Gene expression signatures predictive of subject response to a multi-kinase inhibitor and methods of using the same
A multi-kinase inhibitor and subject technology, applied in biological testing, biochemical equipment and methods, microbial measurement/testing, etc., can solve problems such as instabilities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
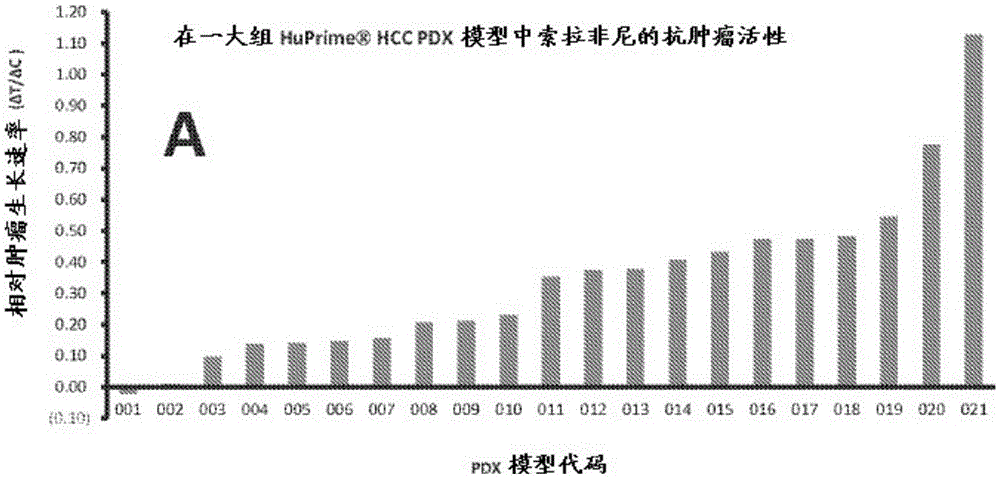
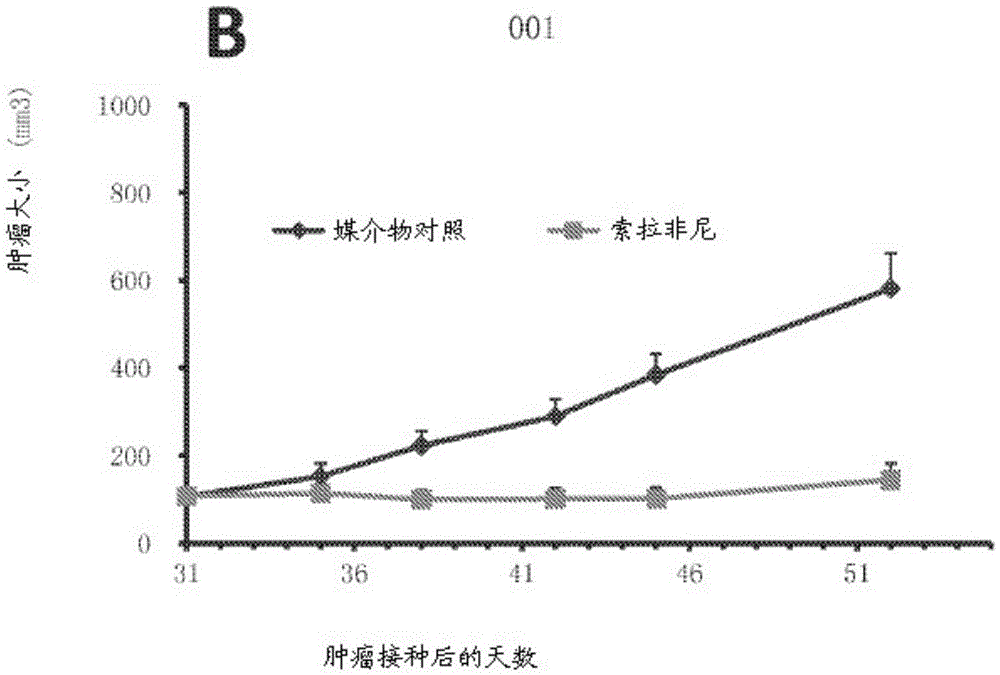
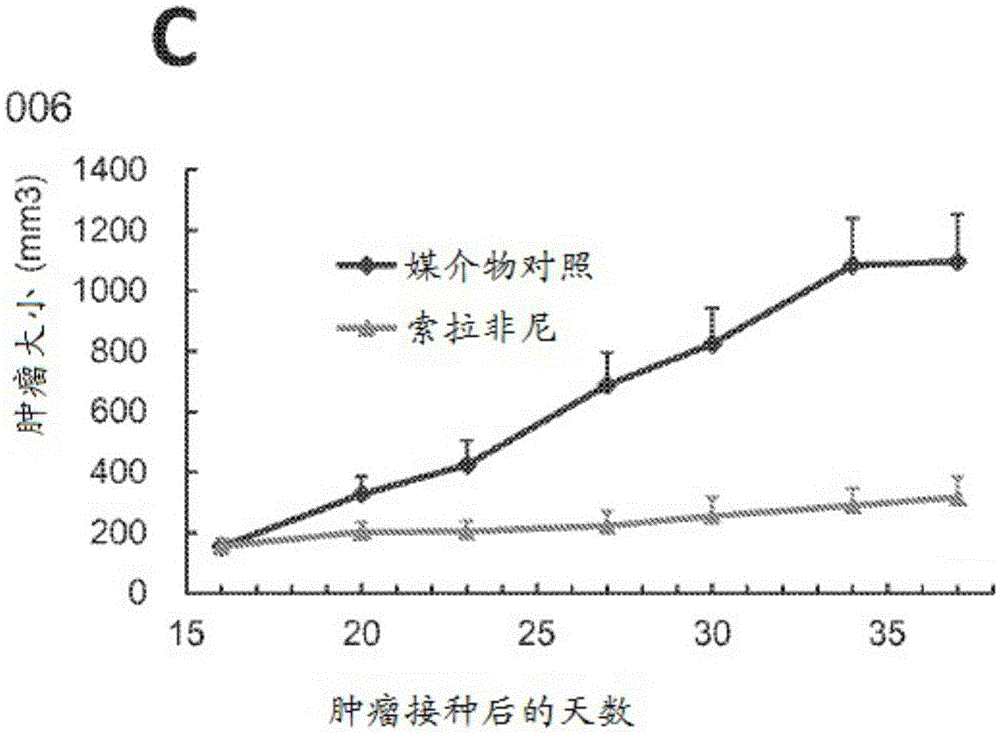
[0113] Example 1. Bulk HCC Models were sensitive or partially sensitive to sorafenib.
[0114] Experimental models that capture the oncogenic mechanisms of human HCC are critical for evaluating HCC therapy and discovering biomarkers that predict patient response. Patient-derived xenograft (PDX) models reflect the patient's histopathology and genetic profile (2-7). Currently, a large group of HCC-PDX models (named or patient avatar). In a clinical trial-like study, sorafenib was tested on a randomized cohort of HCC-HuPrime. The study identified "responders and non-responders." Next, the expression of these models was analyzed using microarray GeneChip technology. By applying statistical analysis, specific molecular markers, or The gene expression signature, consisting of only a few genes, could predict the response of HCC patients to sorafenib and was also suitable for developing companion diagnostics for patient staging in the clinic. This signature can be used a...
Embodiment 2
[0117] Example 2. HCC-PDX can be divided into three main classes based on global gene expression profiling do not
[0118] HCC is a disease with different types. Whole-gene expression profiling of patient tumor samples has revealed that HCC can be divided into three major subtypes. Recently, transcriptome analysis divided HCC into three major categories using different clinical parameters as well as cell differentiation 1 . These categories are S1 (stem-like group, presence of WNT pathway and activation of TGF-β), S2 (activation of MYC and AKT) and S3 (hepatocyte differentiation). HCC-PDX is believed to be a predictive experimental model due to the maintenance of the original patient histopathology and genetic profile 2,3 . This study attempts to test this hypothesis by illustrating that HCC avatars have similar genomic profiles to patient tumors in terms of classification and biological properties.
[0119] First, global gene expression profiling was performed for al...
Embodiment 3
[0125] Example 3. Identification of HCC gene expression signatures predictive of response to sorafenib.
[0126]Using a statistical approach based on linear regression, global gene expression levels and the effect of Sorafenib treatment on HCC-PDX as measured by %ΔT / ΔC as described above were used to identify predictive biomarkers (i.e., gene signatures). ). Based on pre-set statistical criteria, including p-value 4-fold for all tested HCC-PDXs, and no outliers in linear regression analysis, it can be concluded that the composition of 8 genes exhibited good predictive properties signs (eg, Figure 1 and Table 3). It is worth noting that markers with more genes will yield better associations and smaller p-values, while having less practical value in clinical applications. The stringency of the preset criteria will determine the number of marker genes.
[0127] Although these marker genes were only identified using statistical analysis without any filtering before and after ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com