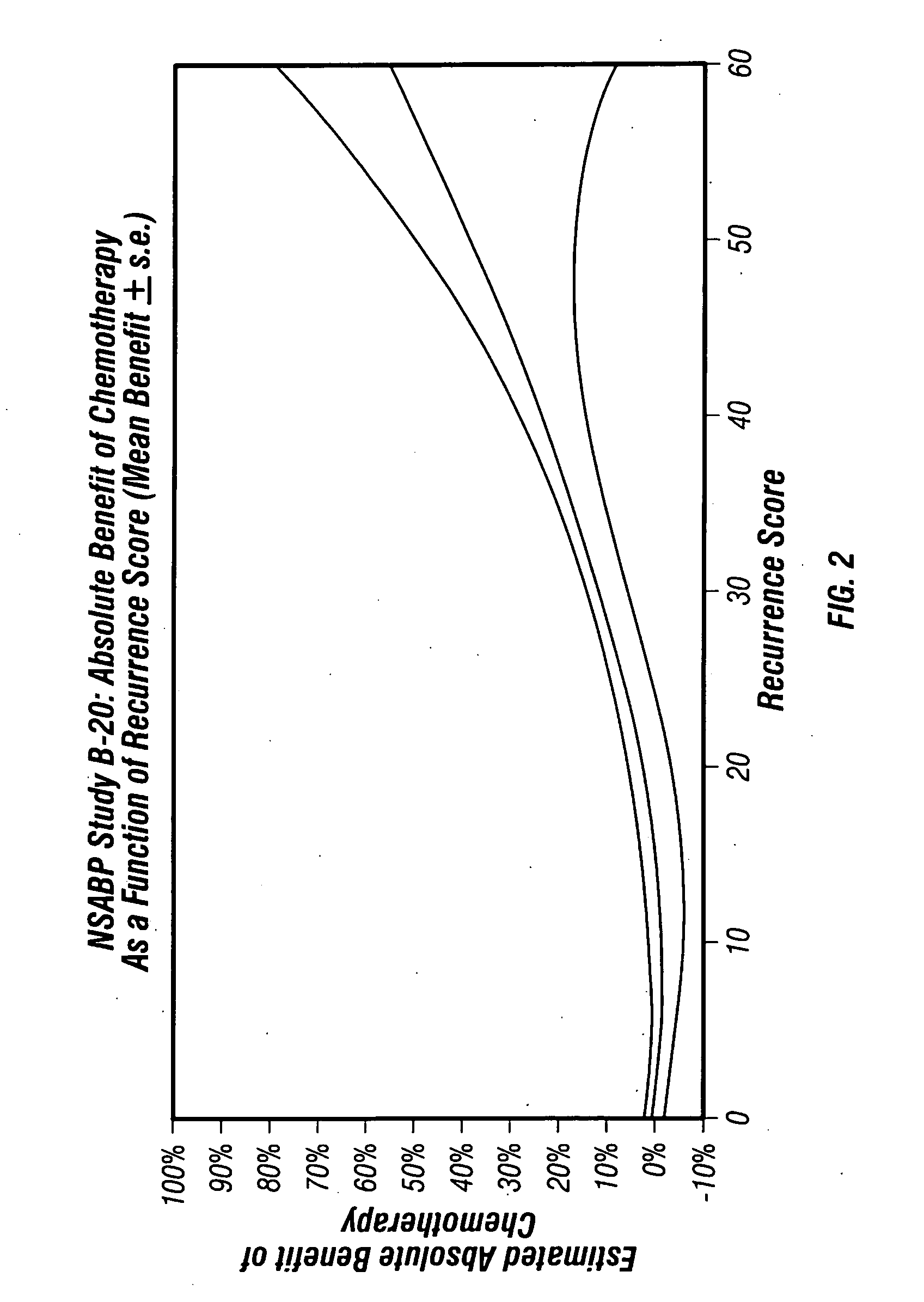
Predicting response to chemotherapy using gene expression markers
ActiveUS20060166230A1Raise the possibilityReduce the possibilityMicrobiological testing/measurementProteomicsCancer researchGene expression
The present invention provides gene expression information useful for predicting whether cancer patients are likely to have a beneficial response to treatment response with chemotherapy.
Owner:GENOMIC HEALTH INC
Methods and Compositions for Detection of Lethal Cell and Uses Thereof
ActiveUS20110053178A1Facilitate decision-makingEarly detectionMicrobiological testing/measurementDisease diagnosisDisease statusDisease injury
The present invention relates to methods and compositions for identifying and detecting lethal cell useful for monitoring disease status and therapy response in various types of cancer patients regardless of the etiological origin of the cancer and uses thereof.
Owner:NATIONAL TSING HUA UNIVERSITY
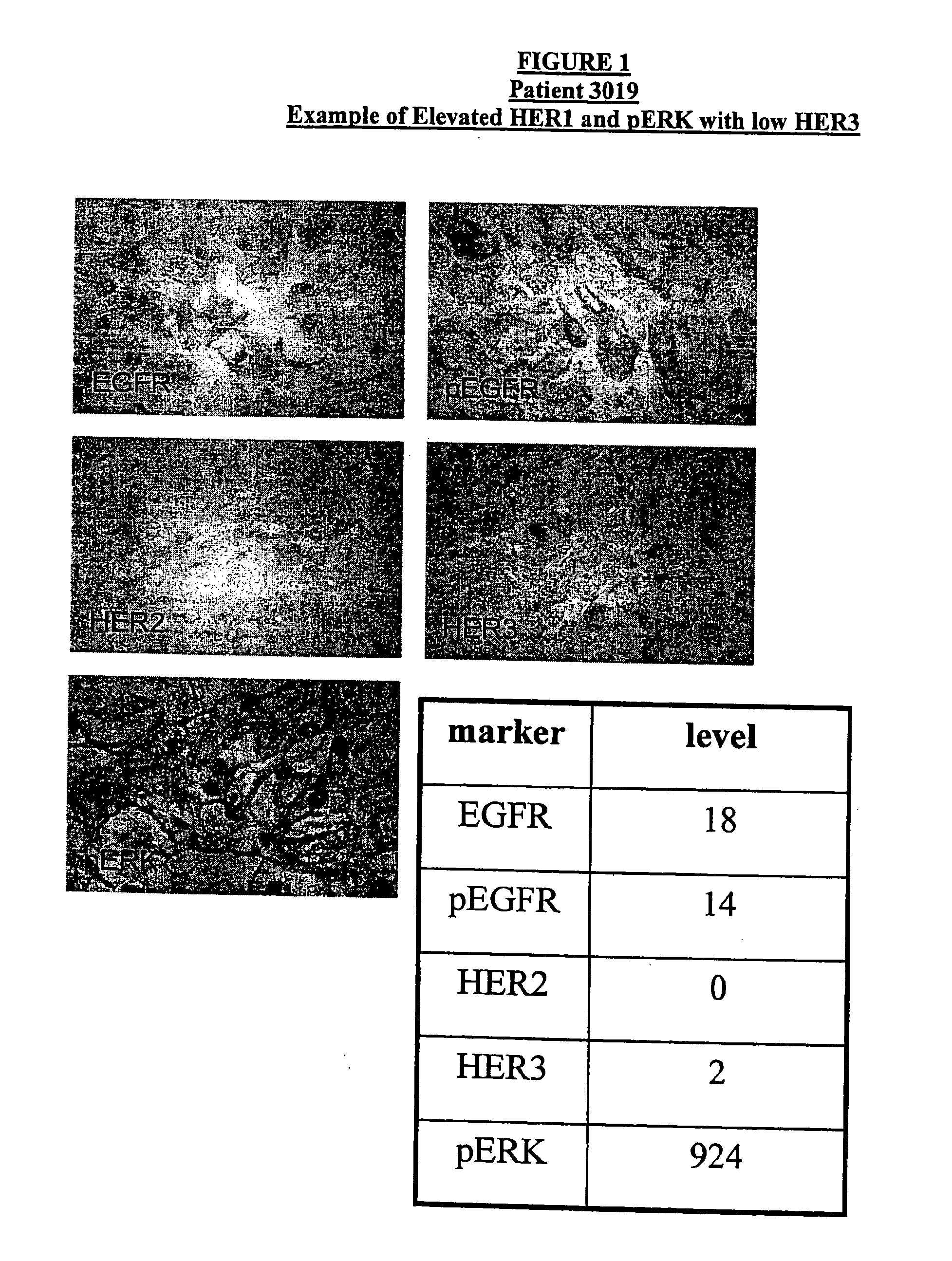
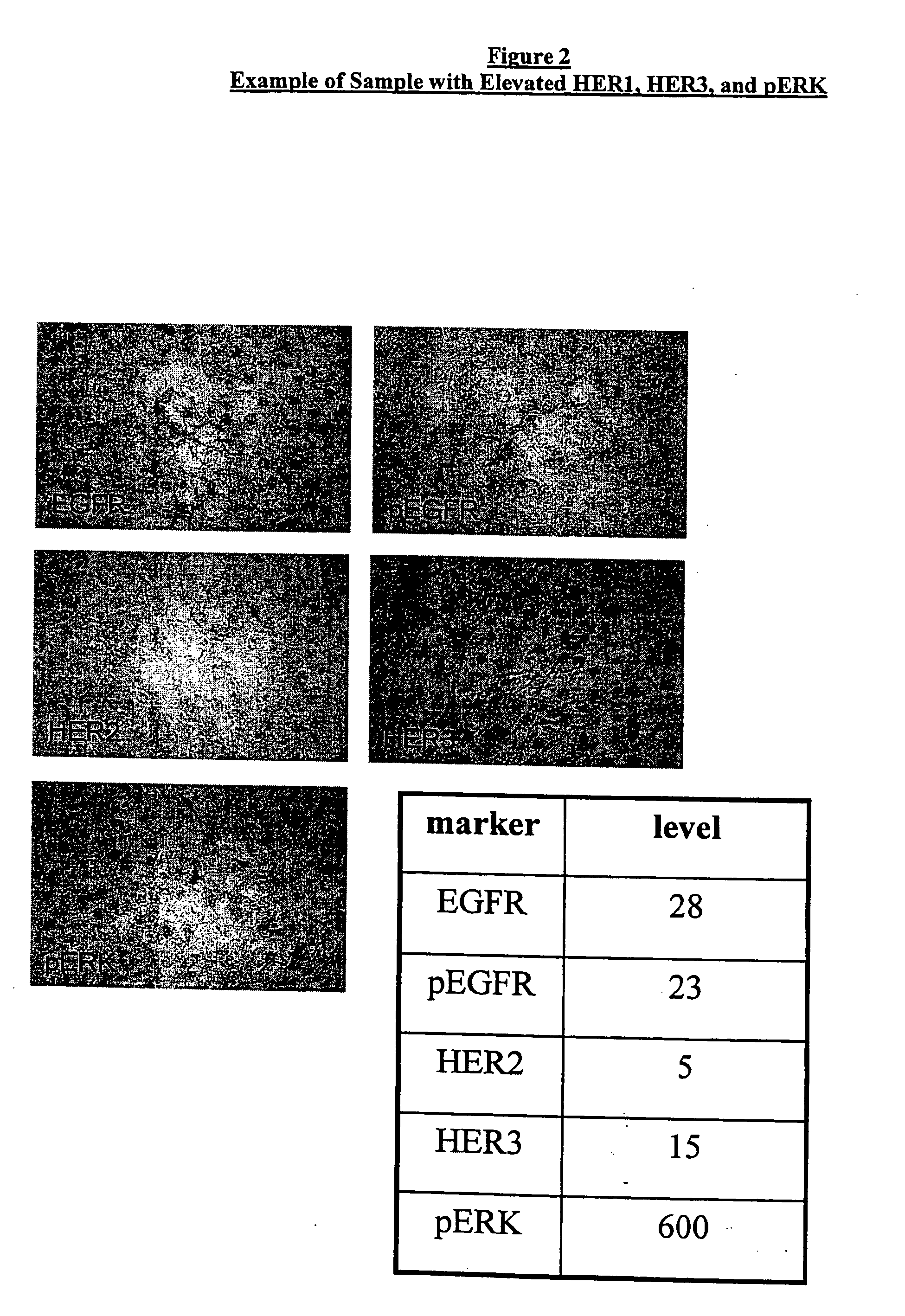
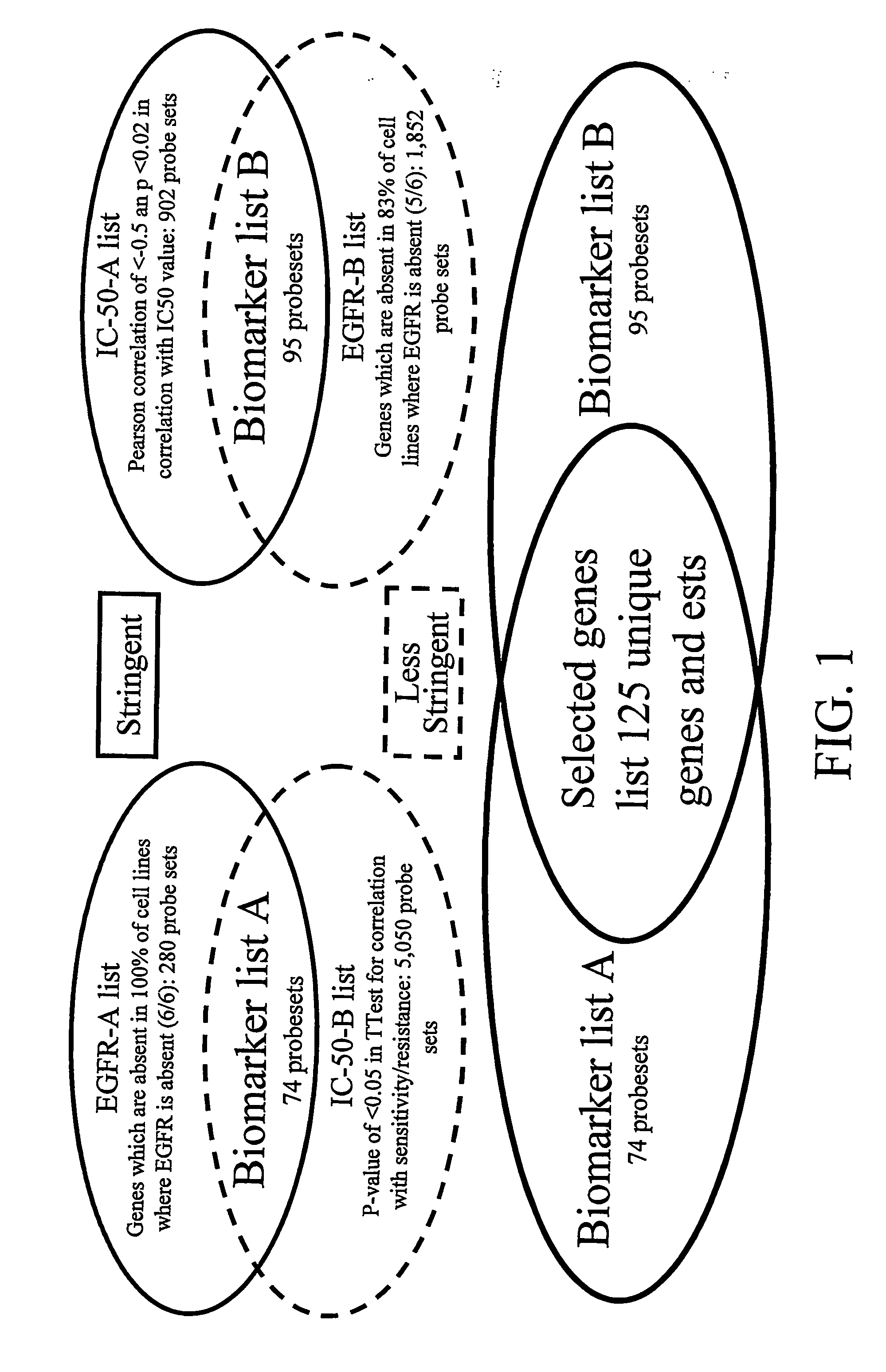
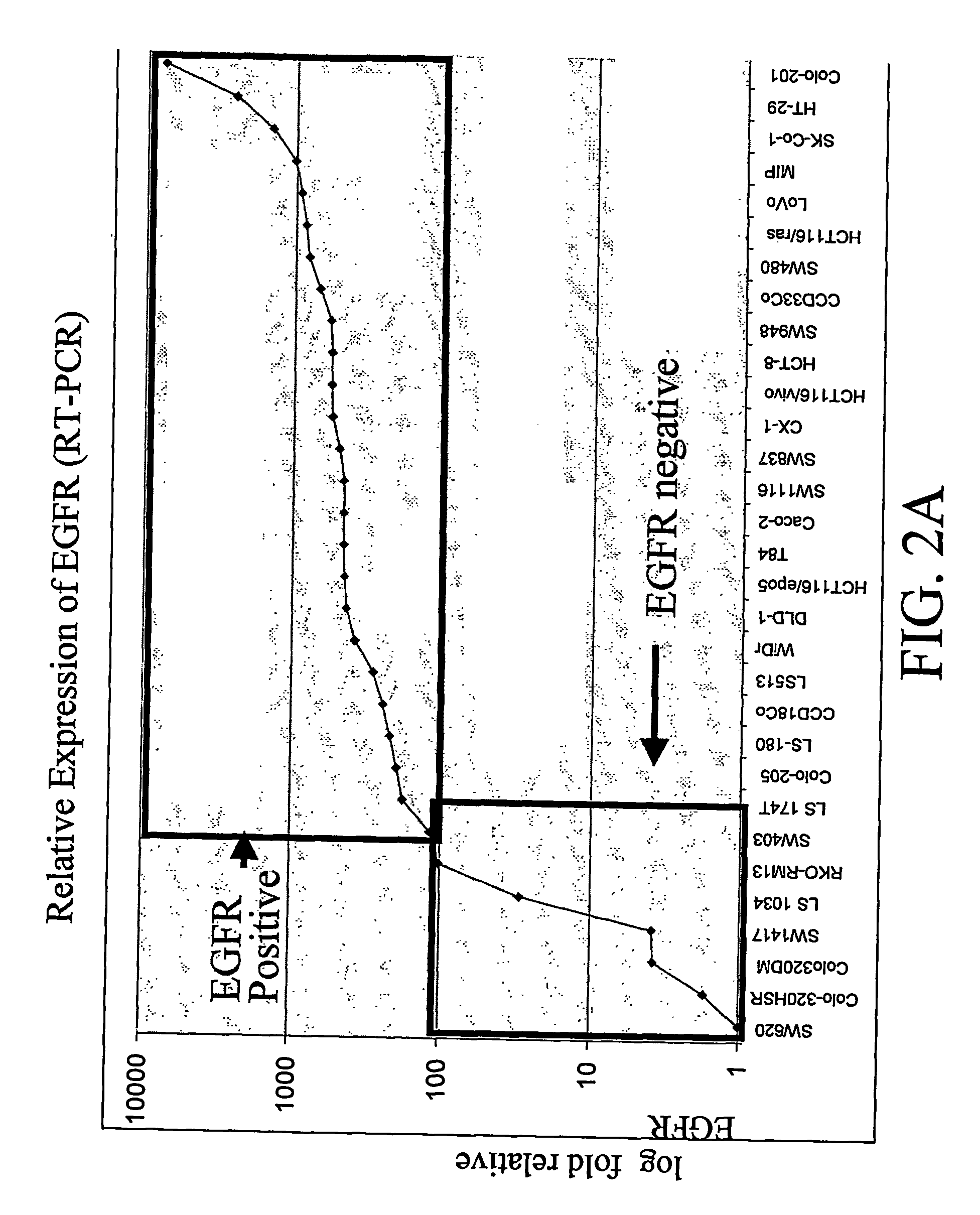
Biomarkers and Methods for Determining Sensitivity to Epidermal Growth Factor Receptor Modulators in Non-Small Cell Lung Cancer
EGFR biomarkers useful in a method for identifying a mammal that will respond therapeutically to a method of treating cancer comprising administering an EGFR modulator, wherein the method comprises (a) exposing a biological sample from the mammal to the EGFR modulator and (b) measuring in the biological sample the level of the at least one biomarker, wherein a difference in the level of the at least one biomarker measured in (b) compared to the level of the biomarker in a mammal that has not been exposed to the EGFR modulator indicates that the mammal will respond therapeutically to the method of treating cancer.
Owner:BRISTOL MYERS SQUIBB CO
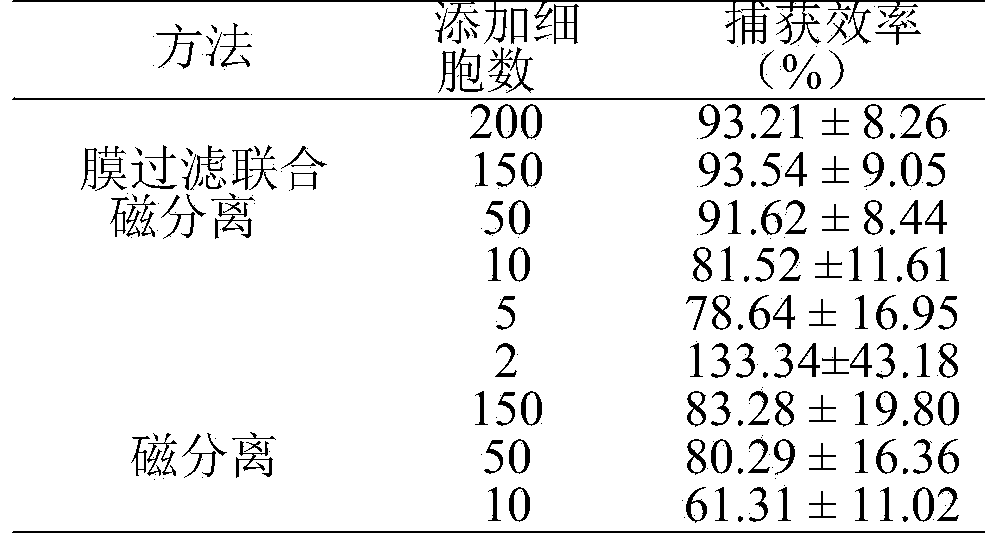
Immunomagnetic microsphere used for capturing circulating tumor cells in peripheral blood
ActiveCN103911345APlay a role in magnifying the sizeHigh selectivityElectrical/wave energy microorganism treatmentTumor/cancer cellsCirculating cancer cellSpecific antibody
The invention discloses an immunomagnetic microsphere used for capturing circulating tumor cells in peripheral blood. The immunomagnetic microsphere comprises a magnetic microsphere, wherein an epithelial cell specific antibody used for specifically recognizing and capturing circulating tumor cells is coupled on the magnetic microsphere. The epithelial cell specific antibody is selected according to a target tumor cell needed to be captured, for example, when the MCF (michigan cancer foundation)-7 cell is needed to be captured, the epithelial cell specific antibody selects an anti-EpCAM (epithelial cell adhesion molecule) antibody. The invention further discloses a method for capturing circulating tumor cells in peripheral blood by utilizing the immunomagnetic microsphere. The immunomagnetic microsphere disclosed by the invention is modified by the epithelial cell specific antibody, so that high-selectivity and high-specificity capturing for the target cell can be realized. The magnetic microsphere can achieve dimension amplifying effect, is used for realizing film separation with high recovery, and further can be used for magnetic separation operation to realize high-purity capturing. The immunomagnetic microsphere and the capturing method thereof can be used for early diagnosis of a cancer patient and prediction of therapeutic response.
Owner:BRILLIANCE BIO TECH CO LTD +1
Method for Predicting Response to Epidermal Growth Factor Receptor-Directed Therapy
ActiveUS20070134252A1Reduced expression levelSugar derivativesPeptide/protein ingredientsCancer therapyCancer research
This invention provides methods for determining or predicting response to cancer therapy in an individual.
Owner:AMGEN INC
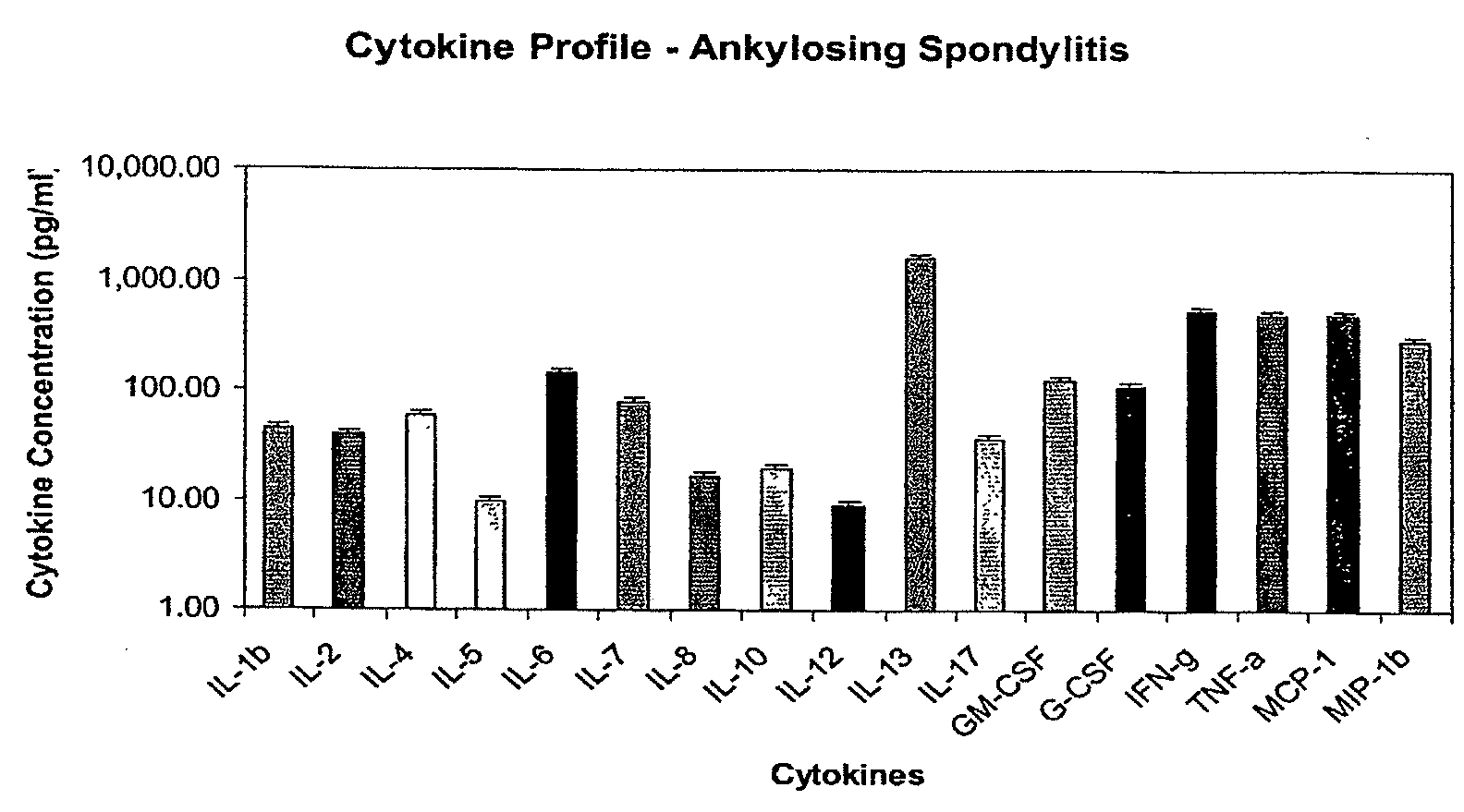
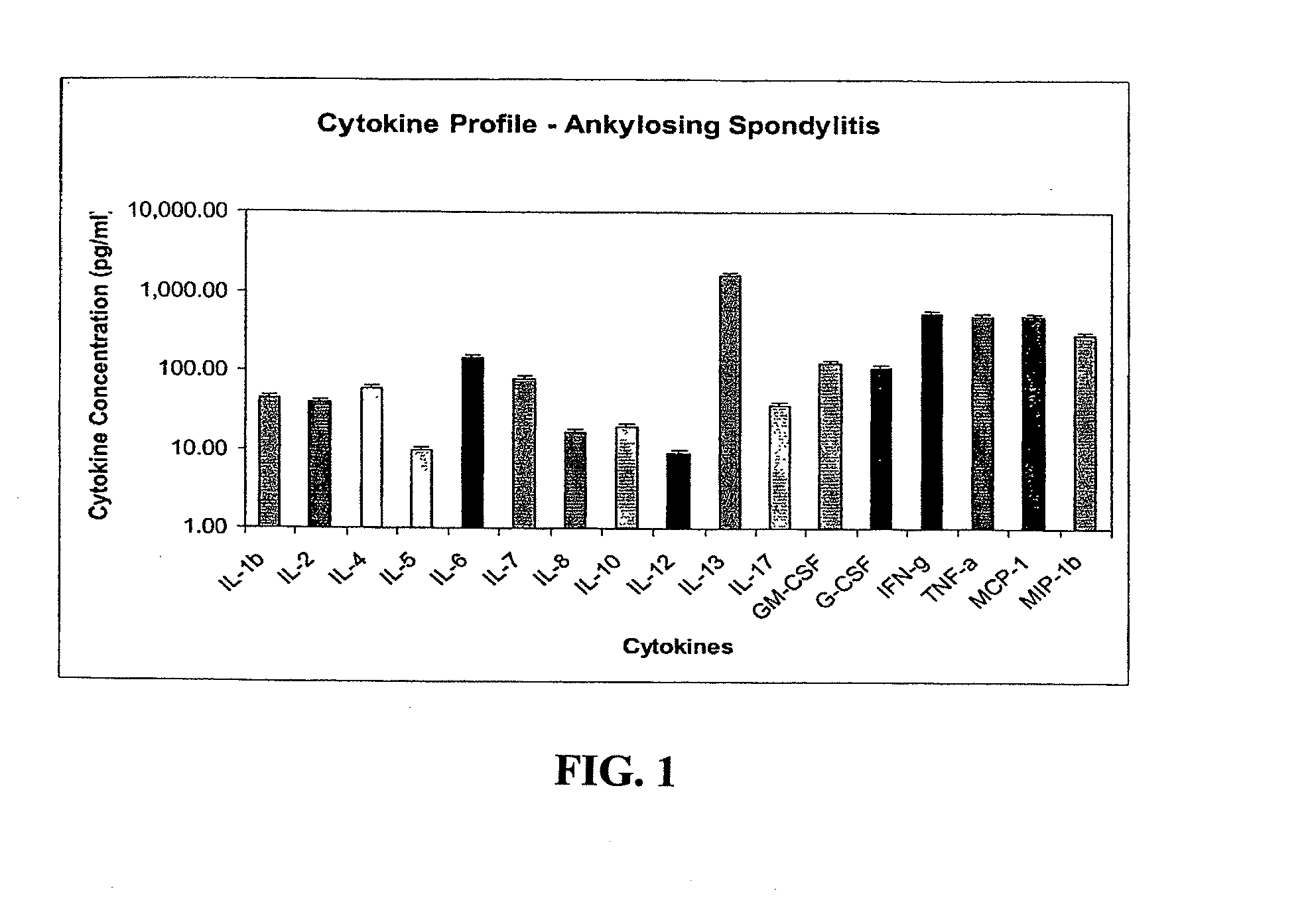
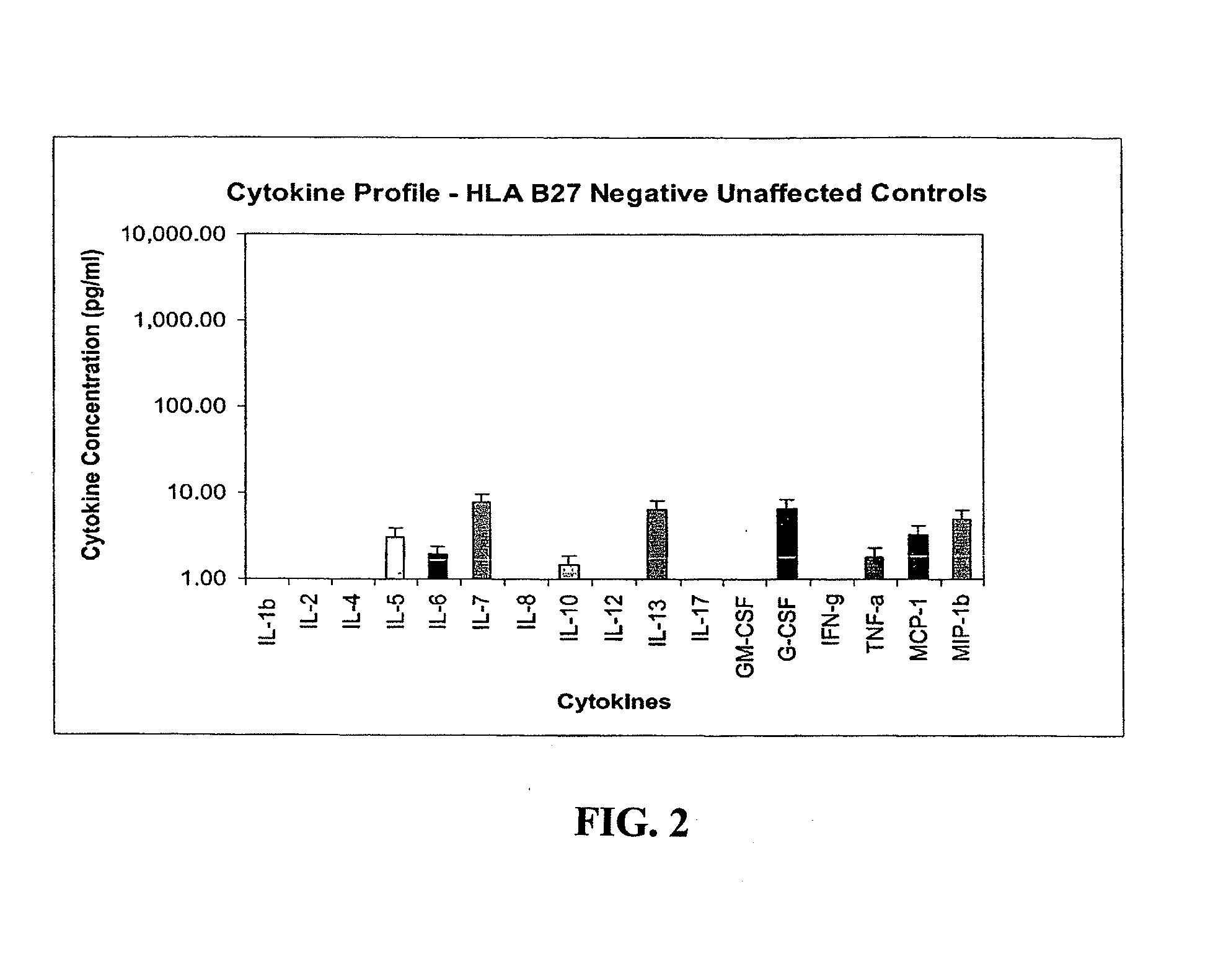
Method of using cytokine assay to diagnose, treat, and evaluate inflammatory and autoimmune diseases
InactiveUS20090325167A1Microbiological testing/measurementLibrary screeningCerebrospinal fluidFactor ii
The invention provides methods for diagnosing, treating, or evaluating inflammatory and autoimmune diseases by sampling peripheral blood, serum, plasma, tissue, cerebrospinal fluit, or other bodily fluids from a human subject having a suspected diagnosis. The sample is analyzed for the presence and amount of certain cytokines, which provides the diagnosis, prognosis or evaluation of therapeutic response.
Owner:THE BOARD OF RGT UNIV OF OKLAHOMA +1
Markers for diagnosis of pulmonary inflammation and methods related thereto
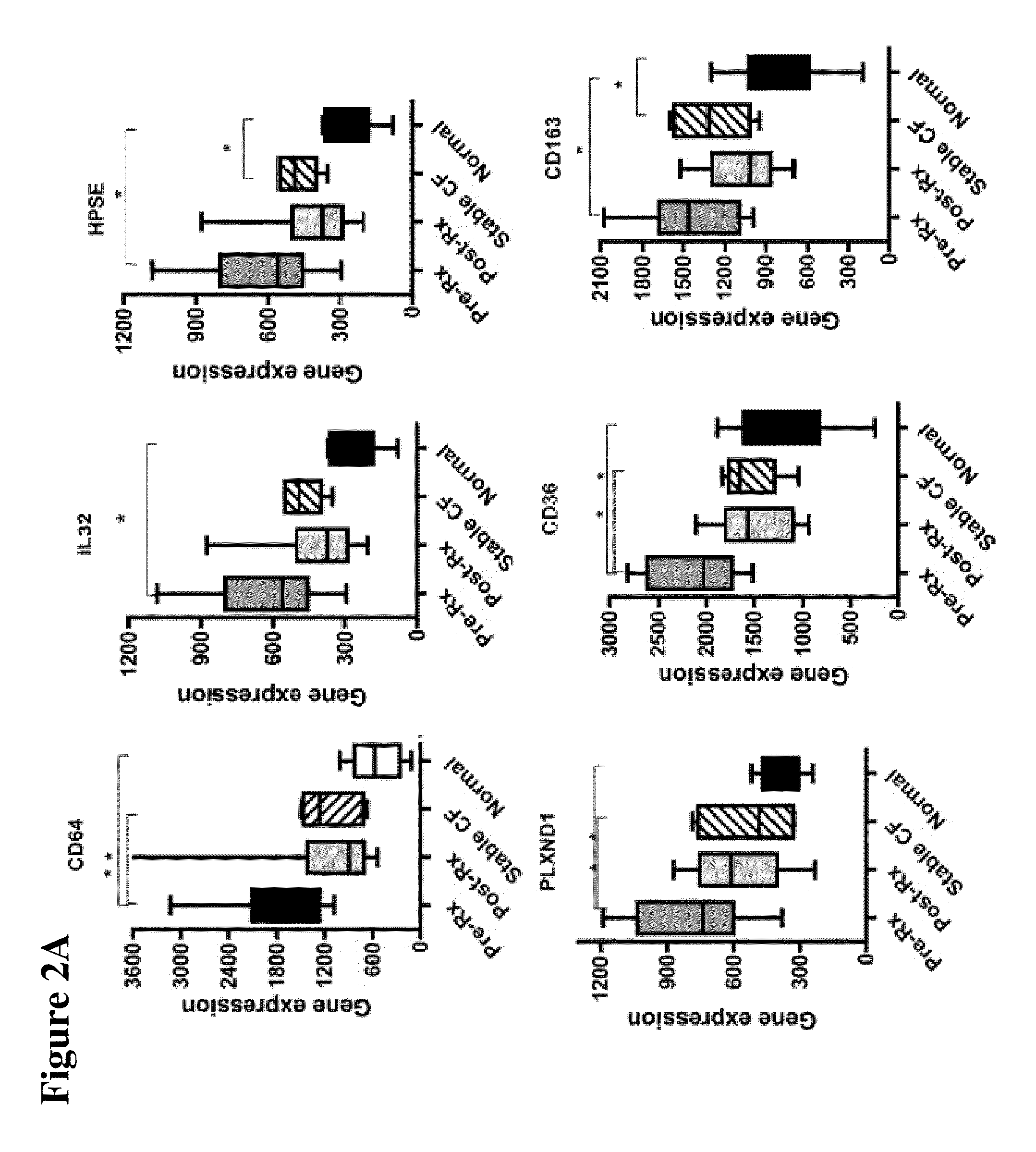
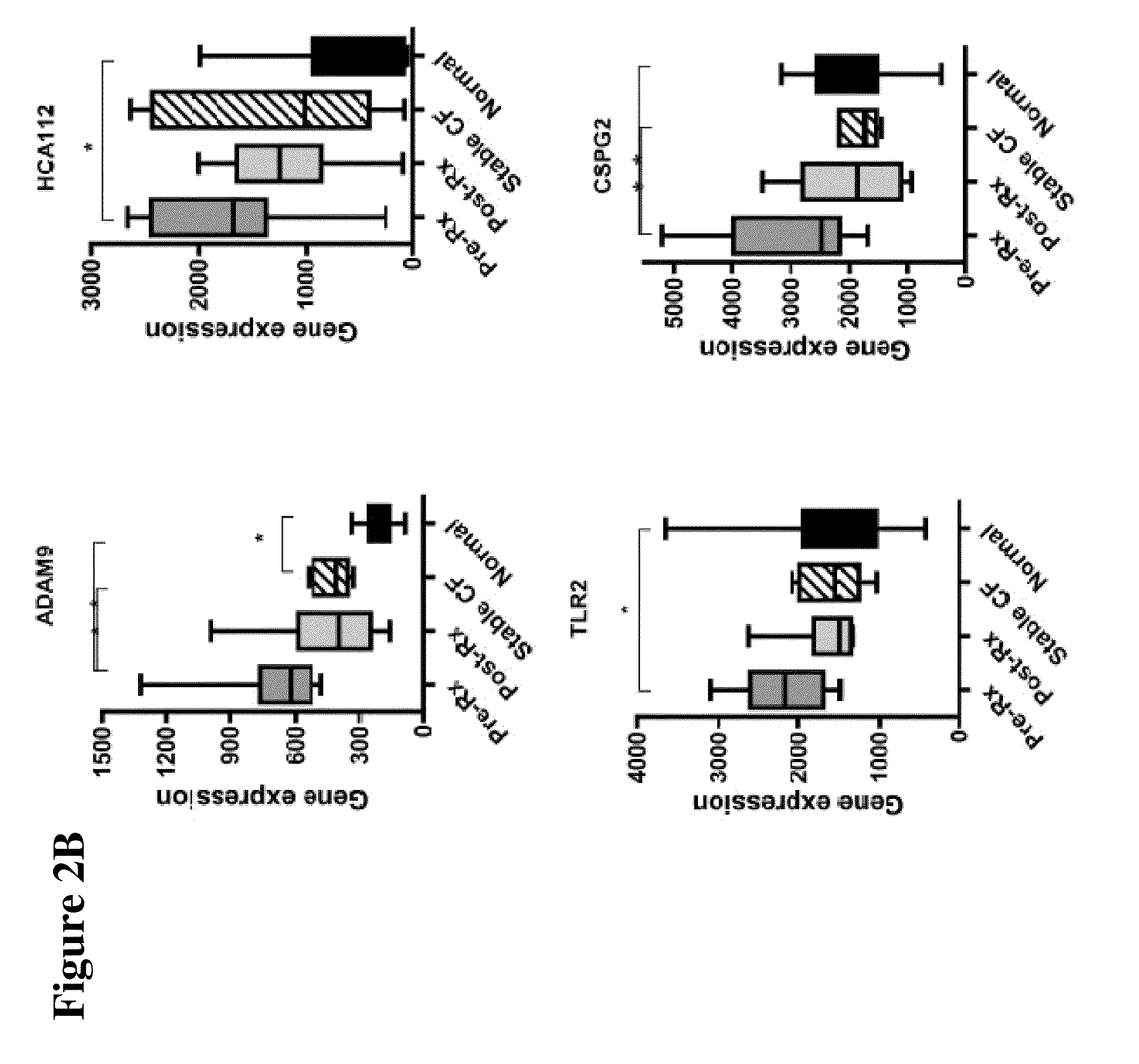
The present invention is related to the novel discovery of a number of genes that were identified as systemic markers of pulmonary inflammation. This discovery allows for development of a novel tool for reliable, rapid and efficient assessment of therapeutic responses and enables design of novel therapies targeted against diseases associated with pulmonary inflammation. In one embodiment, the present invention allows quantification of therapeutic response in patients who have a disease associated with pulmonary inflammation. In preferred embodiments, the genes are CD64, ADAM9, CD36, IL32, HPSE, PLXND1, HCA112, CSPG2, TLR2, and CD163.
Owner:NAT JEWISH HEALTH
Biomarkers and methods for determining sensitivity to epidermal growth factor receptor modulators
EGFR biomakers useful in a method for identifying a mammal that will respond therapeutically to a method of treating cancer comprising administering an EGFR modulator, wherein the method comprises (a) exposing the mammal to the EGFR modulator and (b) measuring in the mammal level of at least one biomaker, wherein a difference in the level in at least one biomaker measured in (b) compared to the level of the biomaker in a mammal that has not been exposed to the EGFR modulator indicates that the mammal will respond therapeutically to the method of treating cancer.
Owner:AMLER LUKAS C +1
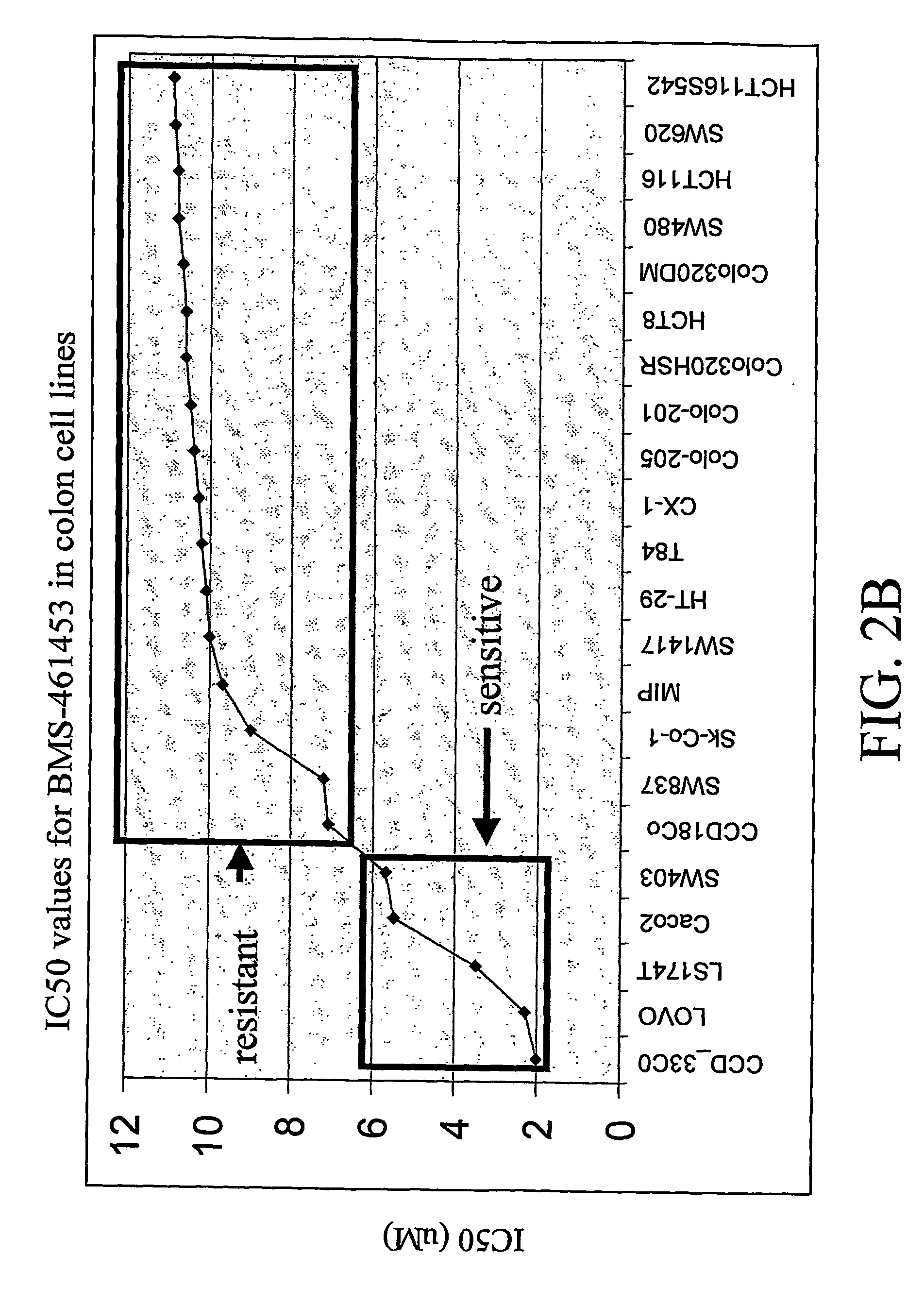
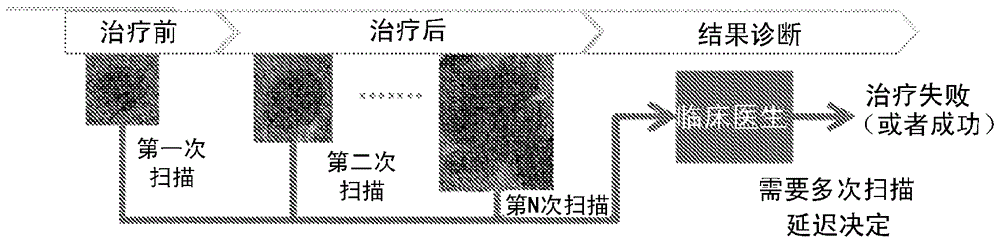
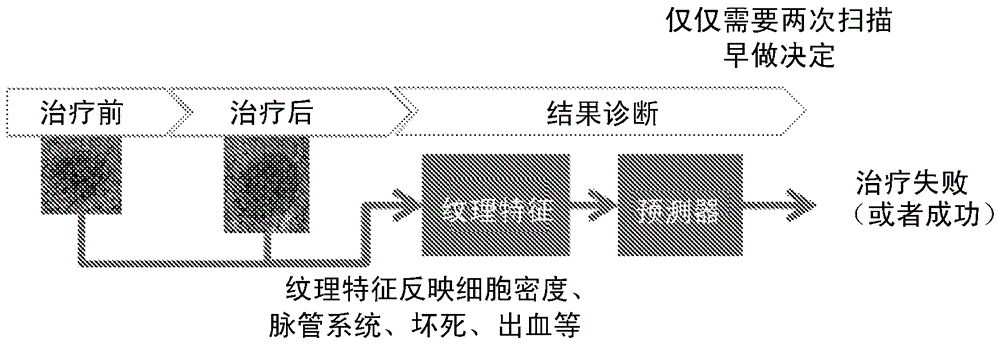
Early therapy response assessment of lesions
For therapy response assessment, texture features are input for machine learning a classifier and for using a machine learnt classifier. Rather than or in addition to using formula-based texture features, data driven texture features are derived from training images. Such data driven texture features are independent analysis features, such as features from independent subspace analysis. The texture features may be used to predict the outcome of therapy based on a few number of or even one scan of the patient
Owner:SIEMENS HEATHCARE GMBH
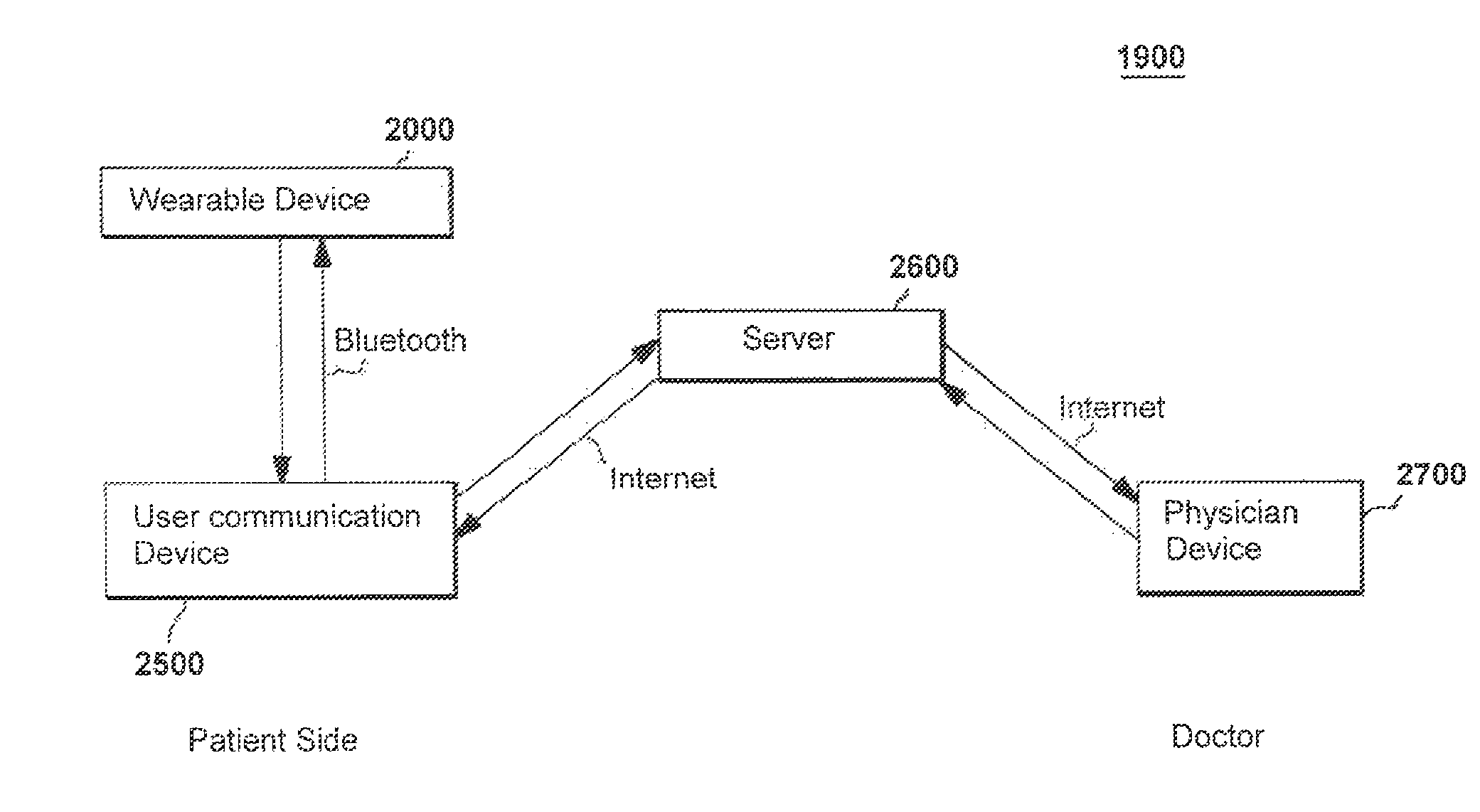
ECG acquisition and treatment-response system for treating abnormal cardiac function
ActiveUS9107571B2Prevent unintended medicatingAvoid overdoseElectrocardiographySensorsMicrocontrollerCardiac functioning
A method and system for detecting and treating abnormal cardiac function. The patient's cardiac activity is substantially continuously monitored via a portable wearable device having electrodes and a microcontroller. Features in the monitored cardiac activity indicative of abnormal cardiac function are automatically detected. In response to the detection of an abnormal detected cardiac function, at least one person is automatically alerted of the detected abnormal cardiac function of the patient, and medication may be automatically caused to be administered to the patient. The portable wearable device preferably includes a plurality of electrodes and a microcontroller in communication with the electrodes, adapted to receive sensed cardiac activity signals and create digital signals enabling identification of at least one cardiac parameter. A remote computer server is in communication with the portable wearable device and compares values of the identified cardiac parameter with a range of normal values for the cardiac parameter.
Owner:CARDIMETRIX
Biomarkers predictive of therapeutic responsiveness to ifnb and uses thereof
InactiveUS20120328567A1Effective monitoringEffectively treat multiple sclerosisNervous disorderPeptide/protein ingredientsRelapsing remittingTherapy response
Methods, assays and kits for the identification, assessment and / or treatment of a subject having multiple sclerosis (MS) (e.g., a patient with relapsing-remitting multiple sclerosis (RRMS)) are disclosed.
Owner:BIOGEN MA INC
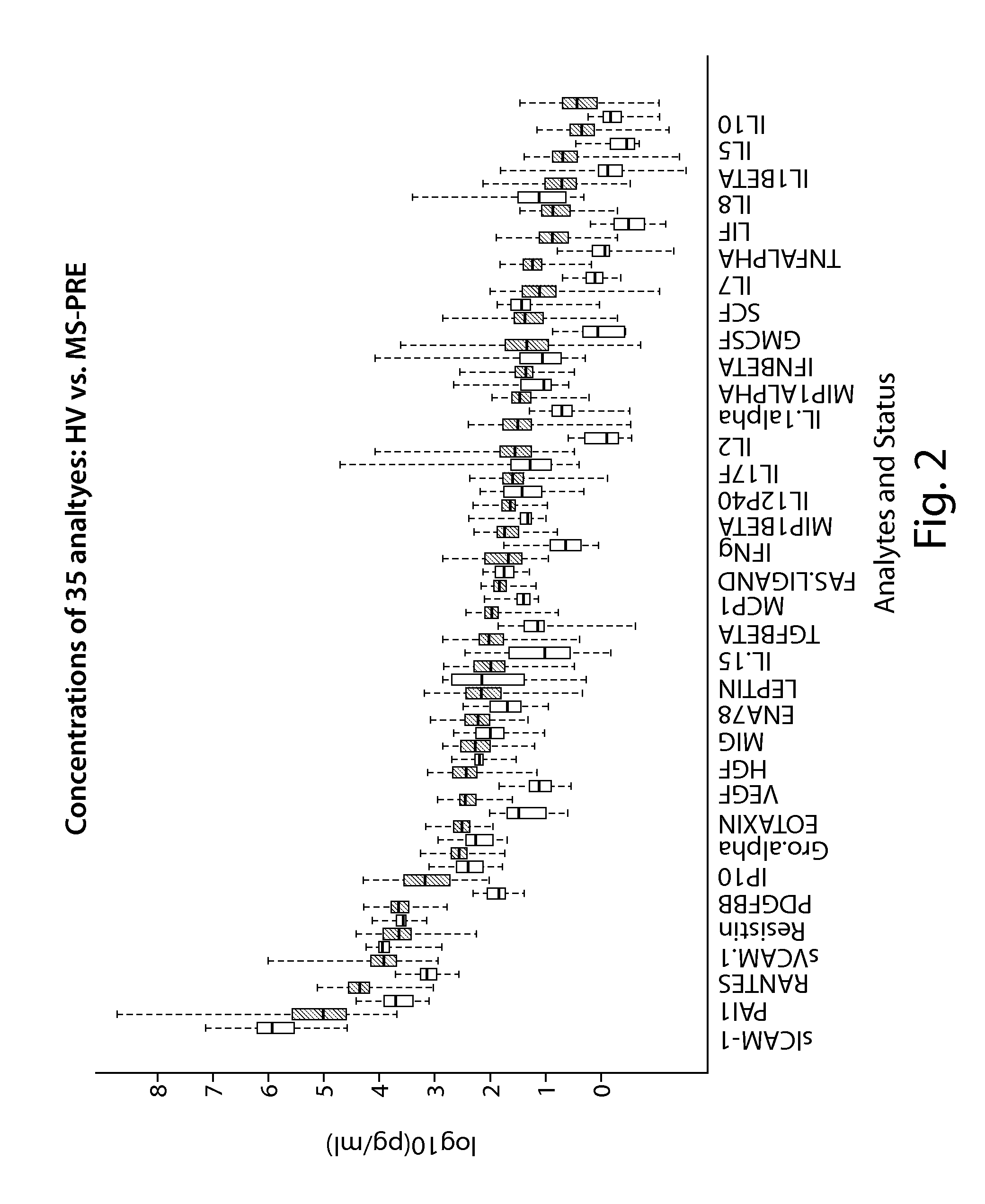
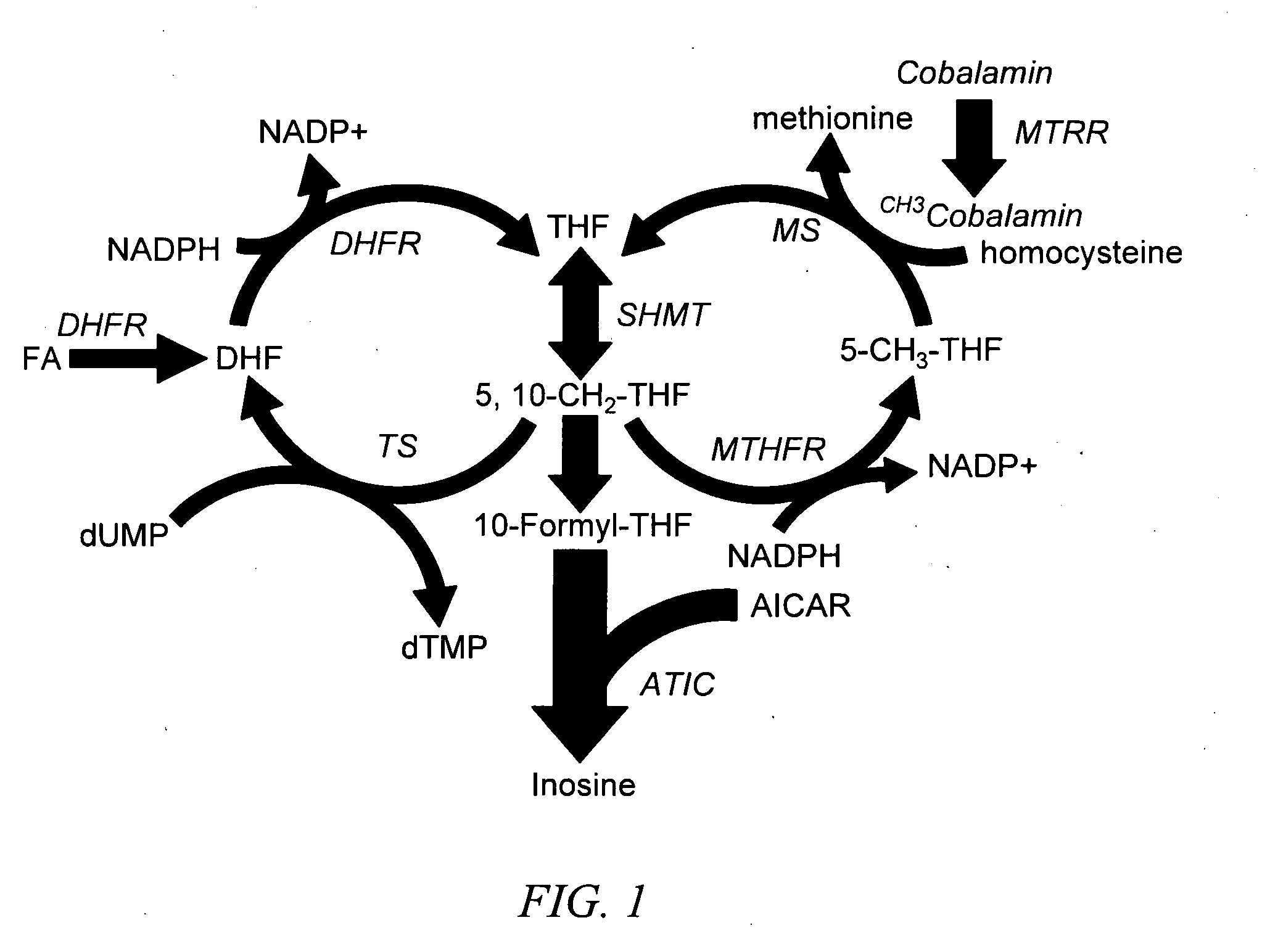
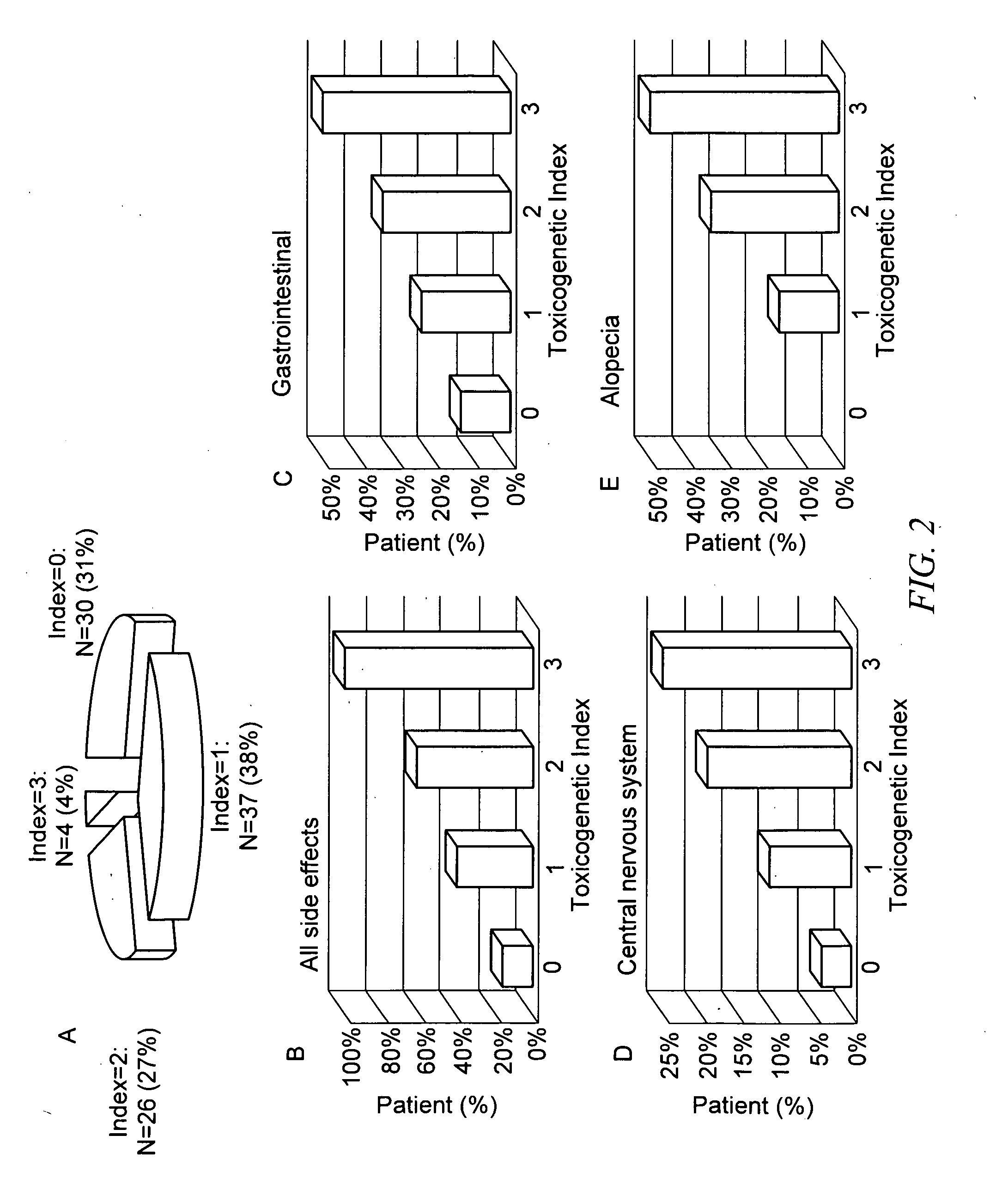
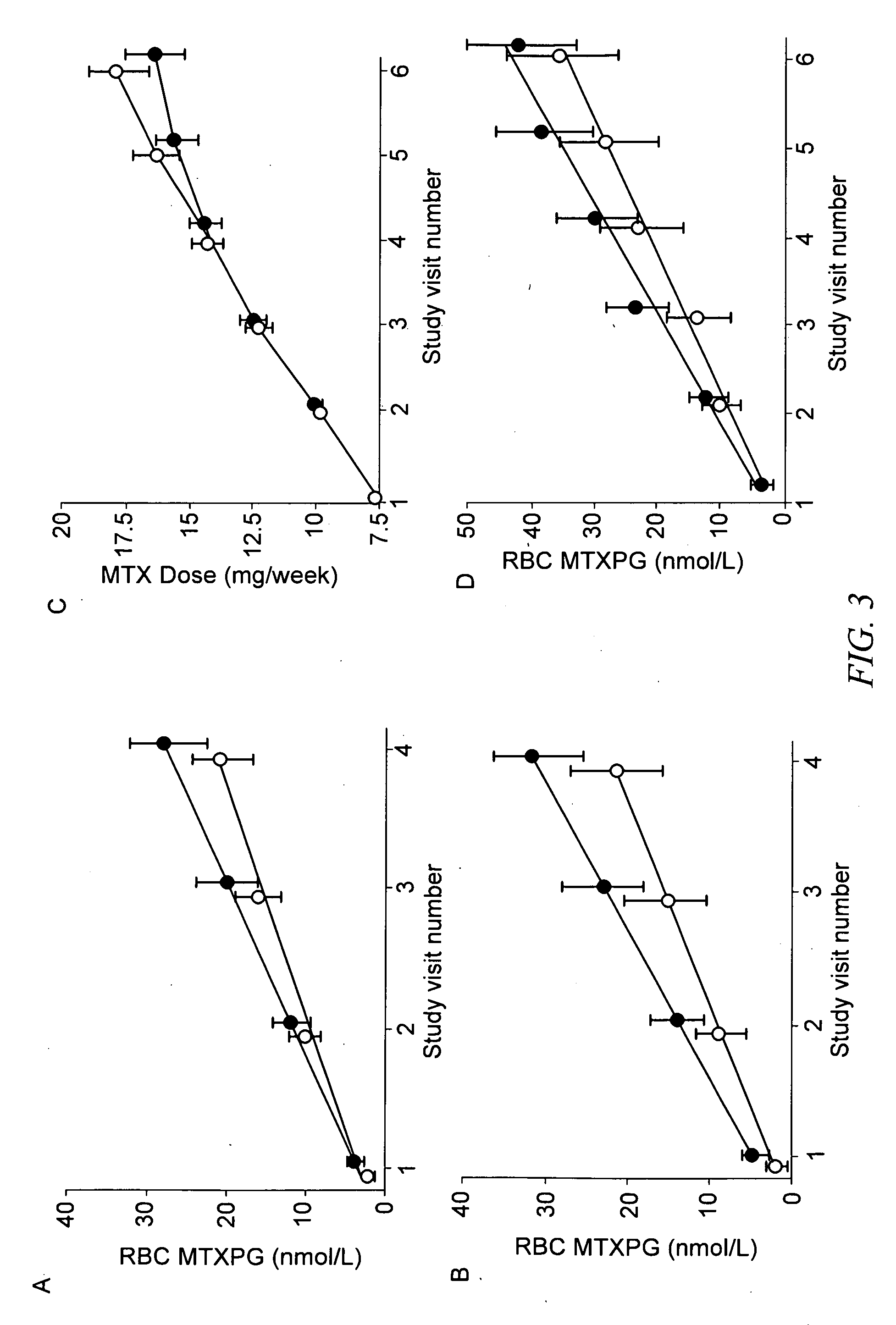
Methods of predicting methotrexate efficacy and toxicity
InactiveUS20100203508A1Therapeutic efficacyEliminate side effectsMicrobiological testing/measurementSide effectMetabolite
Owner:PROMETHEUS LAB
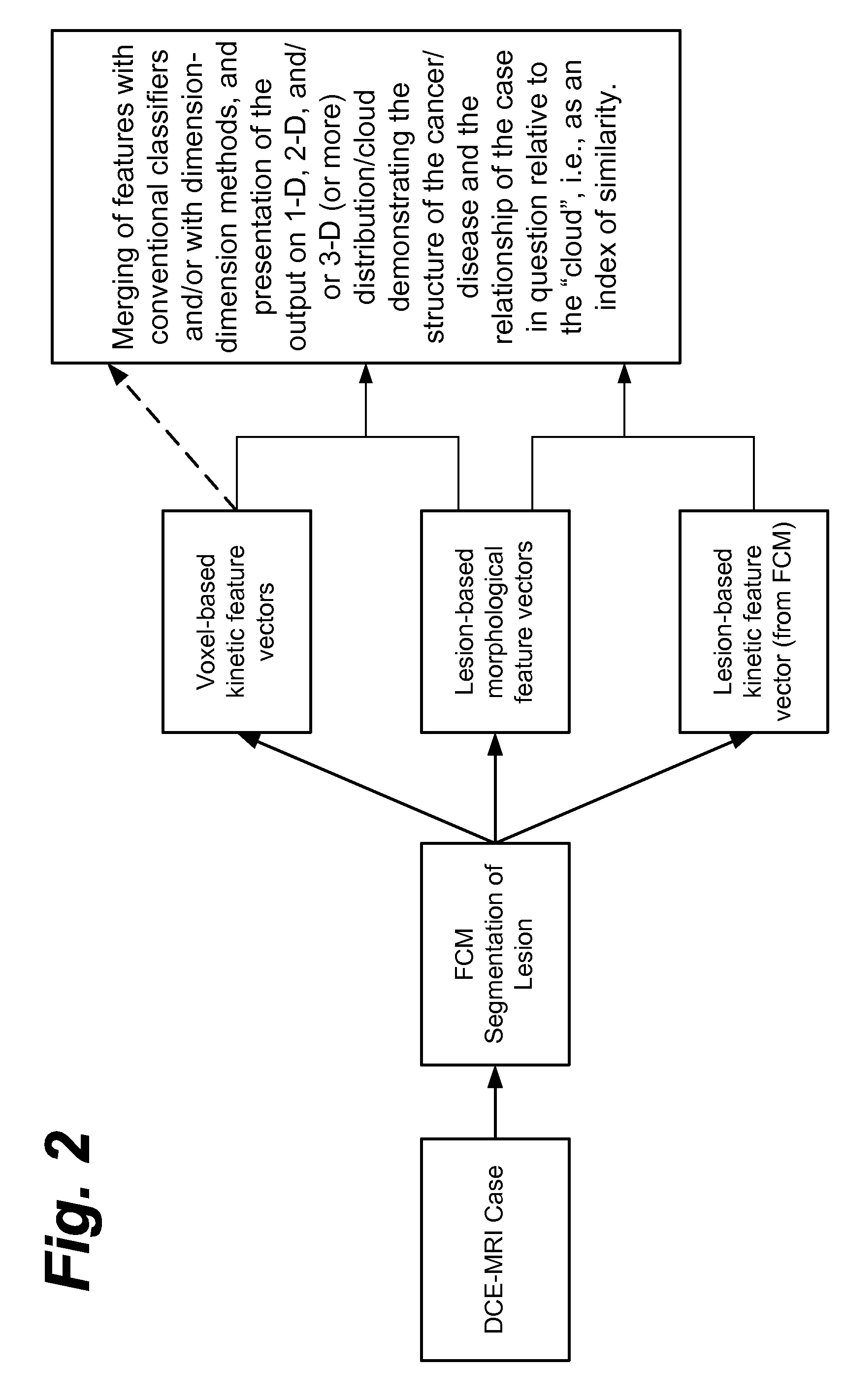
Method, system, software and medium for advanced intelligent image analysis and display of medical images and information
Computerized interpretation of medical images for quantitative analysis of multi-modality breast images including analysis of FFDM, 2D / 3D ultrasound, MRI, or other breast imaging methods. Real-time characterization of tumors and background tissue, and calculation of image-based biomarkers is provided for breast cancer detection, diagnosis, prognosis, risk assessment, and therapy response. Analysis includes lesion segmentation, and extraction of relevant characteristics (textural / morphological / kinetic features) from lesion-based or voxel-based analyzes. Combinations of characteristics in several classification tasks using artificial intelligence is provided. Output in terms of 1D, 2D or 3D distributions in which an unknown case is identified relative to calculations on known or unlabeled cases, which can go through a dimension-reduction technique. Output to 3D shows relationships of the unknown case to a cloud of known or unlabeled cases, in which the cloud demonstrates the structure of the population of patients with and without the disease.
Owner:QLARITY IMAGING LLC
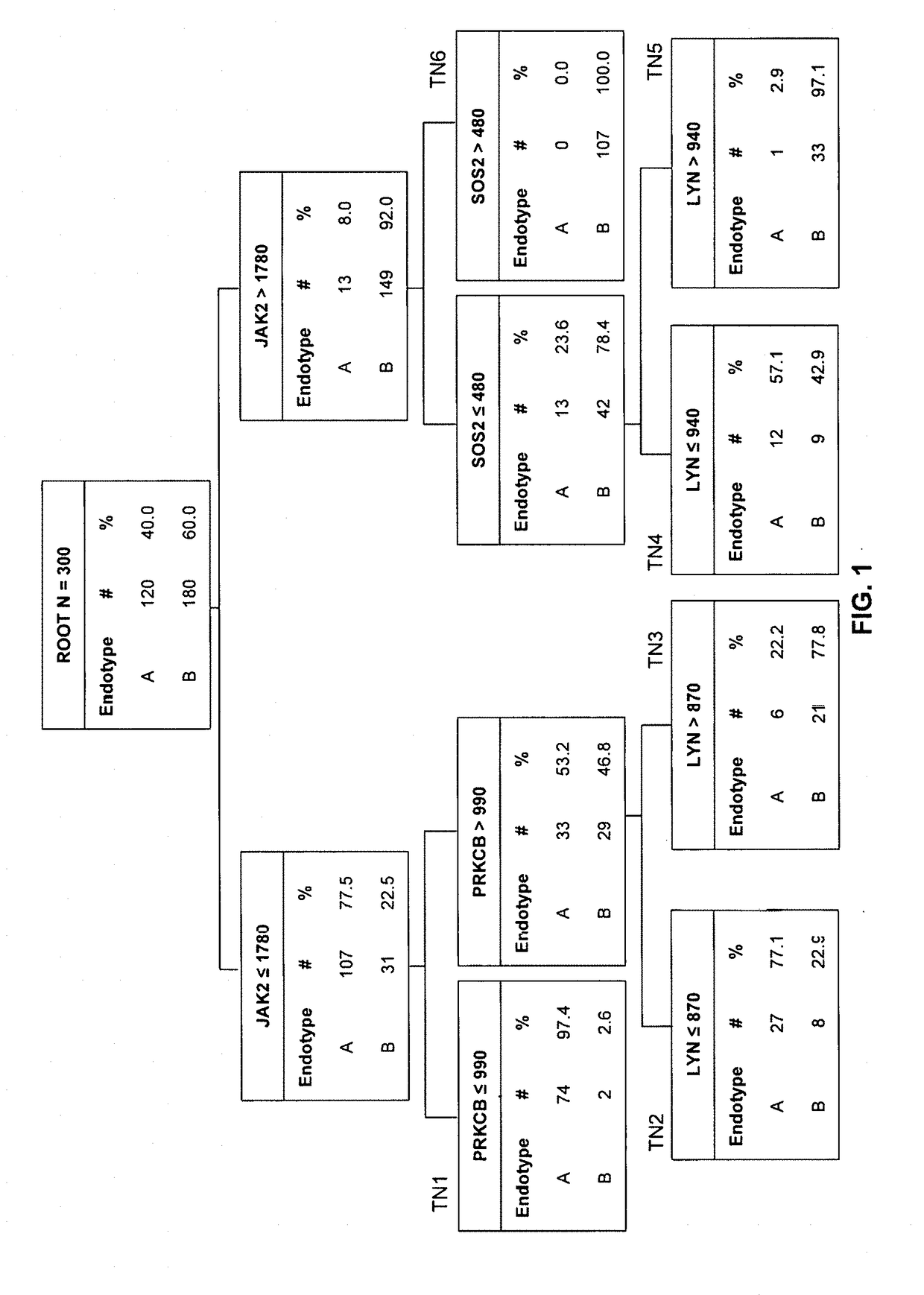
Septic Shock Endotyping Strategy and Mortality Risk For Clinical Application
ActiveUS20190065666A1Promote resultsHigh riskMicrobiological testing/measurementBiostatisticsPediatric patientDeath risk
Methods and compositions disclosed herein generally relate to methods of identifying, validating, and measuring clinically relevant, quantifiable biomarkers of diagnostic and therapeutic responses for blood, vascular, cardiac, and respiratory tract dysfunction, particularly as those responses relate to septic shock in pediatric patients. In particular, the invention relates to identifying two or more biomarkers associated with septic shock in pediatric patients, obtaining a sample from a pediatric patient having at least one indication of septic shock, then quantifying from the sample an amount of two or more of said biomarkers, wherein the level of said biomarker correlates with a predicted outcome.
Owner:UNIVERSITY OF CINCINNATI +1
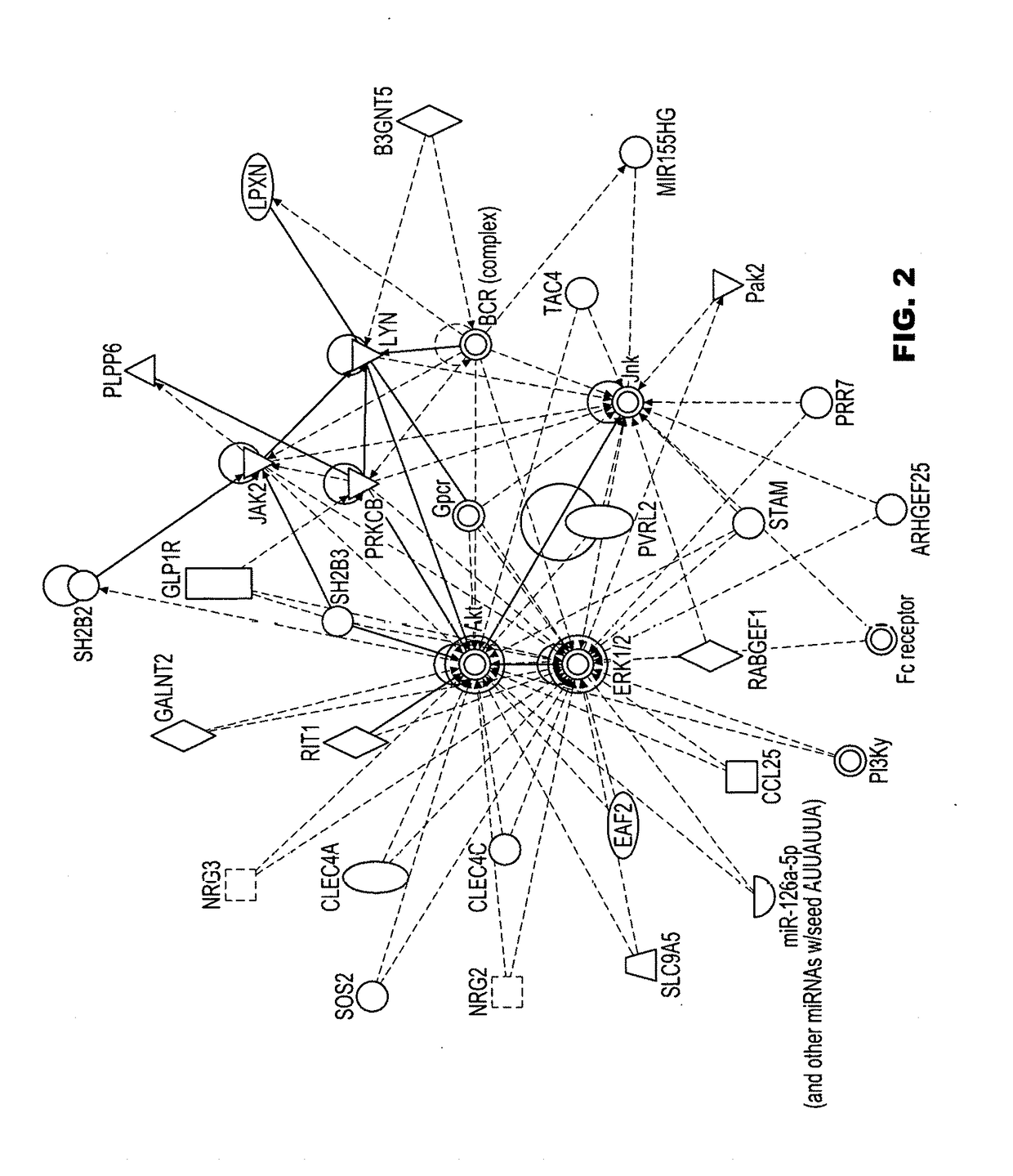
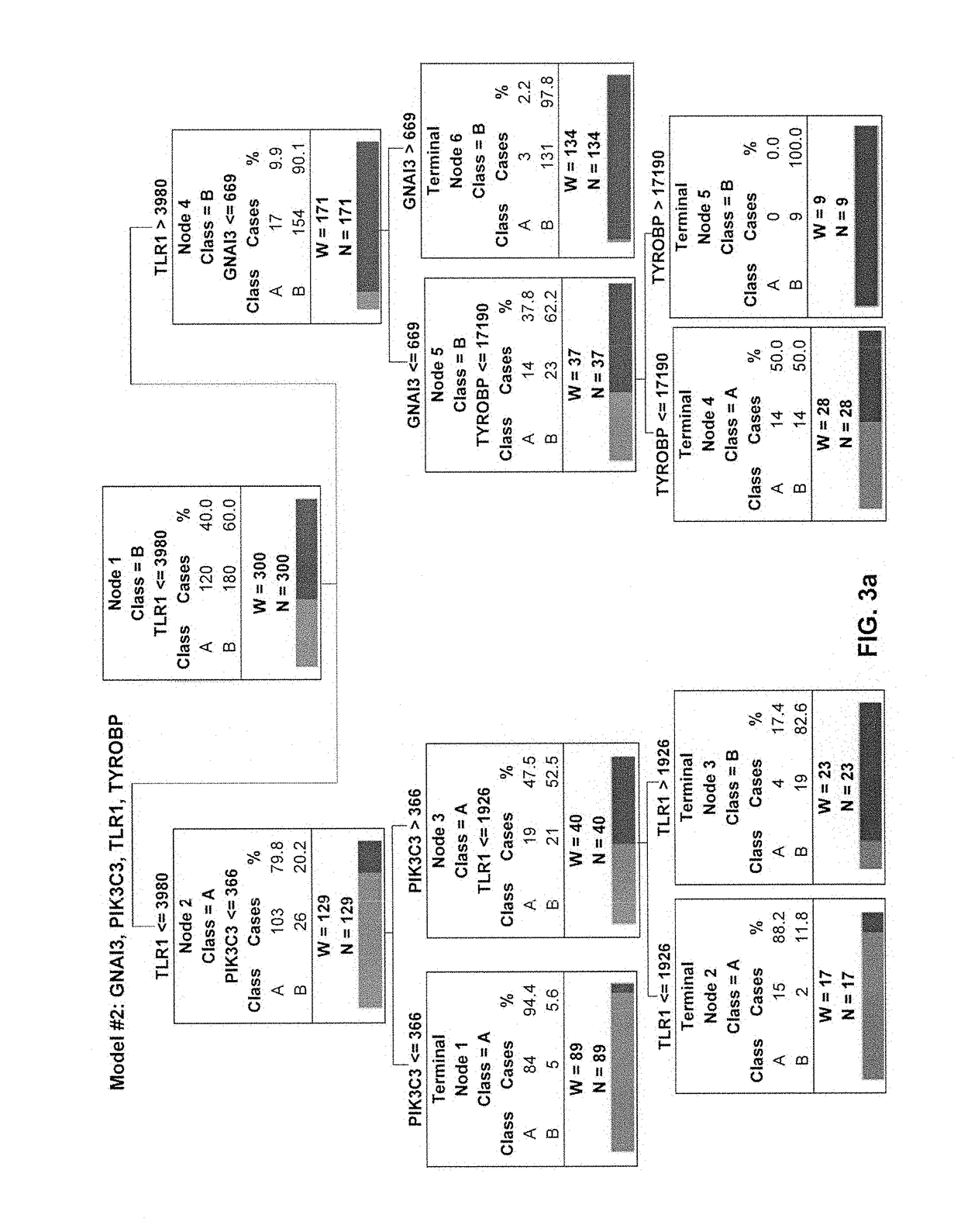
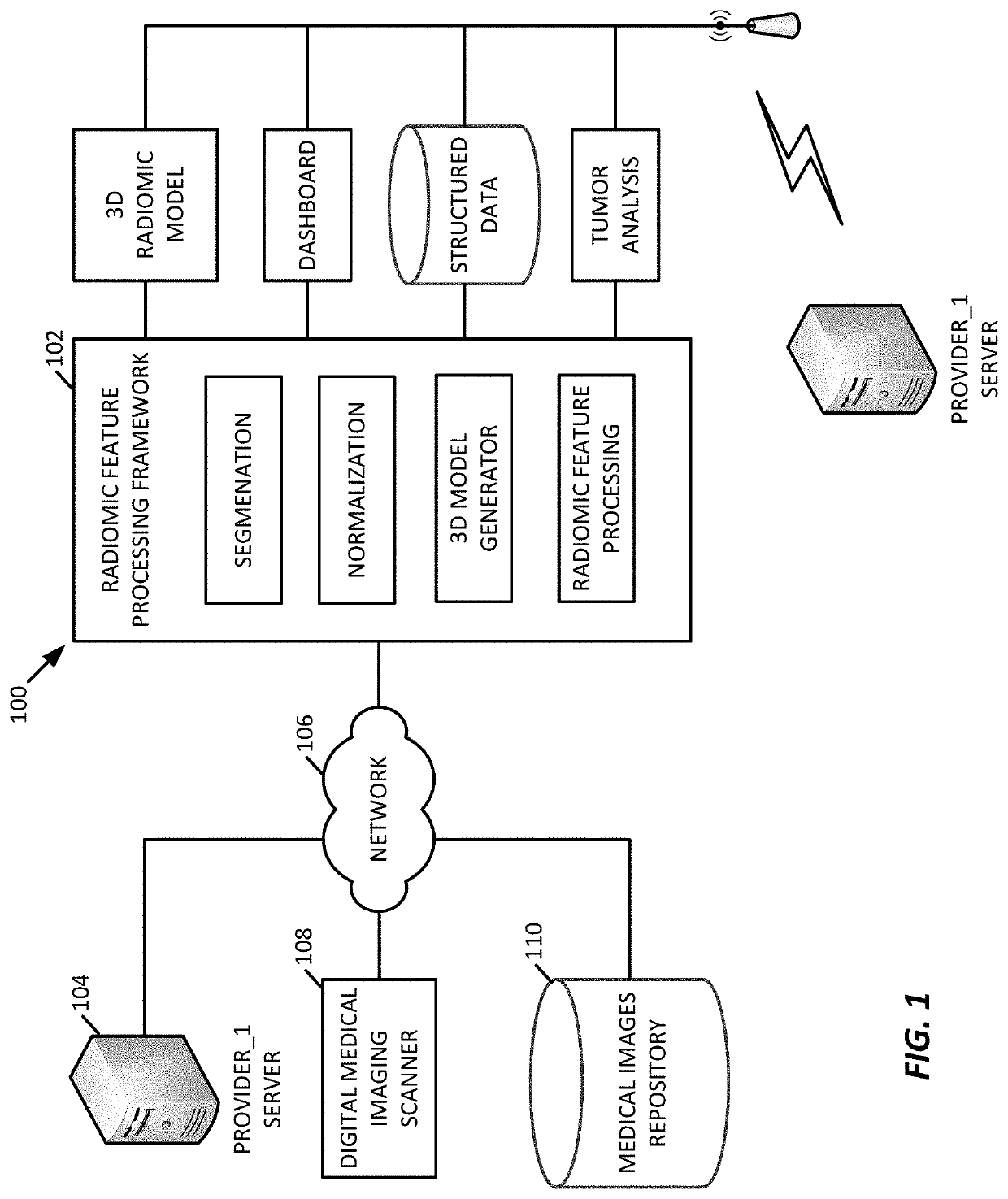
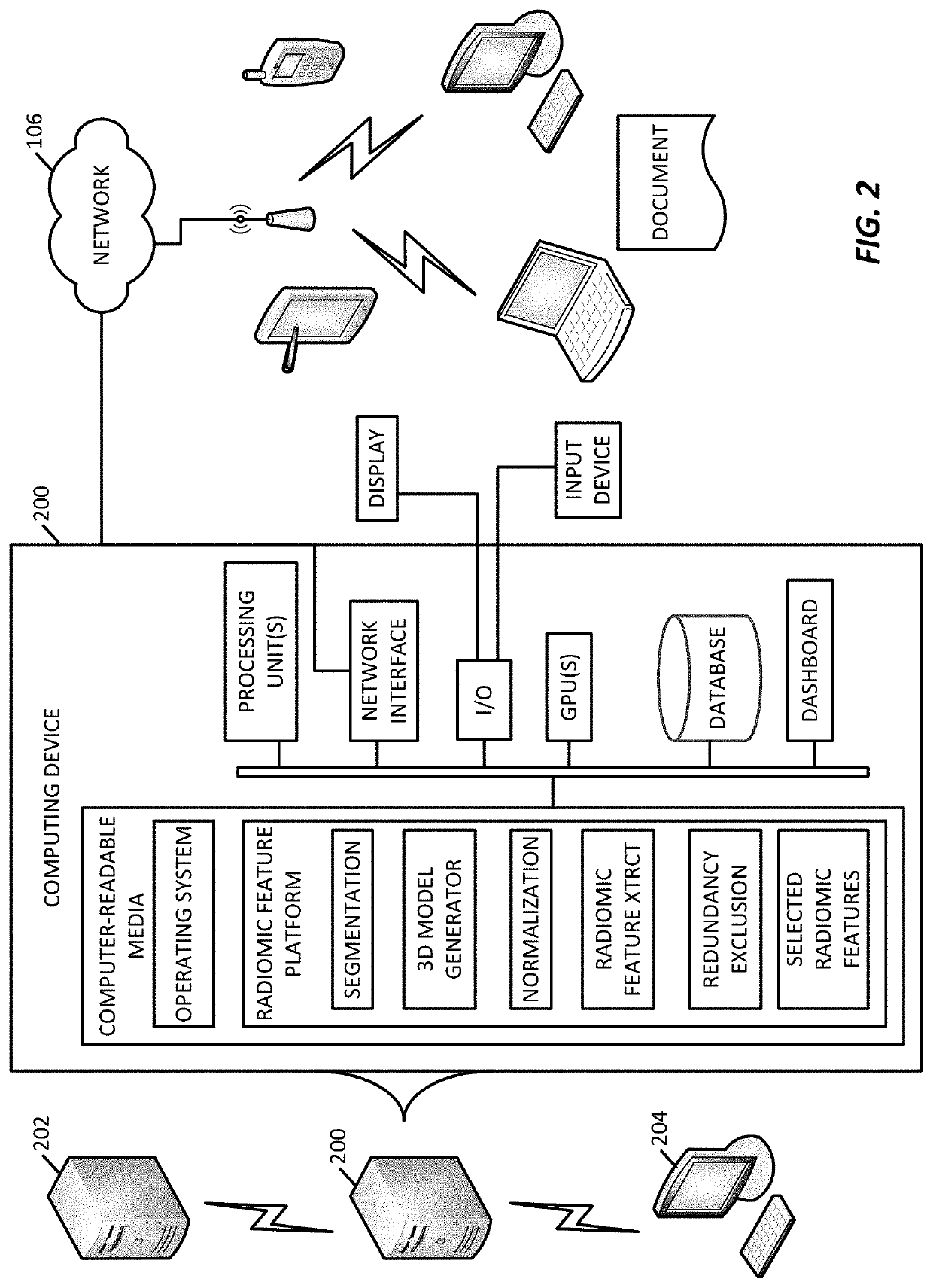
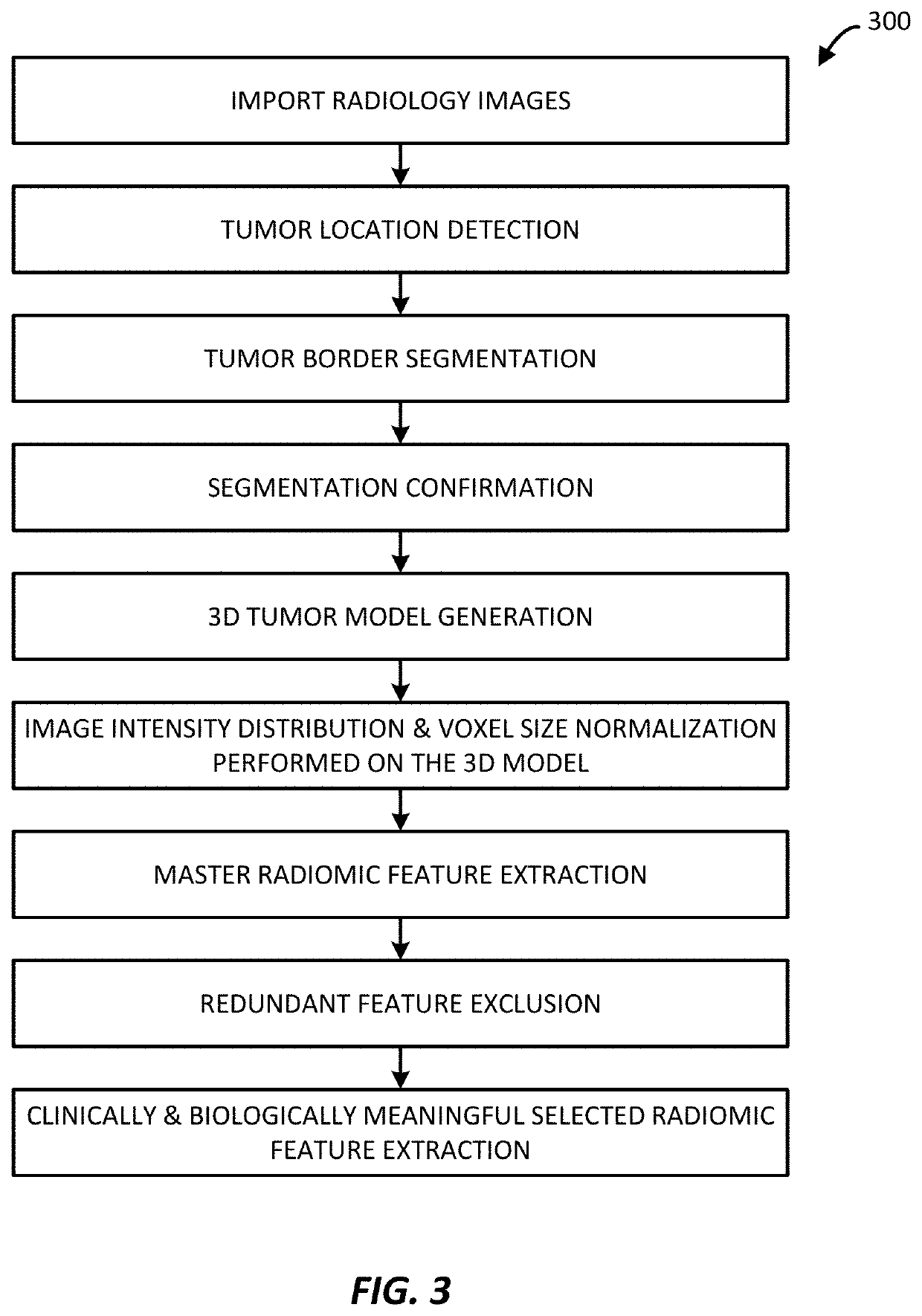
3D radiomic platform for imaging biomarker development
ActiveUS20200005461A1Reduce processing burdenImprove monitoring accuracyImage enhancementImage analysisImaging biomarkerRadiology studies
A platform is provided for generating 3D models of a tumor segmented from a series of 2D medical images and for identifying from these 3D models, radiomic features that may be used for diagnostic, prognostic, and treatment response assessment of the tumor. The radiomic features may be shape-based features, intensity-based features, textural features, and filter-based features. The radiomic features are compared to remove sufficiently redundant features, thereby producing a reduced set of radiomic features, which is then compared to separate genomic data and / or outcome data to identify clinically and biologically significant radiomic features for diagnostic, prognostic, and treatment response assessment, other applications.
Owner:TEMPUS LABS INC
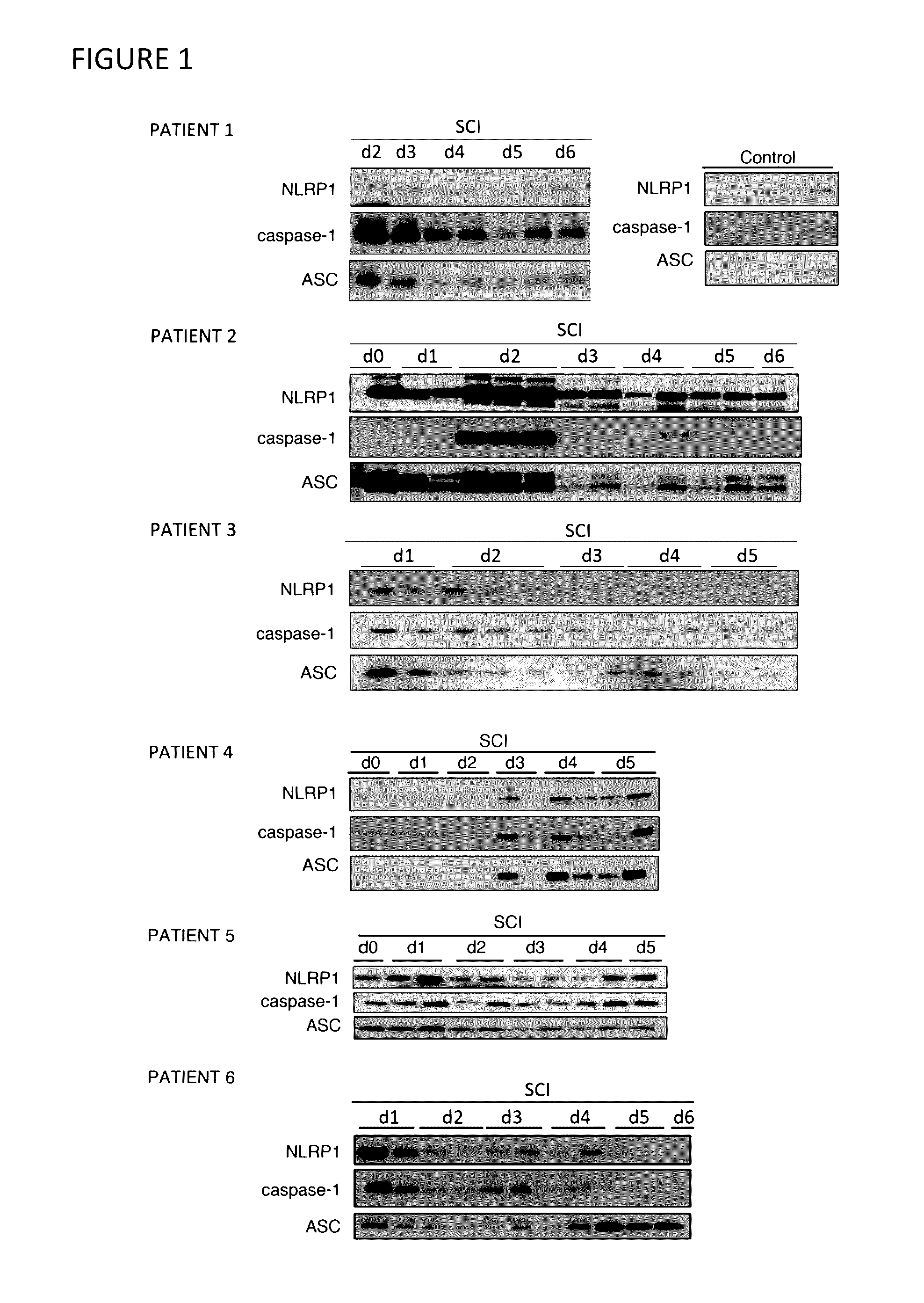
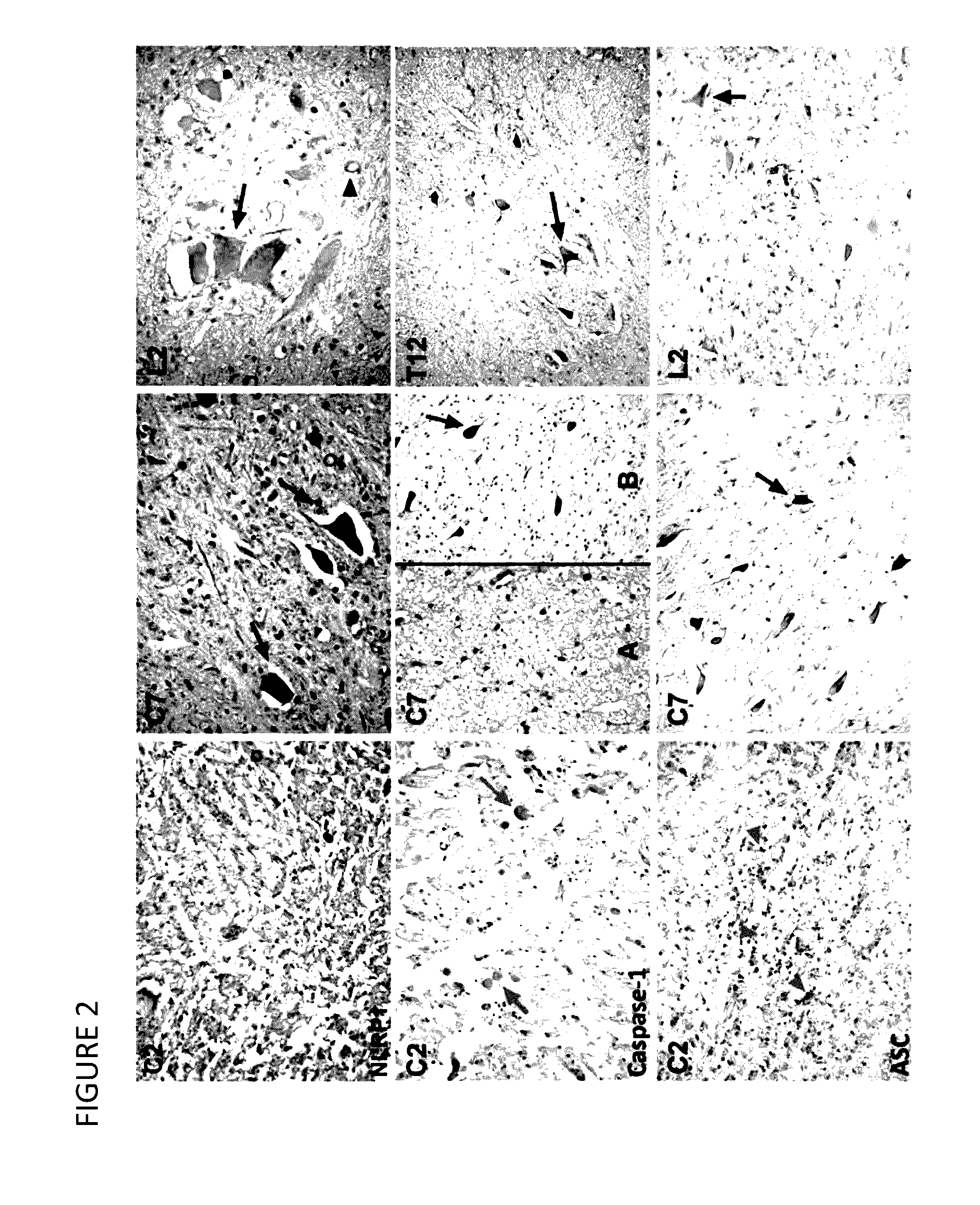
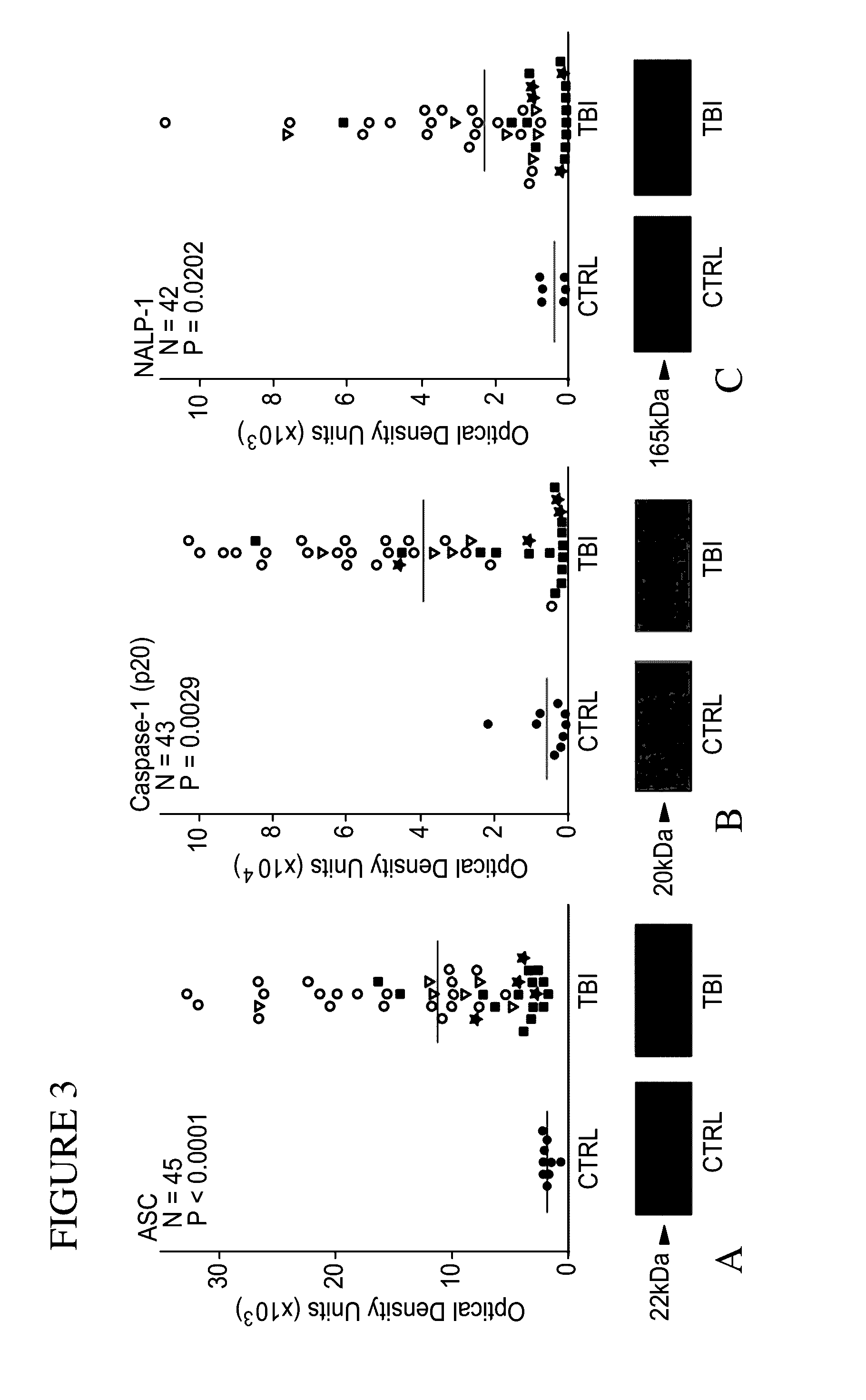
Innate Immune Proteins As Biomarkers for CNS Injury
The present invention provides novel markers of the severity of a central nervous system injury, such as spinal cord injury or traumatic brain injury, in a patient. In particular, protein components of inflammasomes in the cerebrospinal fluid that can be used to assess the serverity of central nervous system injury in a patient are disclosed. Methods of using such protein biomarkers to determine a prognosis, direct treatment and rehabilition efforts, and monitor response to treatment for a patient with a central nervous system injury are also described.
Owner:UNIV OF MIAMI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com