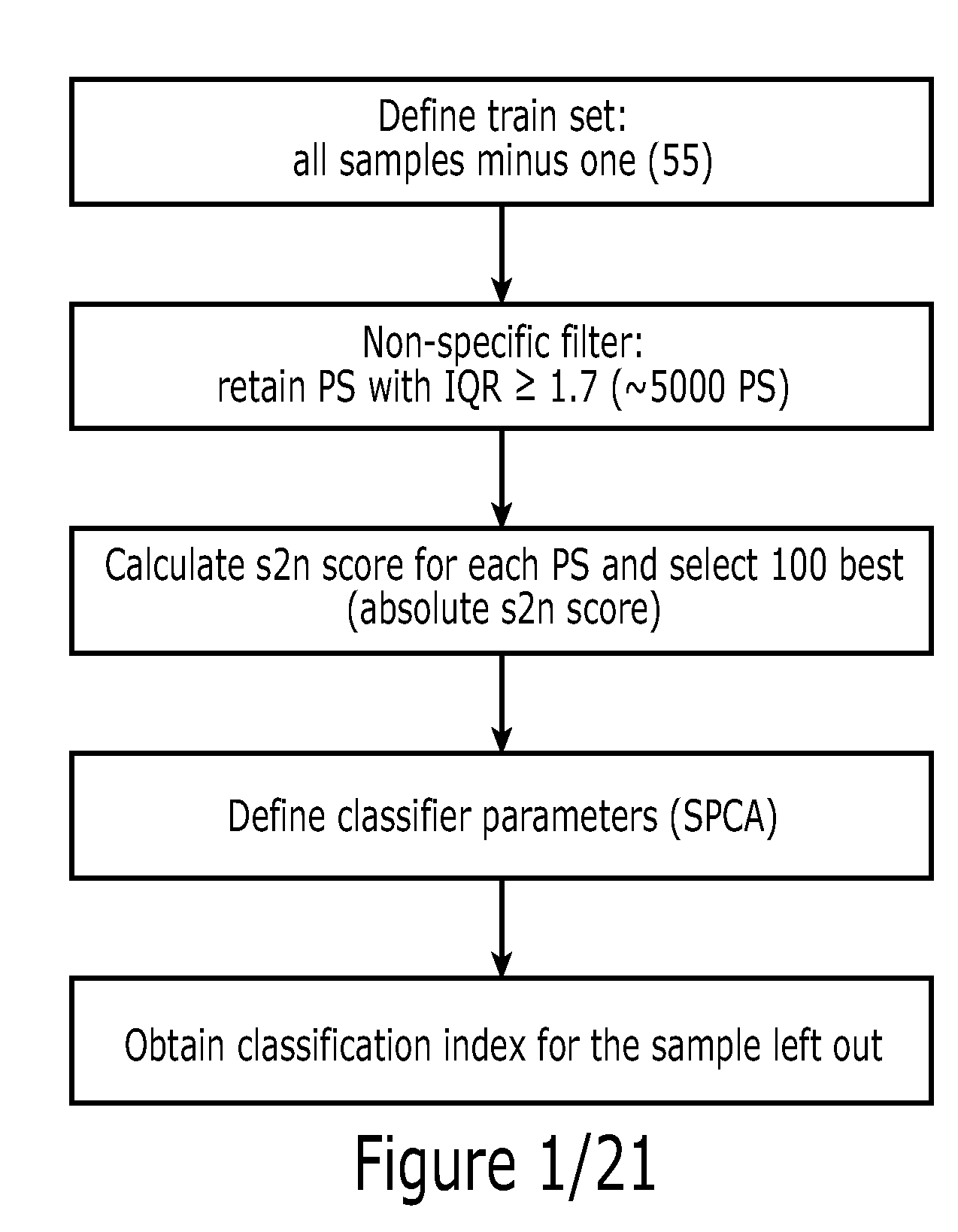
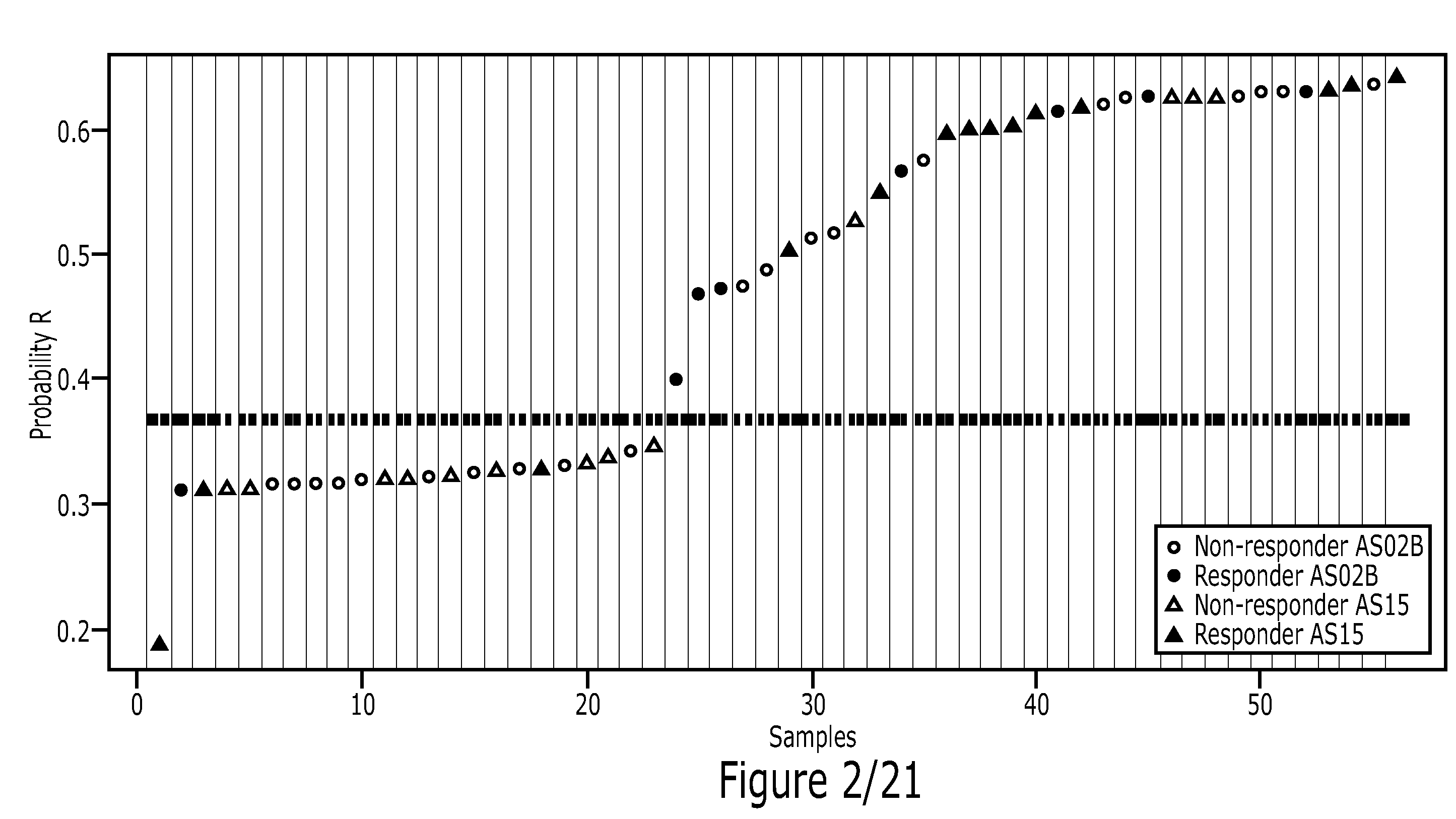
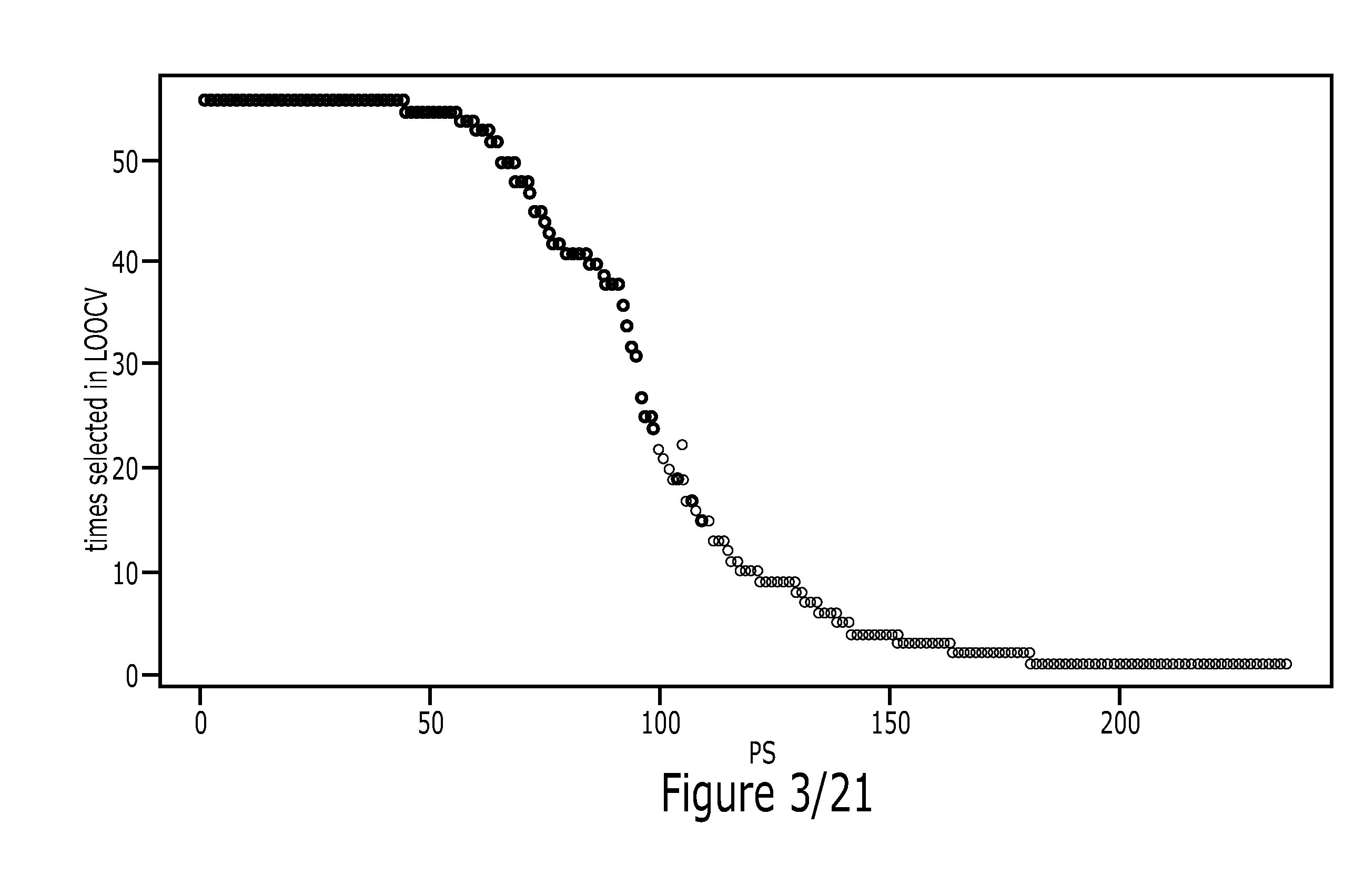
Patents
Literature
45 results about "Non responders" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
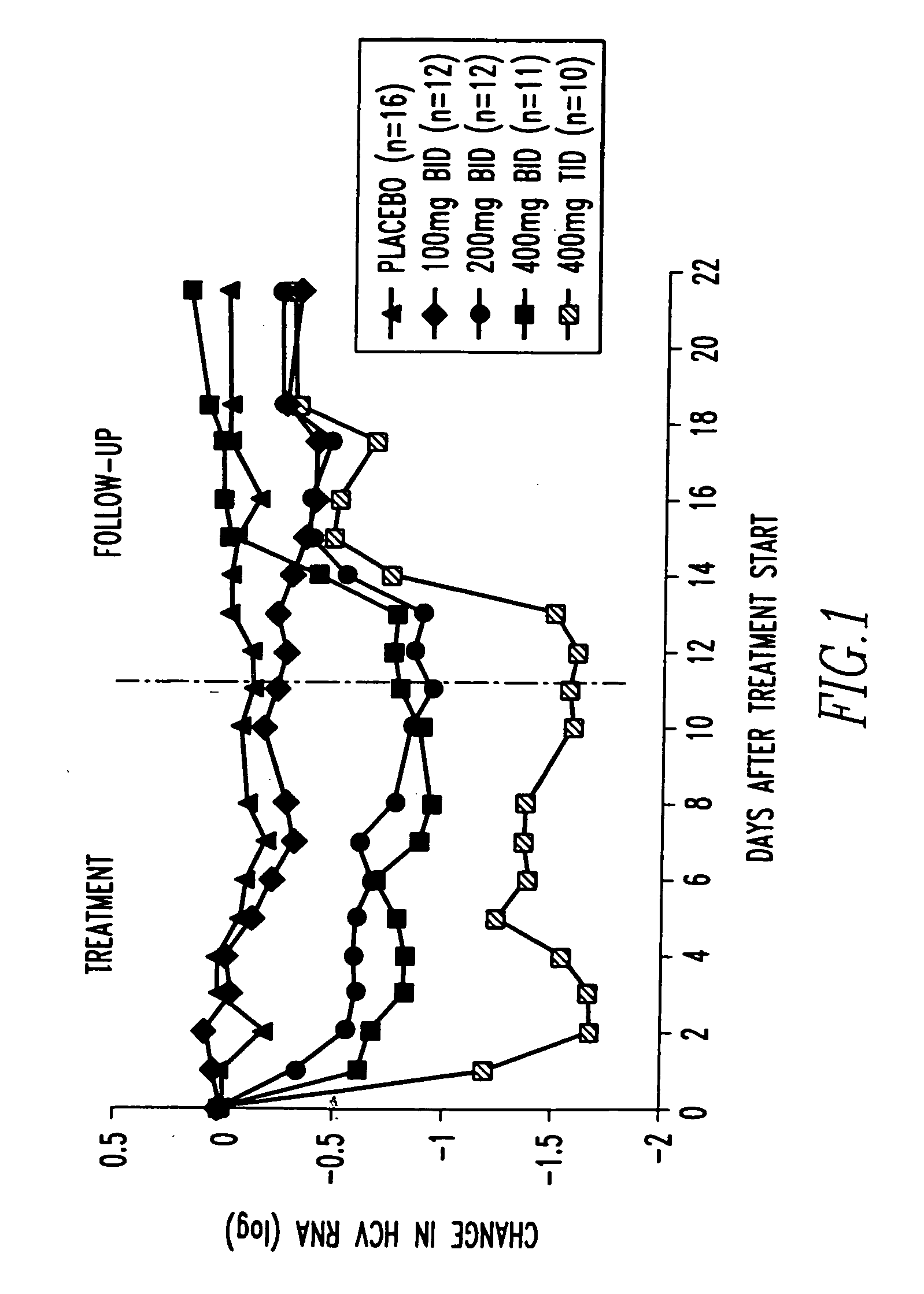
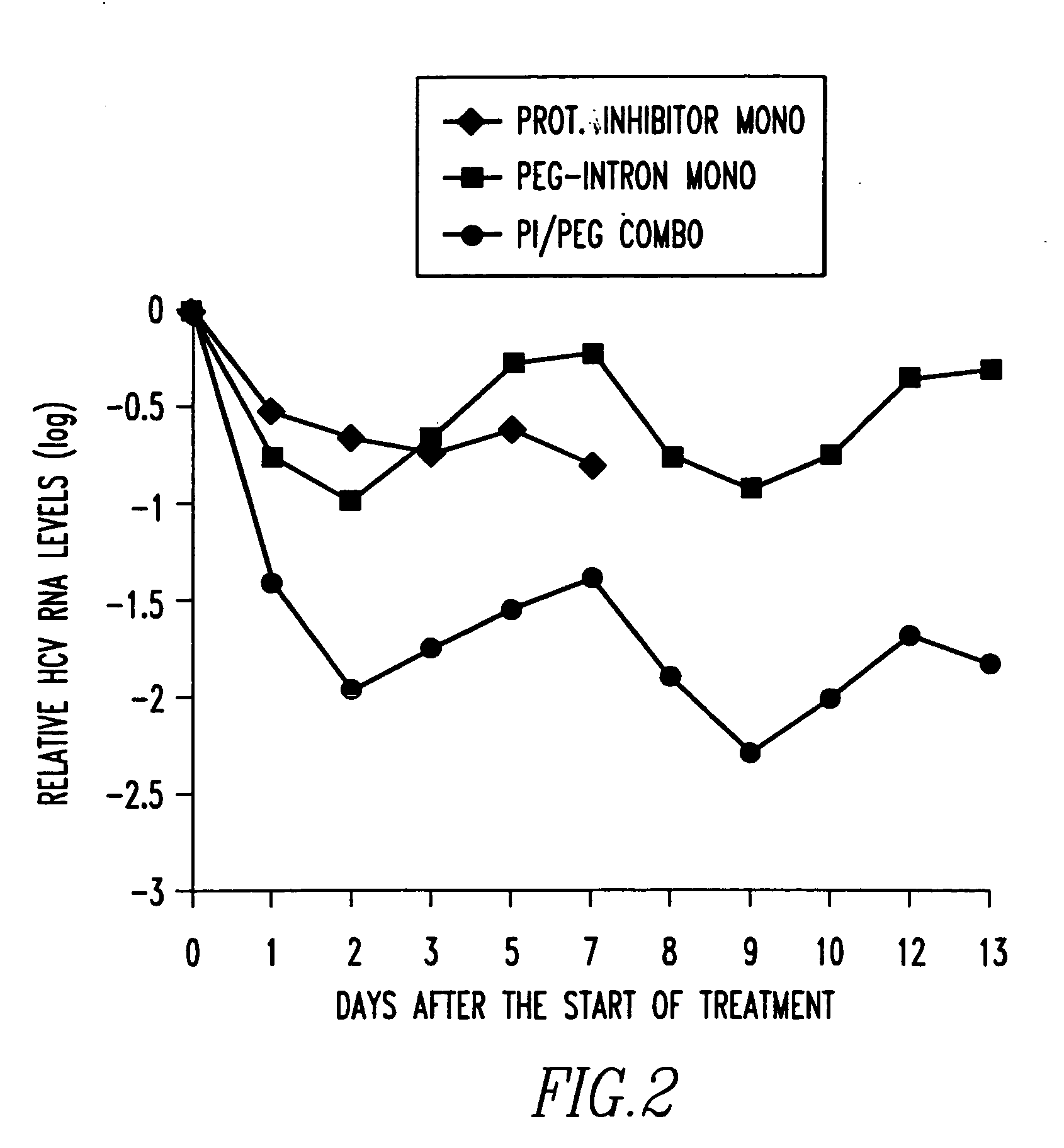
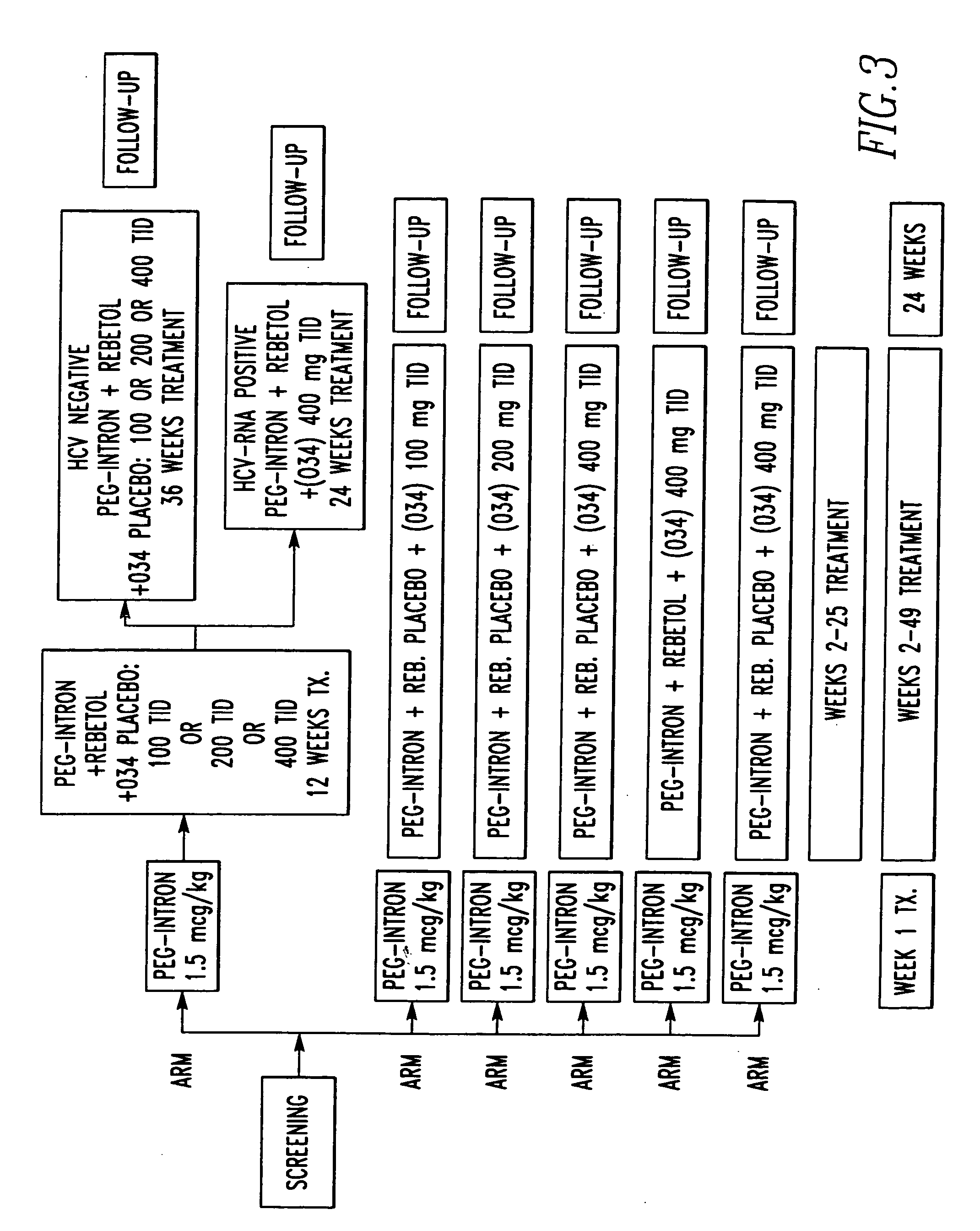
Method of treating interferon non-responders using HCV protease inhibitor
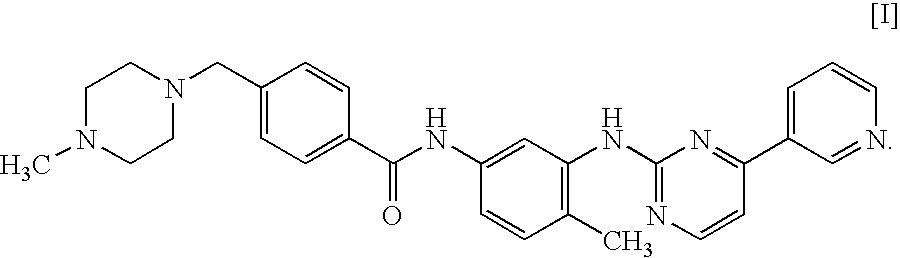
A method of treating, preventing or ameliorating one or more symptoms associated with hepatitis C virus (HCV) in a patient in whom either the HCV is of Genotype 1 and / or the patient was previously treated with interferon and the previous interferon therapy was ineffective to treat the one or more symptoms associated with HCV, comprising administering to such a patient an effective amount of at least one compound of formulae I-XXVI of which the following structural formula is exemplary or a pharmaceutically acceptable salt, solvate or ester thereof. Optional combined administration of said at least one compound with an interferon or pegylated interferon and / or ribaviron is also contemplated.
Owner:SCHERING CORP
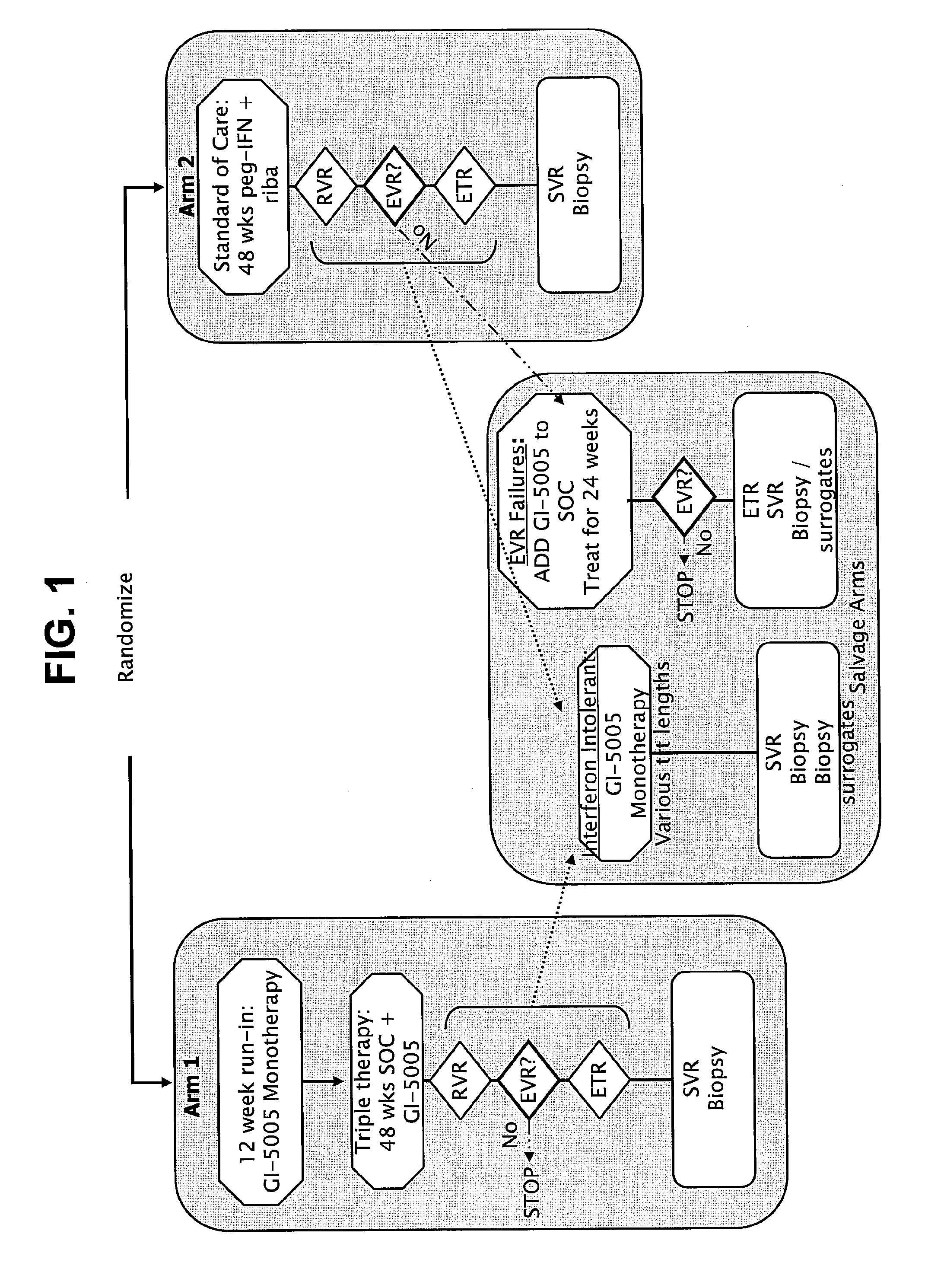
Immunotherapy for chronic hepatitis c virus infection
InactiveUS20110256098A1Increase the number ofReduce in quantitySsRNA viruses positive-sensePeptide/protein ingredientsChronic viral hepatitis CInterferon therapy
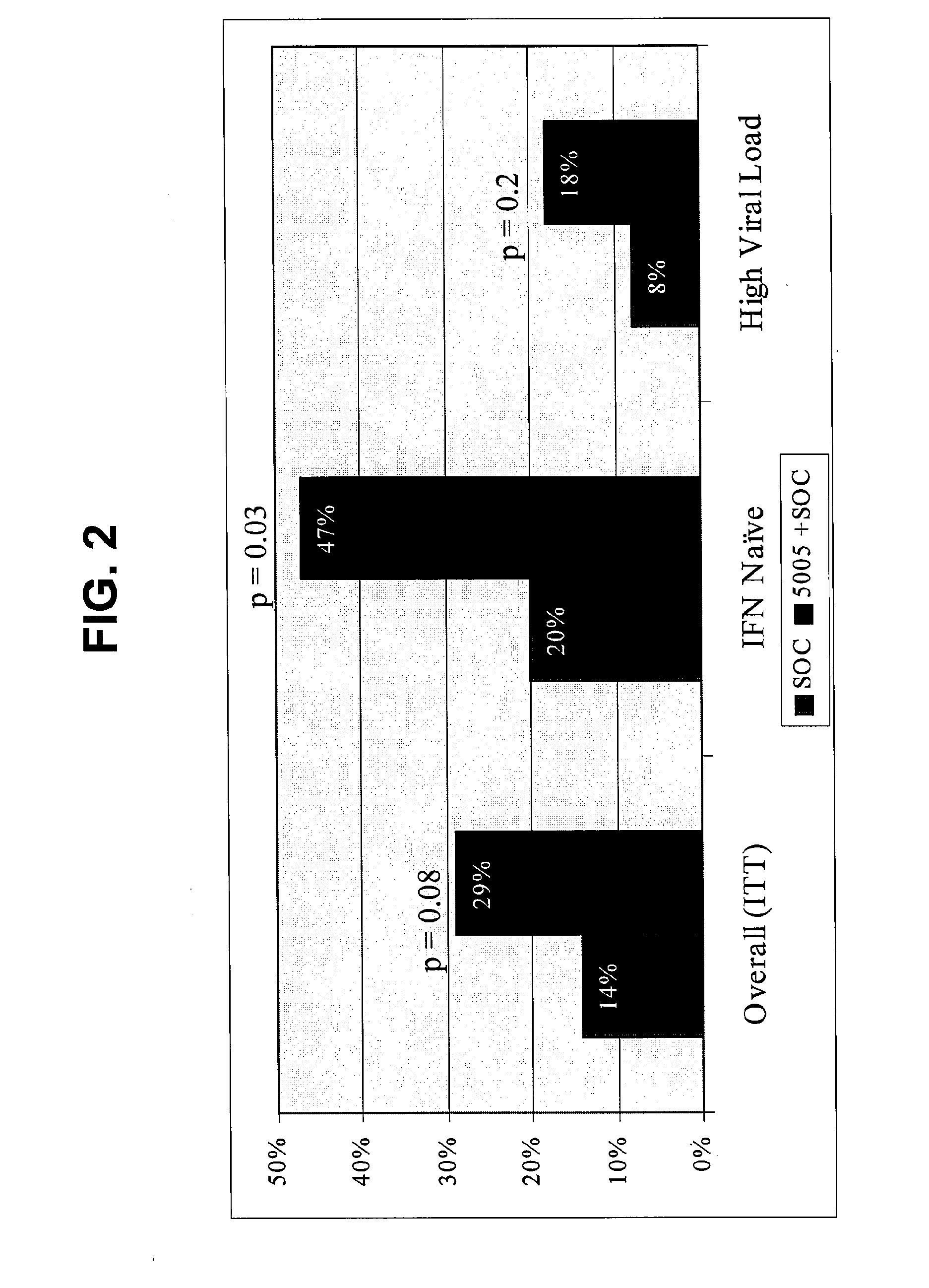
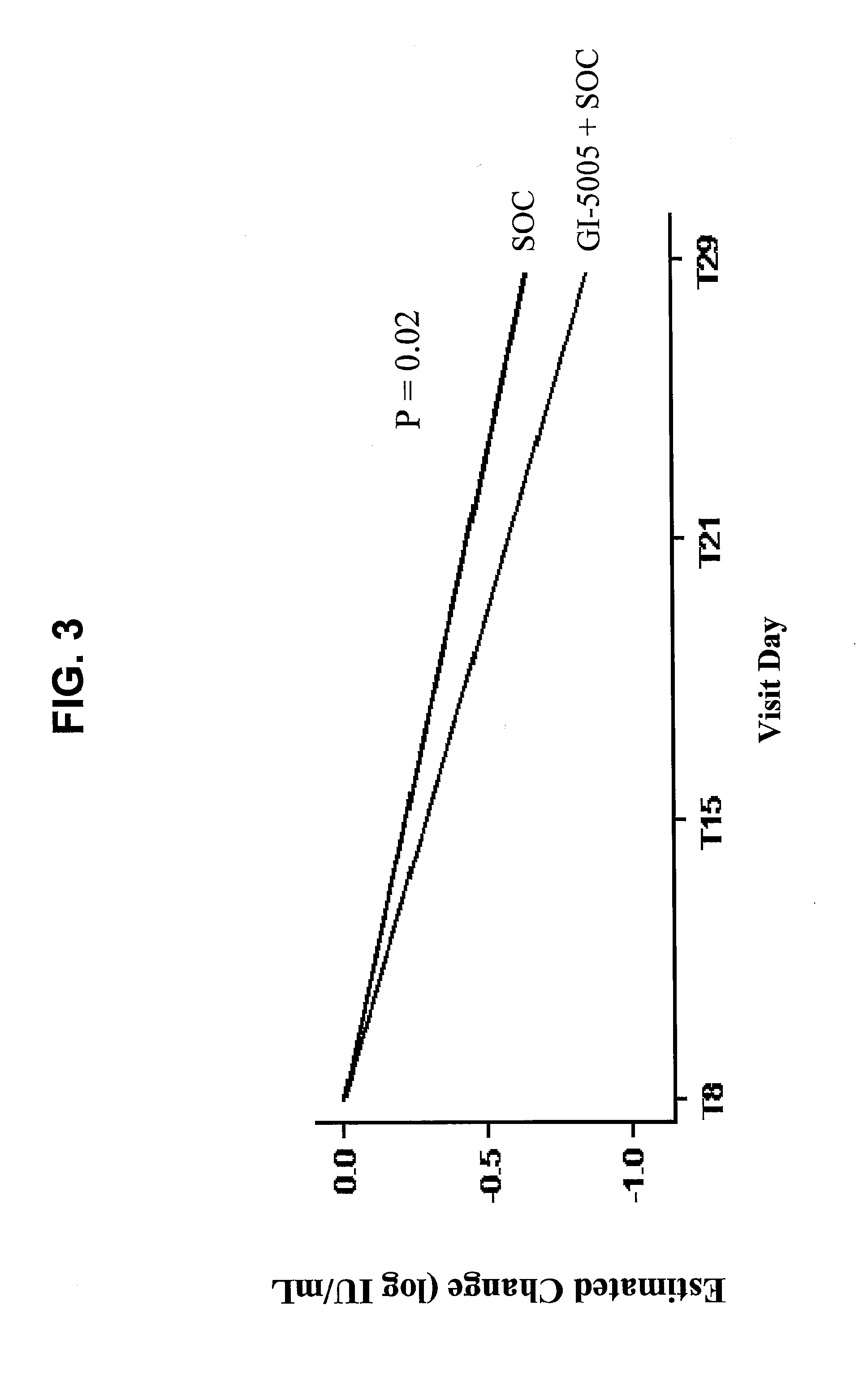
Disclosed are uses of immunotherapeutic compositions in combination with Standard of Care (SOC), or interferon therapy combined with anti-viral therapy, for the improved treatment of chronic hepatitis C virus (HCV) infection and related conditions, including liver function. The compositions, kits and uses of the invention, as compared to the use of SOC therapy alone: improves the rate of early response to therapy as measured by early virologic markers (e.g., RVR and EVR), enlarges the pool of patients who will have sustained responses to therapy over the long term, offers shortened courses of therapy for certain patients, enables “rescue” of patients who are non-responders or intolerant to SOC therapy, improves liver function and / or reduces liver damage in patients, and enables the personalization of HCV therapy for a patient, which can result in dose sparing, improved patient compliance, reduced side effects, and improved long term therapeutic outcomes.
Owner:GLOBE IMMUNE INC
Method for judging sensibility to imatinib
InactiveUS20060246436A1Medical cost incurredWaste of medical incurredMicrobiological testing/measurementRecombinant DNA-technologyImatinib treatmentImatinib
A method of judging whether a patient is sensitive to imatinib or not, in case where the patient is suffering from a disease such as CML to be treated by administration of imatinib, that is, a method for judging whether the administration of imatinib is effective for the therapy of the disease or not, is disclosed. Amounts of a plurality of genes selected from the group consisting of the specific 77 genes in sample cells separated from body are measured; and the measured amounts are compared with those of responders and non-responders to imatinib or a derivative thereof or a pharmaceutically acceptable salt thereof:
Owner:THE UNIV OF TOKYO
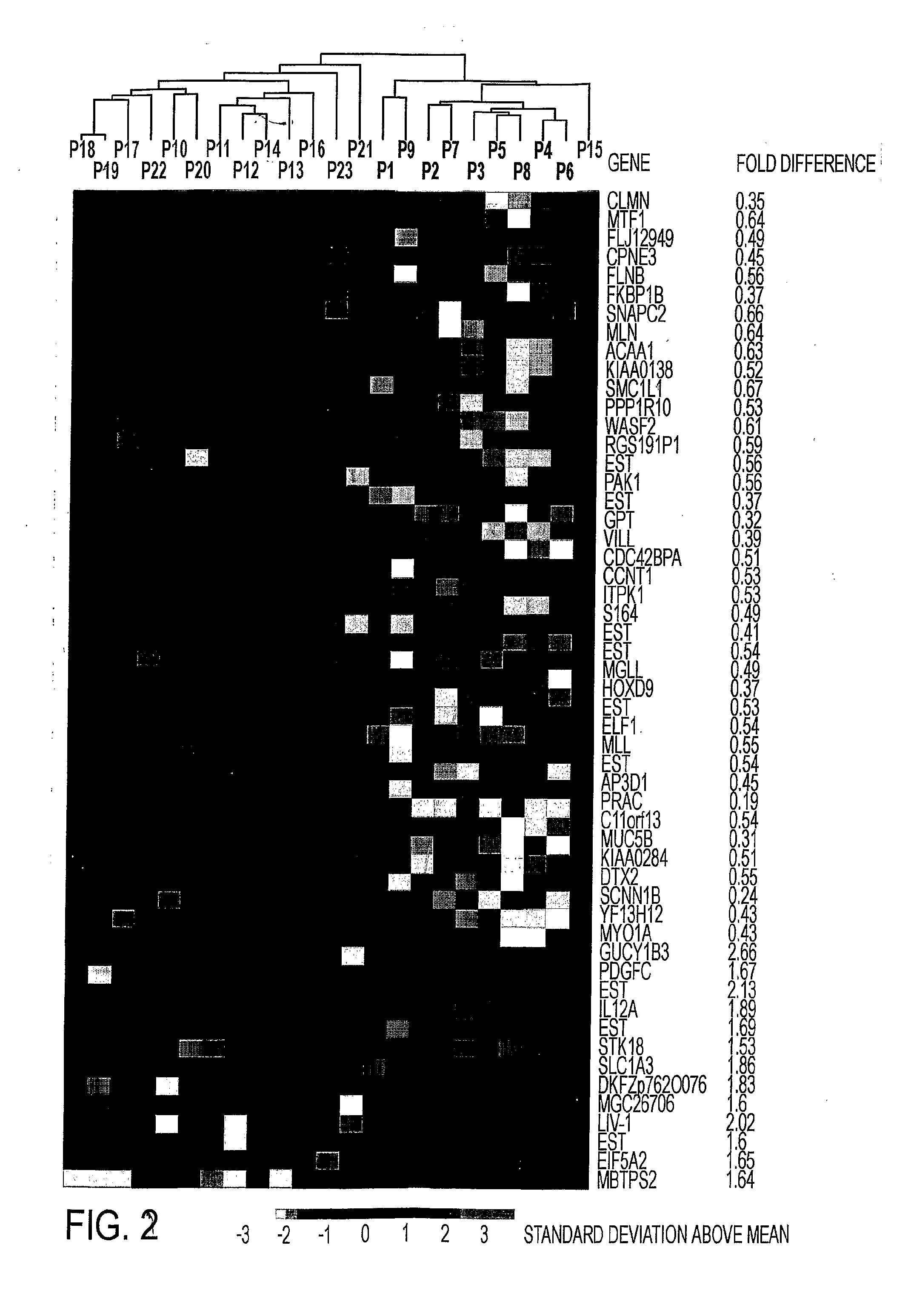
Biomarkers for interferon-alpha response in hepatitis C virus infected patients
InactiveUS20050282179A1More decisionCompound screeningApoptosis detectionNon respondersInterferon alpha
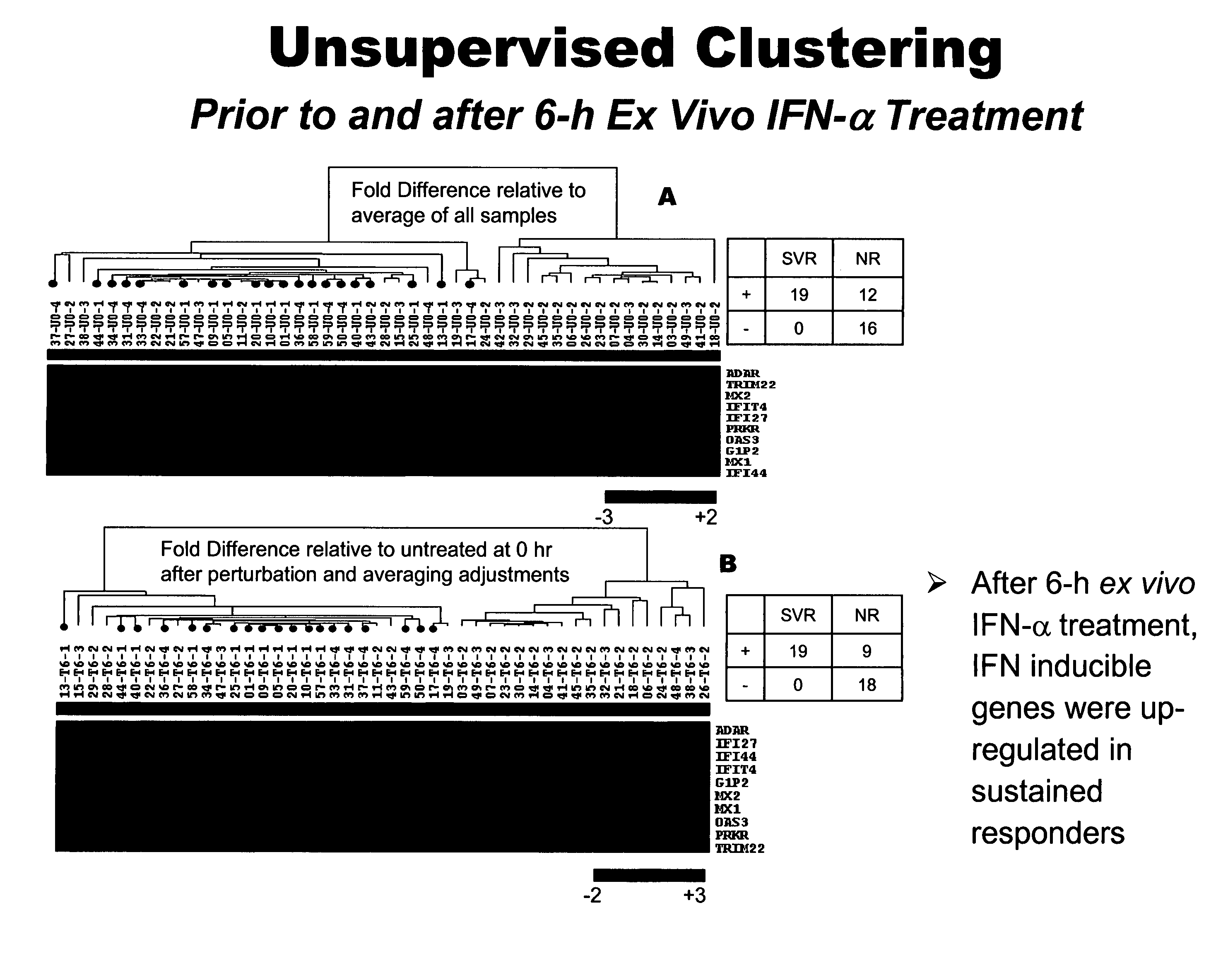
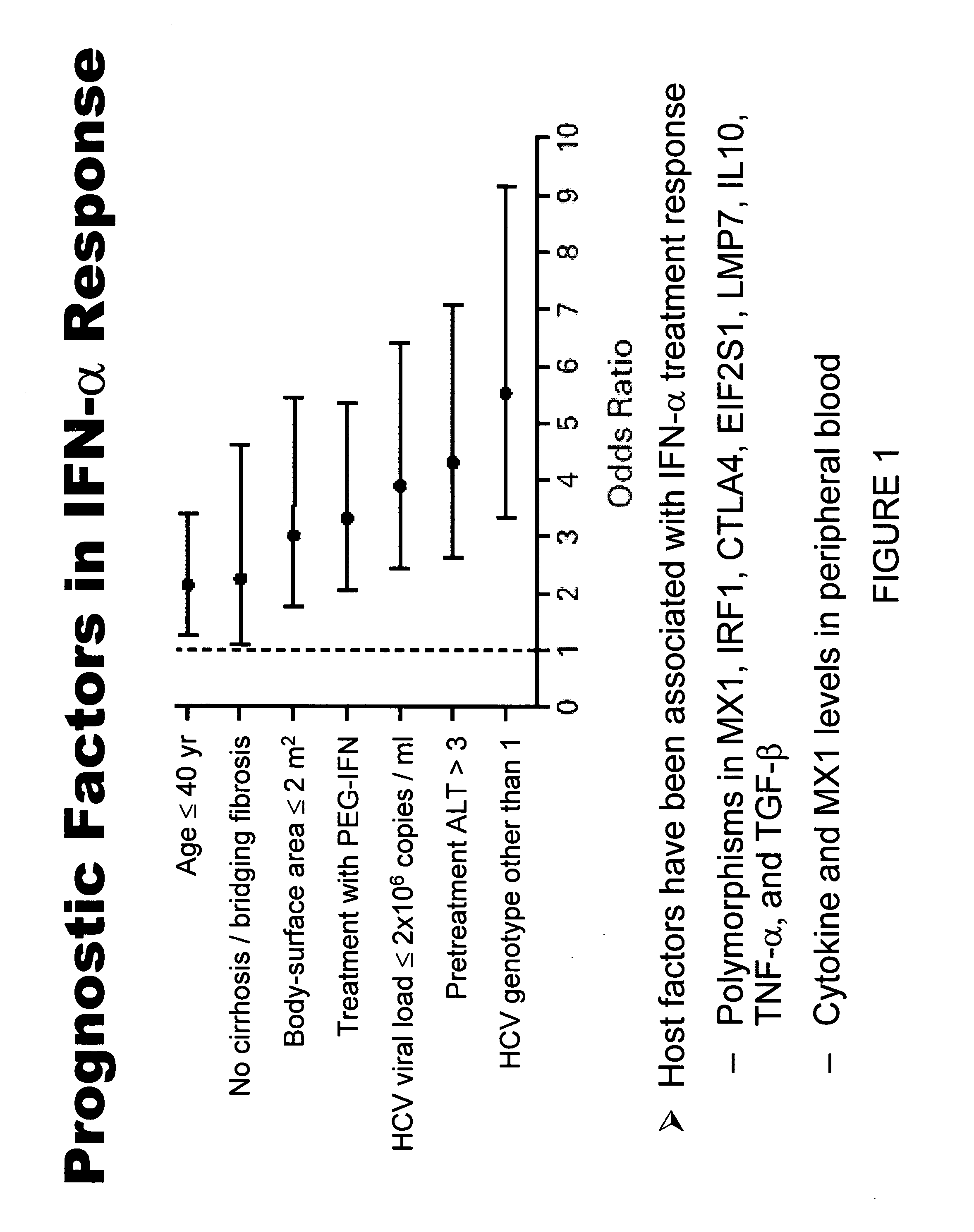
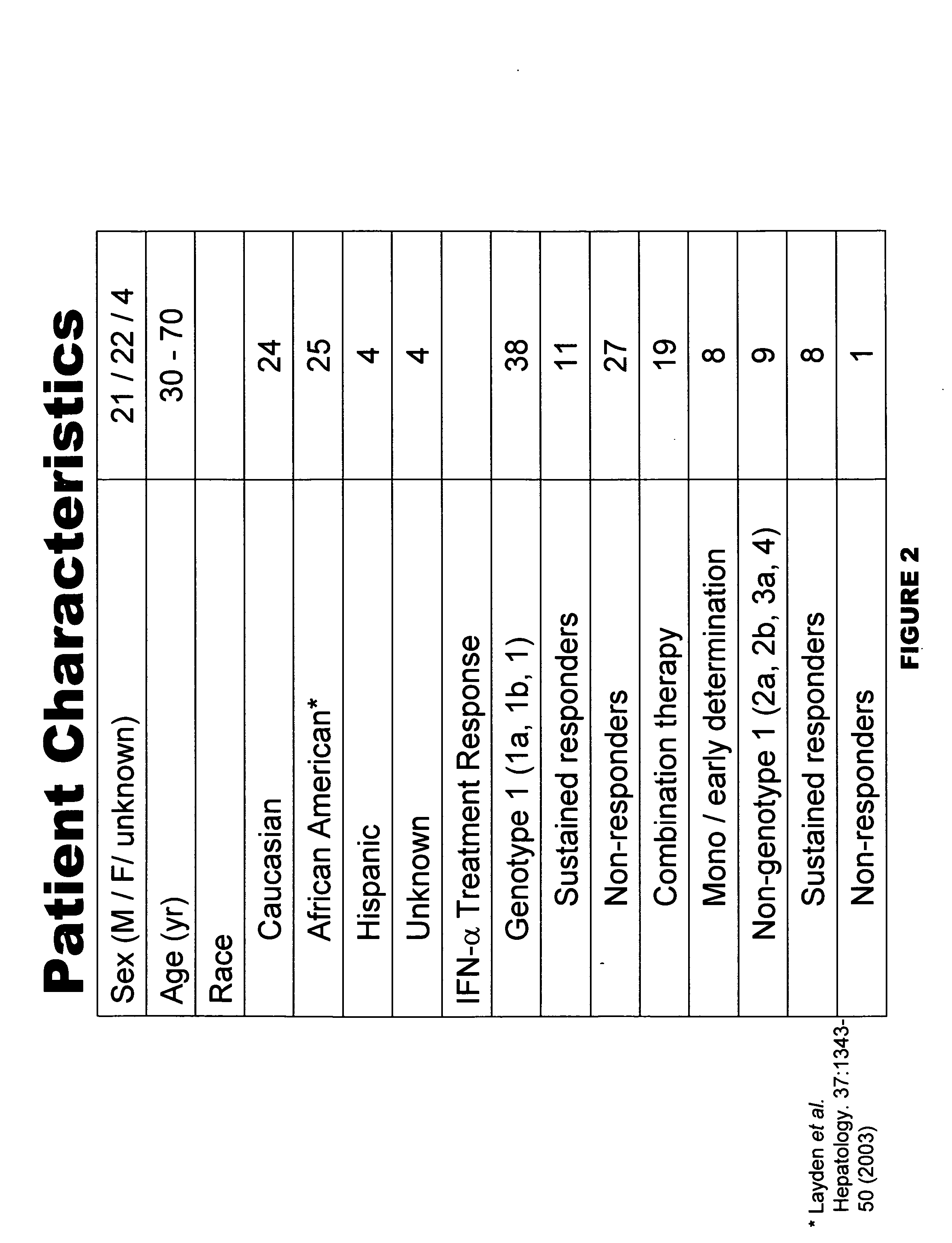
The present invention relates to the identification of prognostic markers useful in determining the response of hepatitis c virus (HCV) infected patients to interferon alpha (IFN-α) treatment. The studies provide biomarkers that can be used to discern sustained responders and non-responders of IFN-α treatment. This information should enable treating physicians to help patients to make more informed decisions.
Owner:APPL BIOSYSTEMS INC
Proteomic Patterns of Cancer Prognostic and Predictive Signatures
The invention provides method for predicting whether a cancer patient will respond to a therapy. Methods of the invention may involve examining protein from a cell of the cancer patient by determining the binding of a panel of antibodies to the protein. Methods of the invention may be used to generate both expression and activation profiles for cells from a cancer patient. Profiles from a cancer patient may then be compared to known profiles for therapy responders and non-responders to predict the individual response of the patient. For example, methods of the invention may be used to determine whether an ovarian or breast cancer patient will respond to a therapeutic protocol.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
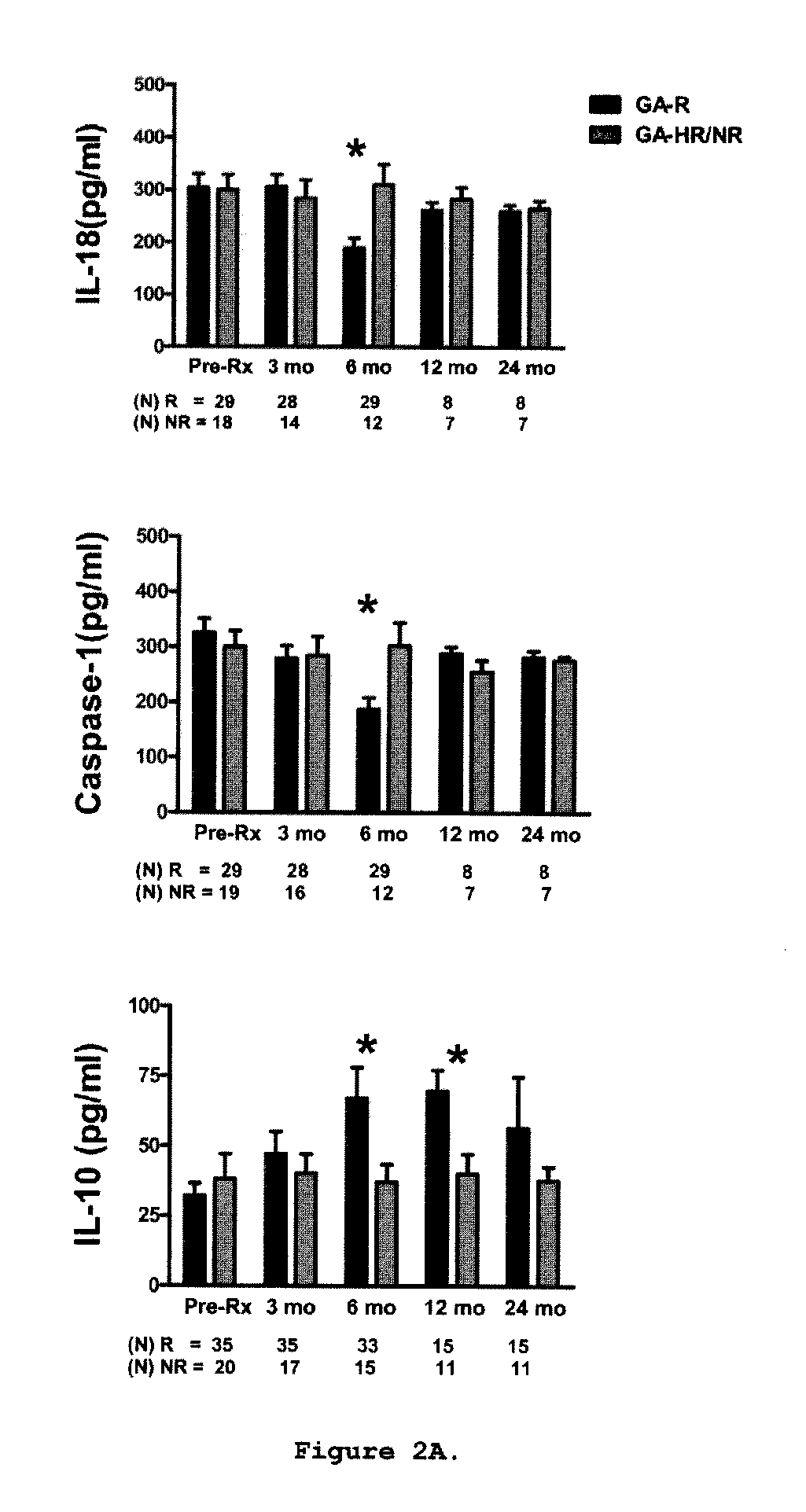
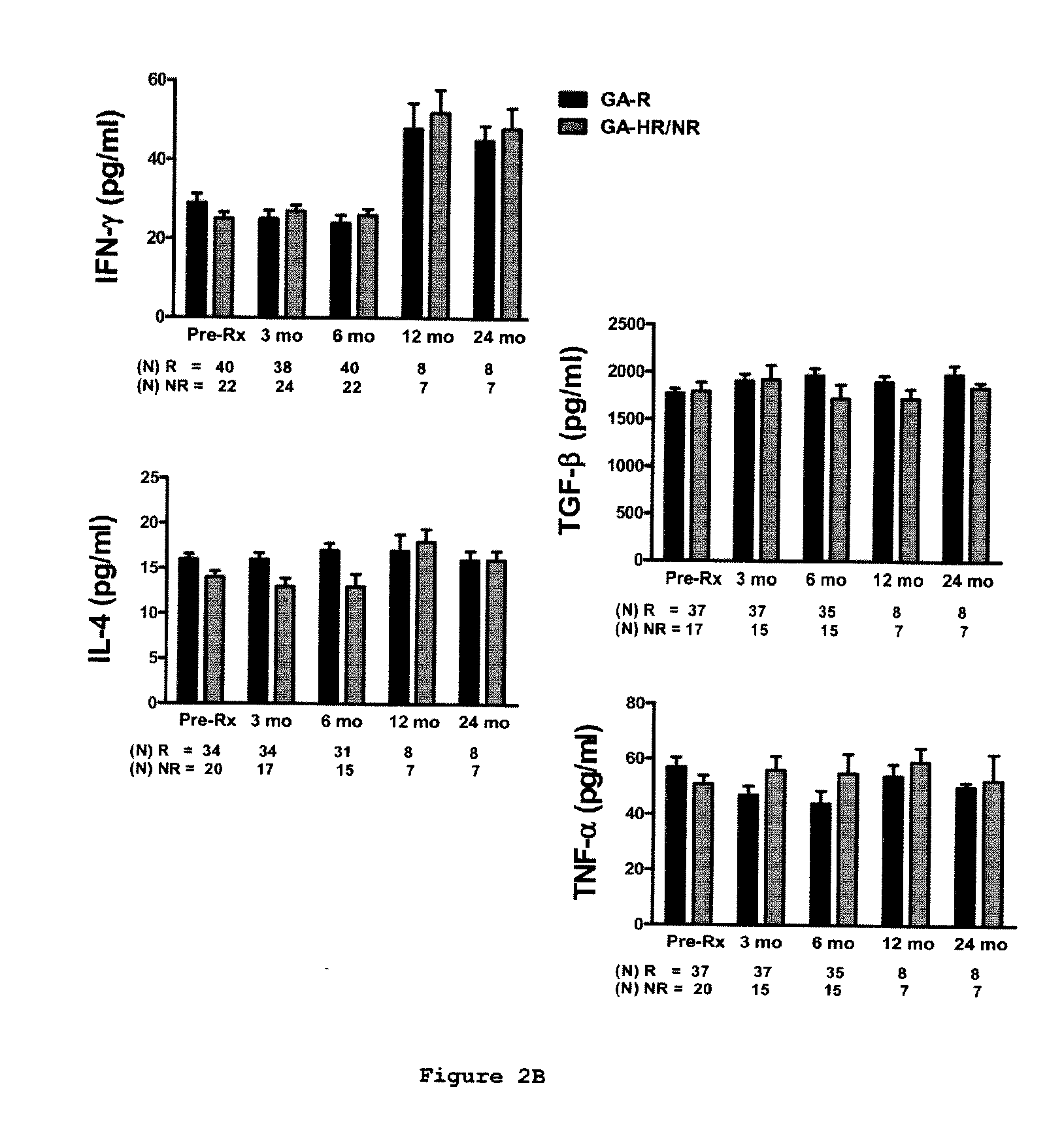
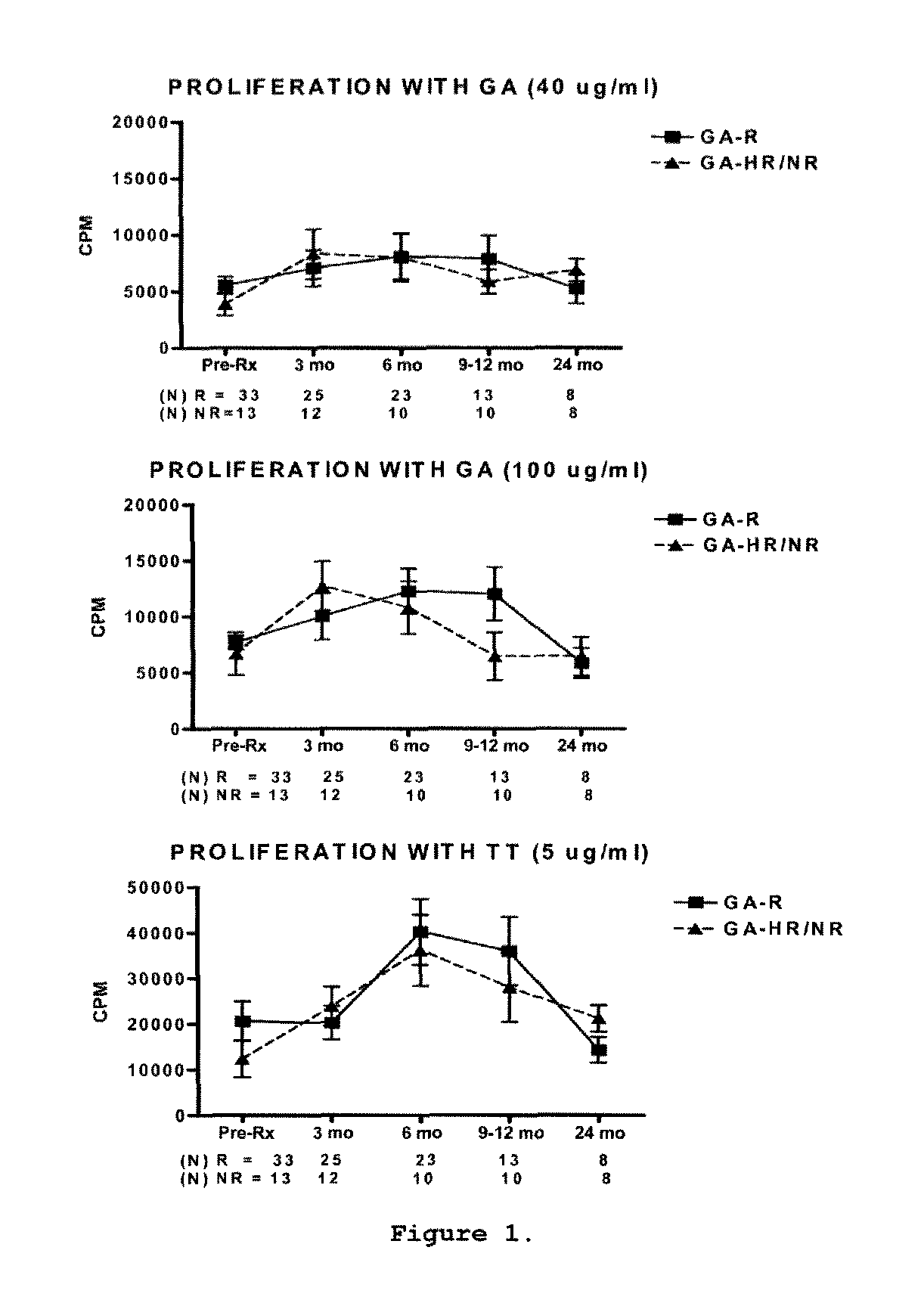
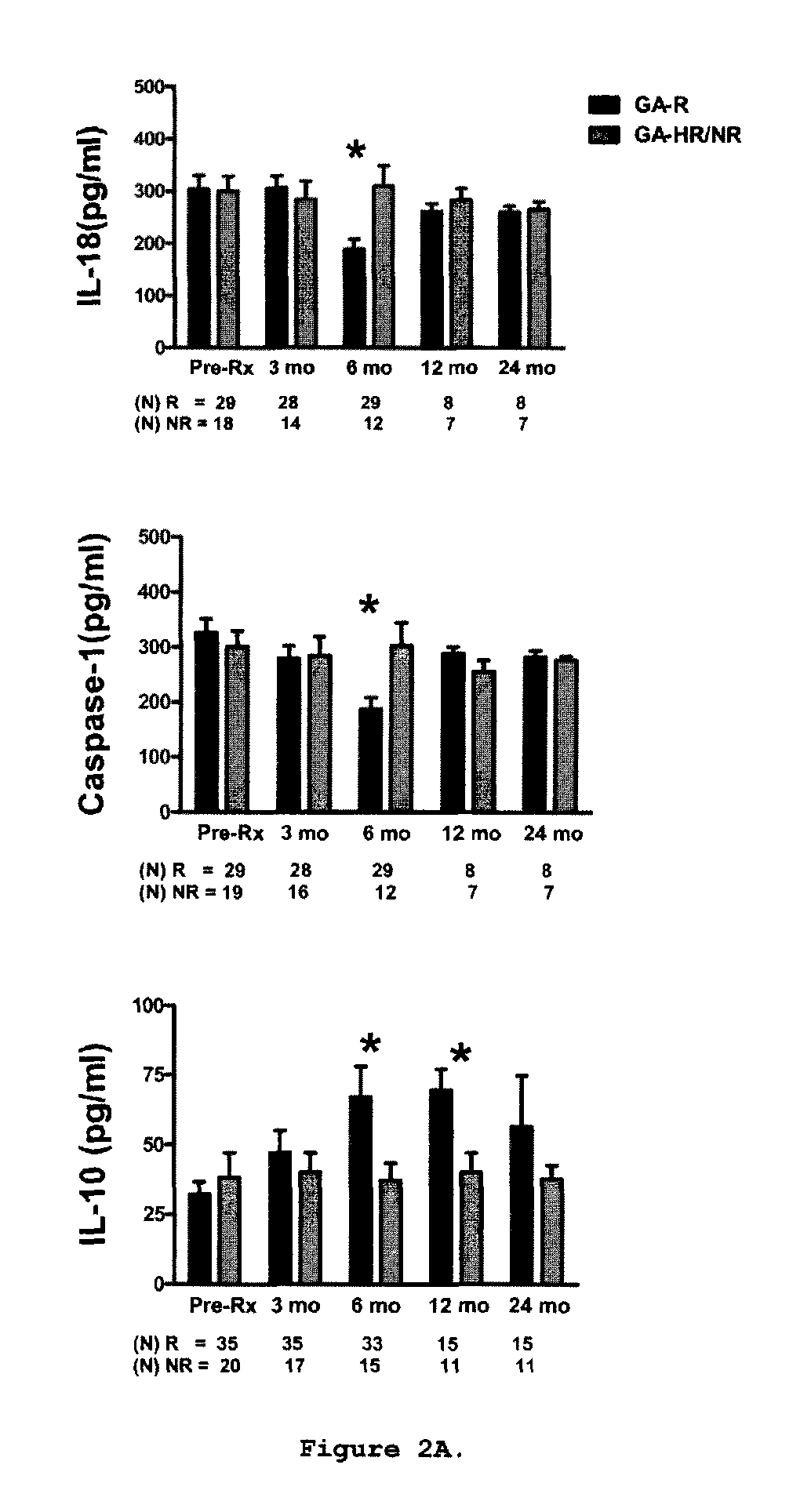
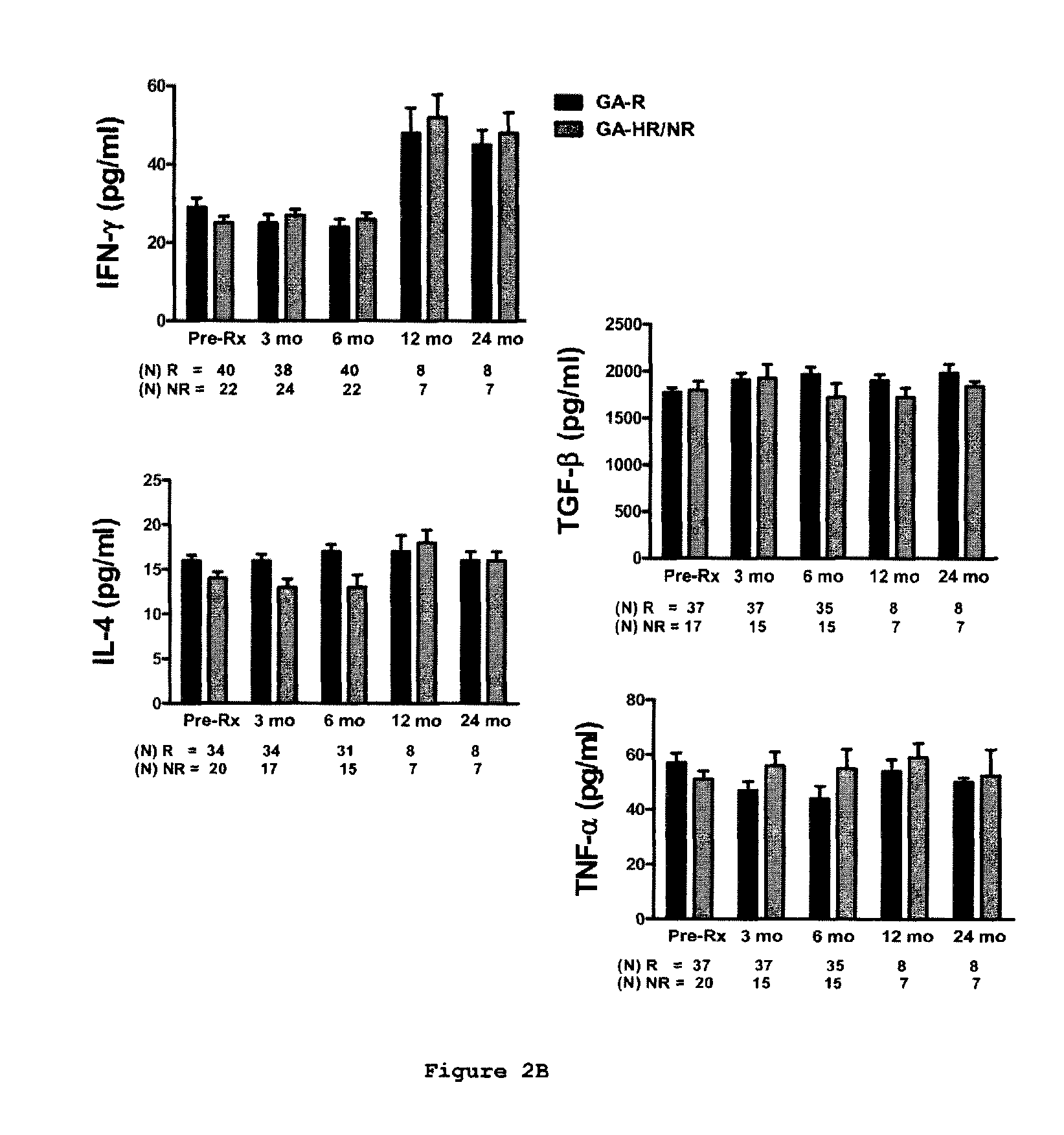
Predictive biomarkers of clinical response to glatiramer acetate therapy in multiple sclerosis
A method for treating a subject afflicted with an autoimmune disease with a pharmaceutical composition comprising glatiramer acetate and a pharmaceutically acceptable carrier, comprising the steps of administering a therapeutic amount of the pharmaceutical composition to the subject, determining whether the subject is a glatiramer acetate responder or a glatiramer acetate hypo- / non-responder by measuring the value of a biomarker selected from the group consisting of IL-10 concentration, IL-17 concentration, IL-18 concentration, TNF-α concentration, BDNF concentration, caspase-1 concentration, IL-10 / IL-18 ratio and IL-10 / IL-17 ratio in the blood of the subject, and comparing the measured value to a reference value for the biomarker to identify the subject as a glatiramer acetate responder or a glatiramer acetate hypo- / non-responder, and continuing the administration if the subject is identified as a glatiramer acetate responder, or modifying treatment of the subject if the subject is identified as a glatiramer acetate hypo- / non-responder.
Owner:TEVA PHARMA IND LTD
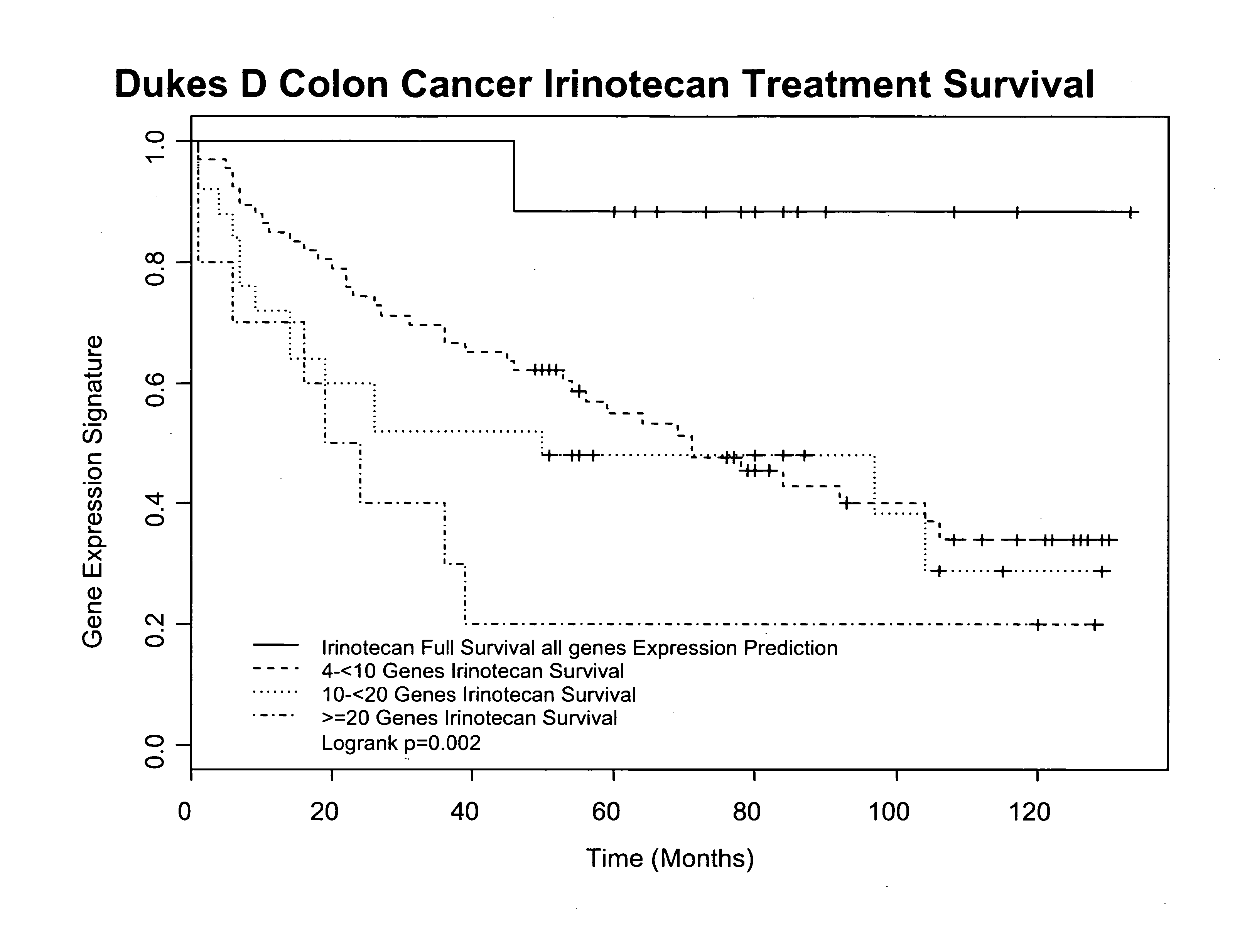
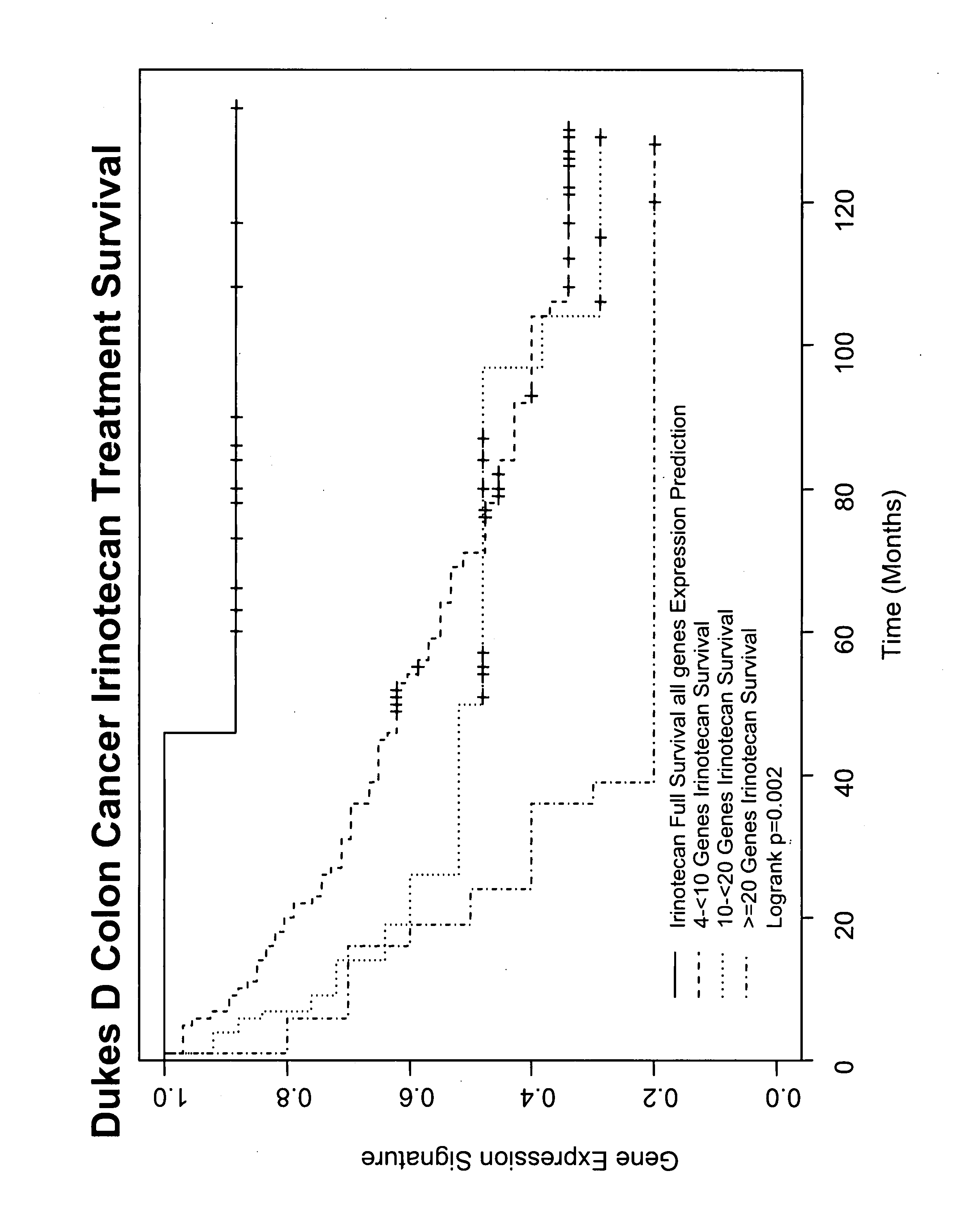
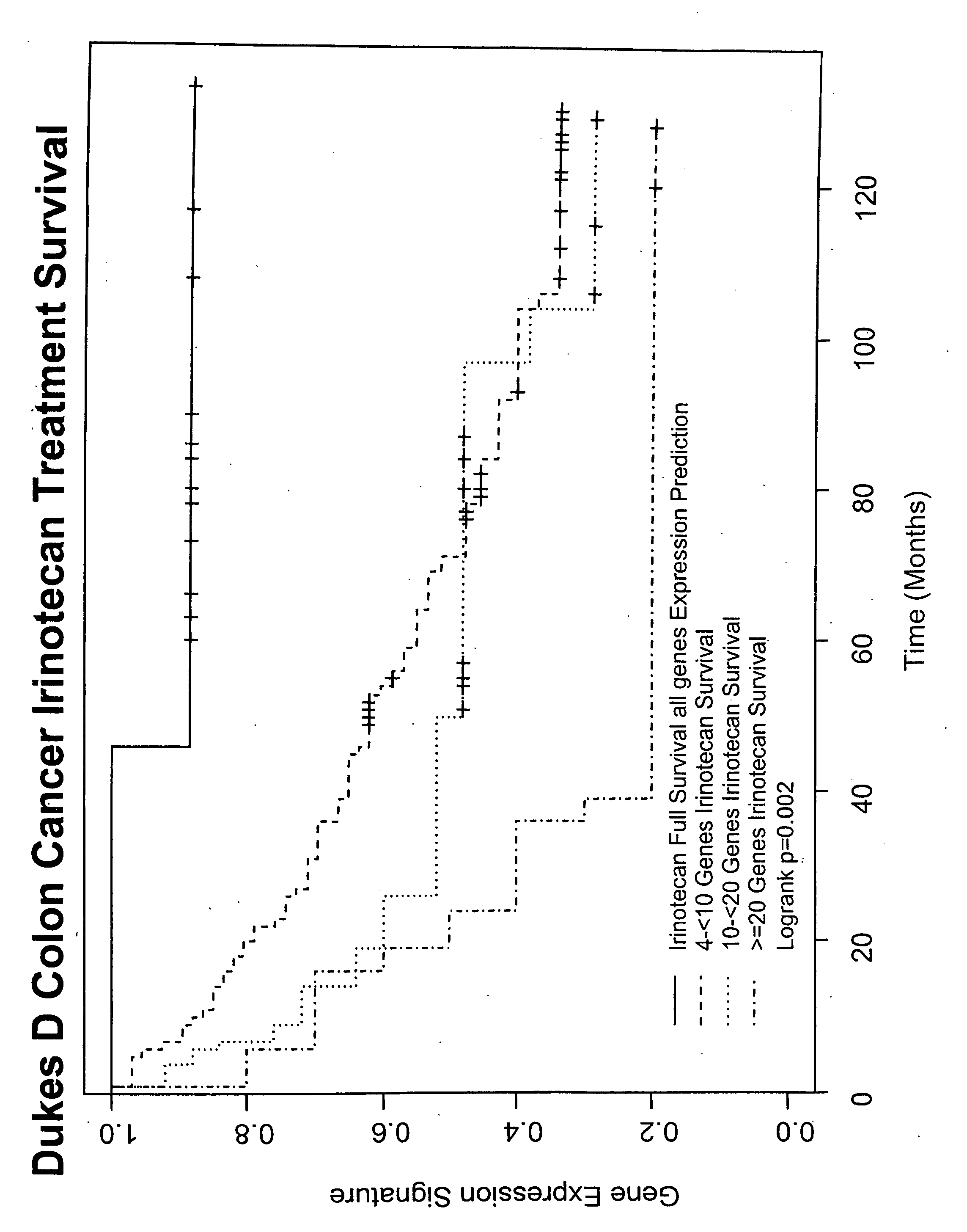
Gene and protein expression profiles associated with the therapeutic efficacy of irinotecan
InactiveUS20080076134A1Microbiological testing/measurementDisease diagnosisProtein expression profileIrinotecan
The present invention includes gene and protein expression profiles indicative of whether a cancer patient is likely to respond to treatment with irinotecan. By identifying such responsiveness, a treatment provider may determine in advance those patients who would benefit from such treatment, as well as identify alternative therapies for non-responders. The present invention further provide methods of using the gene and / or protein expression profiles and assays for identifying the presence of a gene and / or protein expression profile in a patient sample.
Owner:NUCLEA BIOMARKERS
Gene and protein expression profiles associated with the therapeutic efficacy of egfr-tk inhibitors
InactiveUS20090263819A1Microbiological testing/measurementBiological material analysisProtein expression profileNon responders
The present invention provides protein and gene expression profiles indicative of whether a patient afflicted with non-small cell lung cancer is likely to be responsive to treatment with a therapeutic compound that is a EGFR-TK inhibitor. By identifying such responsiveness, a treatment provider may determine in advance those patients who would benefit from such treatment, as well as identify alternative therapies for non-responders. The present invention further provide methods of using the gene and protein expression profiles, and assays for identifying the presence of a gene or protein expression profile in a patient sample.
Owner:NUCLEA BIOMARKERS
In vitro cell-based methods for biological validation and pharmacological screening of chemical entities and biologicals
InactiveUS7198895B2Increase in motilityOrganic active ingredientsBiocidePharmacometricsClinical trial
This patent describes a novel in vitro cell-based method for biological validation and pharmacological screening of drugs, new chemical entities (NCEs) and biologics, which is predictive of in vivo testing for efficacy and adverse events in patients, as occurs in clinical trials. The same method can be used to create an in vitro cell-based assay to identify the ‘right marketed medication for the right patient’ (personalized medicine), and to identify responders / non-responders in ongoing clinical trials with NCEs. In addition this approach can be used to identify new indications for existing medicines and new indications for NCEs that were unsuccessful in their intended uses.
Owner:MOHANLAL RAMON W
Methods of treating a subject afflicted with an autoimmune disease using predictive biomarkers of clinical response to glatiramer acetate therapy in multiple sclerosis
Owner:TEVA PHARMA IND LTD
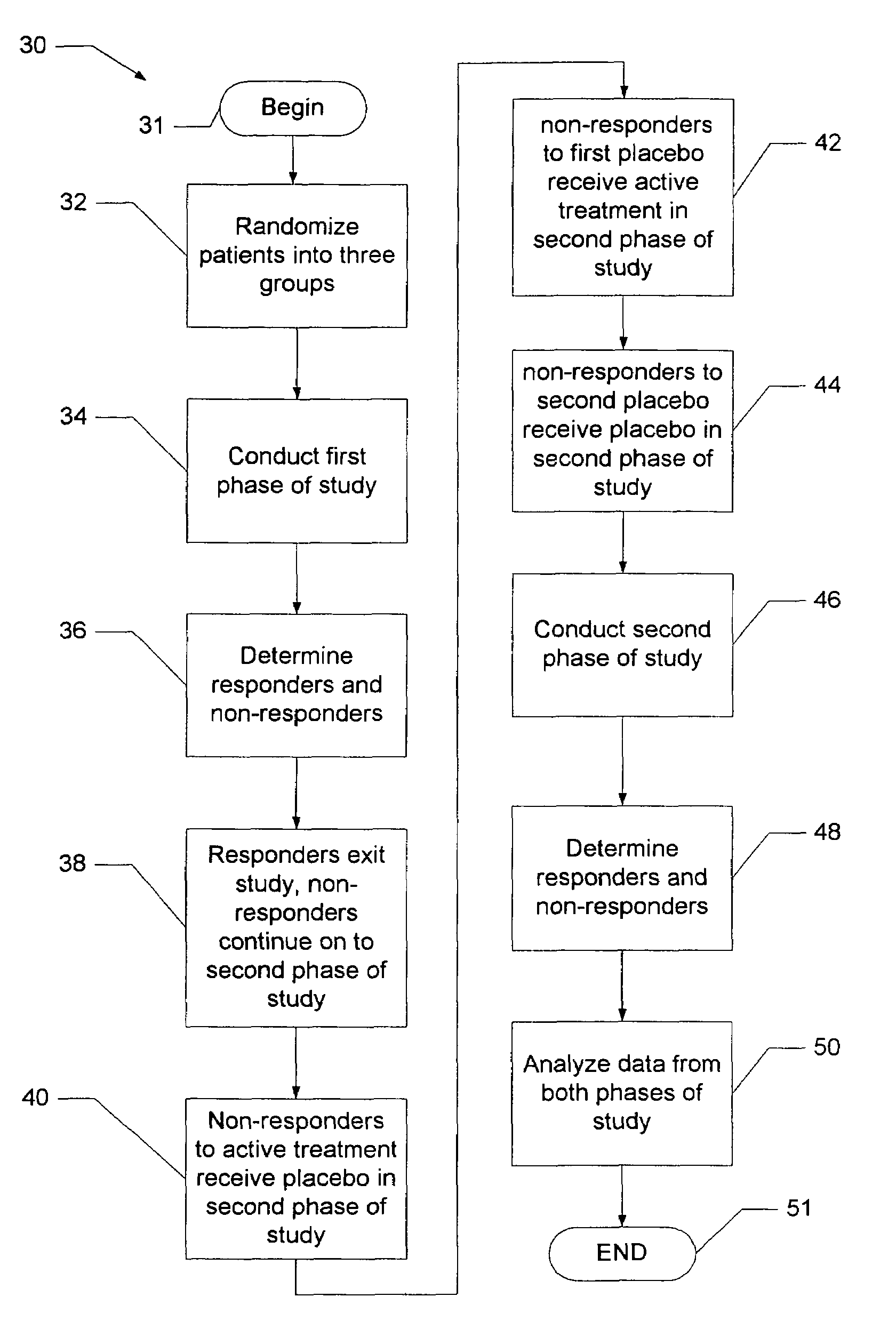
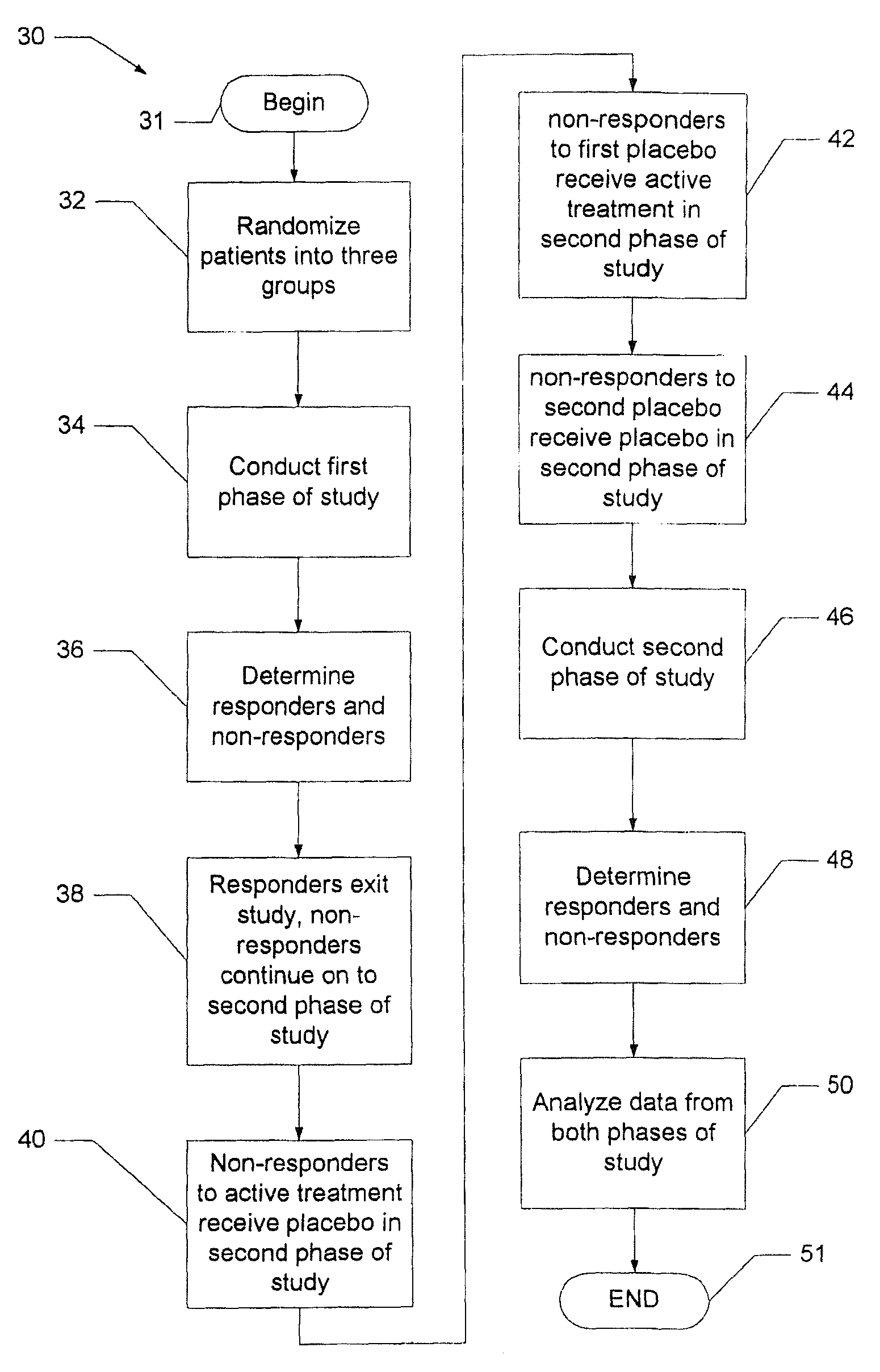
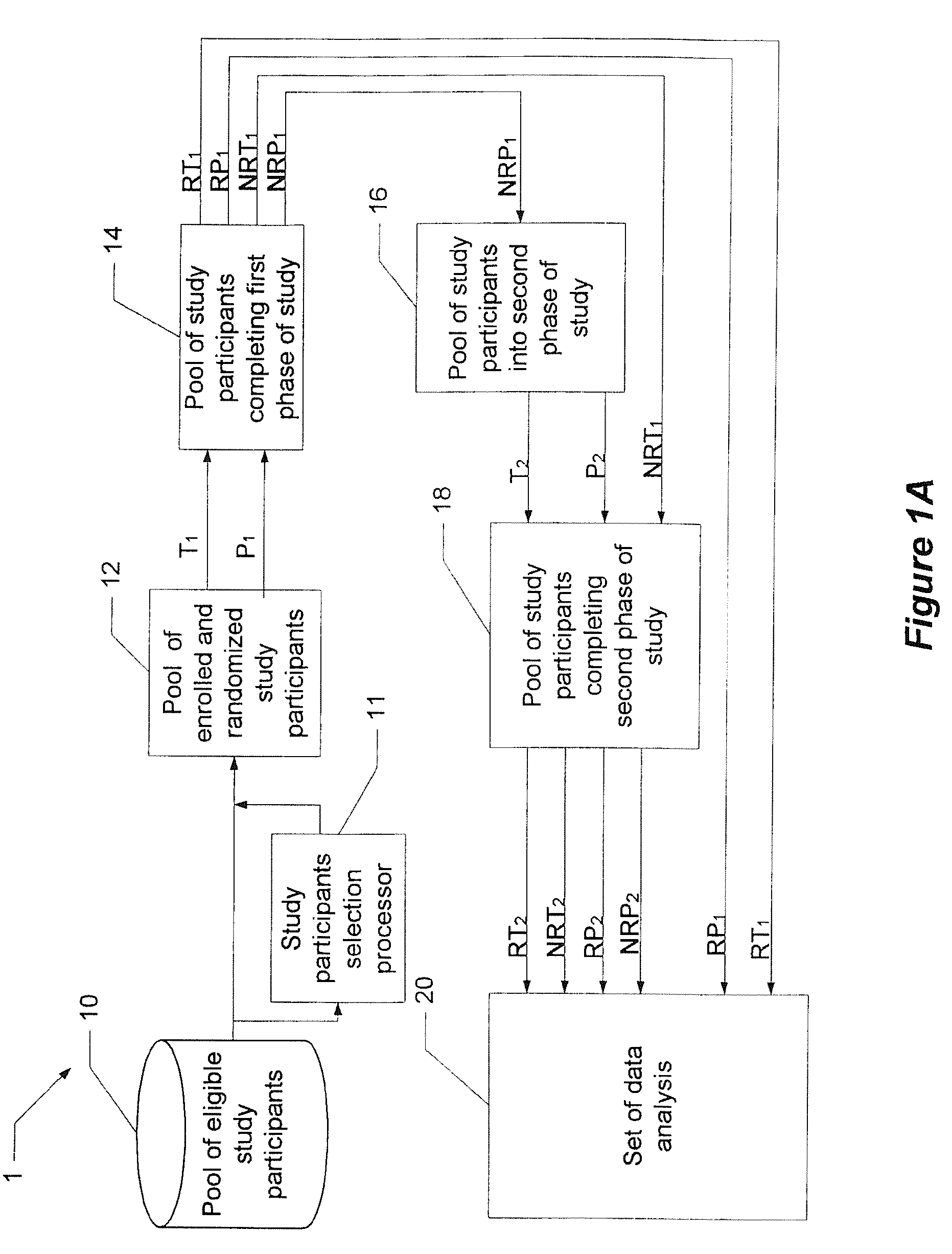
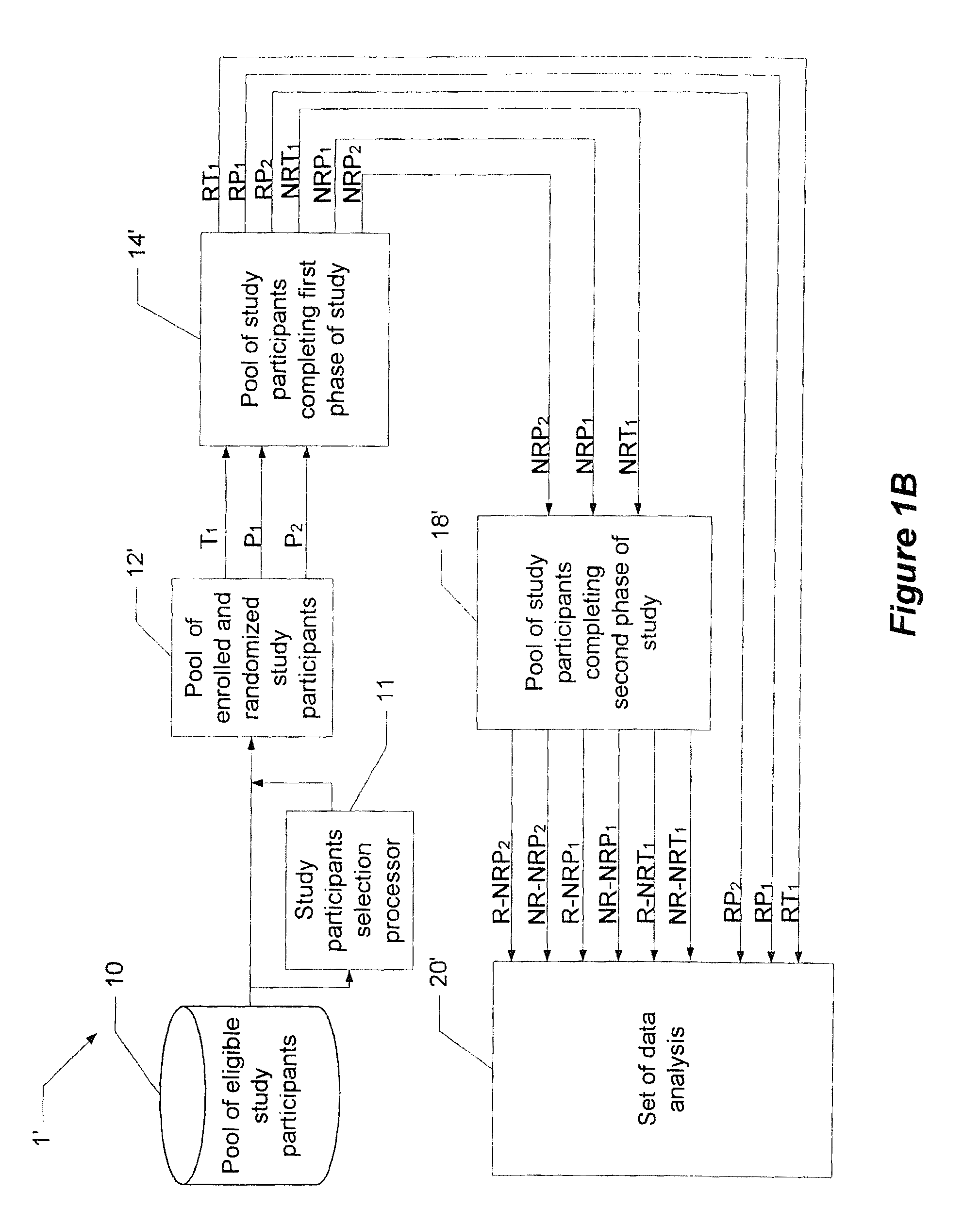
System and method for reducing the placebo effect in controlled clinical trials
ActiveUS7647235B1Reduced placebo effectDetermine effectivenessData processing applicationsTherapiesClinical trialNon responders
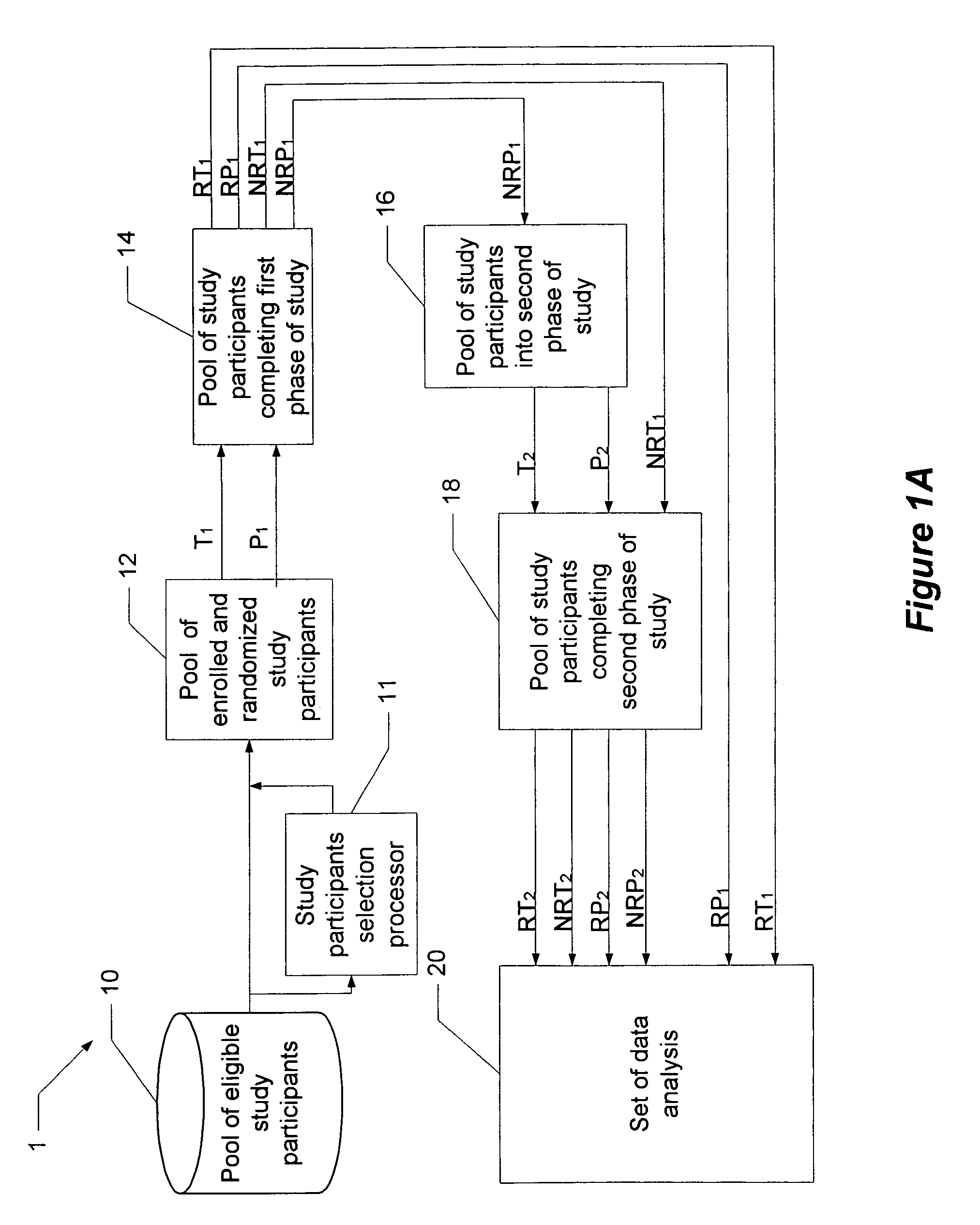
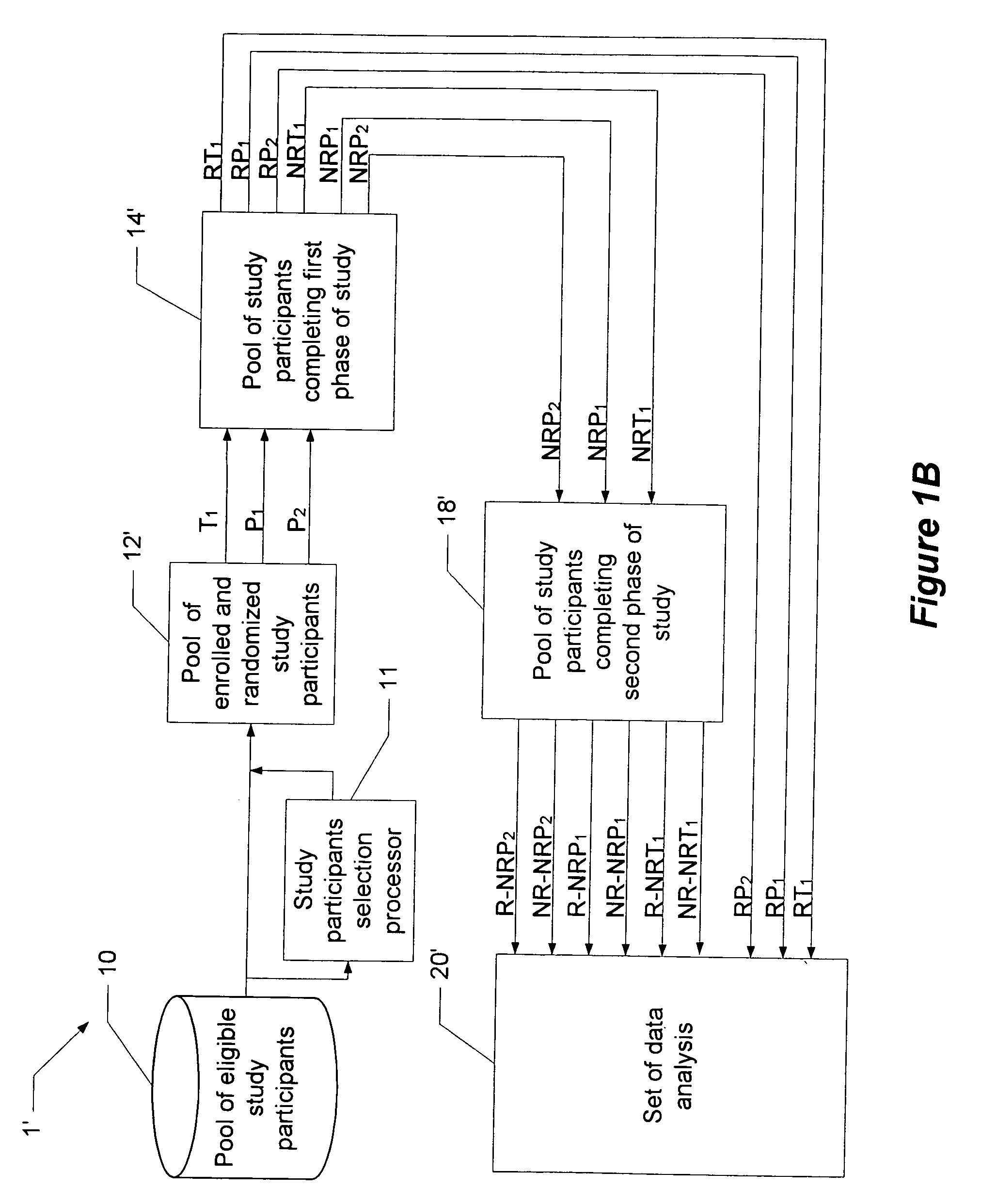
A method and system for performing a clinical trial having a reduced placebo effect is disclosed. The method includes randomizing study participants into three or more treatment groups and performing a first phase of testing on the groups. In a typical embodiment, the first phase of testing includes administering an active treatment to a first group, and administering a placebo to a second group and to a third group. Responders and non-responders are determined for each group. A second phase of testing is then performed. The second phase of testing includes administering the placebo to non-responders in the first group, administering the active treatment to non-responders in the second group, and administering the placebo to non-responders in the third group. The data from the first phase of testing and from the second phase of testing is pooled and analyzed to determine response rates to active treatment and placebo.
Owner:THE GENERAL HOSPITAL CORP
Markers for responsiveness to an erbB receptor tyrosine kinase inhibitor
InactiveUS20060252056A1Improved prognosisEasy to useSugar derivativesMicrobiological testing/measurementReceptor tyrosine kinase inhibitorGene list
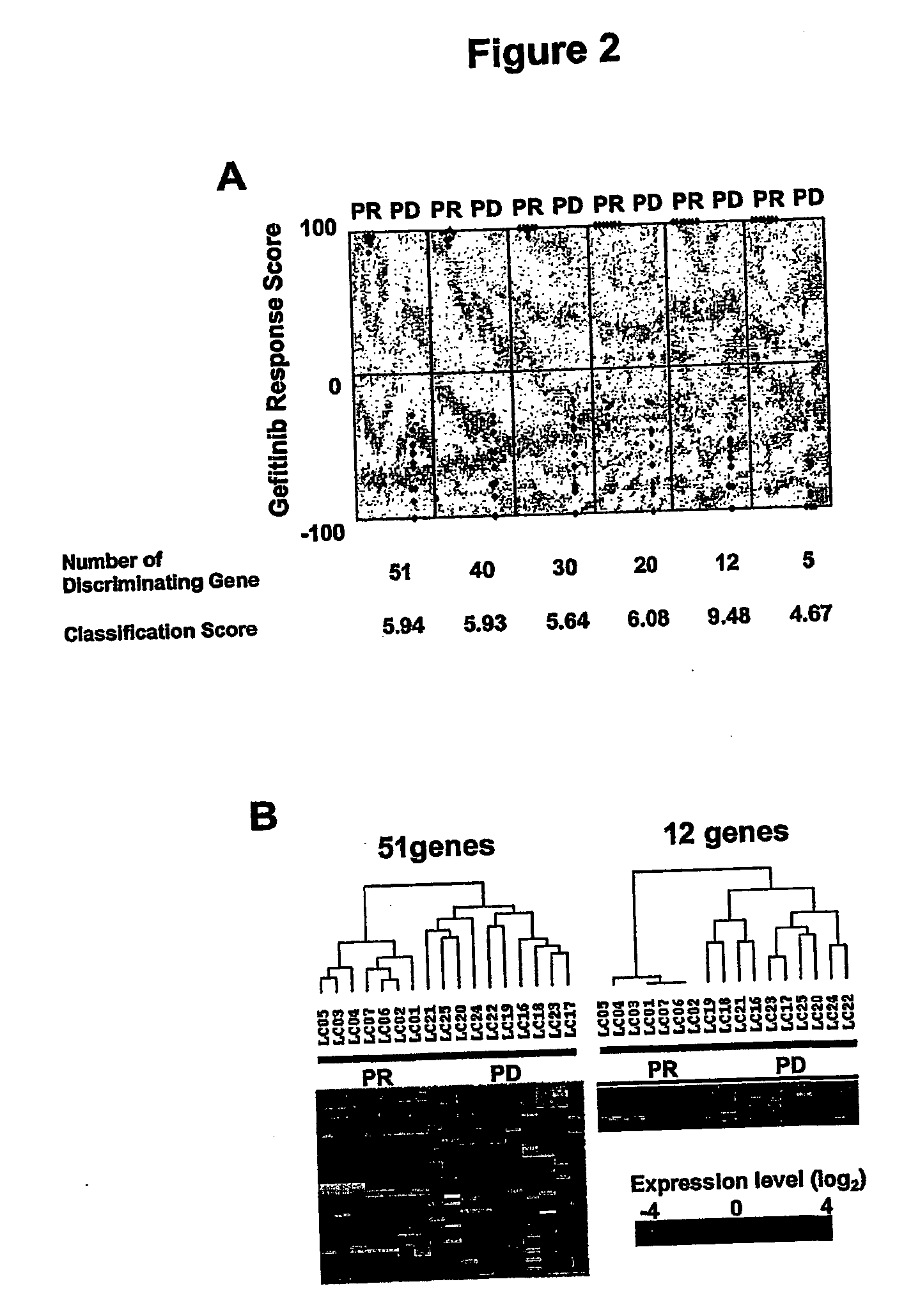
The invention relates to a set of isolated marker genes comprising at least one gene identified as having differential expression as between patients who are responders and non responders to an erbB receptor tyrosine kinase inhibitor; said gene set comprising one or more genes selected from at least the group consisting of the 51 genes listed herein including gene-specific oligonucleotides derived from said genes; and uses of such sets in diagnostic applications.
Owner:ONCOTHERAPY SCI INC
Mucosal gene signatures
InactiveUS20110059445A1Inhibit expressionInhibitory activityMicrobiological testing/measurementImmunoglobulinsMucosal BiopsyUlcerative colitis
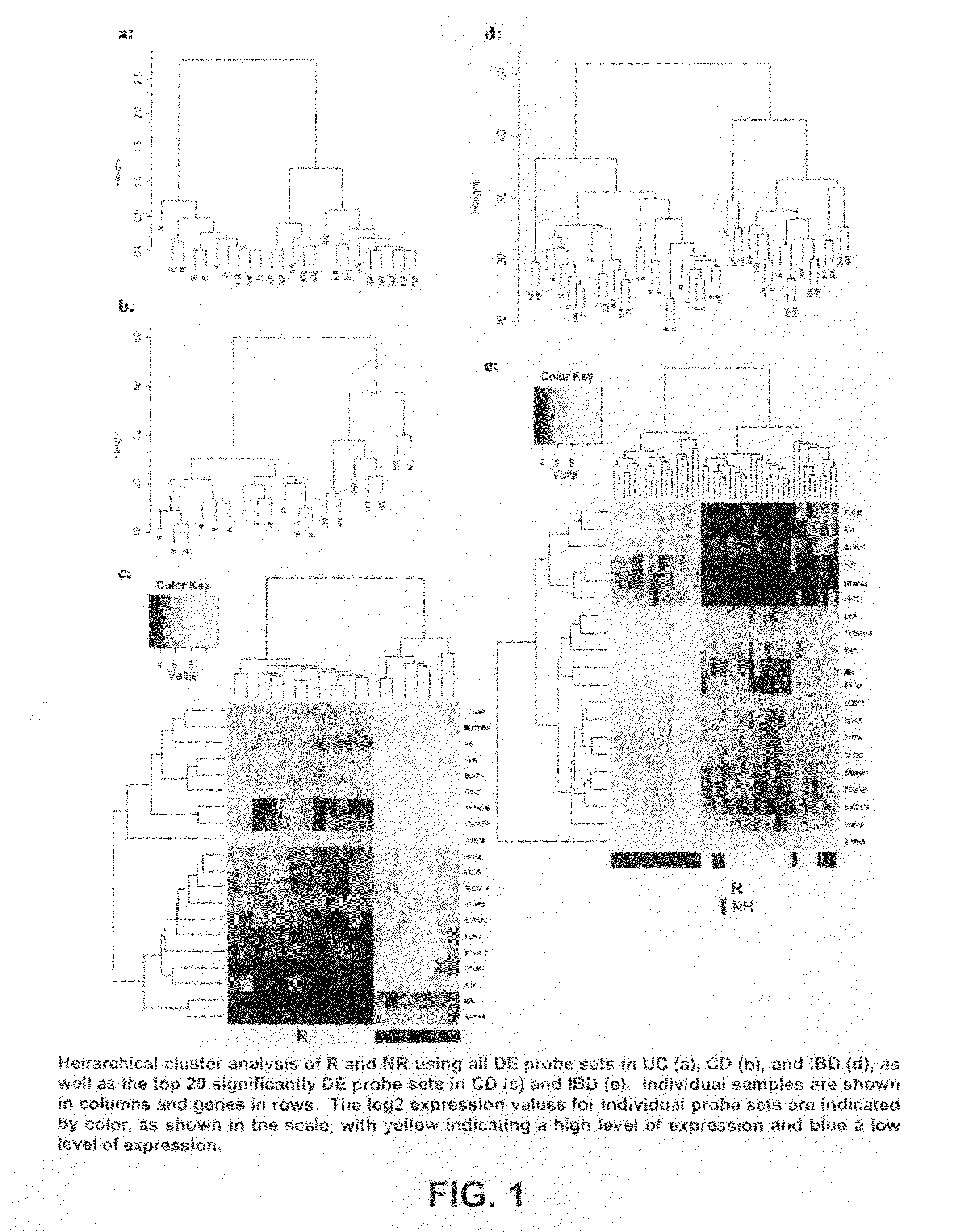
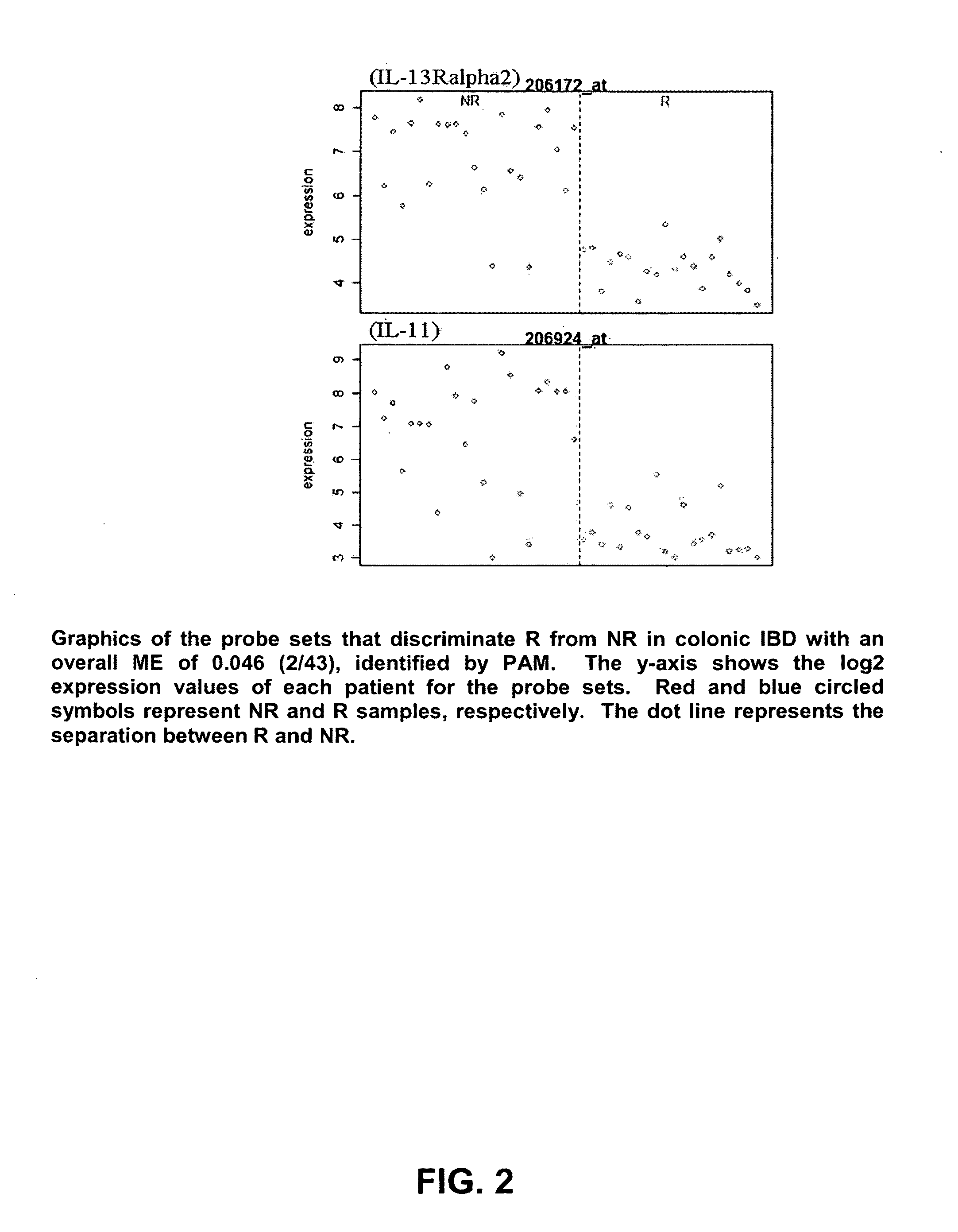
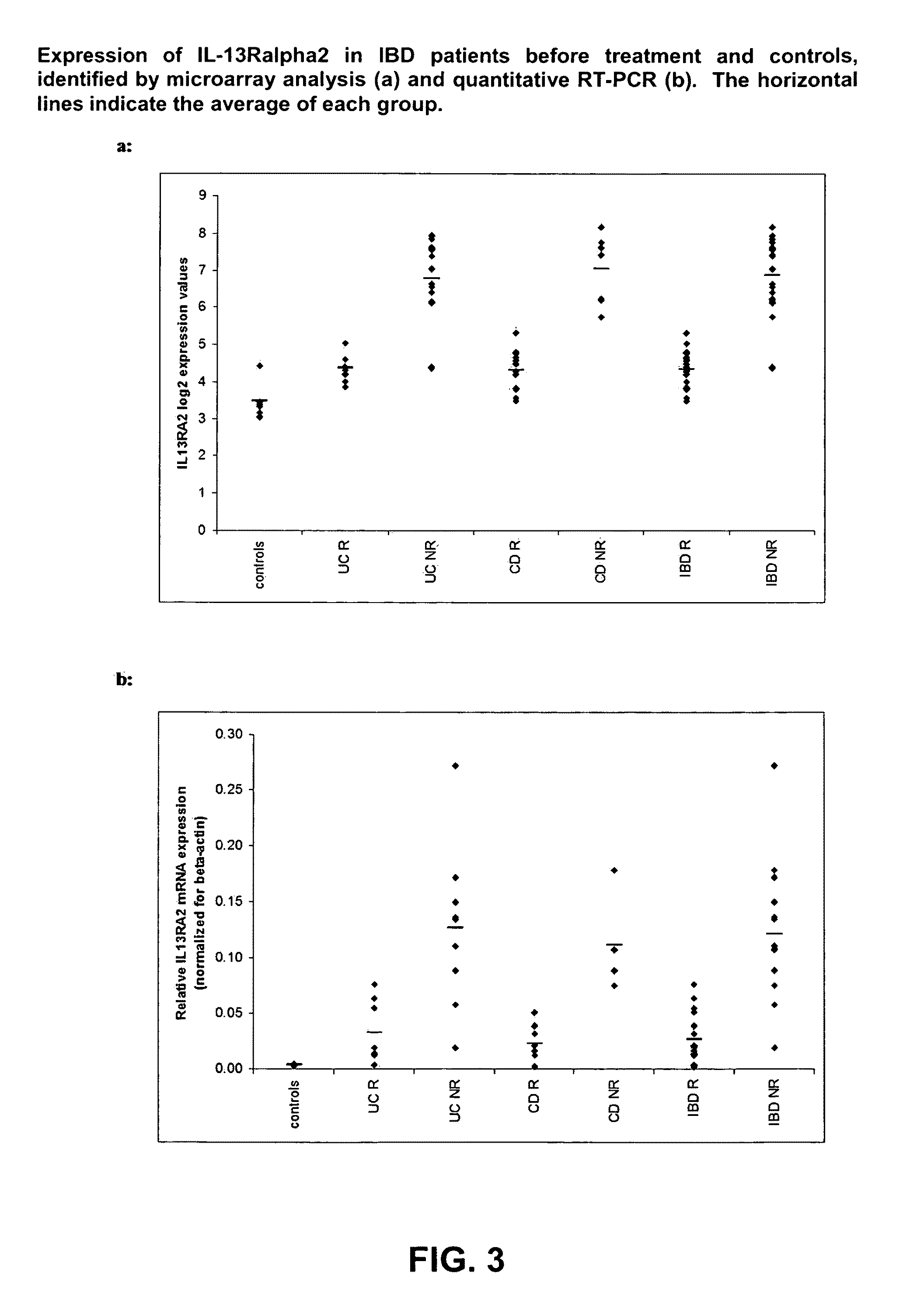
Infliximab (IFX) is an effective treatment for Crohn's disease (CD) and ulcerative colitis (UC) not responding to standard therapy. Thirty percent to forty percent of patients however do not improve and the response is often incomplete. We identified mucosal gene signatures predictive of response to EFX using high-density oligonucleotide arrays. Eight UC patients and twelve CD patients showed healing. In UC, only one probe set was differentially expressed in responders compared with non-responders, i.e., IL-13R(alpha)2. At PAM analysis, two probe sets, representing IL-13Ralpha2 and IL-I 1, separated IBD responders from non-responders with an overall misclassification error rate of 0.046 (2 / 43), with 100% sensitivity and 91.3% specificity. The IL-13R(alpha)2 probe set was a top-ranked probe set in all our analyses using both LIMMA and PAM strategies. Our gene array studies of mucosal biopsies identified IL-13R(alpha)2 in IBD as a predictor of response or non-response to IFX.
Owner:KATHOLIEKE UNIV LEUVEN
Method
InactiveUS20110070268A1Peptide/protein ingredientsLibrary screeningSpecific populationNucleic acid sequencing
Methods for characterisation of patients as responders or non-responders to therapy based on differential expression of one or more genes are provided. Gene expression profiles, microarrays comprising nucleic acid sequences representing gene expression profiles, and new diagnostic kits and methods of treatment are also provided. The kits and methods relate to the treatment of specific populations of, for example, cancer patients, as characterised by their gene expression profile, suffering from MAGE expressing tumours.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Gene array technique for predicting response in inflammatory bowel diseases
InactiveUS20100267575A1Microbiological testing/measurementLibrary screeningReference genesUlcerative colitis
Disclosed are methods for classifying individuals having or suspected of having an inflammatory bowel disease, such as Crohn's Disease or Ulcerative Colitis, as “responders” or “non-responders” to first-line treatment, generally comprising the steps of a) obtaining, a biological sample from the individual, b) isolating mRNA from the biological sample c) determining a gene expression profile from the biological sample; and d) comparing the gene expression profile of the individual to a reference gene expression profile or other suitable control such that changes in expression can be used to stratify individuals and predict efficacy of first-line therapy. A gene expression system is further provided for carrying out these methods.
Owner:CHILDRENS HOSPITAL MEDICAL CENT CINCINNATI
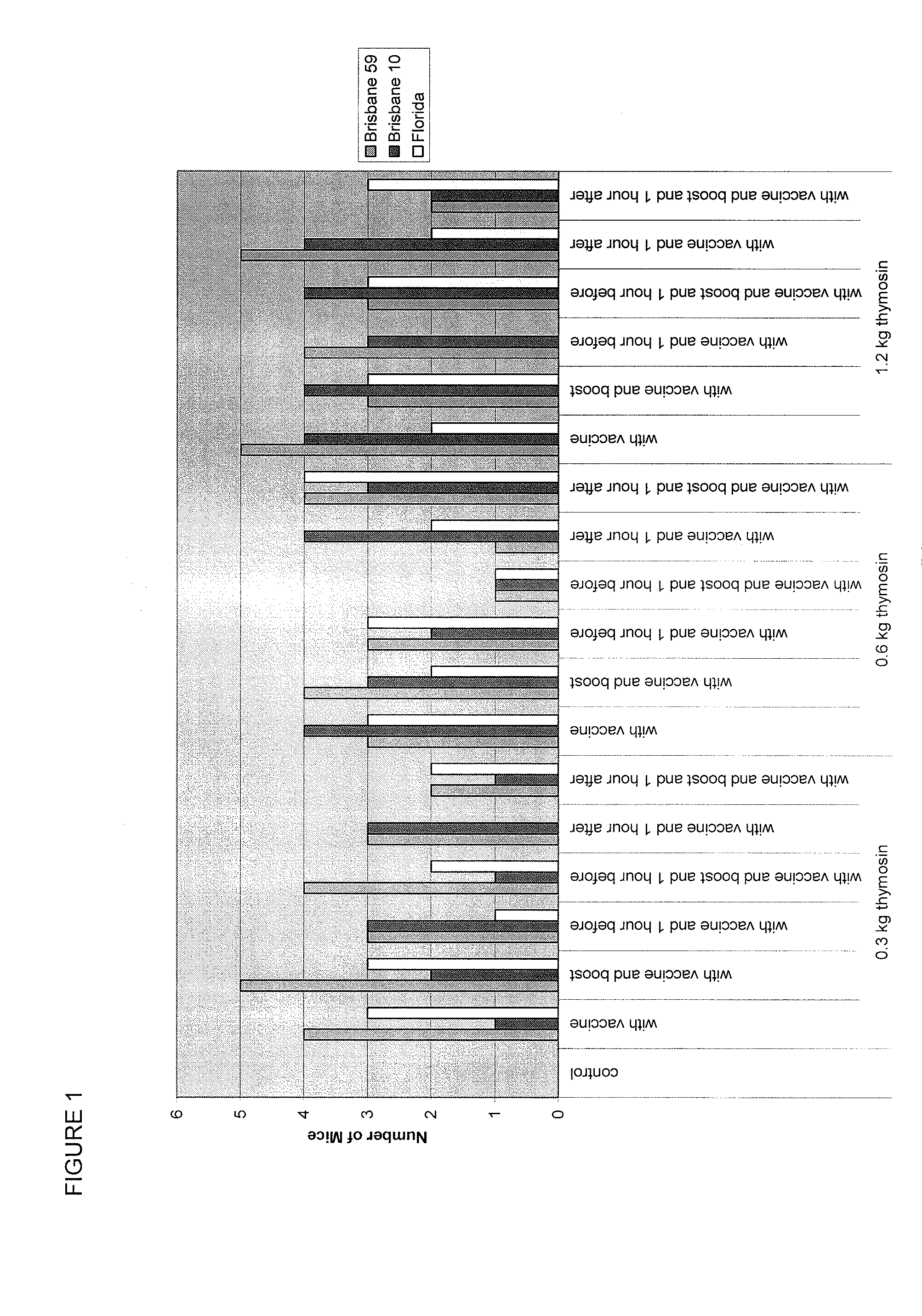
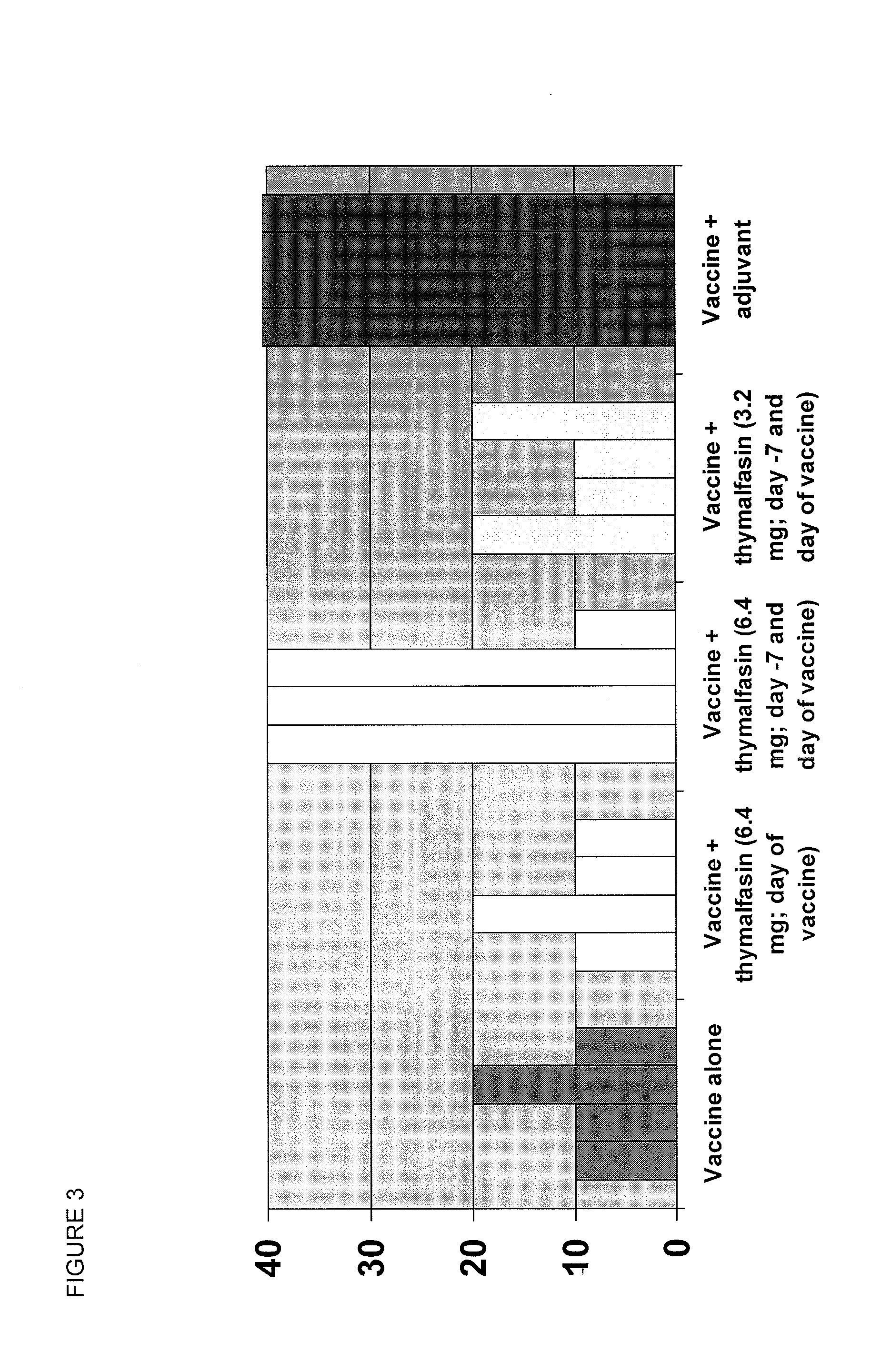
Alpha thymosin peptides as vaccine enhancers
ActiveUS20100285060A1Enhancing vaccine effectivenessEnhance vaccine effectivenessSsRNA viruses negative-senseBacterial antigen ingredientsRegimenImmunodeficiency
The present invention provides methods of vaccination as well as pharmaceutical combinations and kits for enhancing vaccine effectiveness, including for immunodeficient or immunecompromised patients, including non-responders and low-responders to vaccination. As disclosed herein, the invention relates to administering a vaccine and a regimen of thymosin alpha peptide so as to provide higher antibody titers, speed the development of such antibody titers, and / or to provide for a longer duration of such antibody titers, thereby providing a greater protective effect. In another aspect, the invention allows for reducing a vaccine dose, such as an influenza vaccine dose, by administration of a thymosin peptide regimen.
Owner:SCICLONE PHARM INT LTD
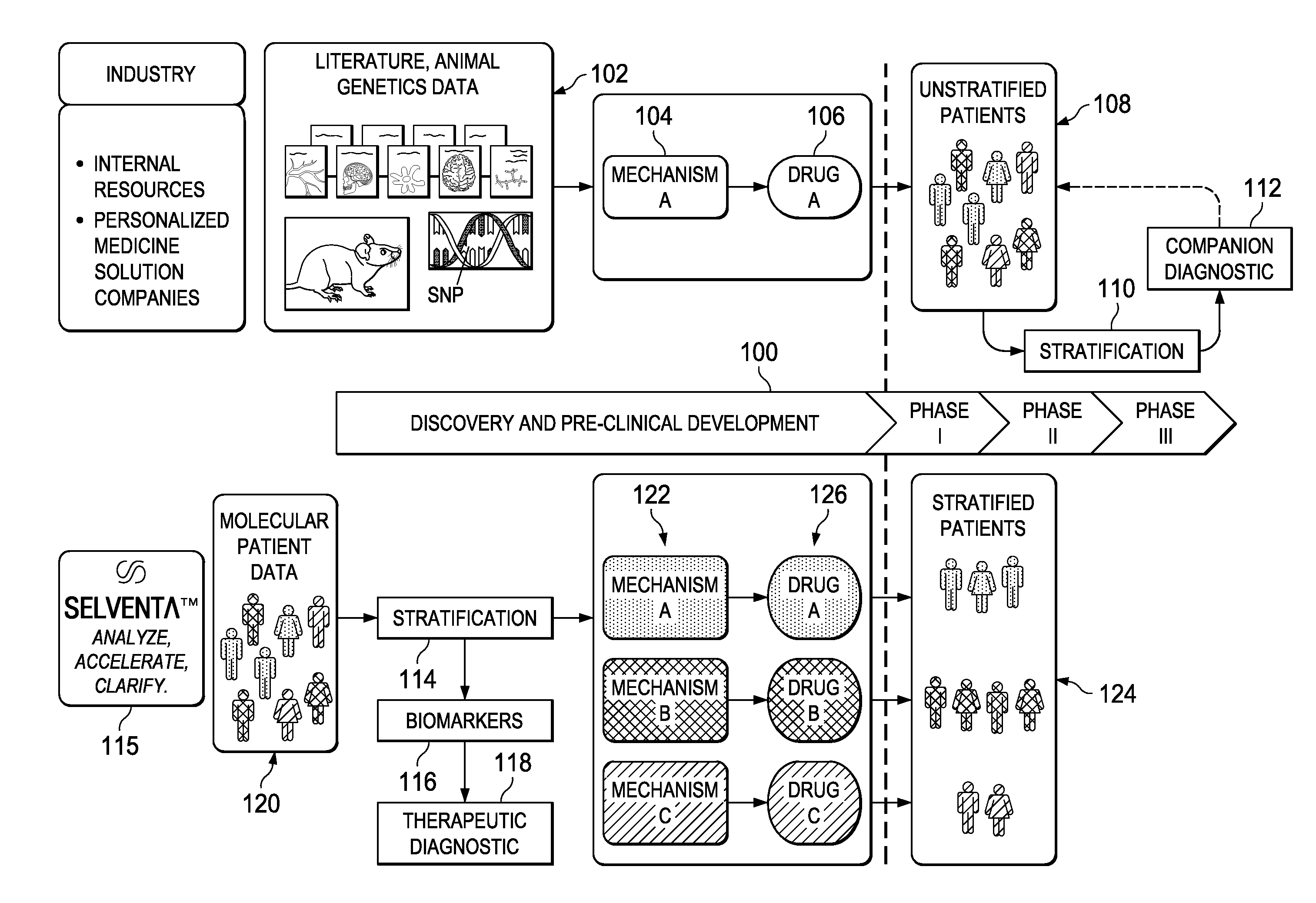
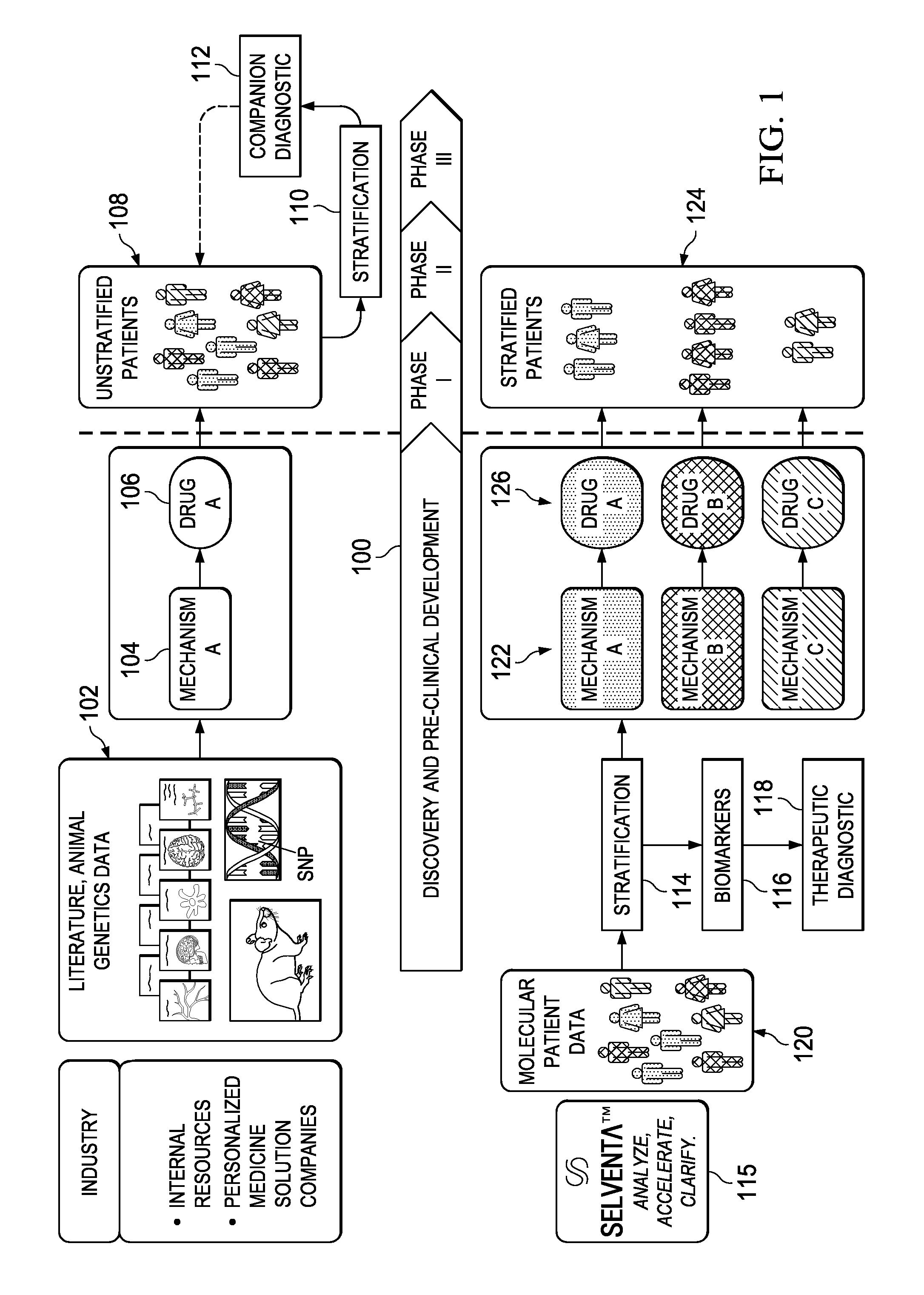
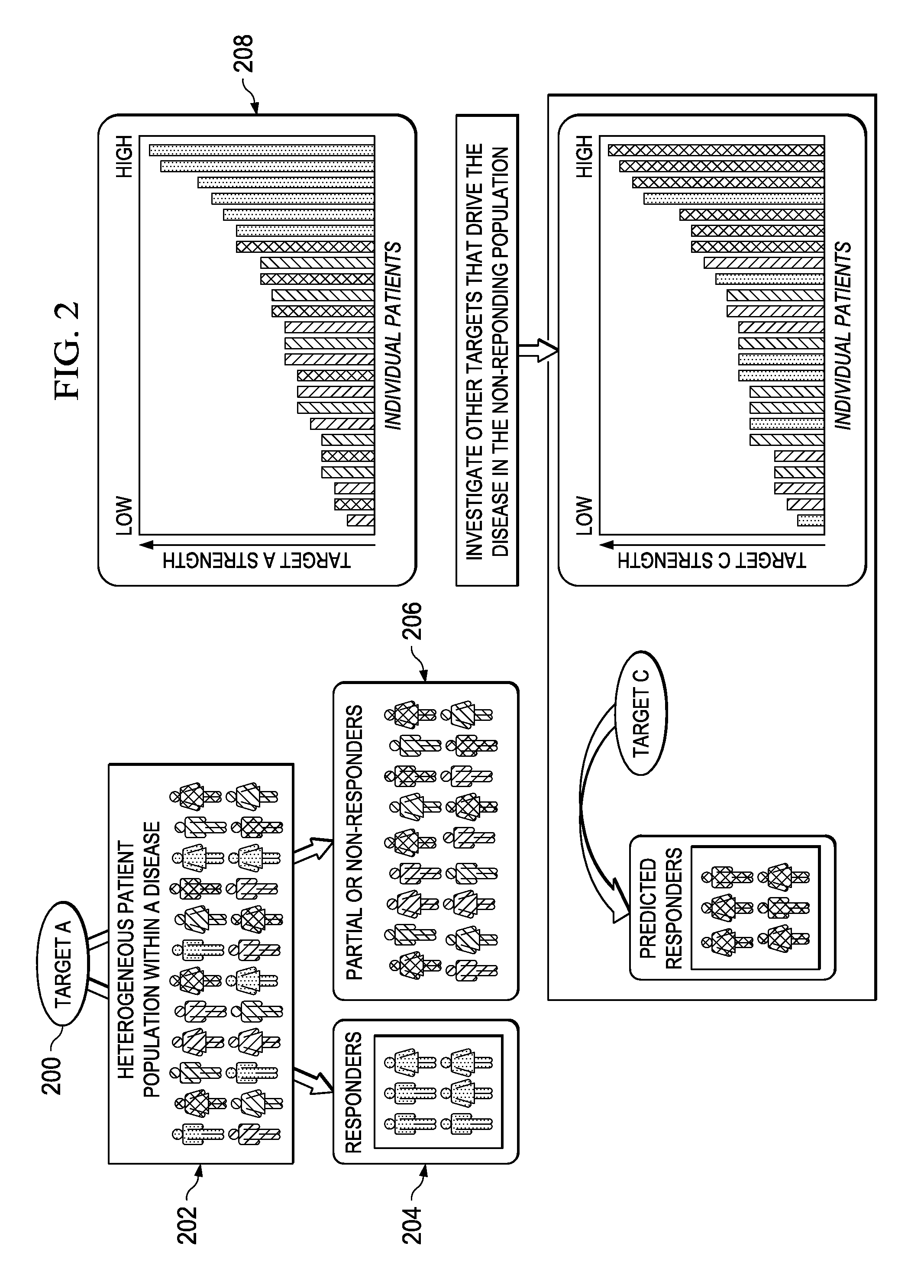
Stratifying patient populations through characterization of disease-driving signaling
InactiveUS20130218581A1UnderstandingIncrease success rateMarket predictionsMedical data miningDiseaseDifferential signaling
A method of stratifying a set of disease-exhibiting patients prior to clinical trial of a target therapy begins by using a molecular footprint derived from a knowledgebase and other patient data to identify genes that are differentially expressed in a direction consistent with increase in the target activity. Therapeutic target “signaling strength” in individual patients of the set is then assessed using the genes identified and a strength algorithm. Based on their therapeutic target signaling strength, the set of disease-exhibiting patients are then stratified along a continuum. One or more gene expressions or other biomarkers may be specified for use in categorizing other disease-exhibiting patient populations. Alternative therapeutic targets are analyzed with respect to the likely non-responders, as evidenced by their differential signaling strength.
Owner:GENSTRUCT
Method for profiling kinase inhibitors
The present invention is concerned with method for pharmacologically profiling compounds using an array of substrates, in particular kinase substrates, immobilized on a porous matrix. This method was found particular useful in the prediction of drug response, i.e. to enable the distinction between responders and non-responders in the treatment of cells, tissues, organs or warm-blooded animals with the compound to be tested, and in compound differentiation.
Owner:JANSSEN PHARMA NV
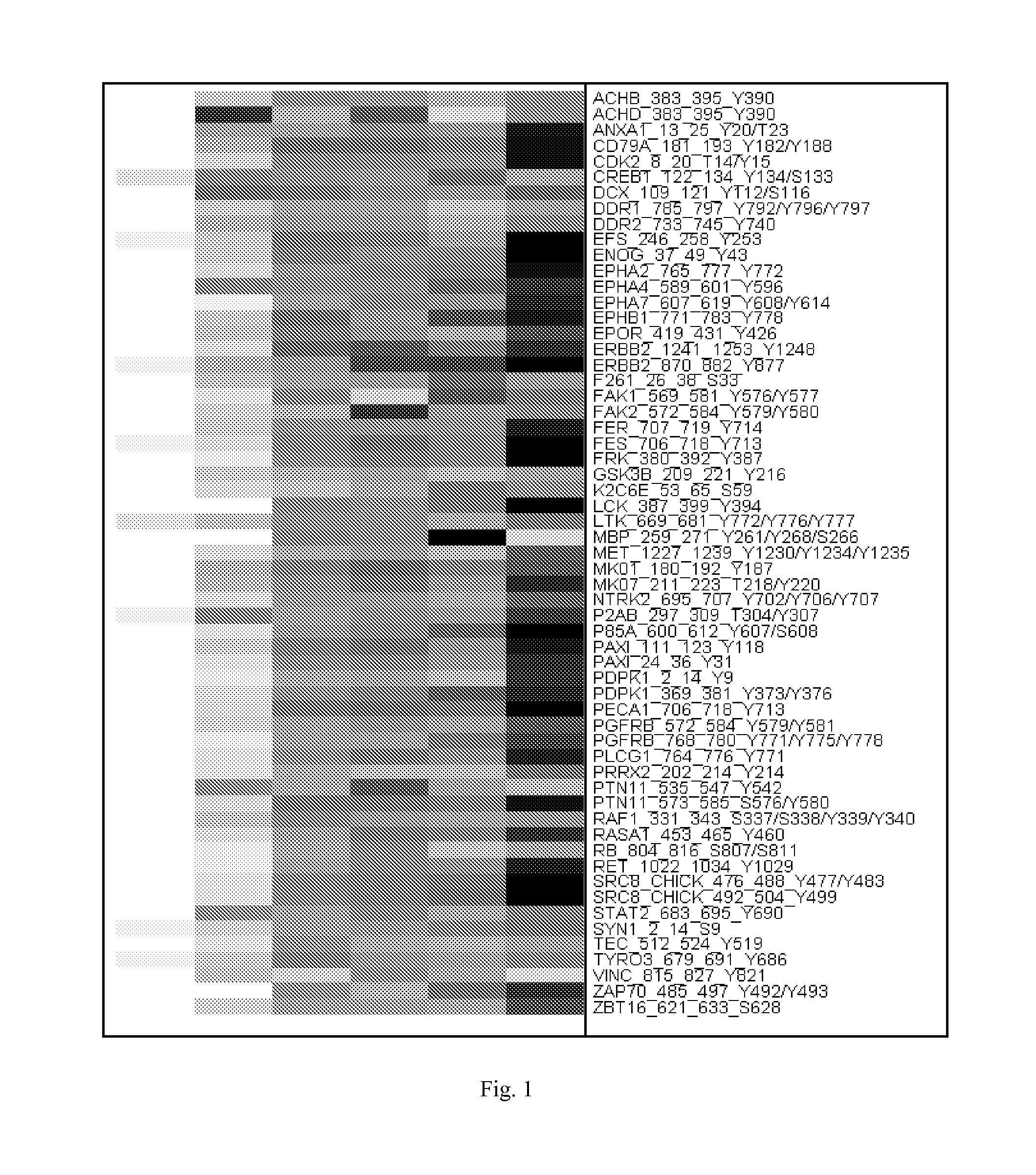
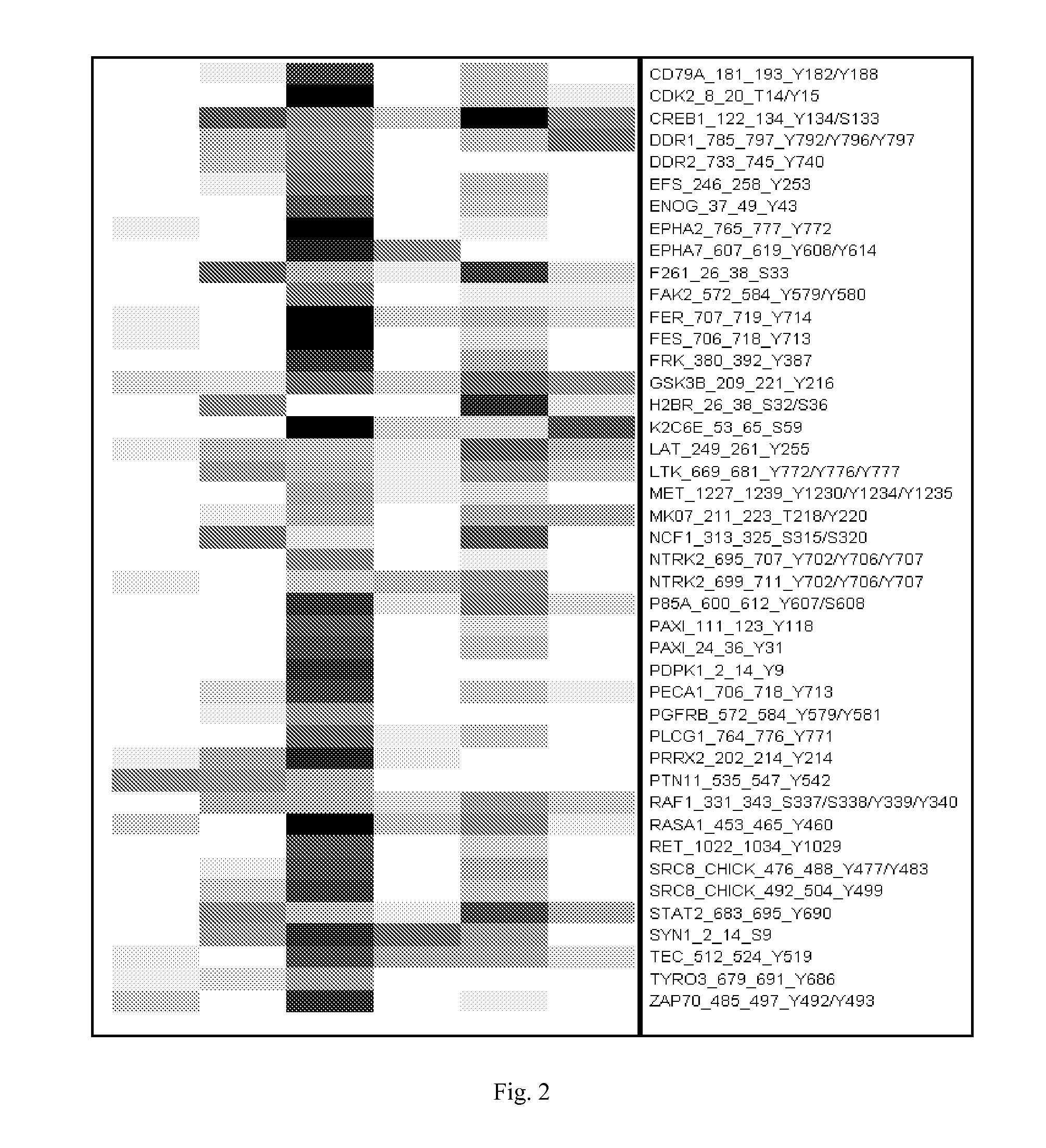
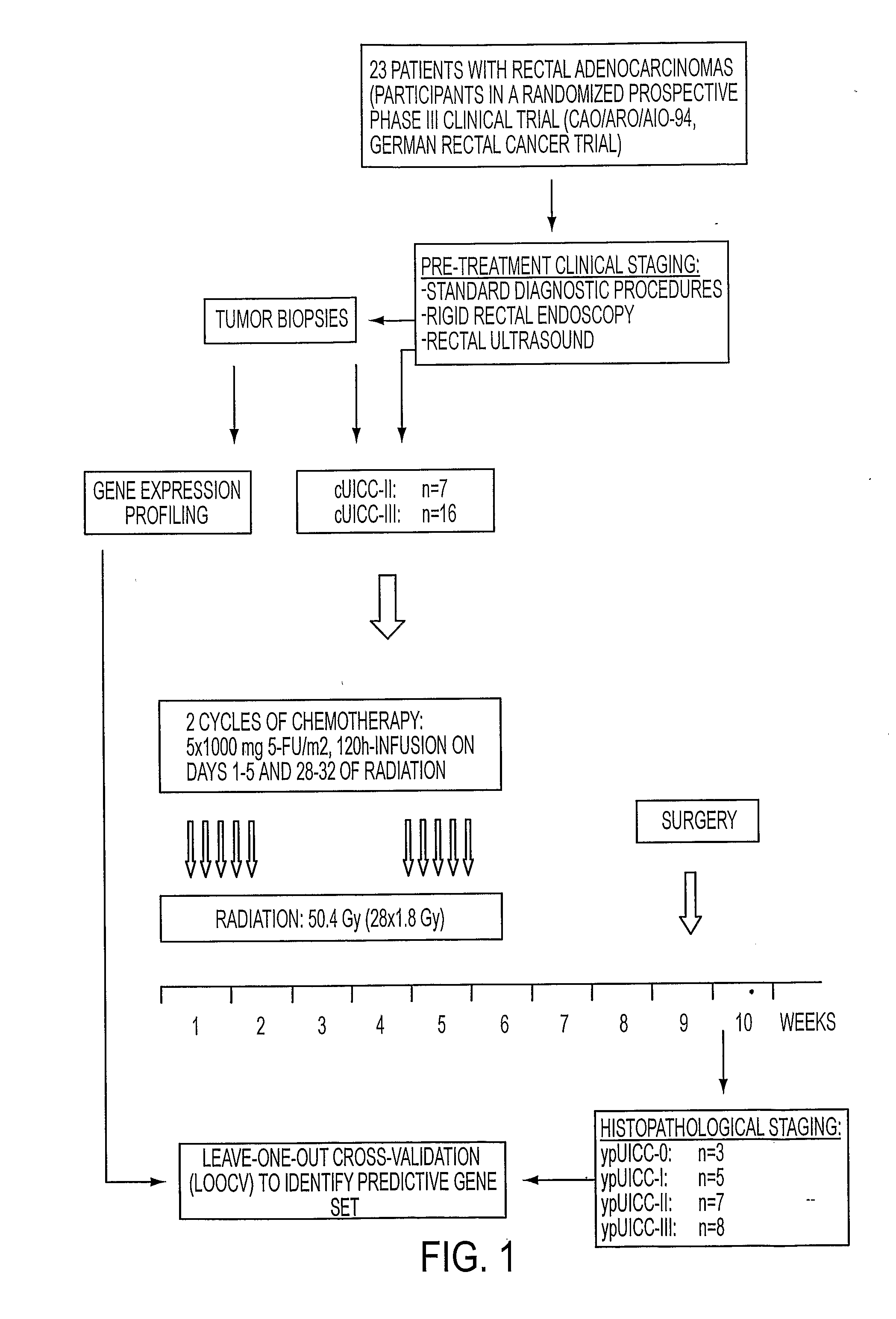
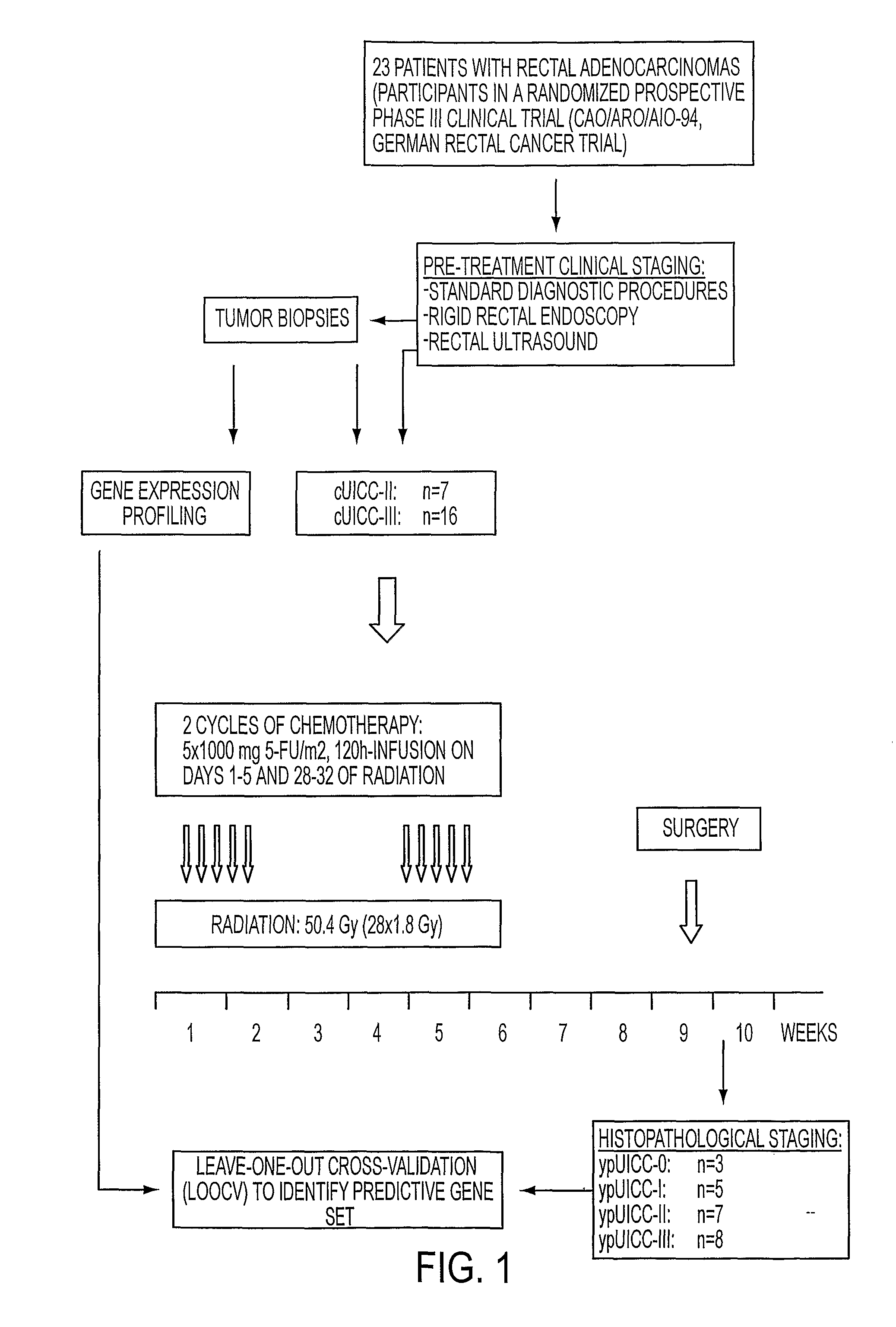
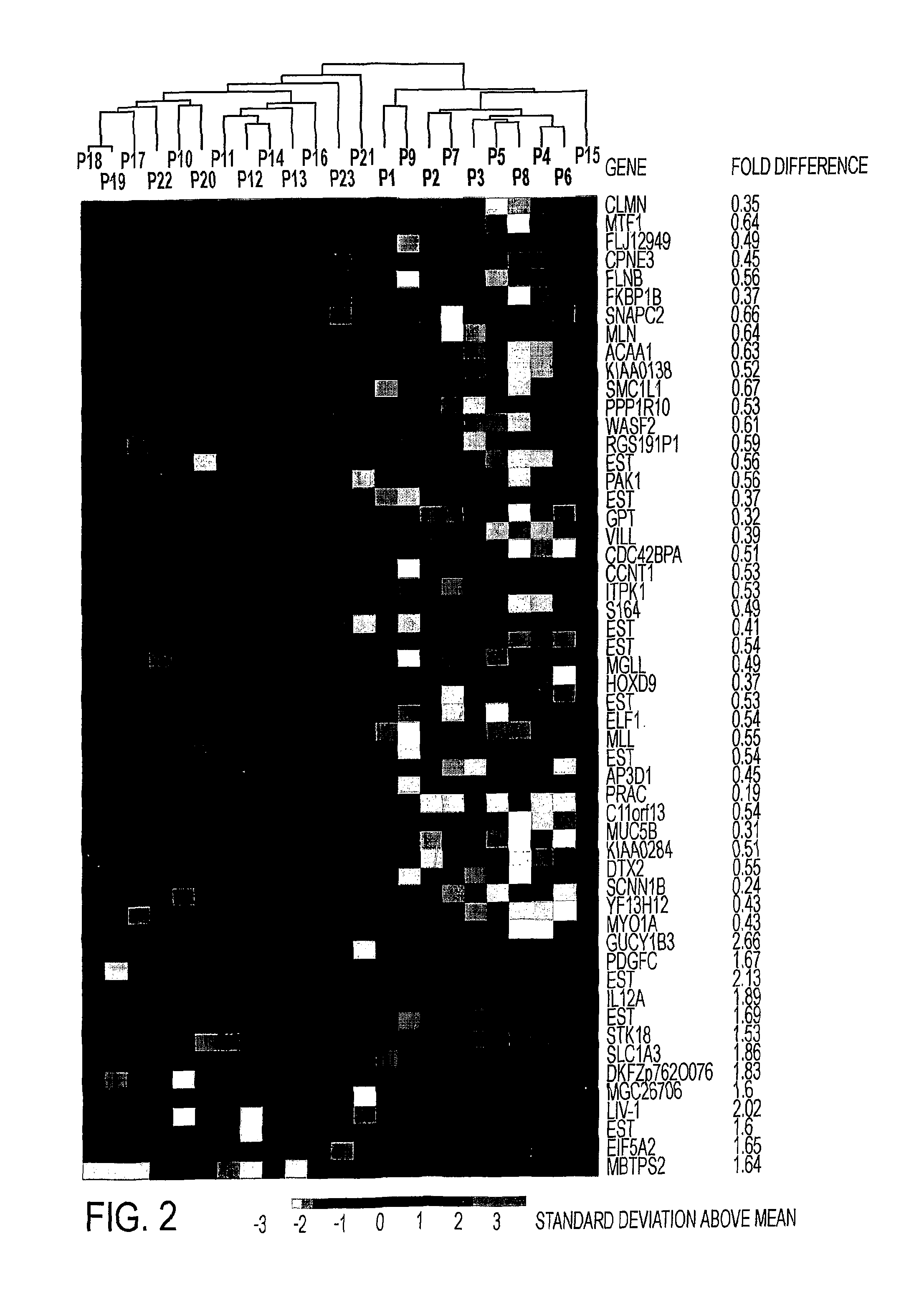
Composition for Detecting the Response of Rectal Adenocarcinomas to Radiochemotherapy
ActiveUS20080020373A1Enhanced hybridizationReduced structureSugar derivativesMicrobiological testing/measurementNon respondersGene
A cDNA array (9984 genes) was used for expression profiling in rectal adenocarcinoma. The expression data were correlated to responsiveness to chemotherapy followed by radiotherapy. A set of 54 genes was found that were differentially expressed in responders vs. non-responders. The genes may be used as prognostic markers for determining whether a rectal adenocarcinoma is responsive to radiochemotherapy.
Owner:UNITED STATES OF AMERICA
System and method for reducing the placebo effect in controlled clinical trials
A method and system for performing a clinical trial having a reduced placebo effect is disclosed. The method includes randomizing study participants into three or more treatment groups and performing a first phase of testing on the groups. In a typical embodiment, the first phase of testing includes administering an active treatment to a first group, and administering a placebo to a second group and to a third group. Responders and non-responders are determined for each group. A second phase of testing is then performed. The second phase of testing includes administering the placebo to non-responders in the first group, administering the active treatment to non-responders in the second group, and administering the placebo to non-responders in the third group. The data from the first phase of testing and from the second phase of testing is pooled and analyzed to determine response rates to active treatment and placebo.
Owner:THE GENERAL HOSPITAL CORP
Predictive mRNA Biomarkers for the Prediction of the Treatment with Methotrexate (MTX)
InactiveUS20170058348A1Rapid and adapted transferCost-effectiveMicrobiological testing/measurementNon respondersOncology
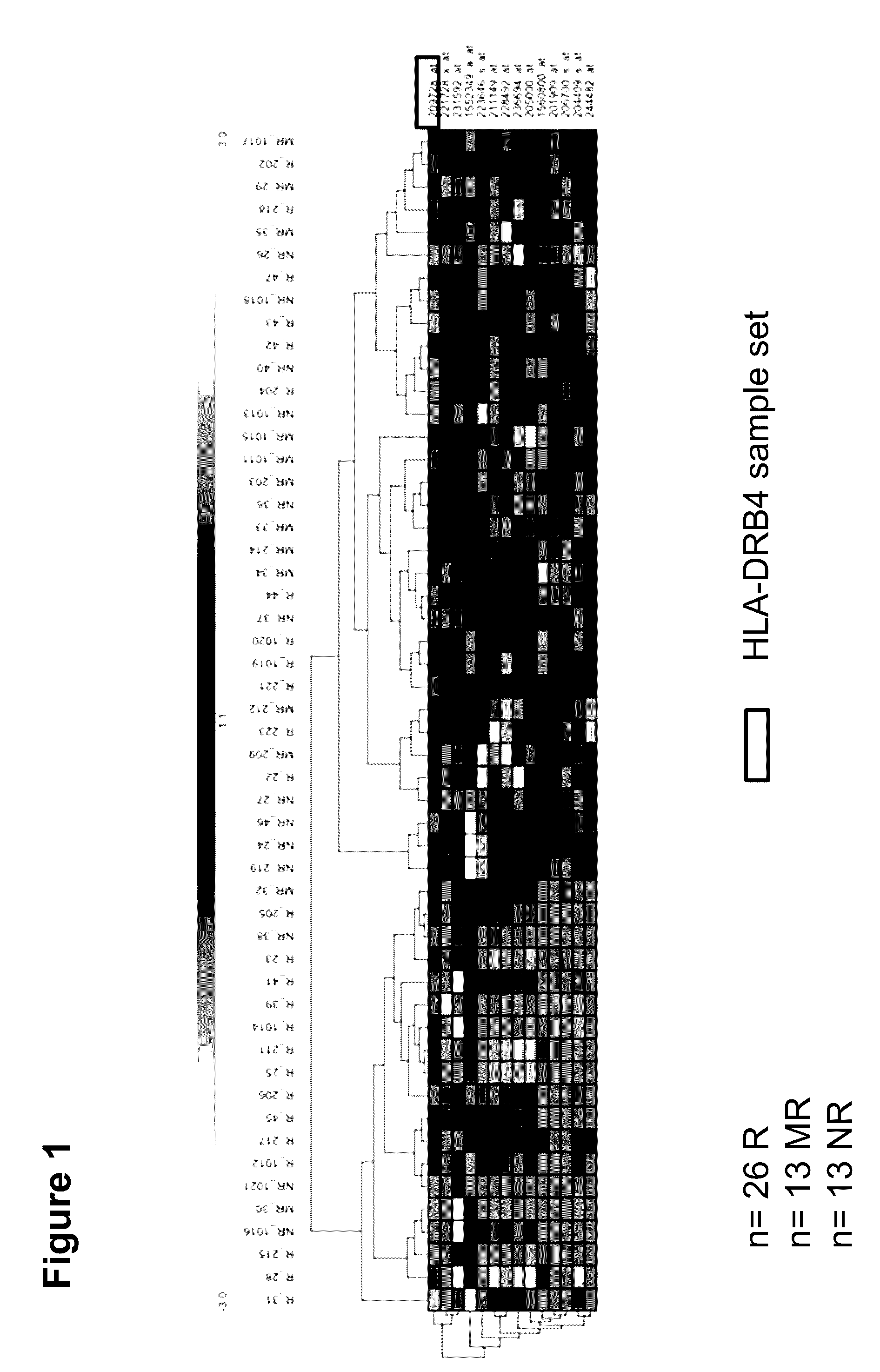
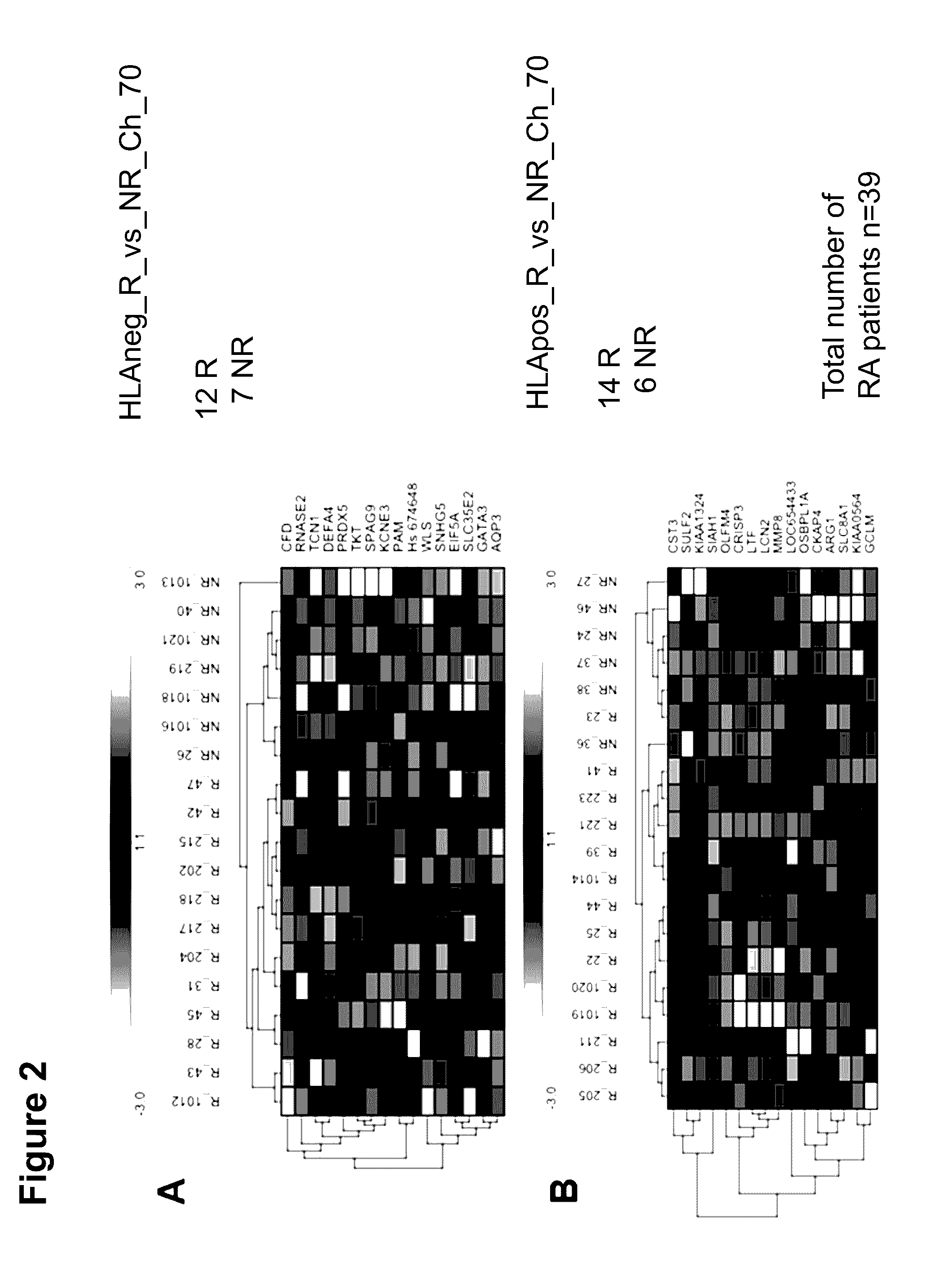
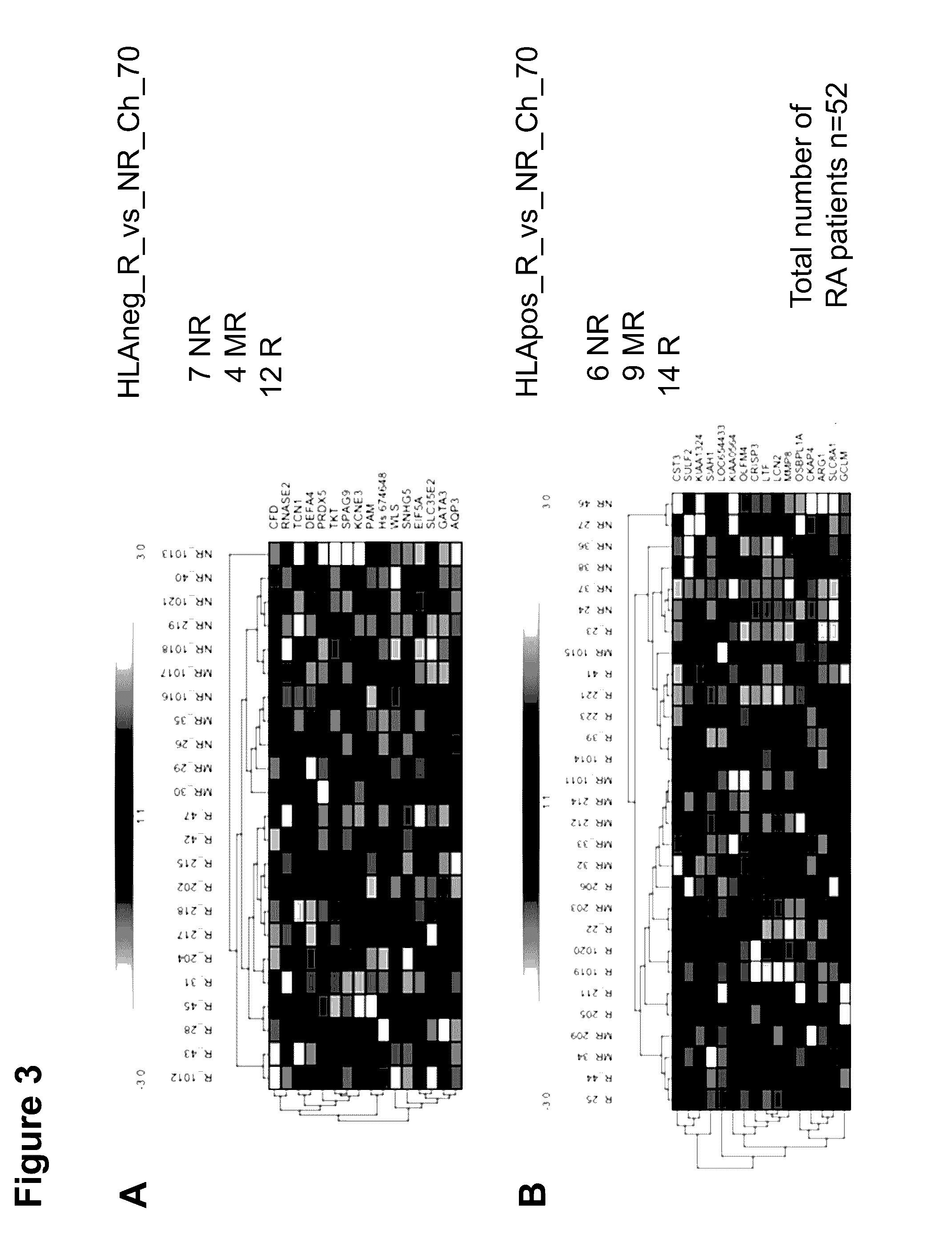
The present invention concerns predictive mRNA biomarkers, which are used in combination with HLA-DRB4 for forecasting the treatment with MTX (methotrexate). The present invention further concerns a method for forecasting the treatment with MTX (methotrexate), comprising detection of the predictive mRNA biomarkers in combination with HLA-DRB4 in patient samples, whereby the patients are classified into responders or non-responders.
Owner:CHARITE UNIVS MEDIZIN BERLIN
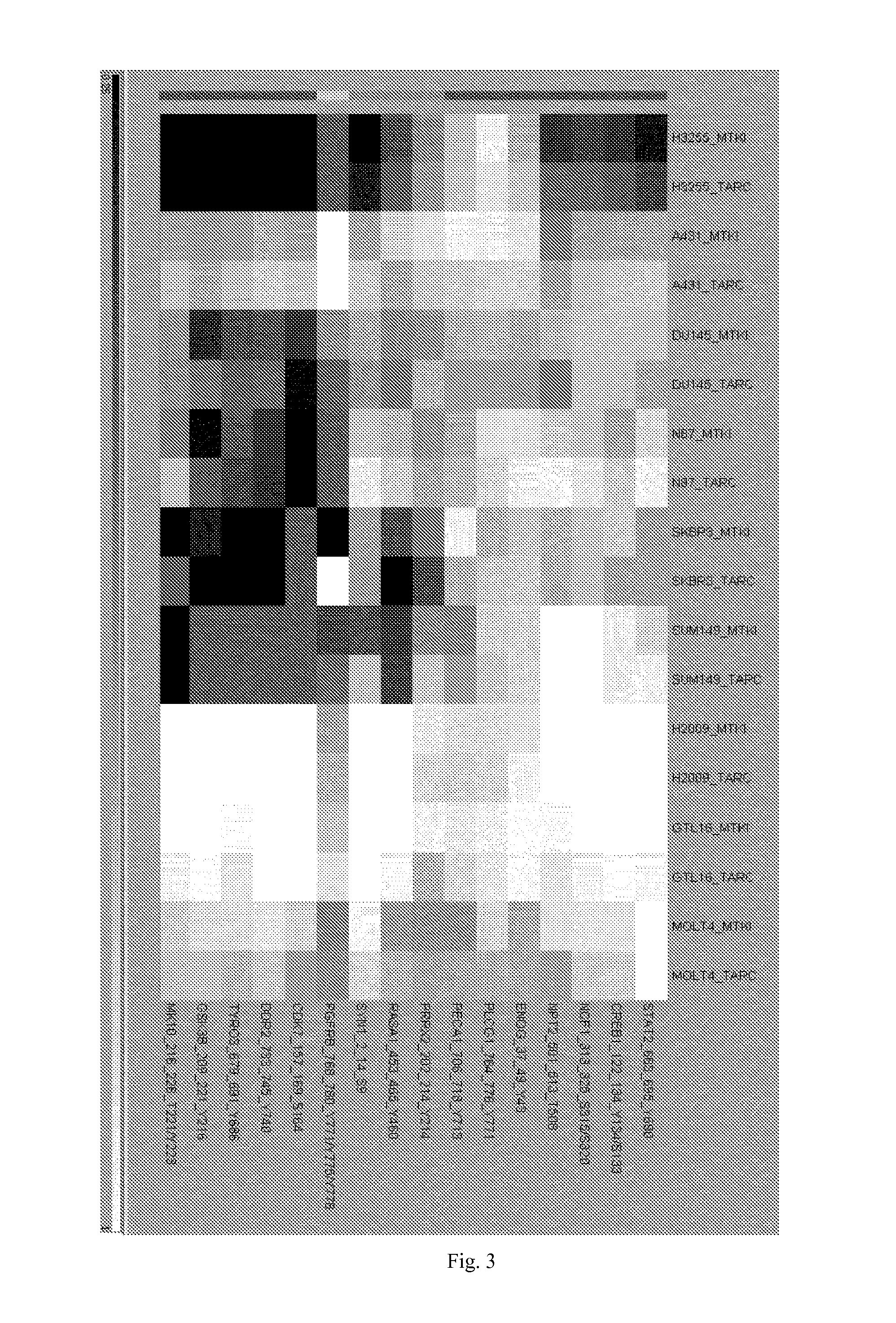
Gene expression signatures predictive of subject response to a multi-kinase inhibitor and methods of using the same
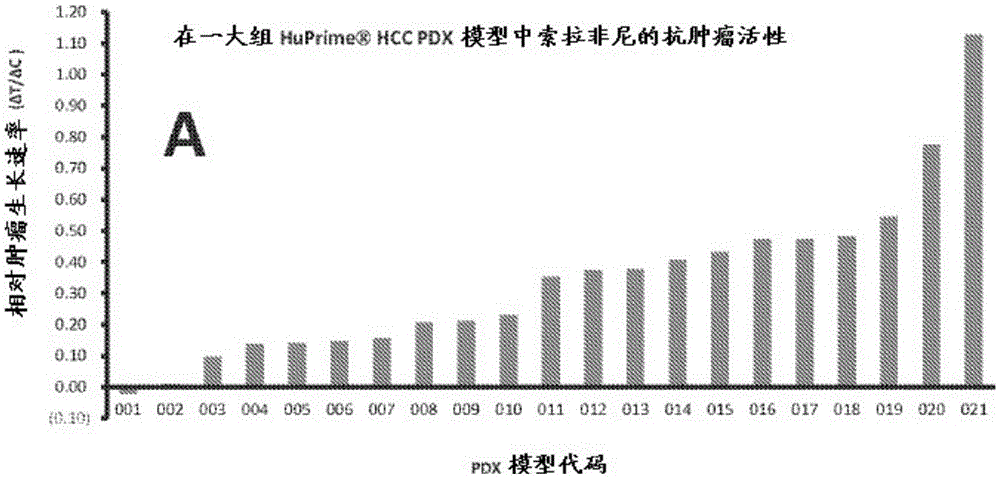
Gene expression signature predictive of cancer patient response to multi-kinase inhibitor is disclosed. Also disclosed are methods predicting the efficacy of the multi-kinase inhibitor for treating cancer in a patient. Also disclosed are methods for distinguishing responders from non-responders to a multi-kinase inhibitor in treating cancer. Also disclosed are methods for treating a cancer patient with a multi-kinase inhibitor.
Owner:CROWN BIOSCI INC TAICANG
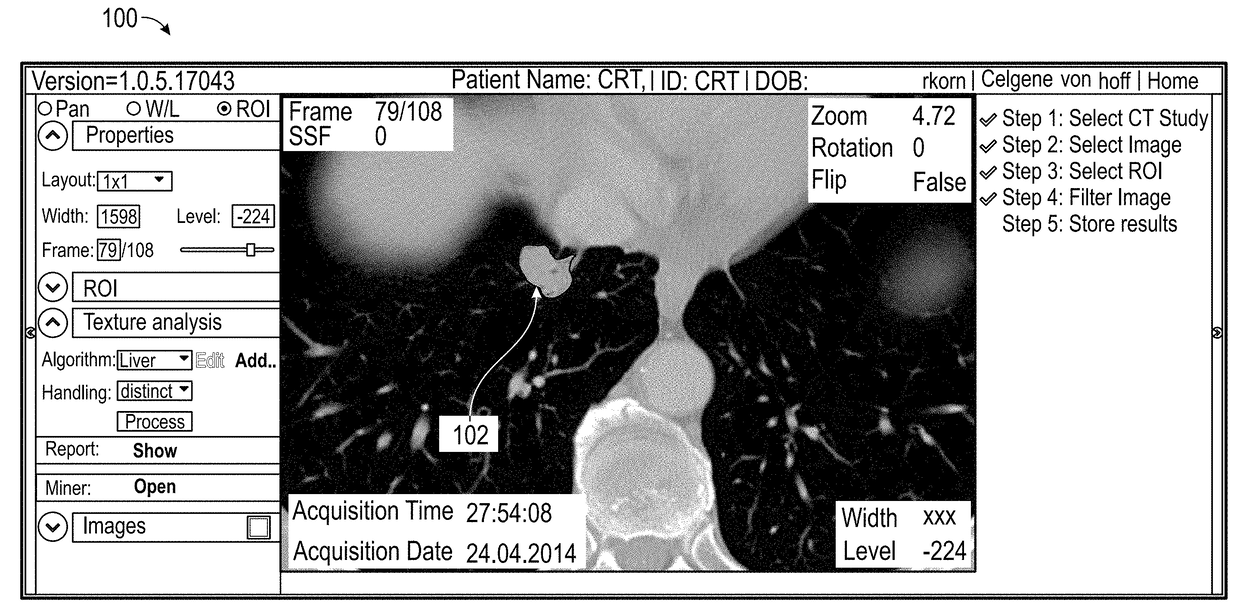
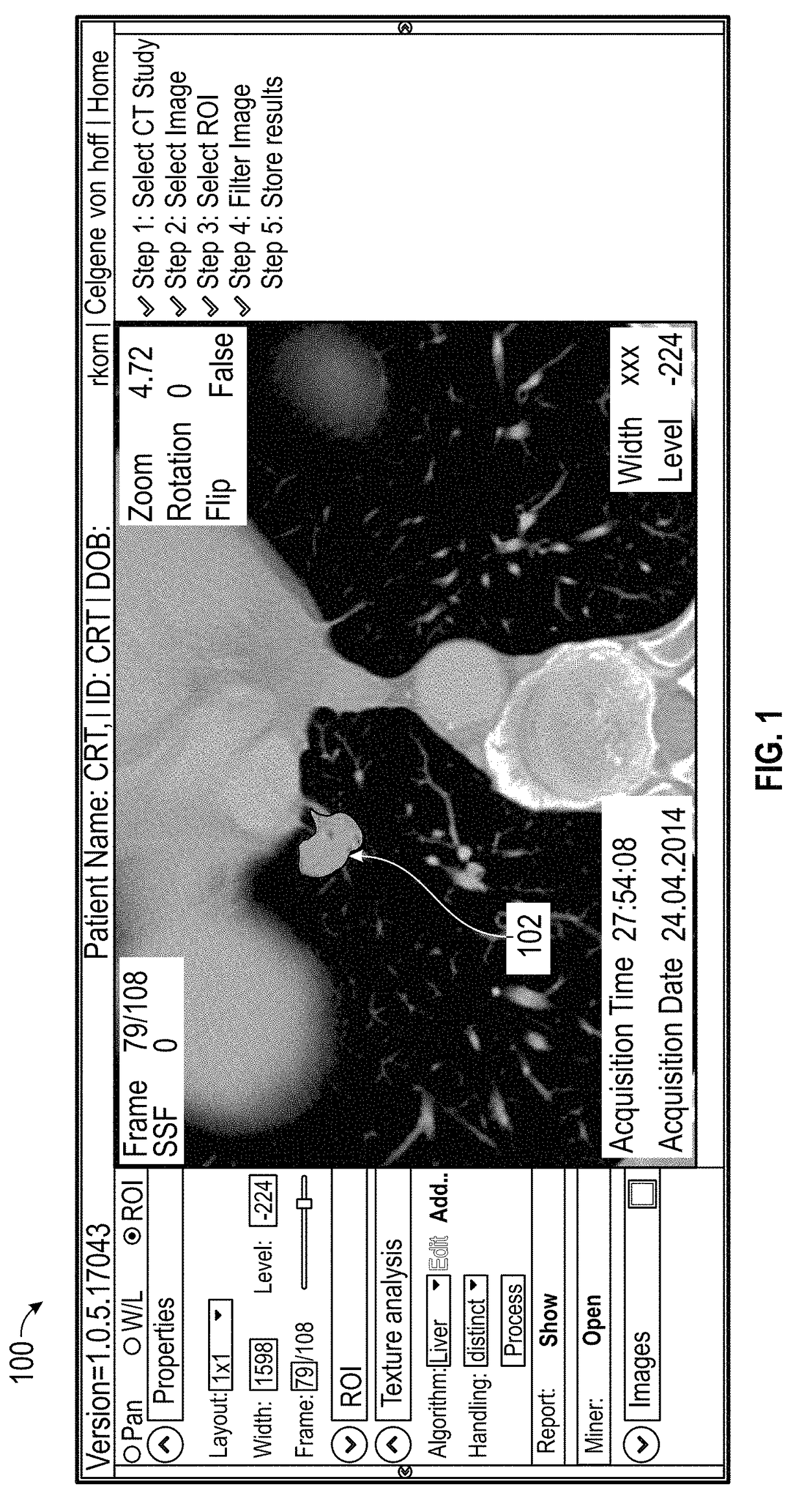
Systems and Methods For Predicting Lung Cancer Immune Therapy Responsiveness Using Quantitative Textural Analysis
ActiveUS20180046750A1Predicting immune therapy responsivenessPrediction of responsivenessBiostatisticsProteomicsPixel densityImmune therapy
Methods and apparatus for predicting responsiveness to immune therapy in lung cancer. The method includes the steps of: identifying a first population of known responders and a second population of known non-responders; processing imaging data for the first and second populations using quantitative textural analysis (QTA); generating, for each member of both populations, quantitative metrics using the QTA; performing logistical regression on the quantitative metrics for both populations to yield a predictive signature expressed in the form of Y=Mx+B where x comprises mean pixel density; performing QTA on a lung cancer scan for a subsequent patient; comparing the predictive signature to one or more relevant metrics associated with the subsequent patient; and predicting responsiveness to immune therapy for the subsequent patient based on the comparison.
Owner:IMAGING ENDPOINTS II LLC
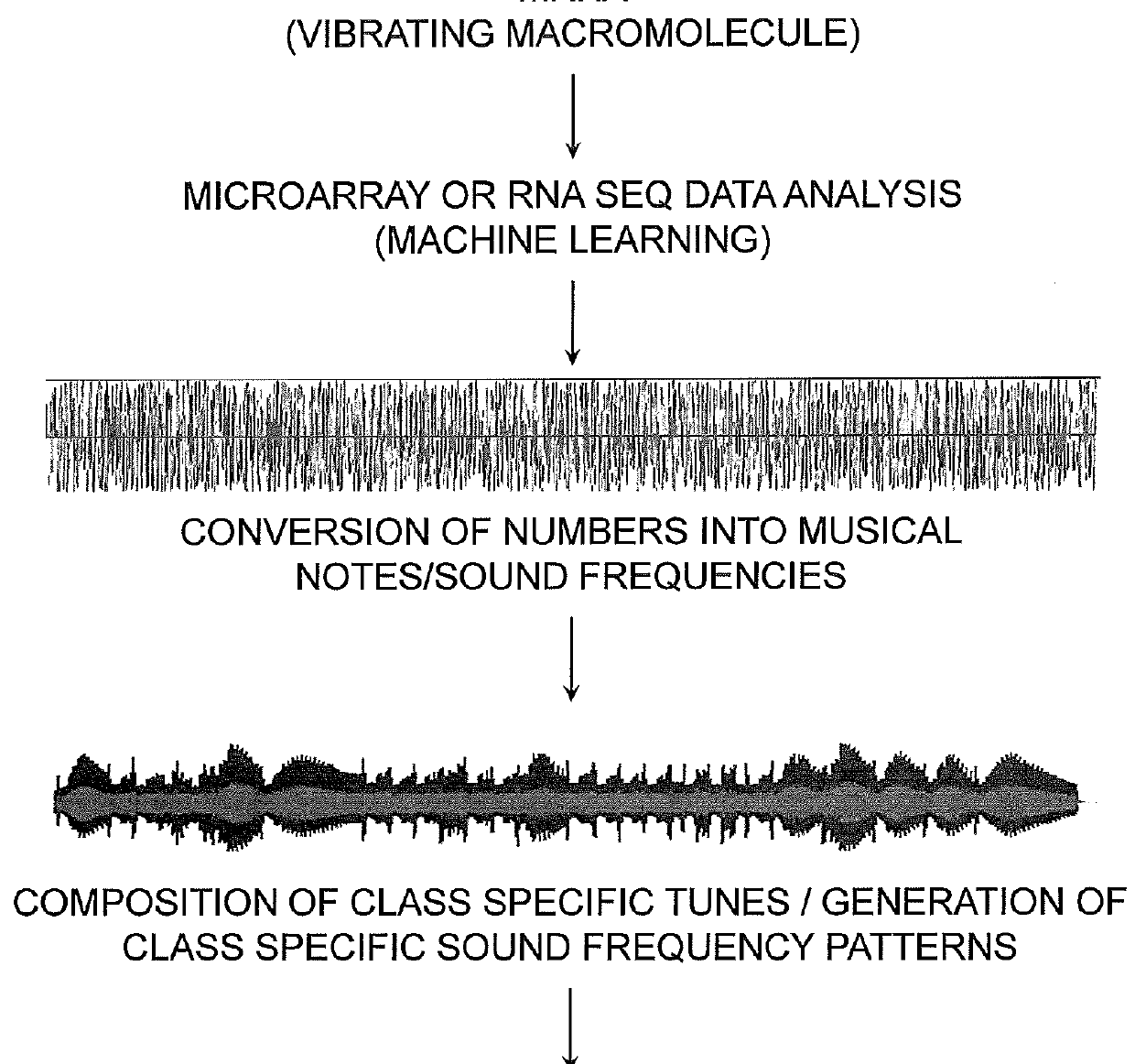
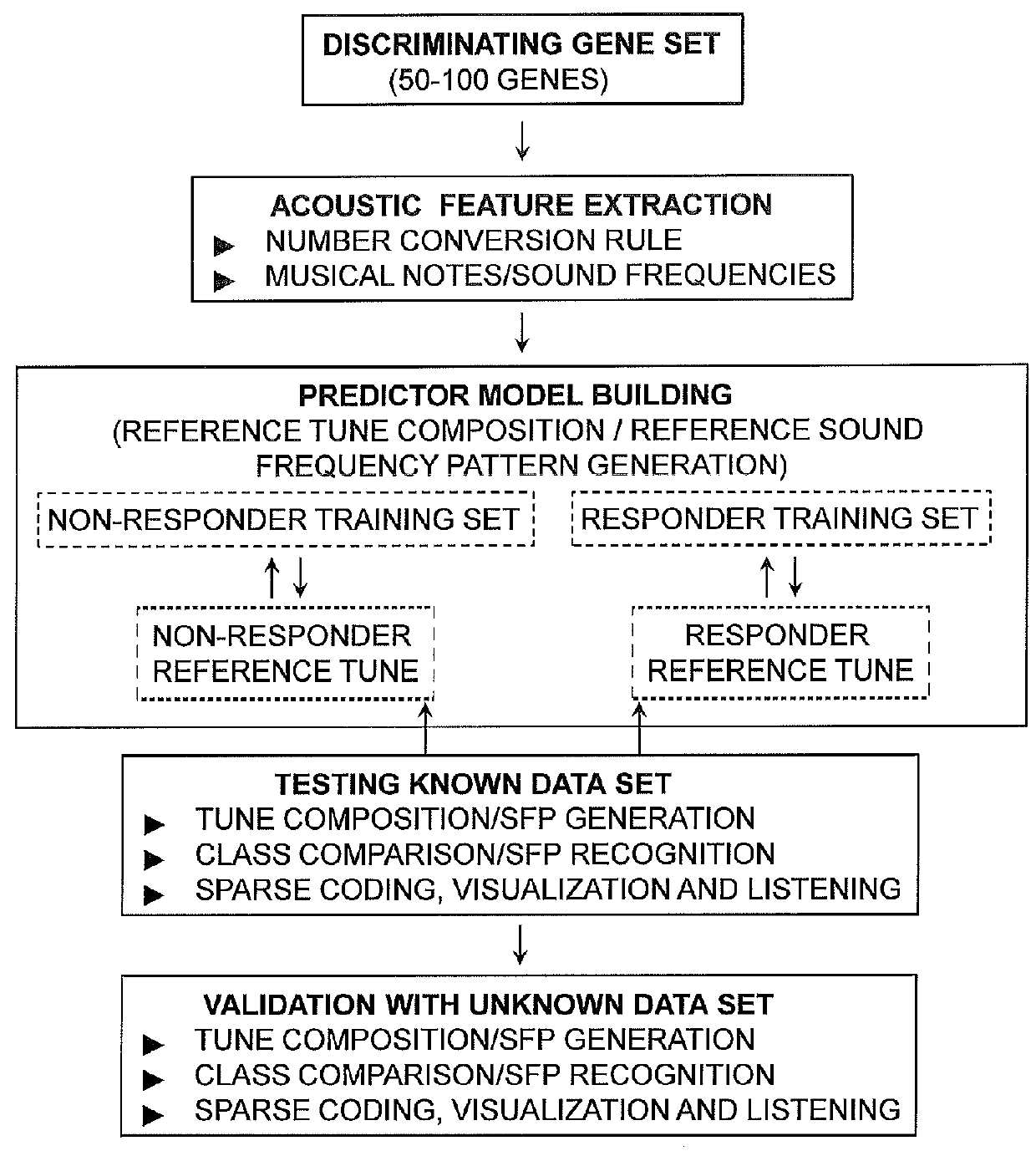
A mathematical musical orchestral method for predicting classes of patients for medical treatment
ActiveUS20160055309A1Improve forecast accuracySmall sample sizeData processing applicationsHealth-index calculationNon respondersMedical treatment
Owner:RUTGERS THE STATE UNIV
Metabolites for oral health and uses thereof
The present invention provides various methods of using metabolite profiles correlated with periodontal disease or a health oral status for the diagnosis of periodontal disease, identification of responders and, or non-responders to therapeutic agents for periodontal disease, a method to test the efficacy of test compounds to prevent periodontal disease. The present invention also provides for a dentifrice composition containing an effective amount of a metabolite therapeutic agent which brings about a greater change in metabolite levels compared to a control dentifrice composition.
Owner:COLGATE PALMOLIVE CO
Assay for screening antidepressants
InactiveUS20110296540A1Reduce expressionIncrease heightMicrobiological testing/measurementBiological testingNon respondersArtificial cerebrospinal fluid
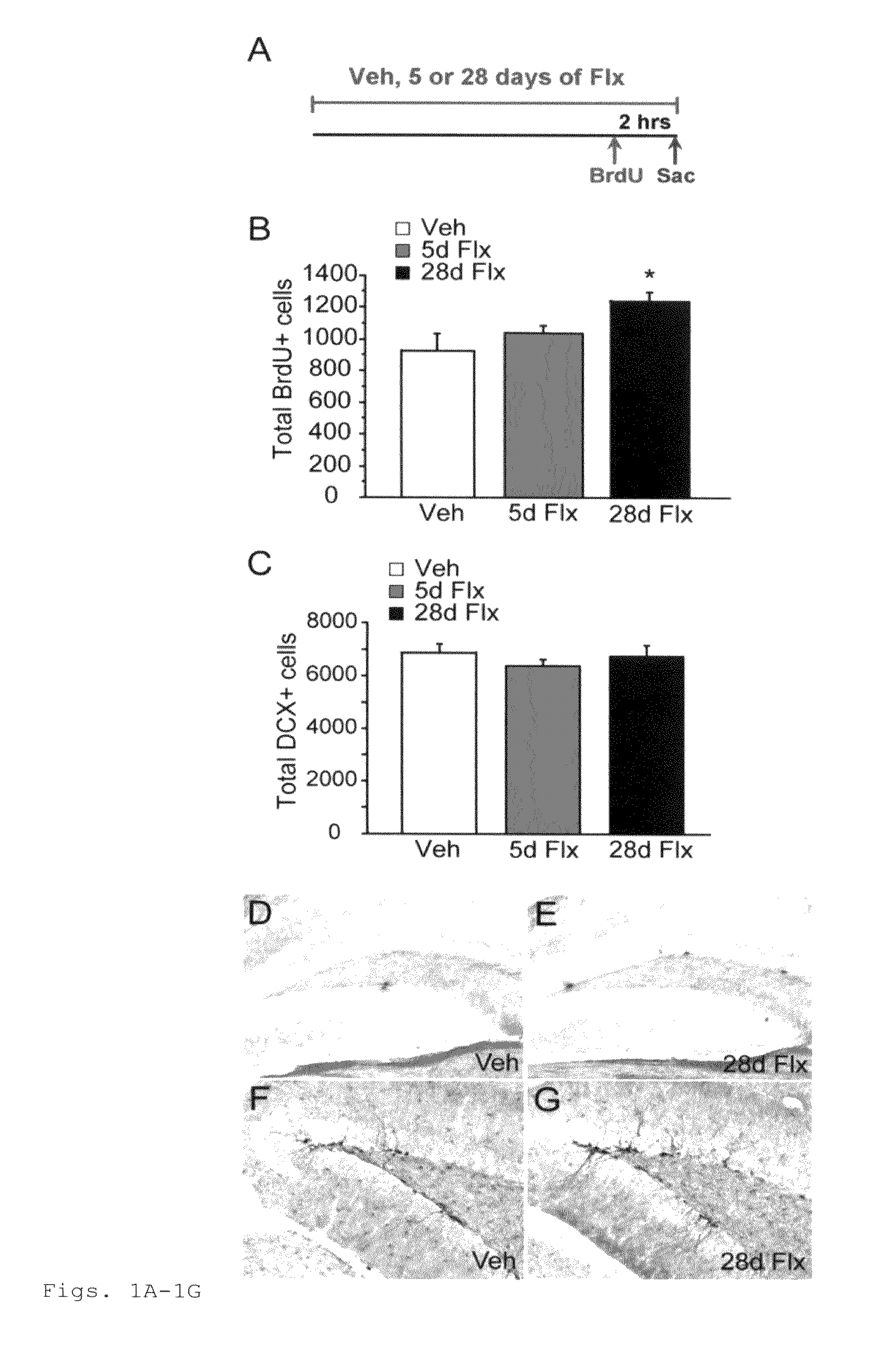
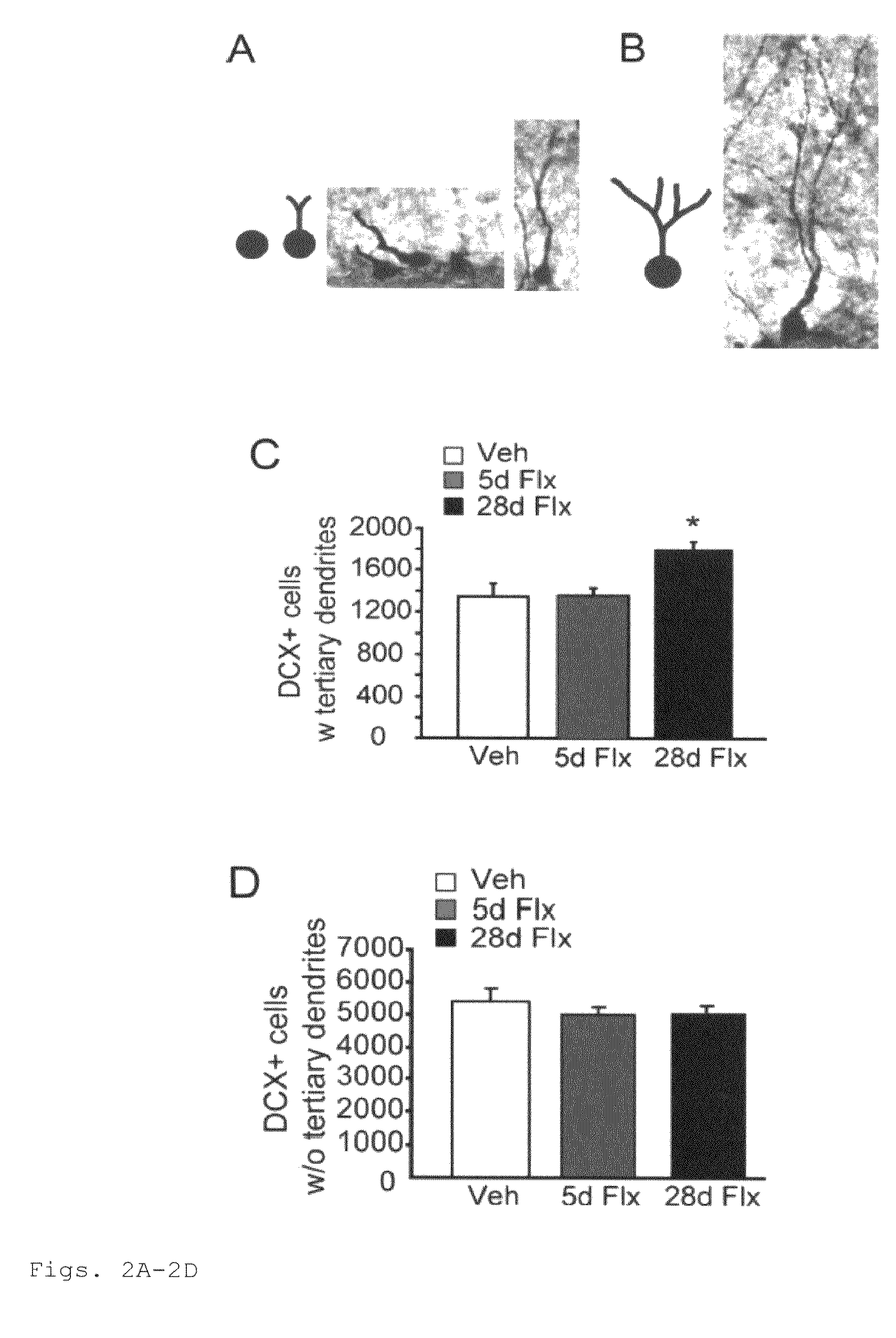
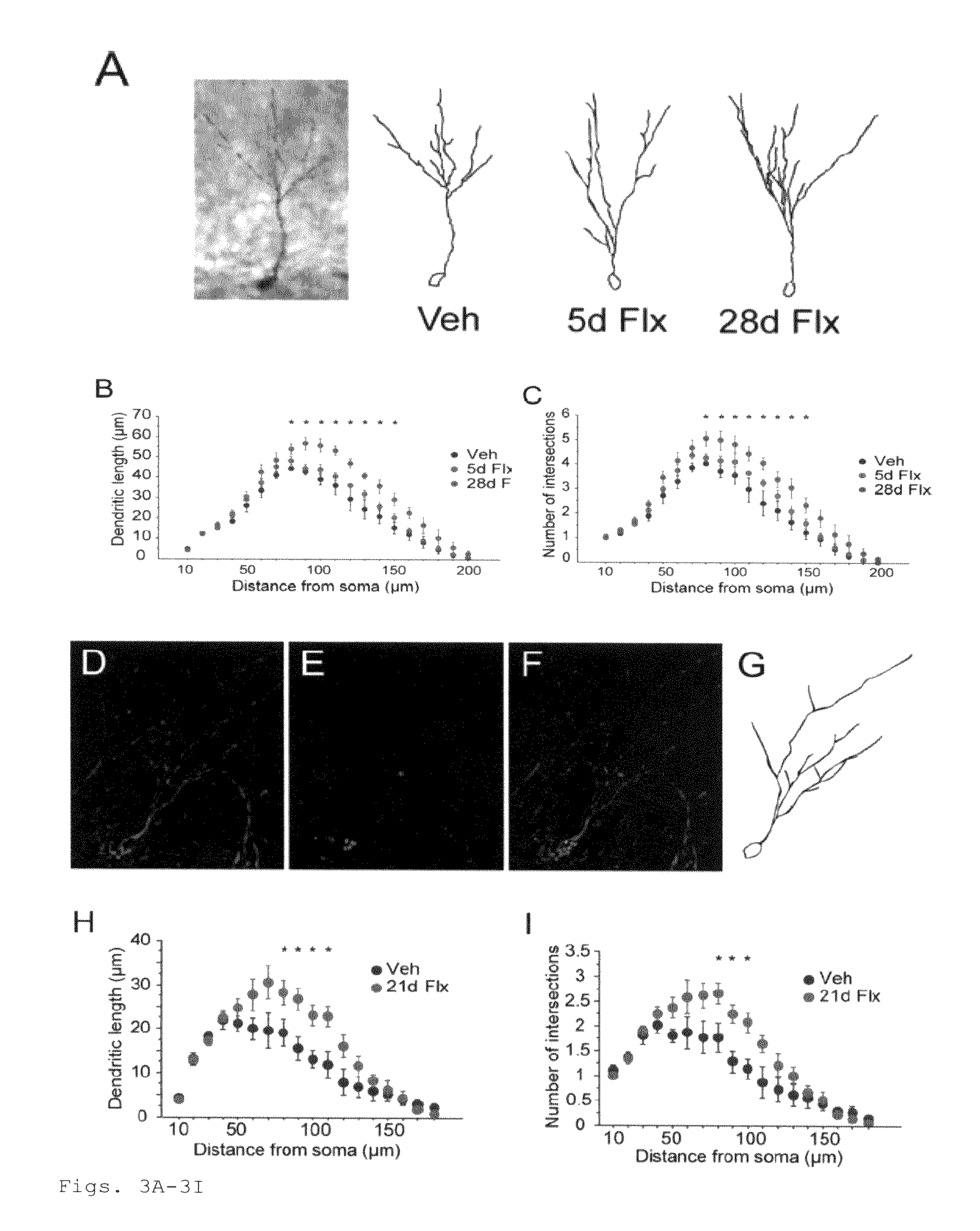
This invention provides a method for identifying a small molecule as an antidepressant, a method for identifying a small molecule as an anxiolytic, and a method for identifying a small molecule as able to increase dendritic arborization, decrease expression of an immaturity marker, increase expression of a maturity marker, or enhance artificial cerebrospinal fluid-type long-term potentiation in central nervous system. This invention also provides a transgenic mouse model for SSRI-non-responders.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Gene and protein expression profiles associated with the therapeutic efficacy of irinotecan
InactiveUS20090047684A1Microbiological testing/measurementDepsipeptidesIrinotecanProtein expression profile
The present invention includes gene and protein expression profiles indicative of whether a cancer patient is likely to respond to treatment with irinotecan. By identifying such responsiveness, a treatment provider may determine in advance those patients who would benefit from such treatment, as well as identify alternative therapies for non-responders. The present invention further provide methods of using the gene and / or protein expression profiles and assays for identifying the presence of a gene and / or protein expression profile in a patient sample.
Owner:NMDX LLC
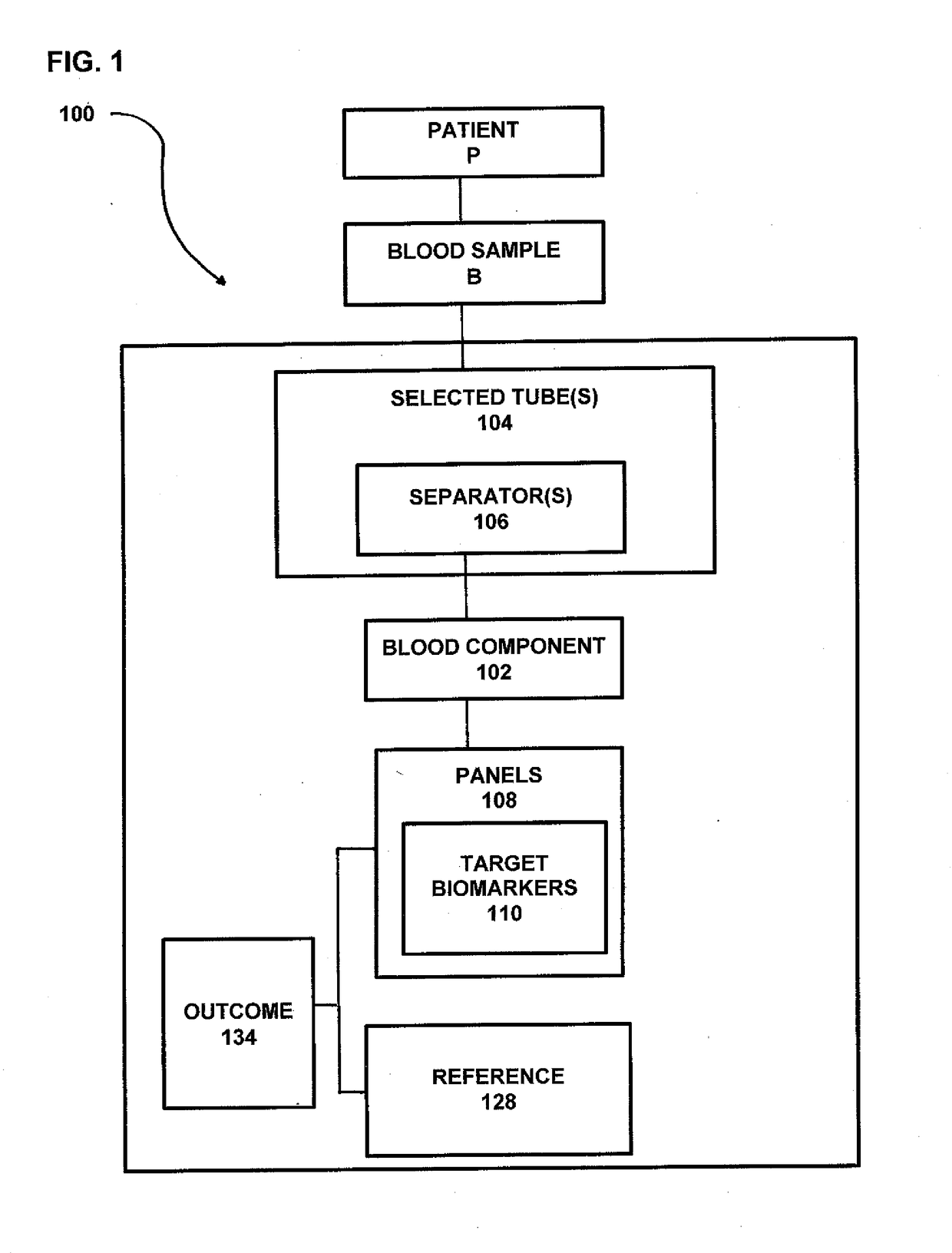
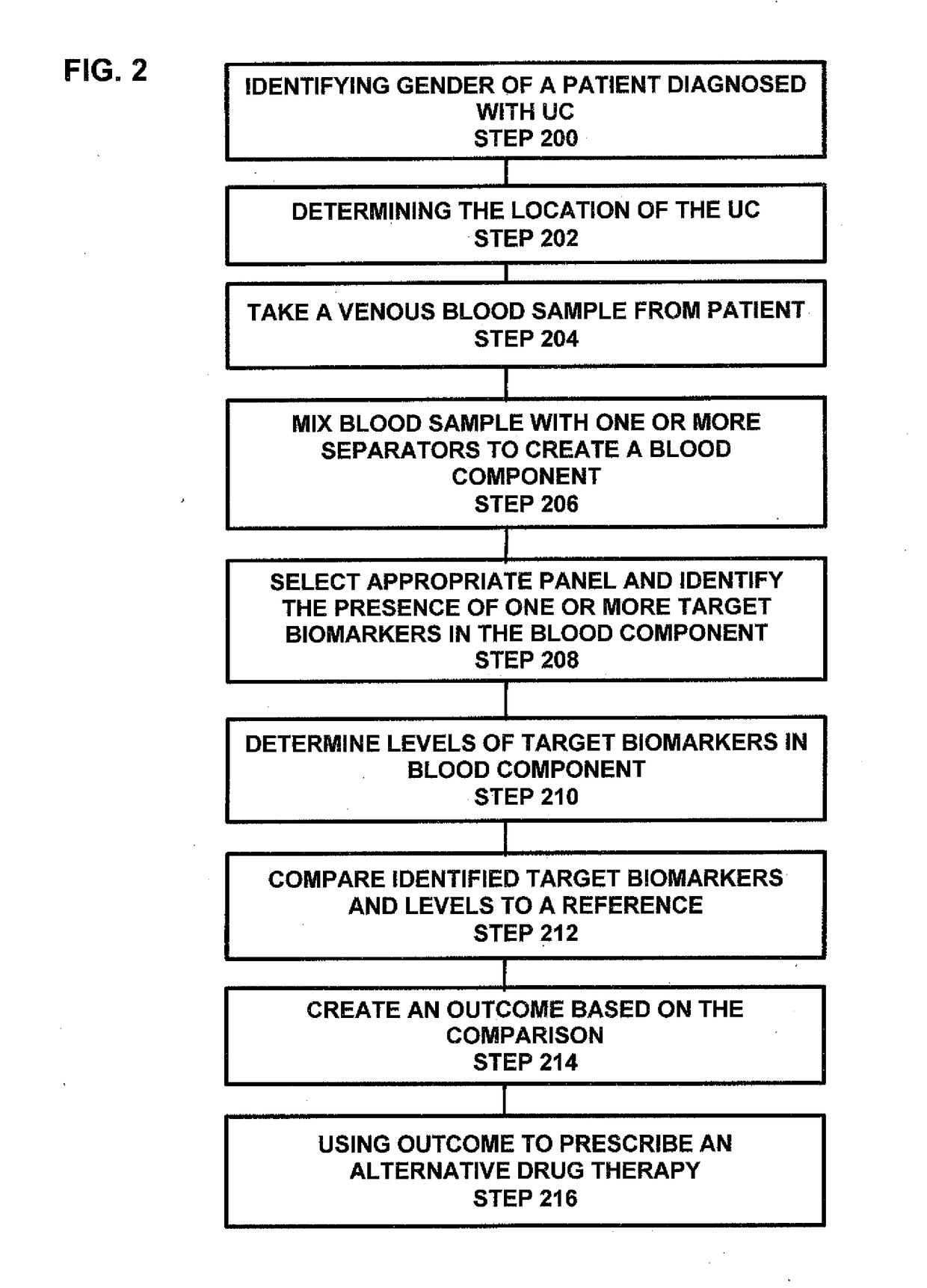
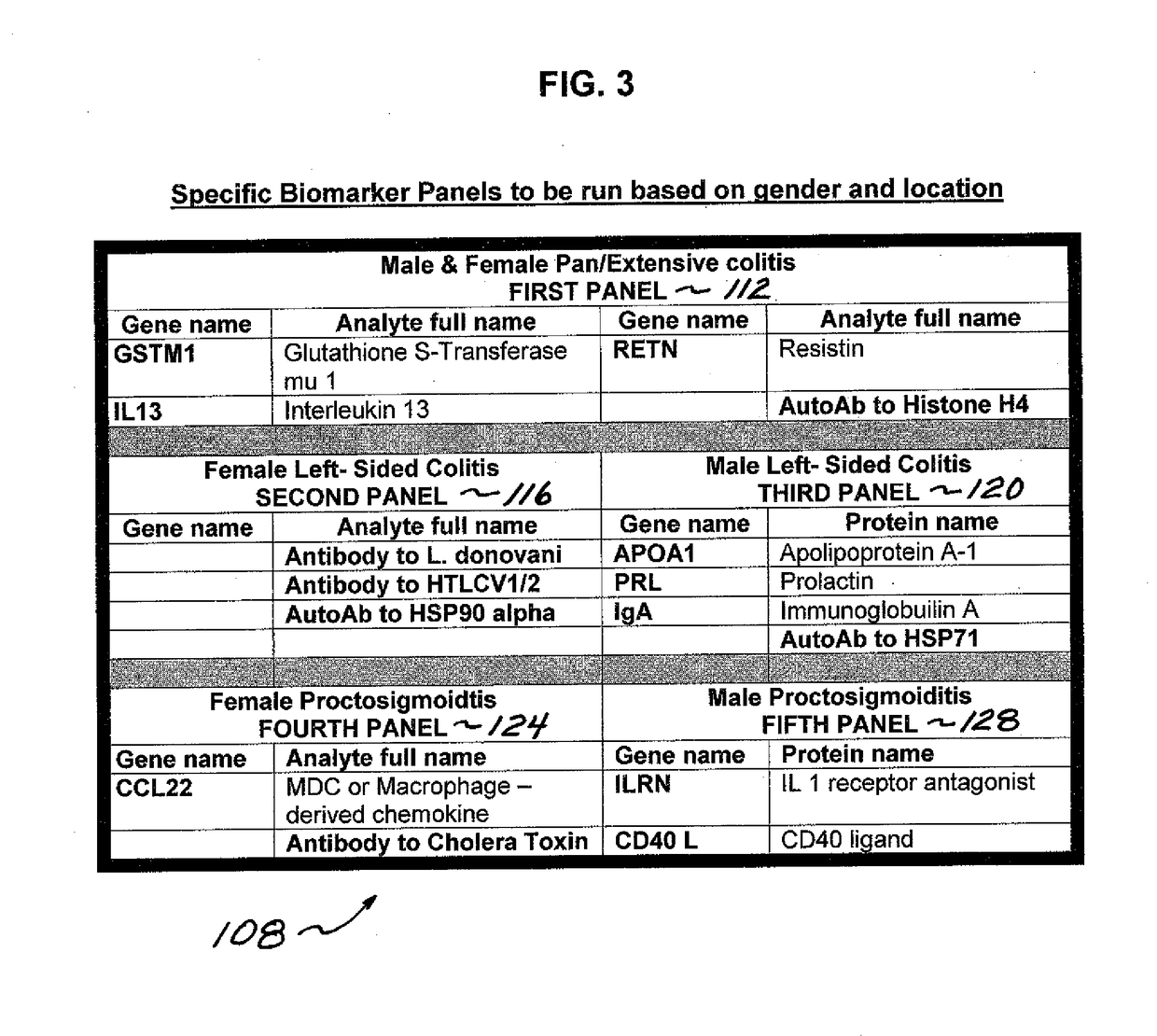
Process and system for predicting responders and non-responders to mesalamine treatment of ulcerative colitis
ActiveUS20170261492A1Good effectFaster and effective disease controlOrganic active ingredientsDisease diagnosisMedicineNon responders
A process and system directed to a more effective, individual based treatment regimen which is built on clinical identified target biomarkers associated with gender differential responses to mesalamine, and includes one or more panels of target biomarkers that distinguishes mesalamine response differences between genders and determines the efficacy of mesalamine for patients being treated for various UC conditions and effectively identifies and validates novel drug targets for new UC therapeutics, new diagnostics and diagnostics standards for UC therapeutic strategies.
Owner:MUSIDORA BIOTECH LLC
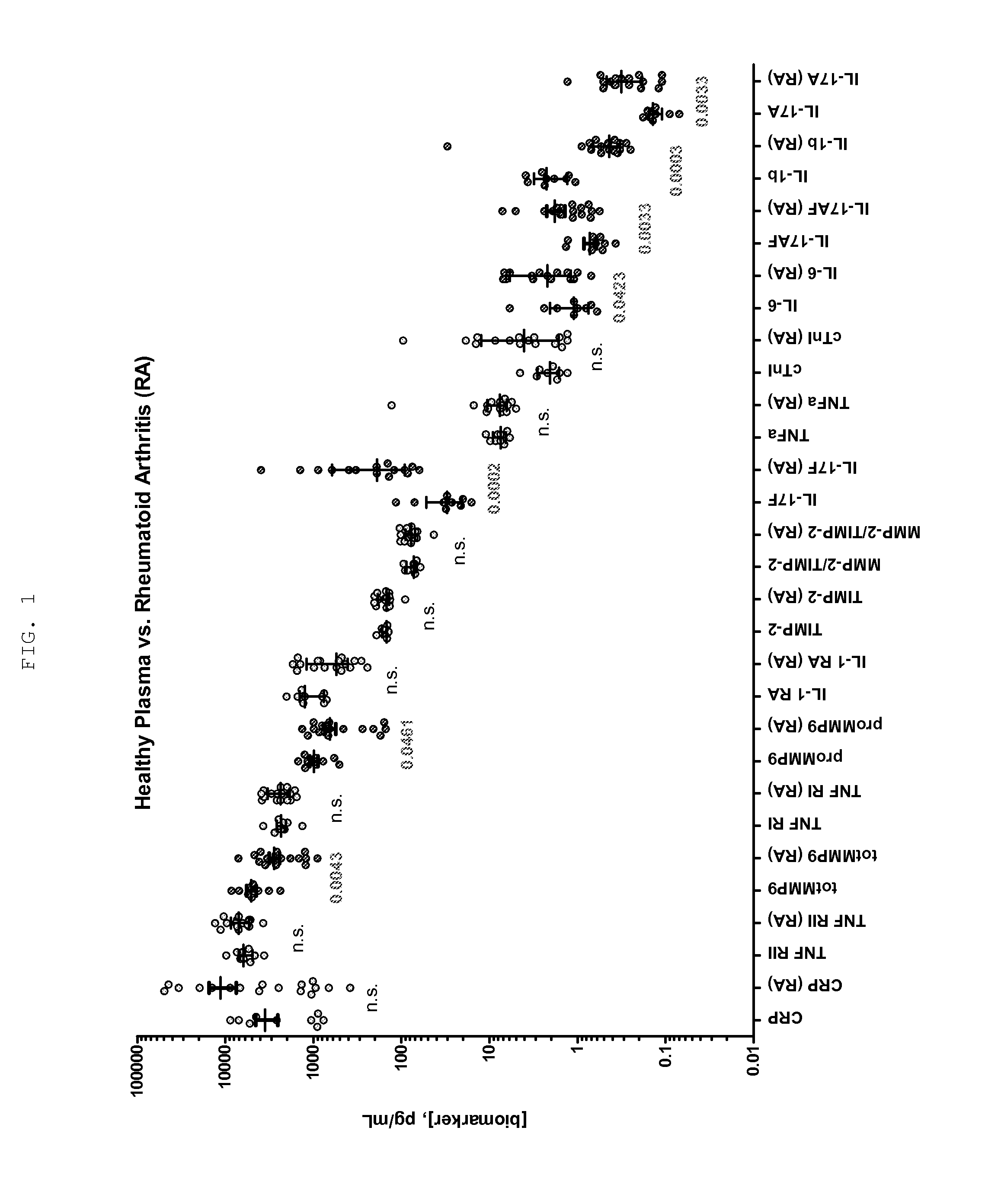
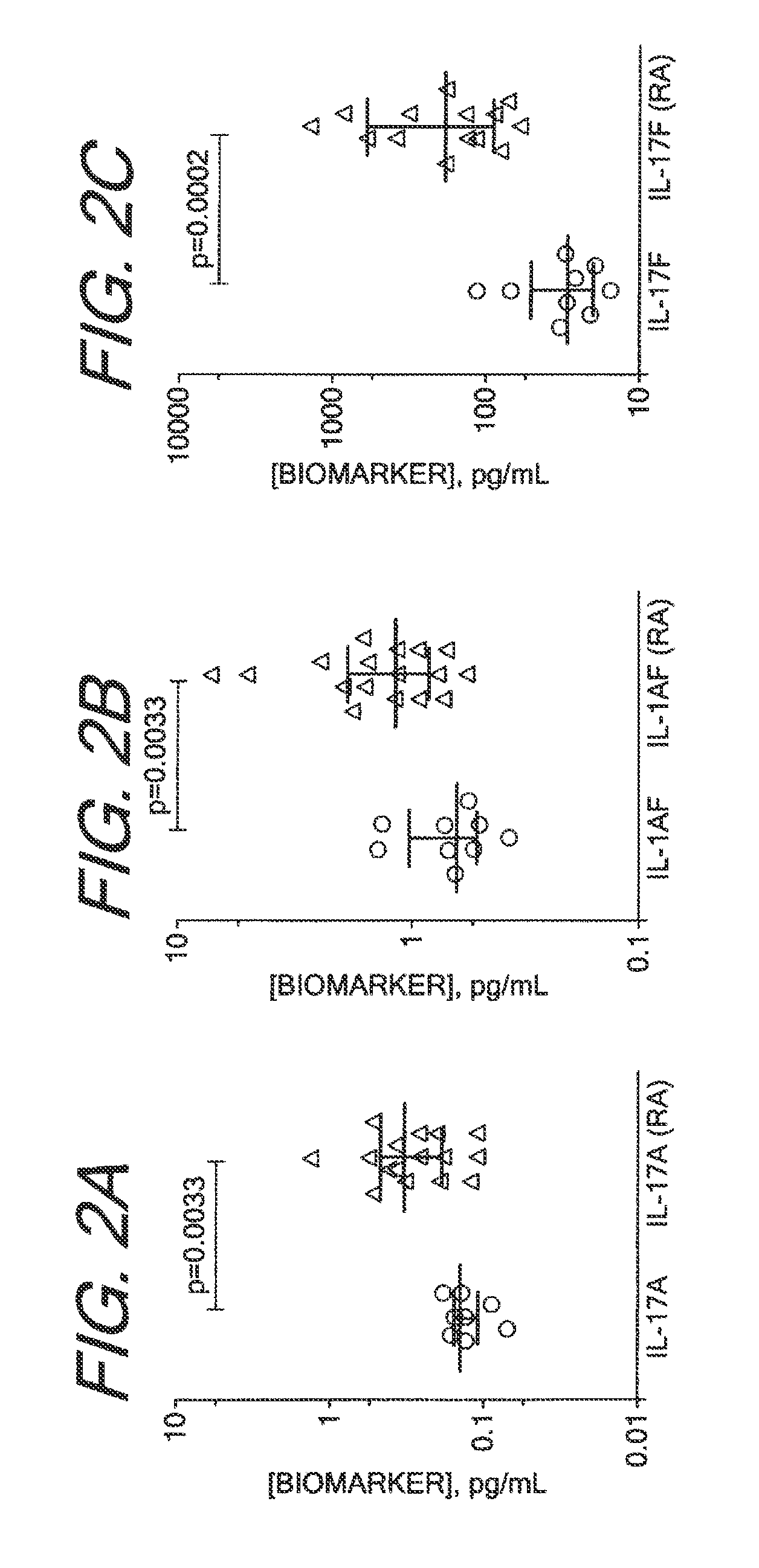
Methods for Diagnosing, Staging, Predicting Risk for Developing and Identifying Treatment Responders for Rheumatoid Arthritis
Disclosed are methods for diagnosing, staging, and predicting risk for developing rheumatoid arthritis and other inflammatory diseases, and methods for identifying treatment responders and non-responders.
Owner:SINGULEX
Composition for detecting the response of rectal adenocarcinomas to radiochemotherapy
A cDNA array (9984 genes) was used for expression profiling in rectal adenocarcinoma. The expression data were correlated to responsiveness to chemotherapy followed by radiotherapy. A set of 54 genes was found that were differentially expressed in responders vs. non-responders. The genes may be used as prognostic markers for determining whether a rectal adenocarcinoma is responsive to radiochemotherapy.
Owner:UNITED STATES OF AMERICA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com