Gene and protein expression profiles associated with the therapeutic efficacy of irinotecan
a technology which is applied in the field of gene and protein expression profiles associated with the therapeutic efficacy of irinotecan, can solve the problems that irinotecan often experience recurrence of disease or disease-related death
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
examples
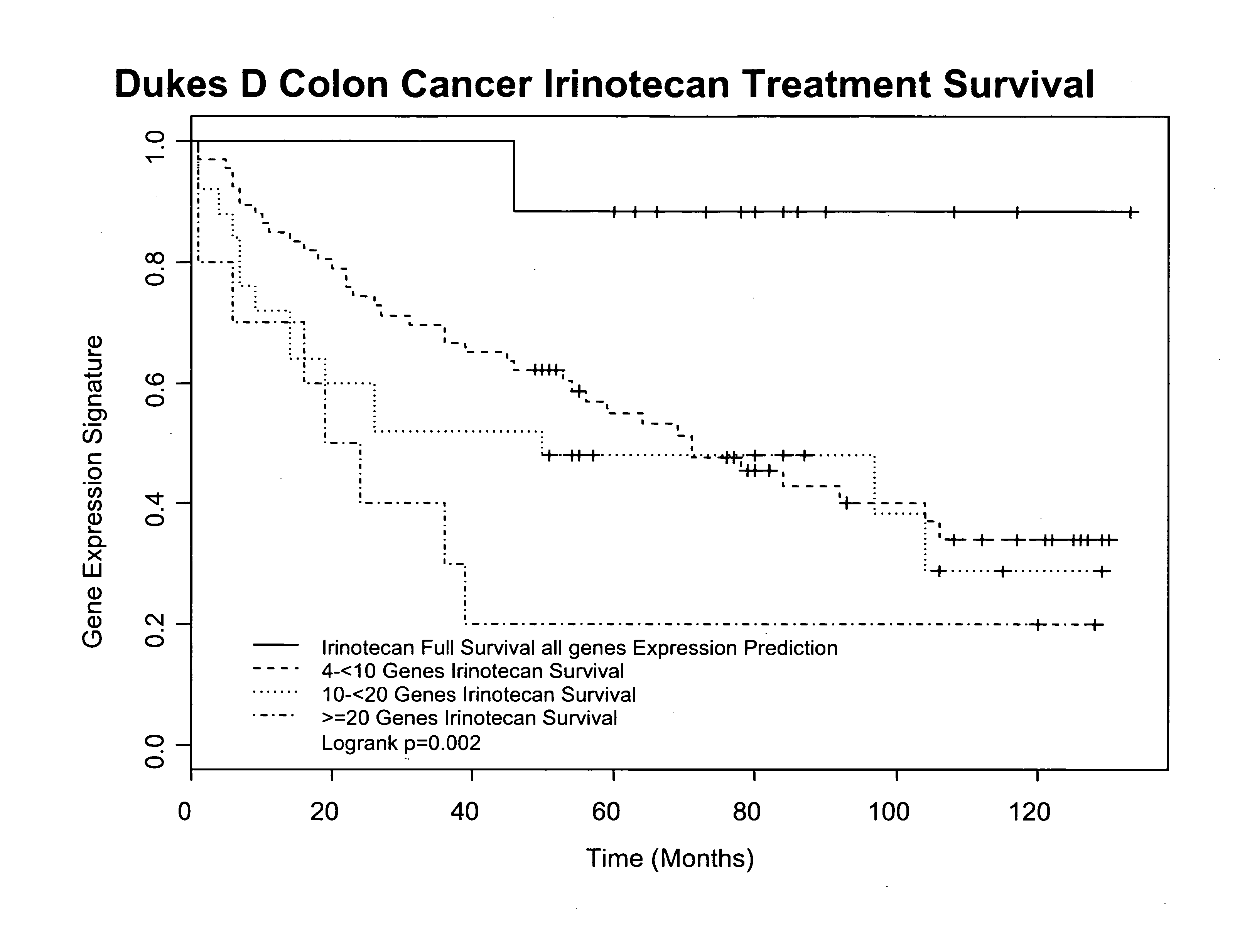
[0080] A series of prognostic factors were tested in order to validate the efficacy of the gene / protein expression profile (GPEP) of the present invention for predicting the therapeutic response of irinotecan therapy. The expression levels of these factors, consisting of the twenty-two (22) proteins in the present GPEP listed in Table 2 (which includes seventeen differentially expressed proteins and five reference proteins), was determined by an immunohistochemical methodology in biopsy tissue samples obtained from late-stage colon cancer patients whose treatment with irinotecan had been successful, as well as samples from patients whose treatment was unsuccessful, e.g., who had experienced late recurrence (LRec) or disease-related death (DRD) associated with the therapy. For purposes of selecting the patients for the study, irinotecan therapy was determined to have failed if a recurrence was present within three years of diagnosis.
[0081] According to the current prescribing inform...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com