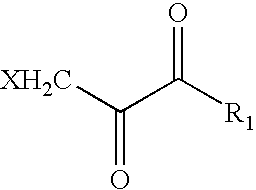
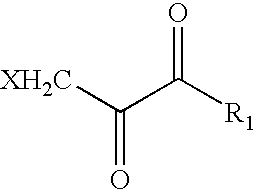
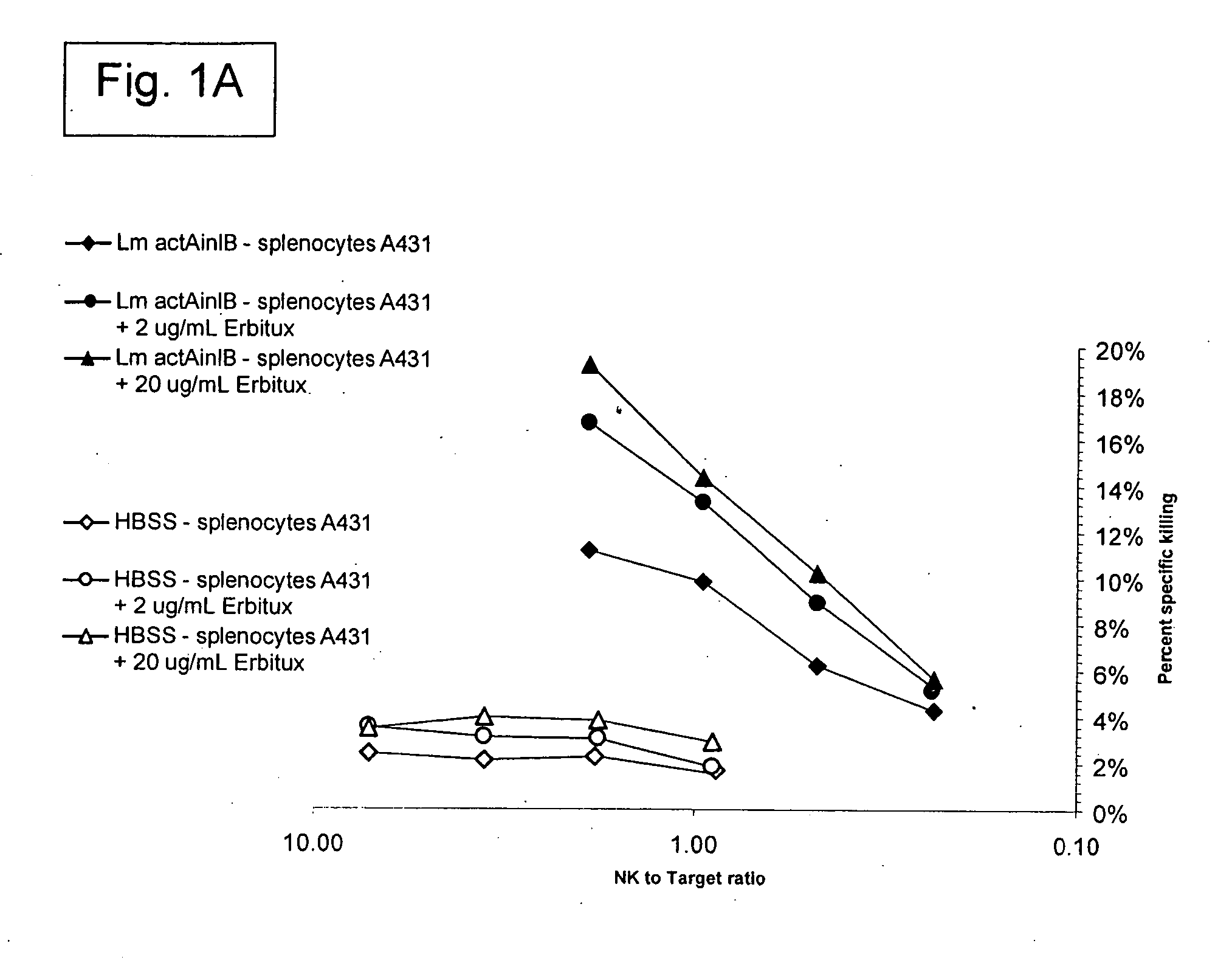
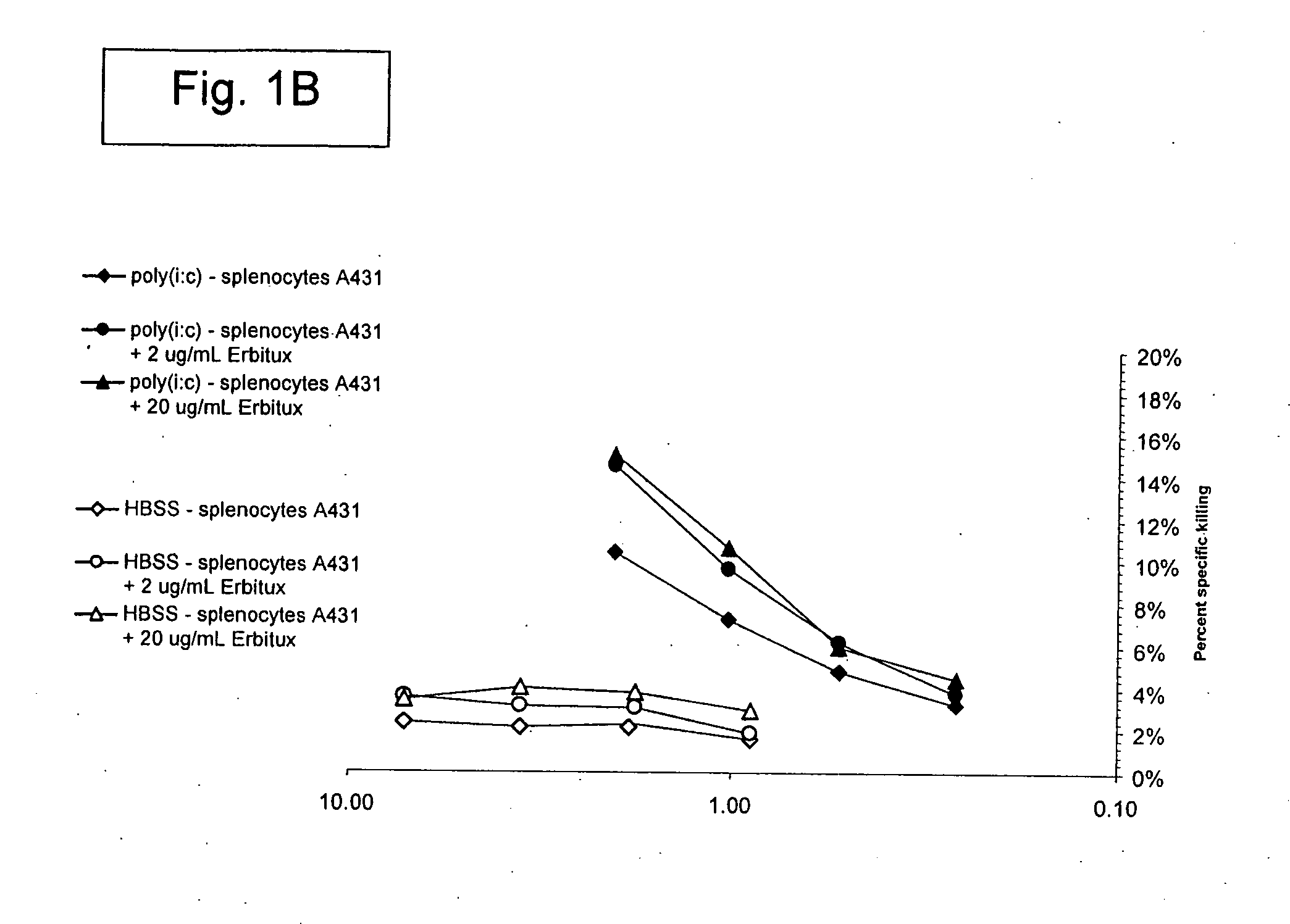
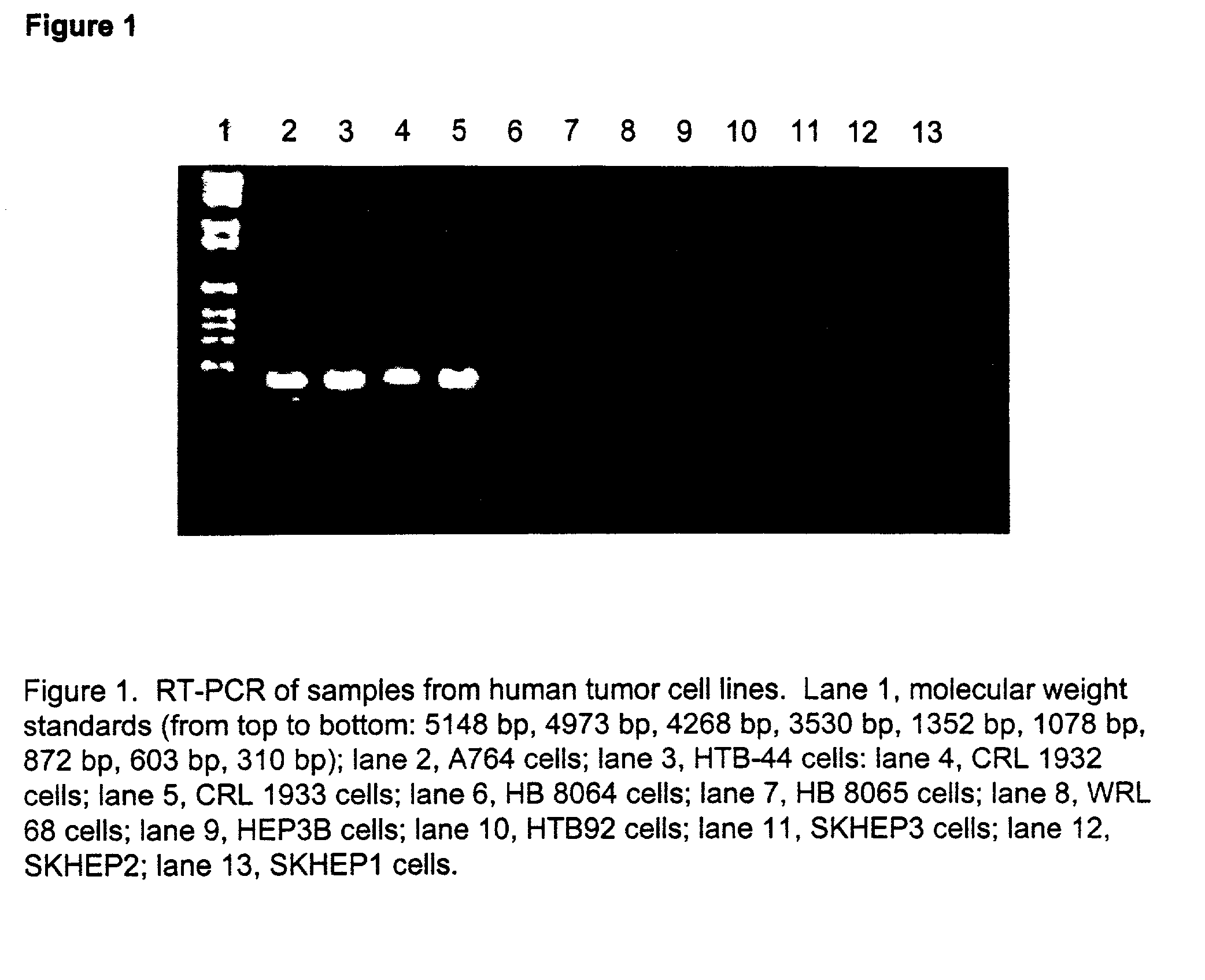
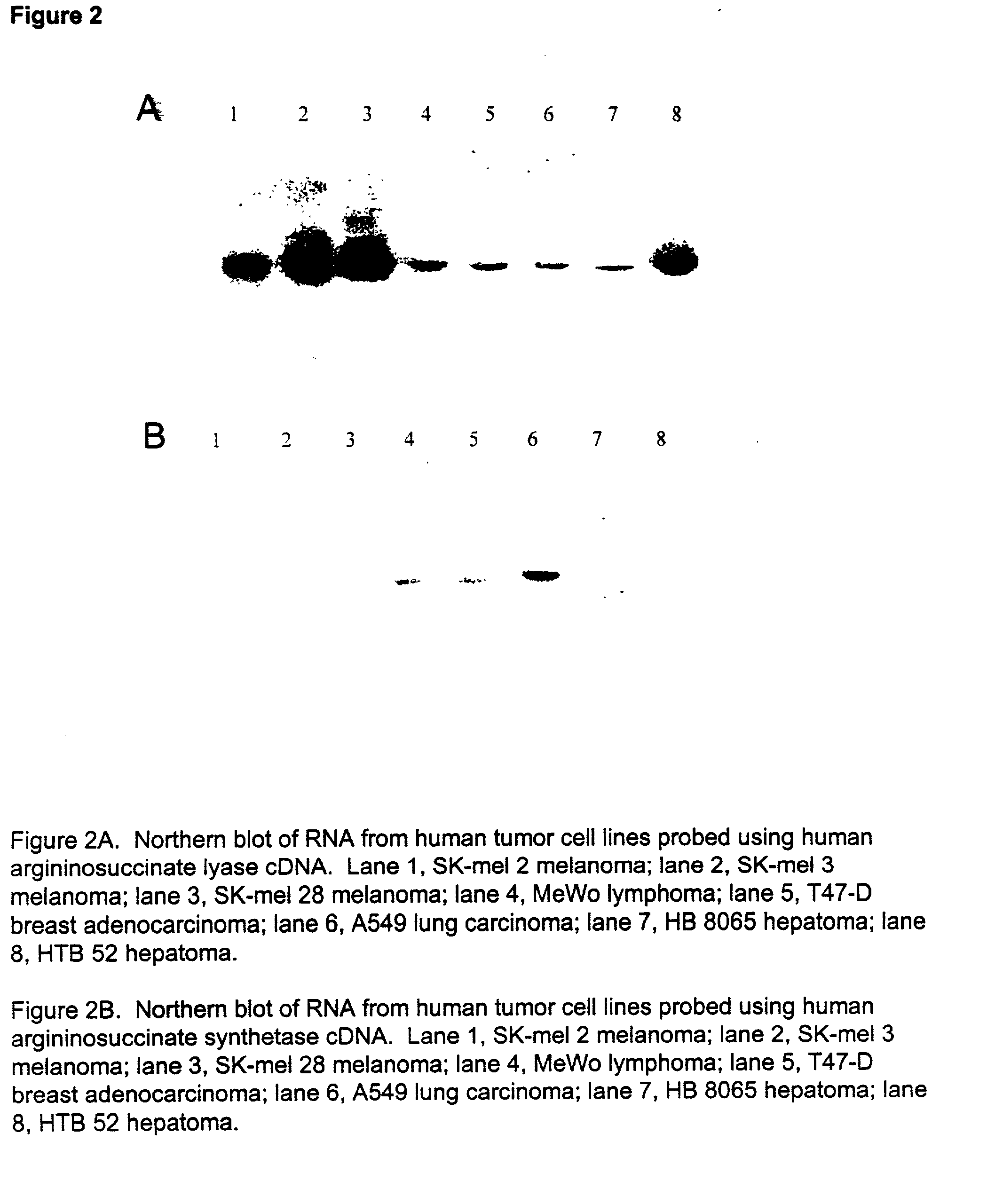
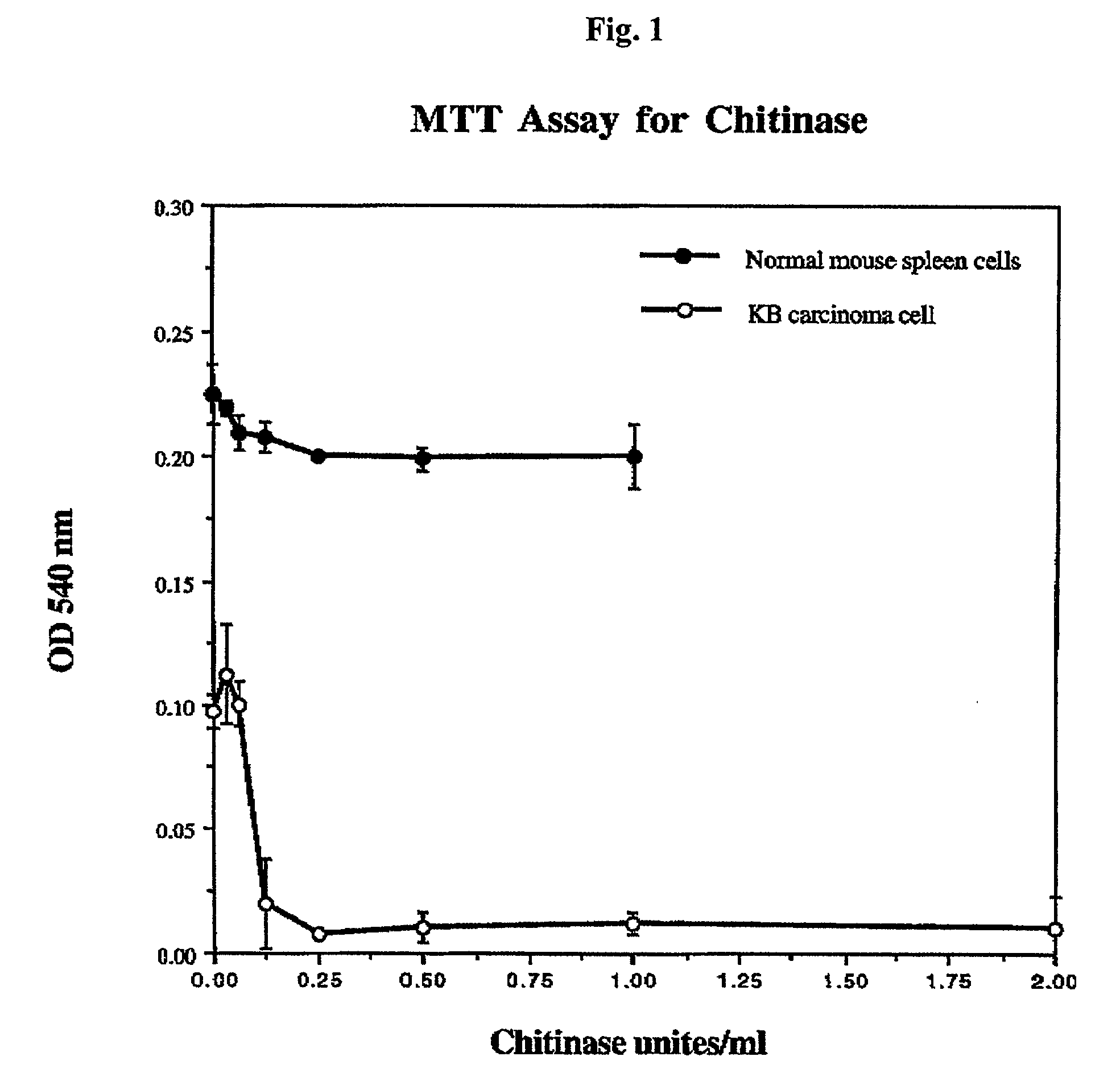
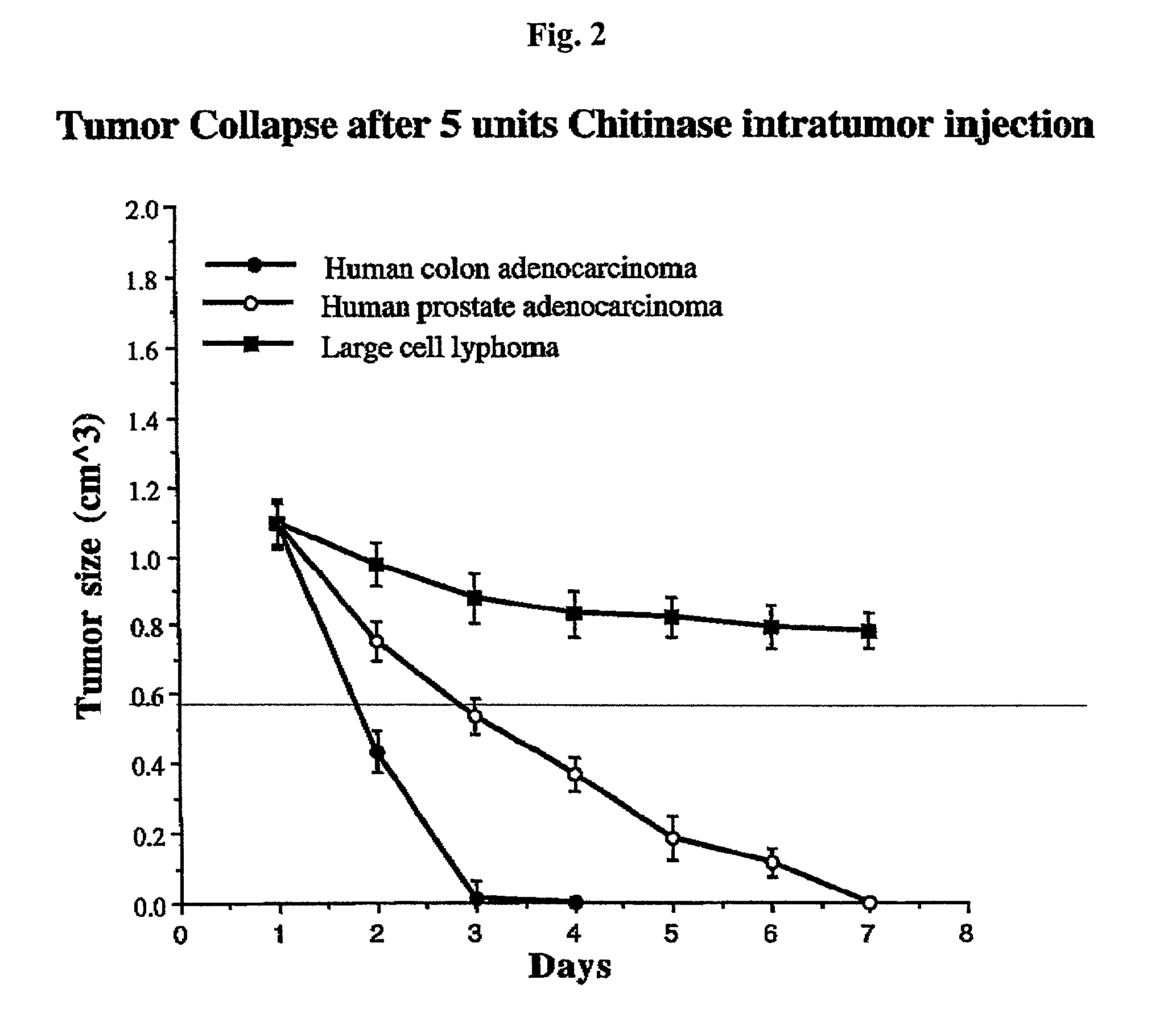
Patents
Literature
229 results about "Carcinomatoses" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Apparatus and method for treatment of malignant tumors
InactiveUS6866624B2Increase powerIncrease temperatureElectrotherapyMicrowave therapyAbnormal tissue growthBrachytherapy
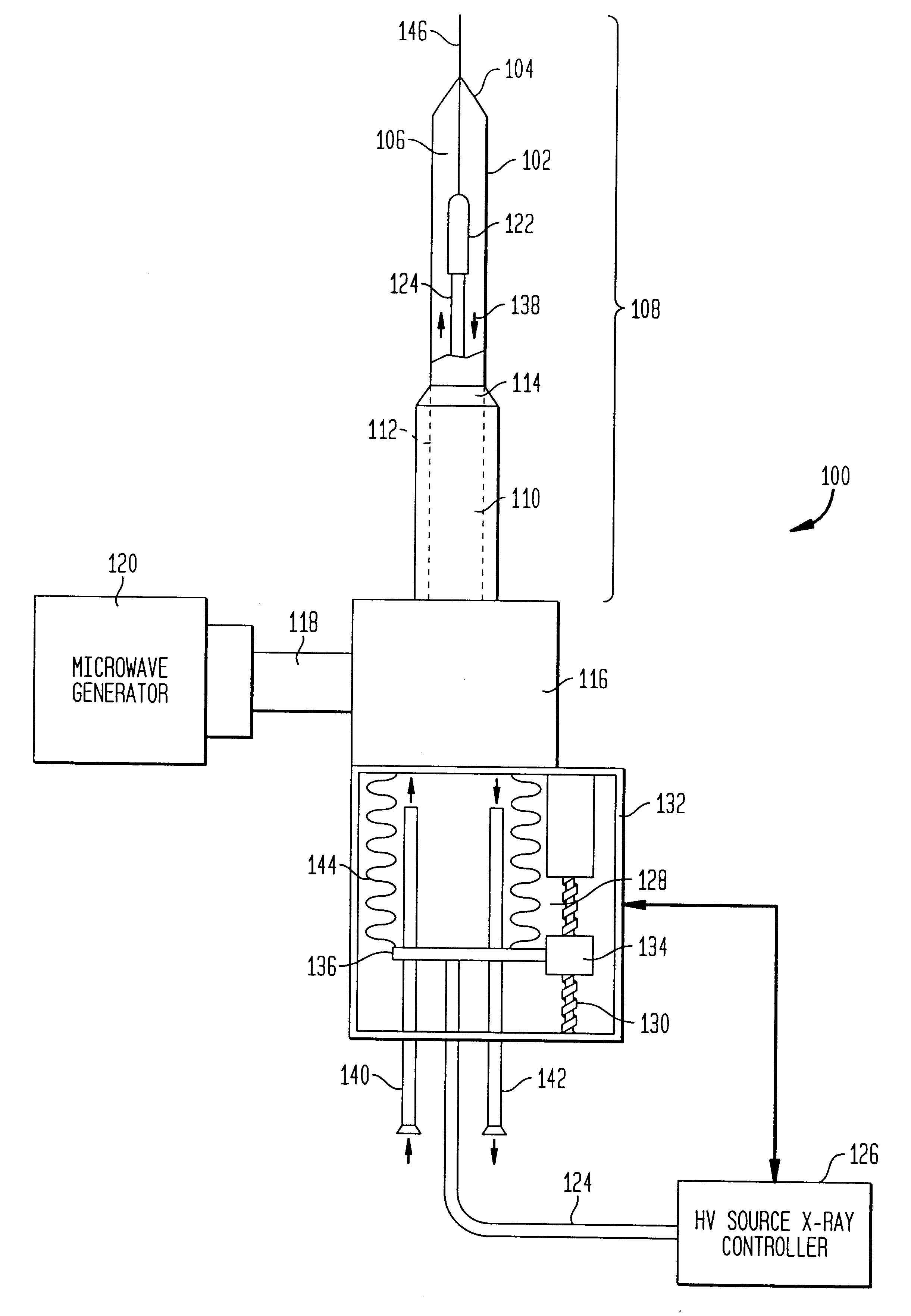
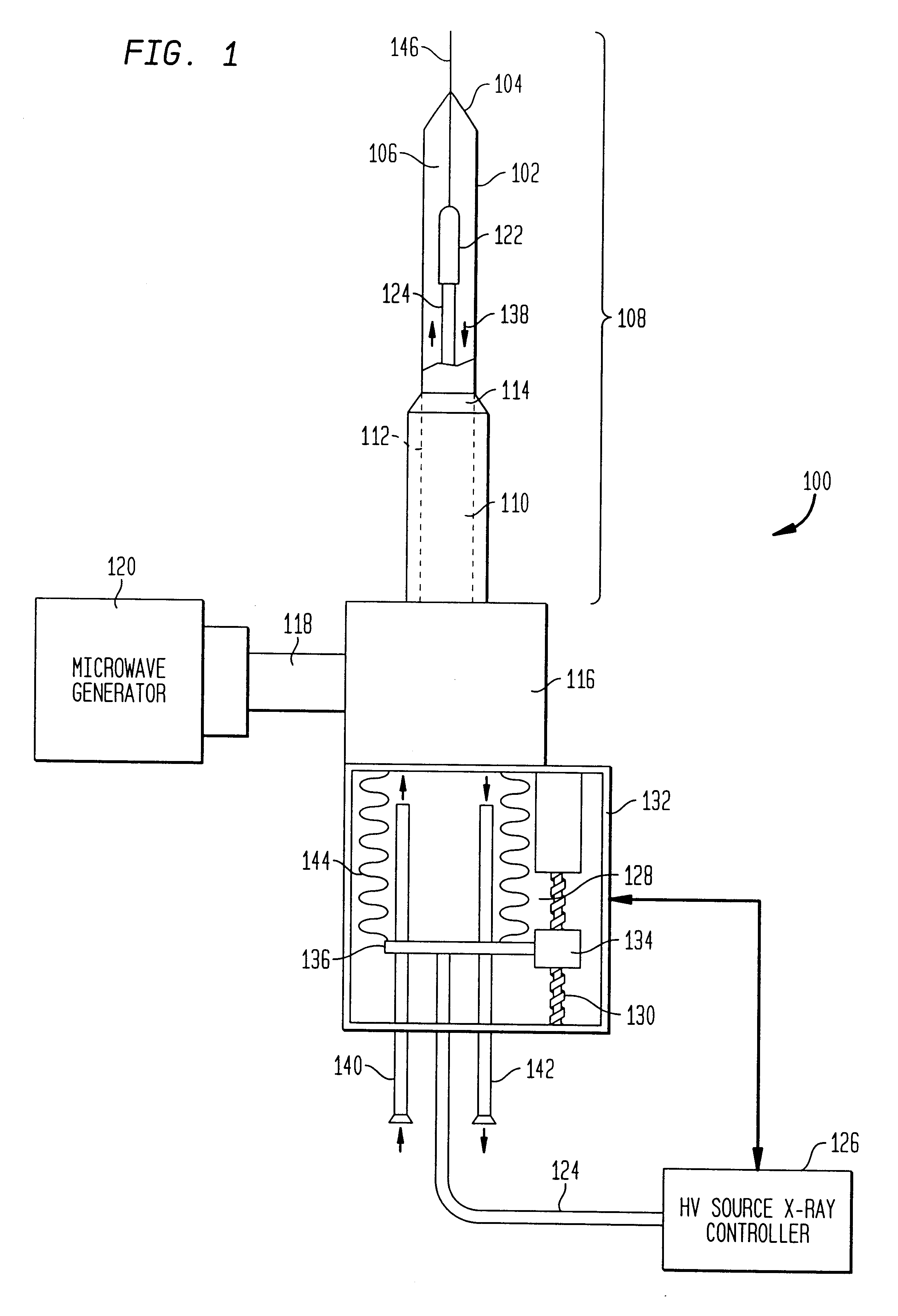
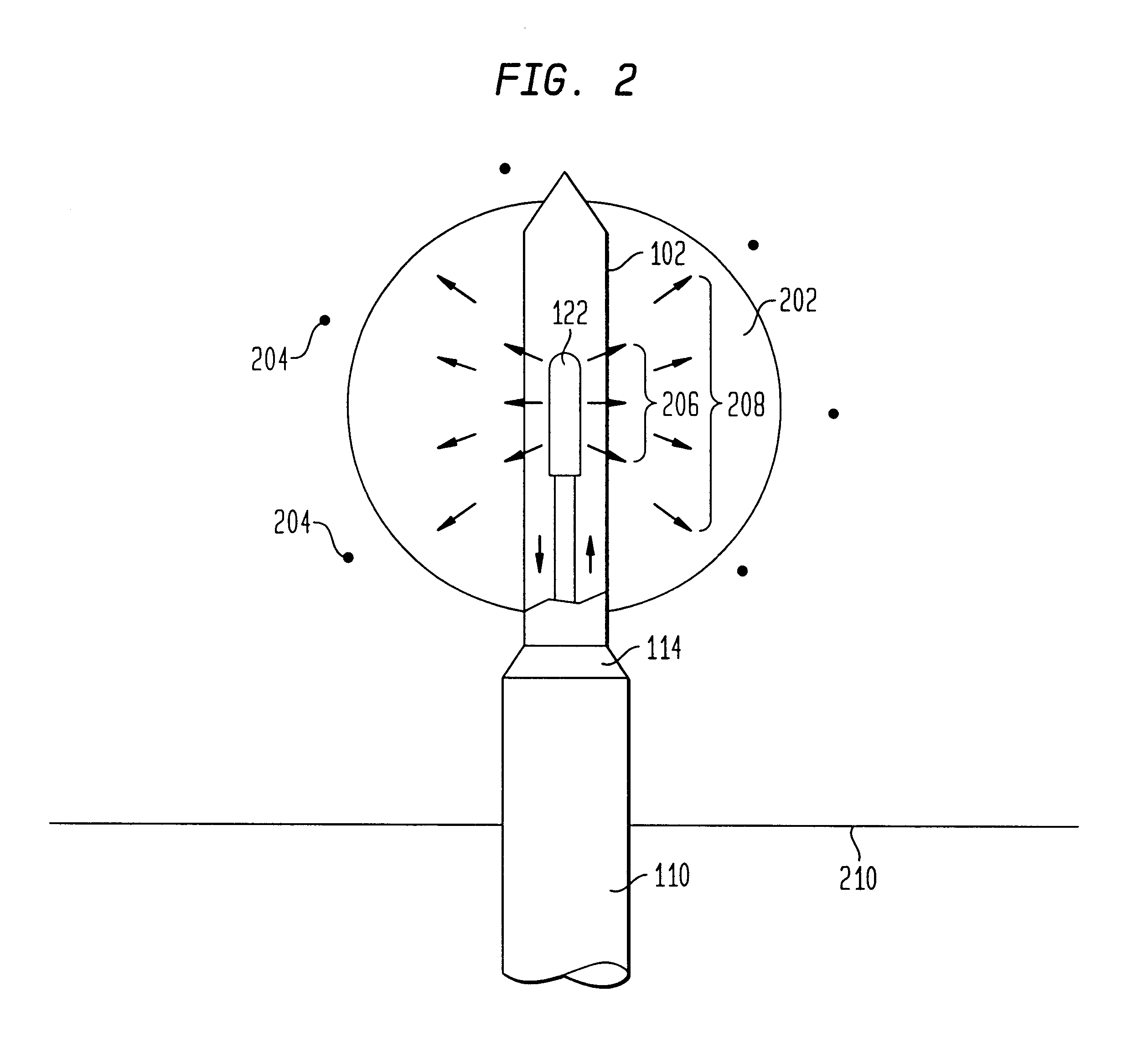
The present invention relates to a device for simultaneously treating a tumor or cancerous growth with both hyperthermia and X-ray radiation using brachytherapy. The device includes a needle-like introducer serving as a microwave antenna. Microwaves are emitted from the introducer to increase the temperature of cancerous body tissue. The introducer is an inner conductor of a coaxial cable. The introducer contains a hollow core which houses an X-ray emitter. The X-ray emitter is connected to a high voltage miniature cable which extends from the X-ray emitter to a high voltage power source. The X-ray emitter emits ionizing radiation to irradiate cancerous tissue. A cooling system is included to control the temperature of the introducer. Temperature sensors placed around the periphery of the tumor monitor the temperature of the treated tissue.
Owner:MEDTRONIC AVE
Medical stent provided with inhibitors of atp synthesis
A stent provided with a composition having at least one type of inhibitor of ATP synthesis, optionally together with at least one inhibitor of the pentose phosphate pathway is disclosed. The medical stent is useful for treating stenosis and preventing restenosis in vascular ducts and for treating cancerous tumors present in ducts, resectioned cavities and scars and any disorder arising from the proliferation of cells in ducts or cavities.
Owner:INTERSTITIAL THERAPEUTICS
Antibody-mediated enhancement of immune response
InactiveUS20070190063A1Reduces conditionEnhance affinityBacterial antigen ingredientsDigestive systemCancerAntigen binding site
Provided are reagents and methods for administering an attenuated bacterium and a binding compound for treating a cancerous or infectious condition, where the binding compound comprises an antibody or an antigen binding site derived from an antibody.
Owner:ANZA THERAPEUTICS INC
Markers for cancer detection
InactiveUS20120039811A1Microbiological testing/measurementLibrary screeningGastrointestinal Tract CancersOncology
The present invention relates to methods for detecting, prognosing and staging cancers, in particular cancers of the gastrointestinal tract. The methods of the invention comprise detecting specific protein markers in a tissue of interest, wherein the detected levels thereof may be indicative of pre-cancerous or cancerous tissue, or the stage or prognosis of a cancer. Further provided are methods of treating cancer, and cancer detection kits.
Owner:TECHNION RES & DEV FOUND LTD
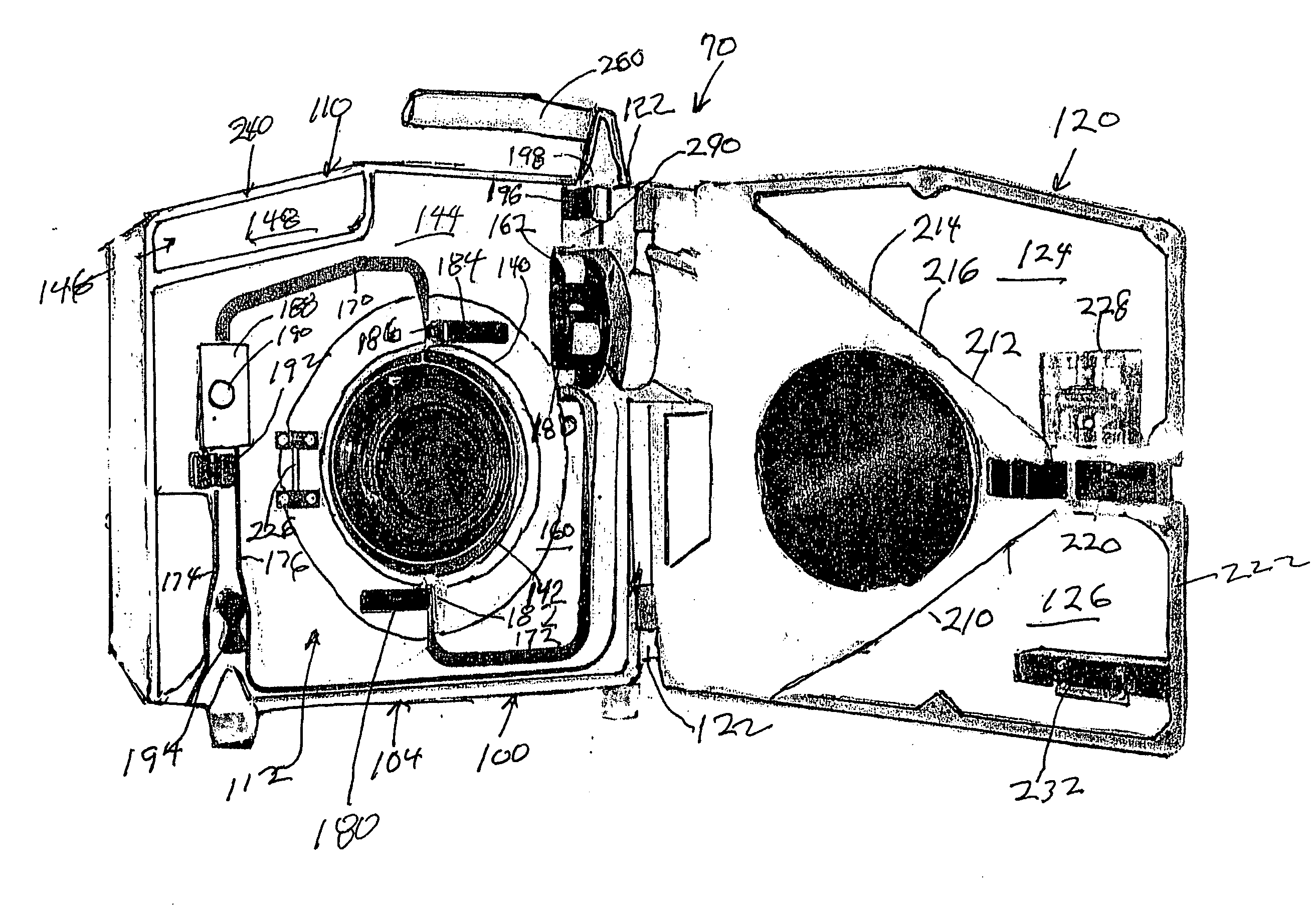
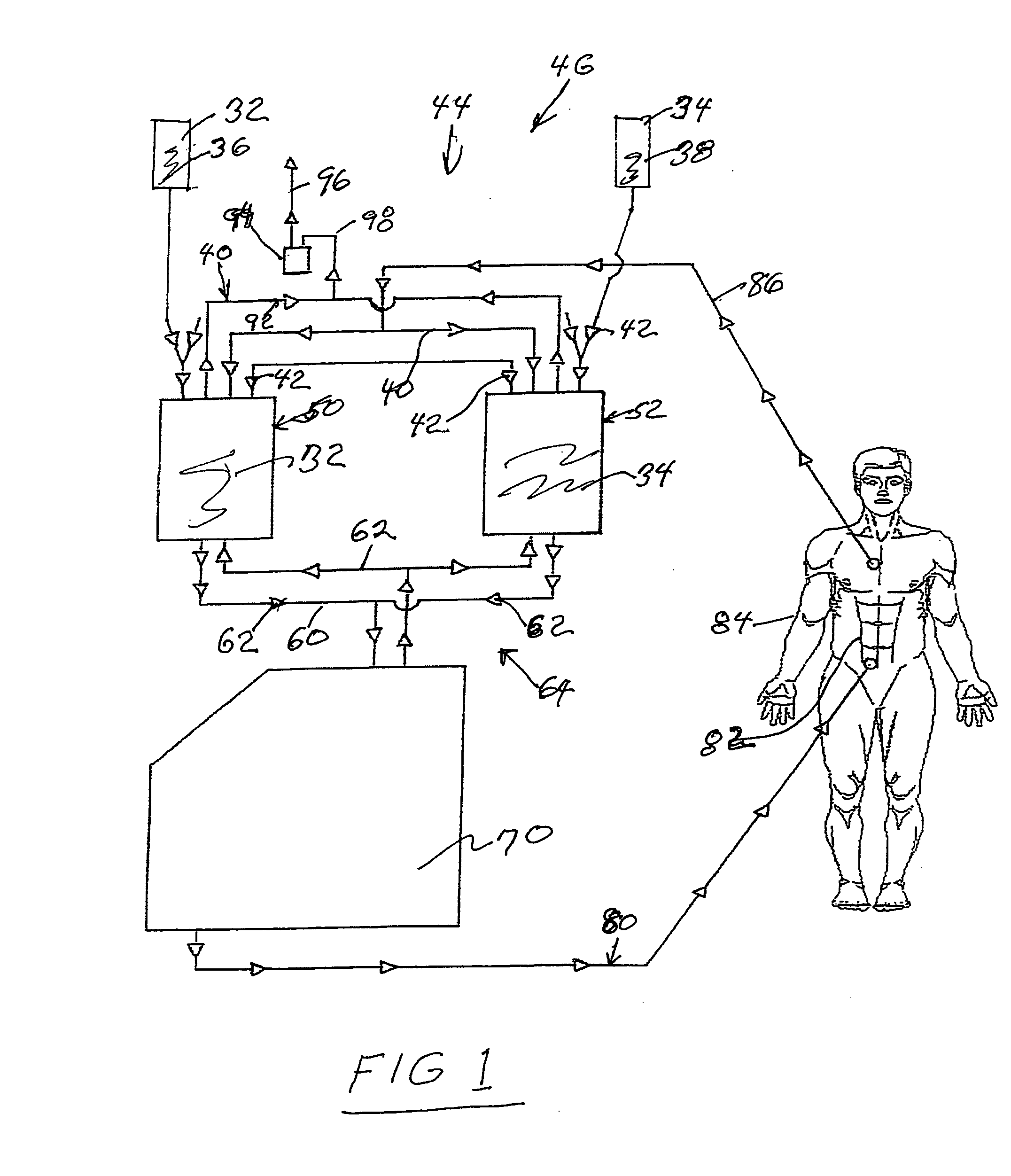
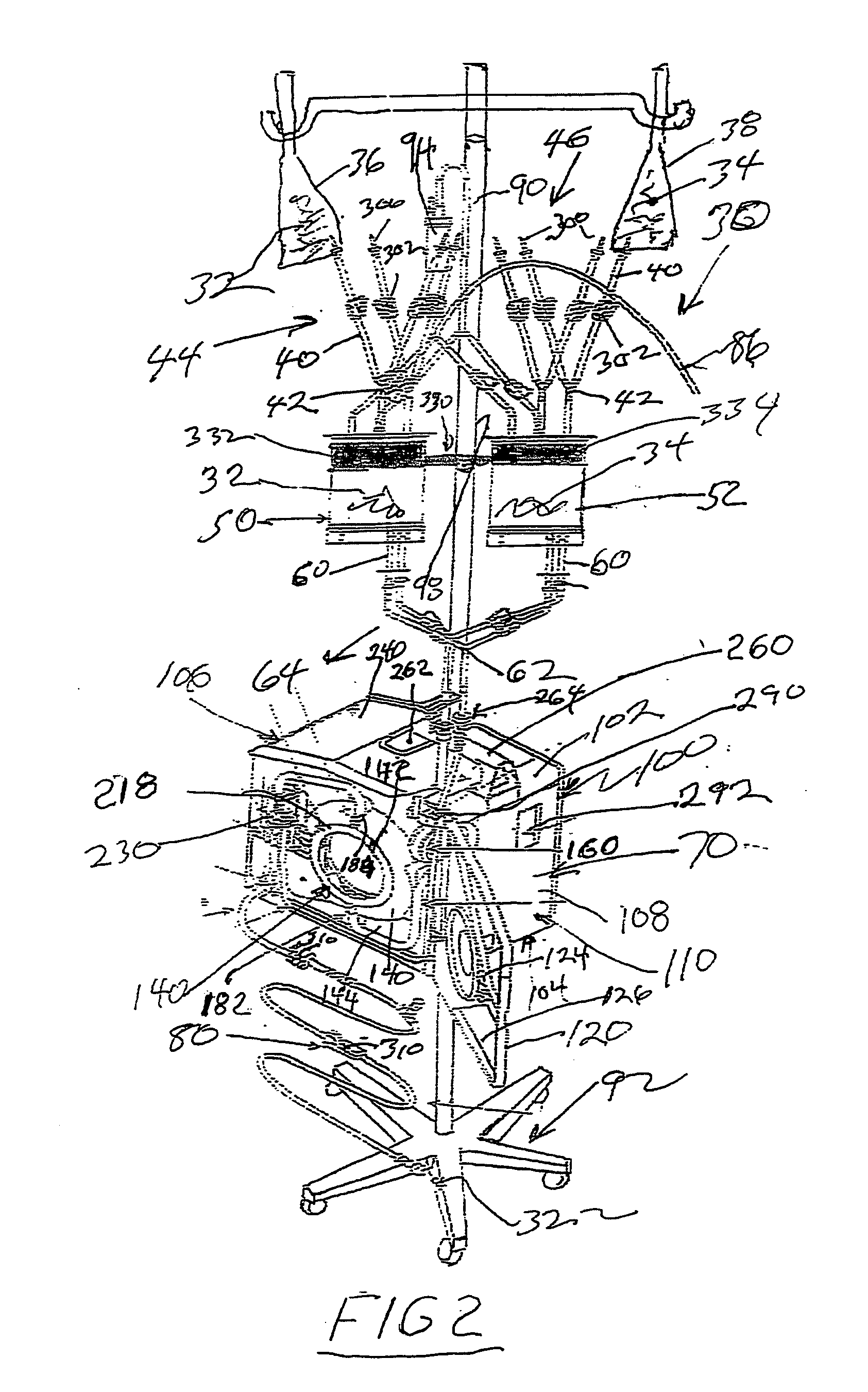
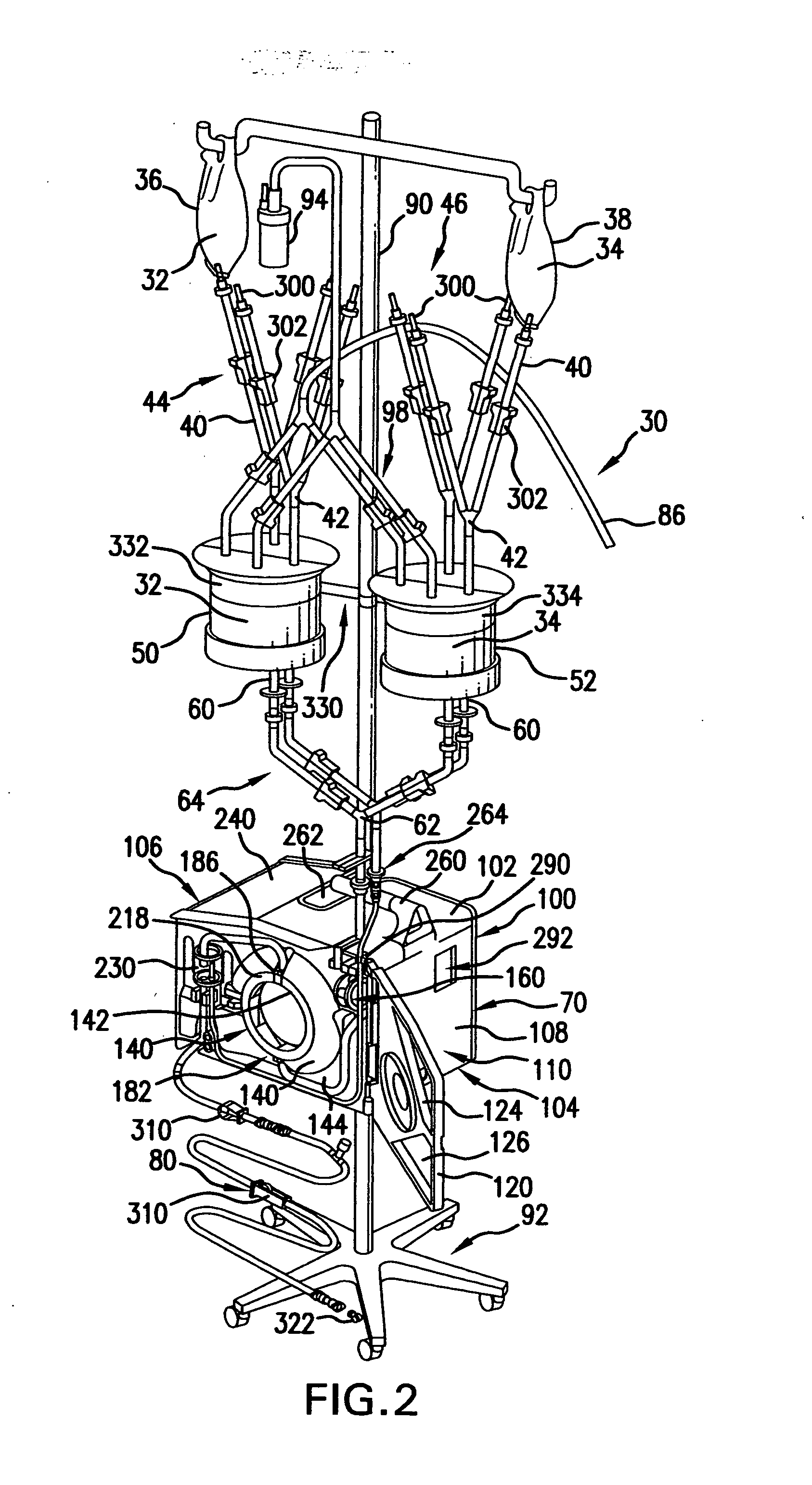
Hyperthermia, system, method and components
ActiveUS20090043256A1Effective and safe for patientInfusion devicesMedical devicesIntensive care medicineHigh body temperature
An IV pole mountable, therapeutic infusate processing device is incorporated into a hypothermia system to receive therapeutic fluid(s), such as normal saline, peritioneal dialysis solution, or other crystalloid solution, to heat such therapeutic fluid(s) a few degrees centigrade above normal body temperature and to direct the resulting heated infusate to and through a selected anatomical portion of a patients body to raise the temperature of that body portion so as to affect any cancerous or other tumors that may be located therein. The processing device is provided with touch screen controls and visual indicators to facilitate its proper use; while the system further includes temperature and pressure sensors to monitor the hyperthermia processing to insure patient safety.
Owner:BELMONT INSTR LLC
High molecular anticarcinogenic prodrug, its preparing method and use
ActiveCN101045163ALymphatic targetingTargetedOrganic active ingredientsPeptide/protein ingredientsMolecular precursorDrainage lymph nodes
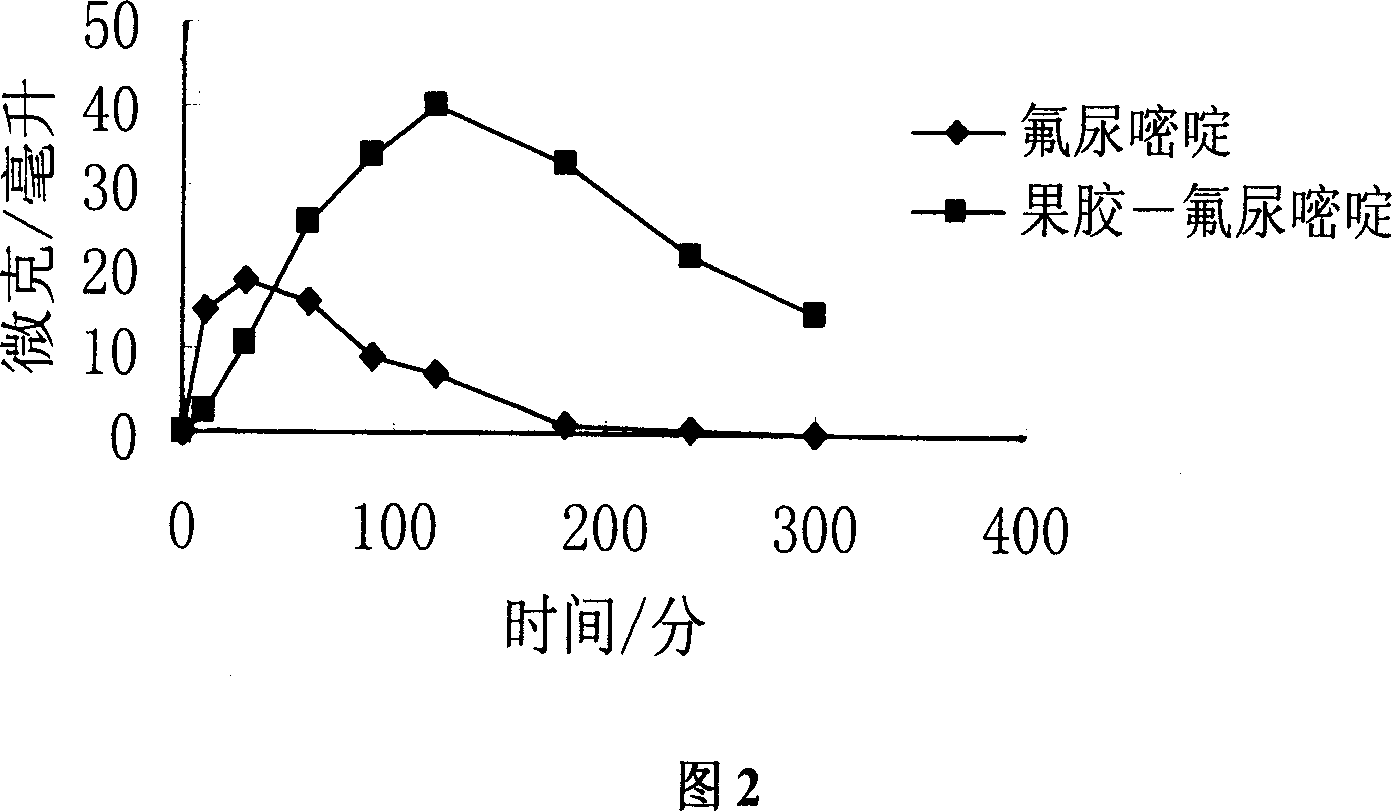
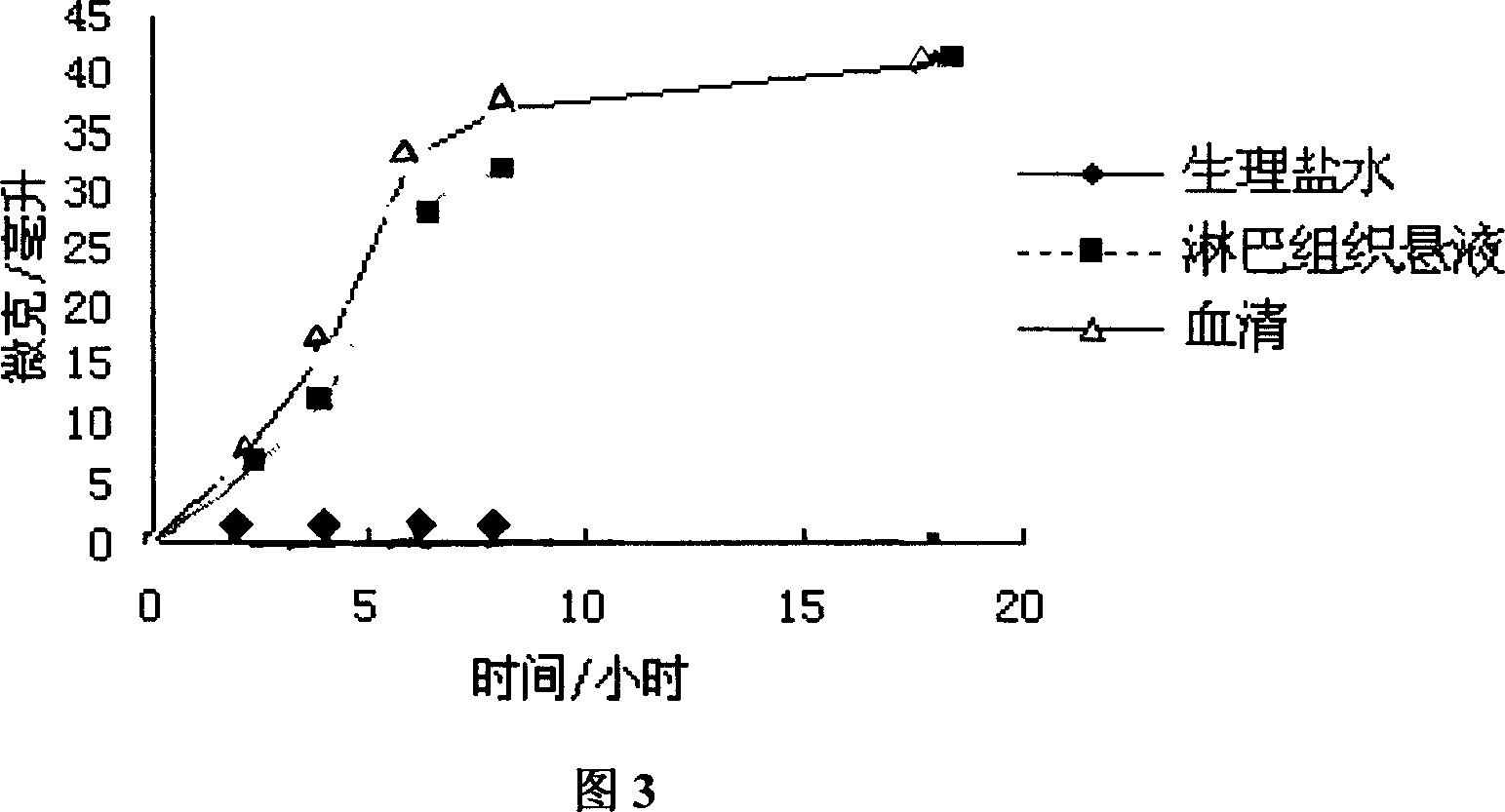
An anticancer high-molecular precursor medicine C-Spacer-P, where P is high- molecular pectin, C is an anticancer medicine containing amino radical or hydroxy radical, and Spacer is the spacing radical, is prepared by bonding the amino or hydroxy of an anticancer medicine with the hydroxy, carboxy, or hydroxymethyl of high- molecular pectin via spacing radical. It is an injection suitable for the solid cancer, and cancerous ascites or hydrothorax.
Owner:SICHUAN YINGRUI PHARMA TECH CO
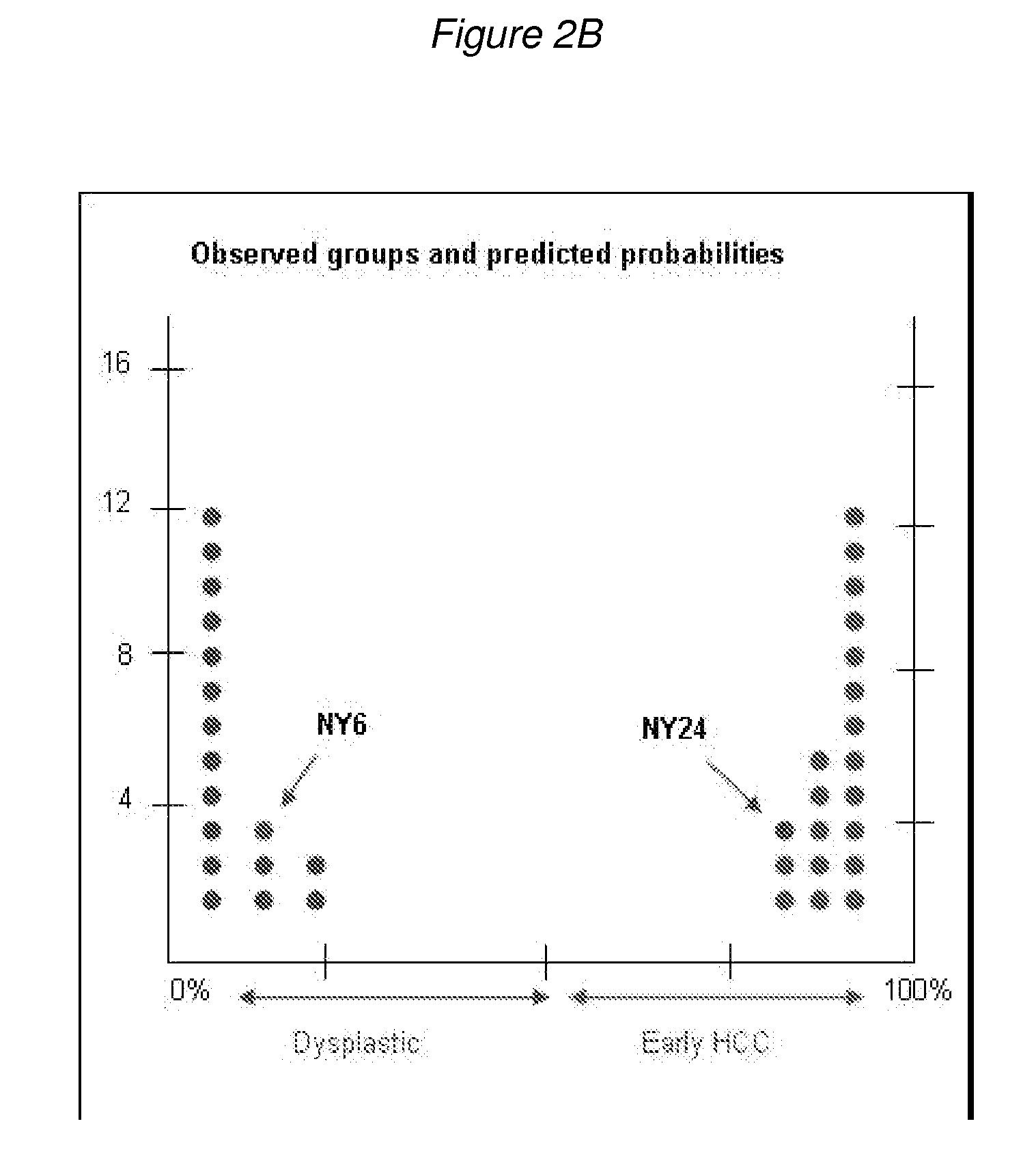
Methods and compositions for the diagnosis for early hepatocellular carcinoma
ActiveUS20080038736A1Microbiological testing/measurementBiological testingCyclin D1Early Hepatocellular Carcinoma
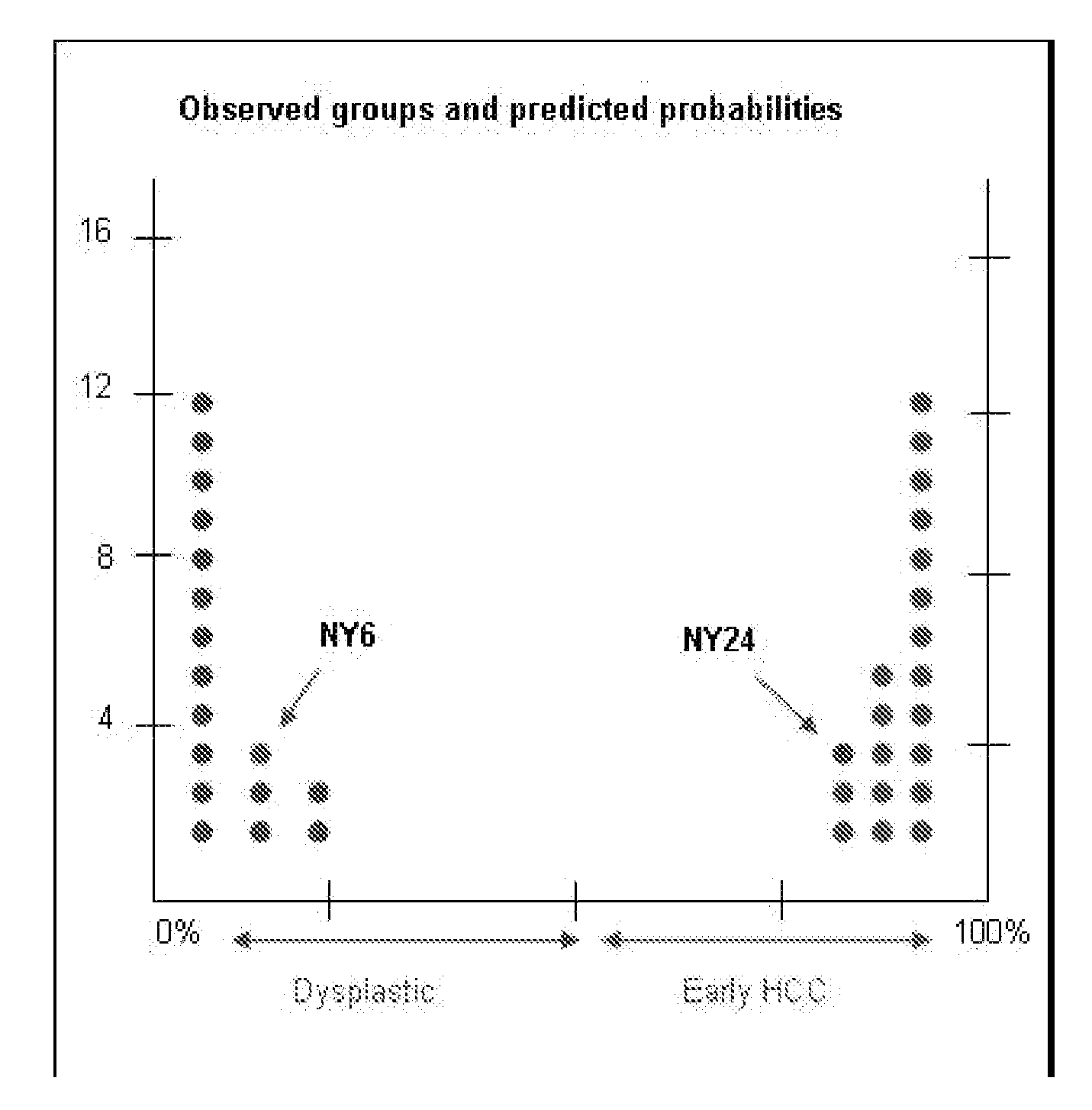
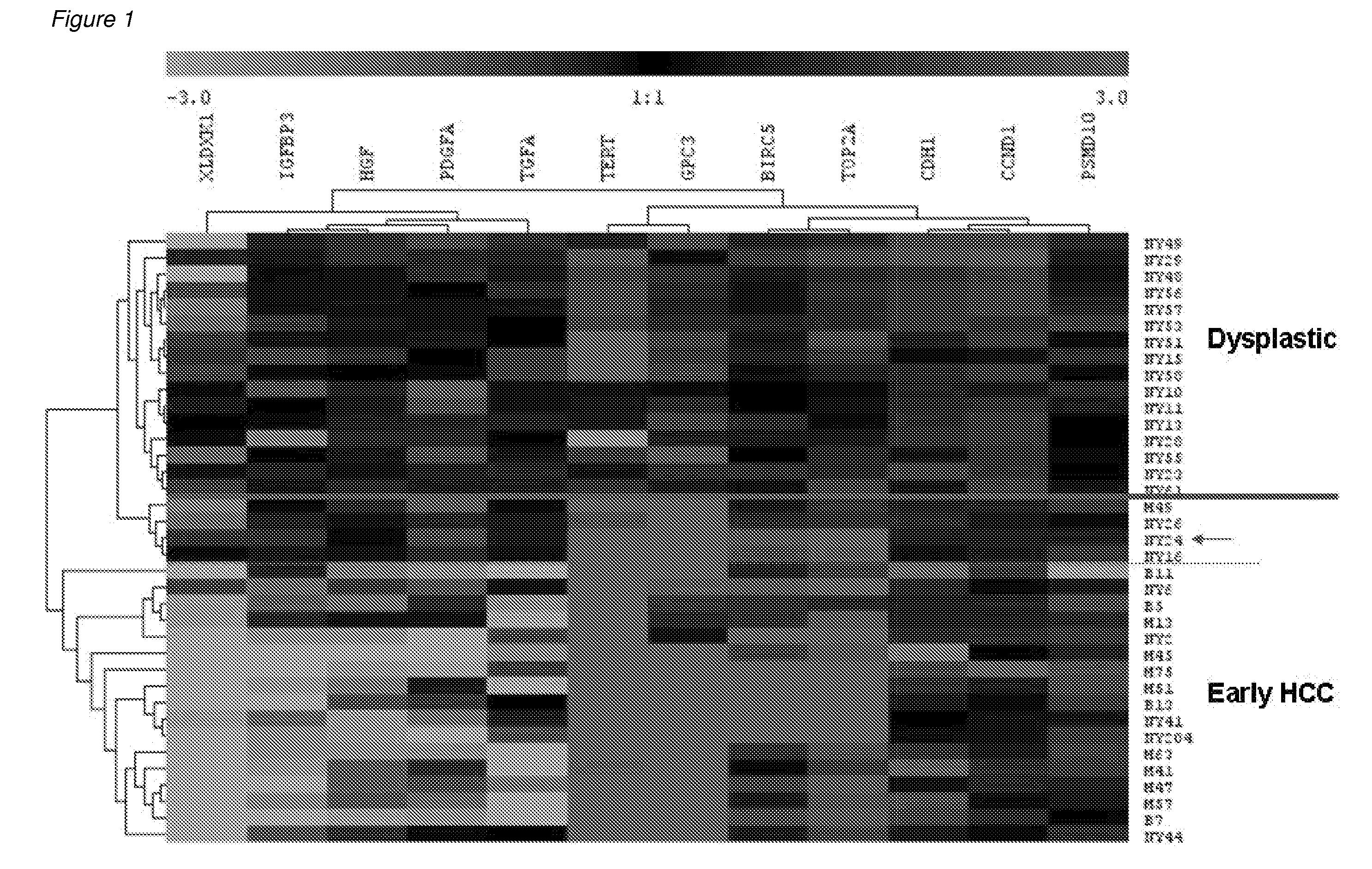
Methods and compositions are provide to allow discrimination of dysplastic nodules from early HCC nodules. More specifically, it has been determined that TERT, GPC3, gankyrin, survivin, TOP2A, LYVE1, Ecadherin, IGFBP3, PDGFRA, TGFA, cyclin D1 and HGF are differentially expressed in HCC as compared to normal liver cells and liver cells that have dysplastic, non-cancerous nodules.
Owner:MT SINAI SCHOOL OF MEDICINE
Tumor-Targeted nanodelivery systems to improve early MRI detection of cancer
ActiveUS20070134154A1Ultrasonic/sonic/infrasonic diagnosticsDispersion deliveryTumor targetNuclear medicine
The present invention is in the fields of drug delivery, cancer treatment and diagnosis and pharmaceuticals. This invention provides a method of making antibody- or antibody fragment-targeted immunoliposomes for the systemic delivery of molecules to treat and image diseases, including cancerous tumors. The invention also provides immunoliposomes and compositions, as well as methods of imaging various tissues. The liposome complexes are useful for encapsulation of imaging agents, for example, for use in magnetic resonance imaging. The specificity of the delivery system is derived from the targeting antibodies or antibody fragments.
Owner:GEORGETOWN UNIV
Aptamer-based targeted delivery microRNA nanometer carrier as well as preparation method and application thereof
InactiveCN104784703AEasy to prepareLow toxicityOrganic active ingredientsGenetic material ingredientsAptamerChemical synthesis
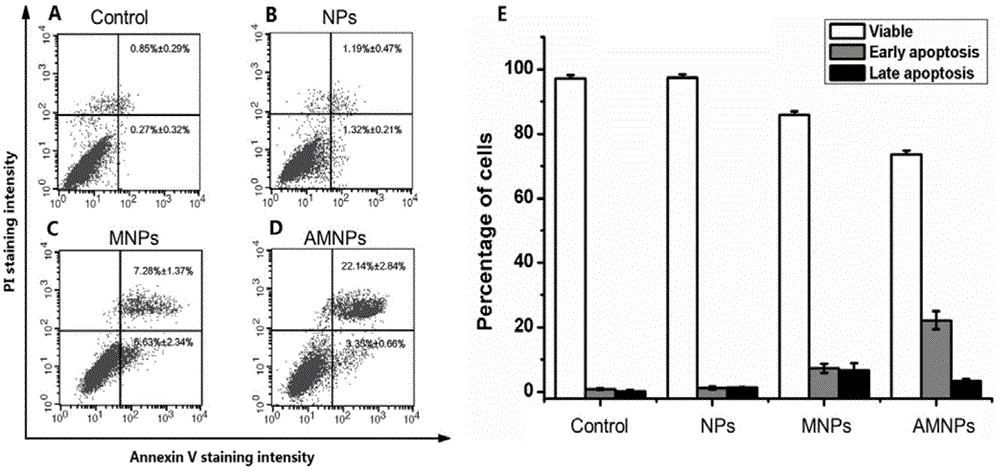
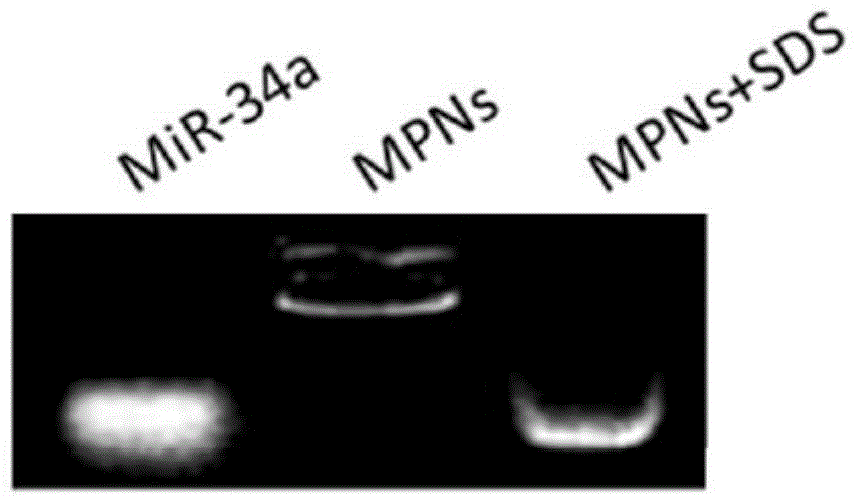
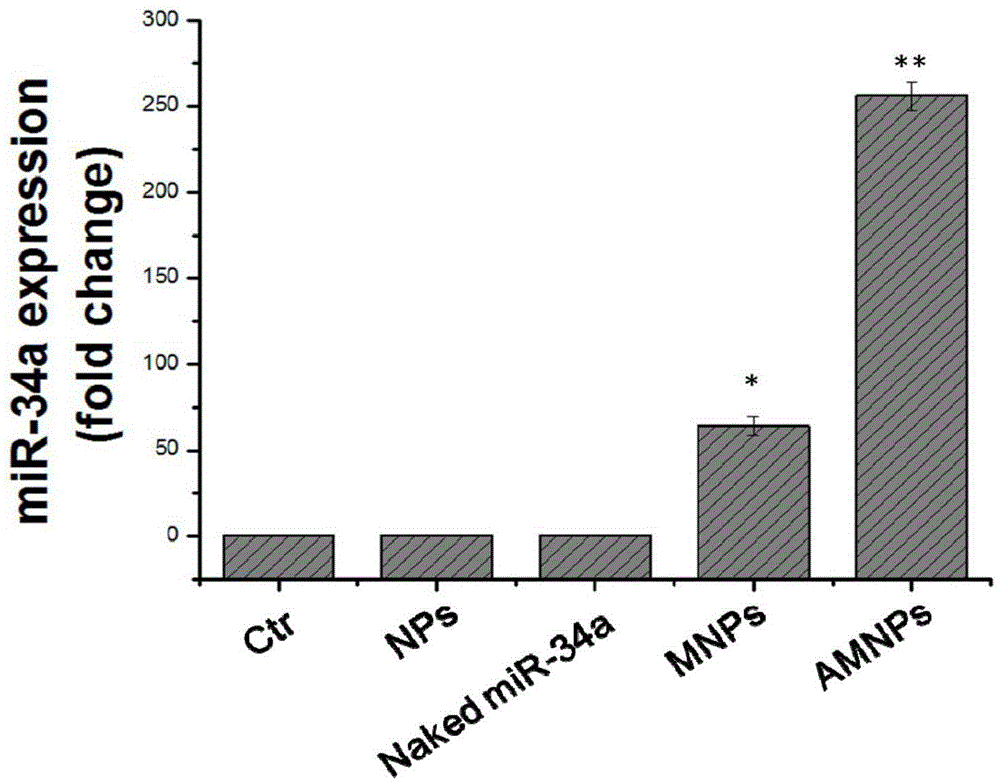
The invention relates to an aptamer-based targeted delivery microRNA nanometer carrier as well as a preparation method and application thereof. A targeting molecule is a sulfydryl-modified MUC-1molecule, and can be combined with cancer-inhibiting microRNA-34a, so that a MicroRNA-nanodiamond-protamine complex (MNPs) becomes AMNPs with an active targeting function; through electrostatic interaction, a negatively-charged aptamer-microRNA (Apt-miR) chimera is adsorbed to a cationic protamine-nanodiamond carrier to form a trinary compound carrier. The therapeutic effect of a nanotransport system on a non-small cell lung cancer is researched; the Apt-miR chimera is low in immunogenicity; the modified MUC-1 aptamer has high chemical stability in vivo, and can be chemically synthesized easily; in order to improve the transfection efficiency, a protamine-modified nanodiamond material (NPs) is used for helping miRNA to increase accumulation in a tumor part through permeability and retention enhancing effects.
Owner:BEIJING UNIV OF TECH
4-(piperrazin-1-yl)-pyrrolidin-2-one compounds as monoacylglycerol lipase (MAGL) inhibitors
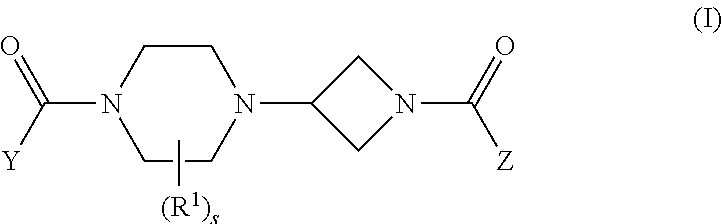
ActiveUS20160318864A1Superior MAGL inhibitory actionNervous disorderOrganic chemistryHuntingtons choreaDepressant
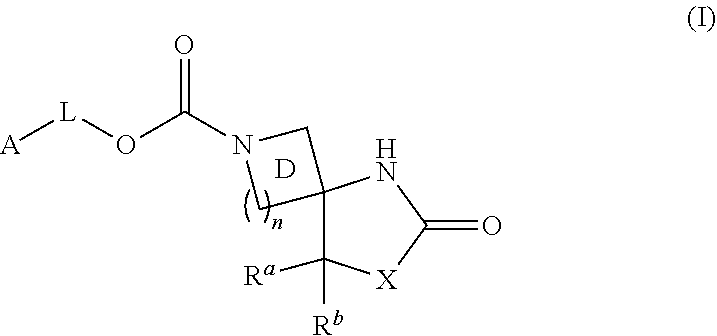
The present invention aims to provide a compound having an MAGL inhibitory action, and useful as a prophylactic or therapeutic agent for neurodegenerative diseases (e.g., Alzheimer's disease, Huntington's disease, Parkinson's disease, amyotrophic lateral sclerosis, traumatic brain injury, glaucoma, multiple sclerosis and the like), anxiety disorder, pain (e.g., inflammatory pain, carcinomatous pain, nervous pain and the like), epilepsy and the like. The present invention relates to a compound represented by the formula (I):wherein each symbol is as described in the DESCRIPTION, or a salt thereof.
Owner:TAKEDA PHARMACEUTICALS CO LTD
Injection of flurbiprofen and its preparing method
InactiveCN1621035AEasy to prepareQuick resultsOrganic active ingredientsAntipyreticSolventLong lasting
The present invention is flurbiprofen ester injection as antiphlogistic and analgetic medicine and its preparation process. Main medicine and supplementary material including solvent, emulsifier, isotonic agent, buffering agent and pH regulator are mixed to compound the injection. Compared with available oral flurbiprofen preparation, the flurbiprofen ester injection has even high curative effect, even fast action, long lasting effect and less stomach mucous membrane damage and other negative effect. The present invention is used in treating chronic rheumarthritis, etc and is especially suitable for relieve post-operational pain and pain caused by cancer, and it has high effect and no addiction.
Owner:梁建华
Compositions, cell constructs, and methods of making and using the same
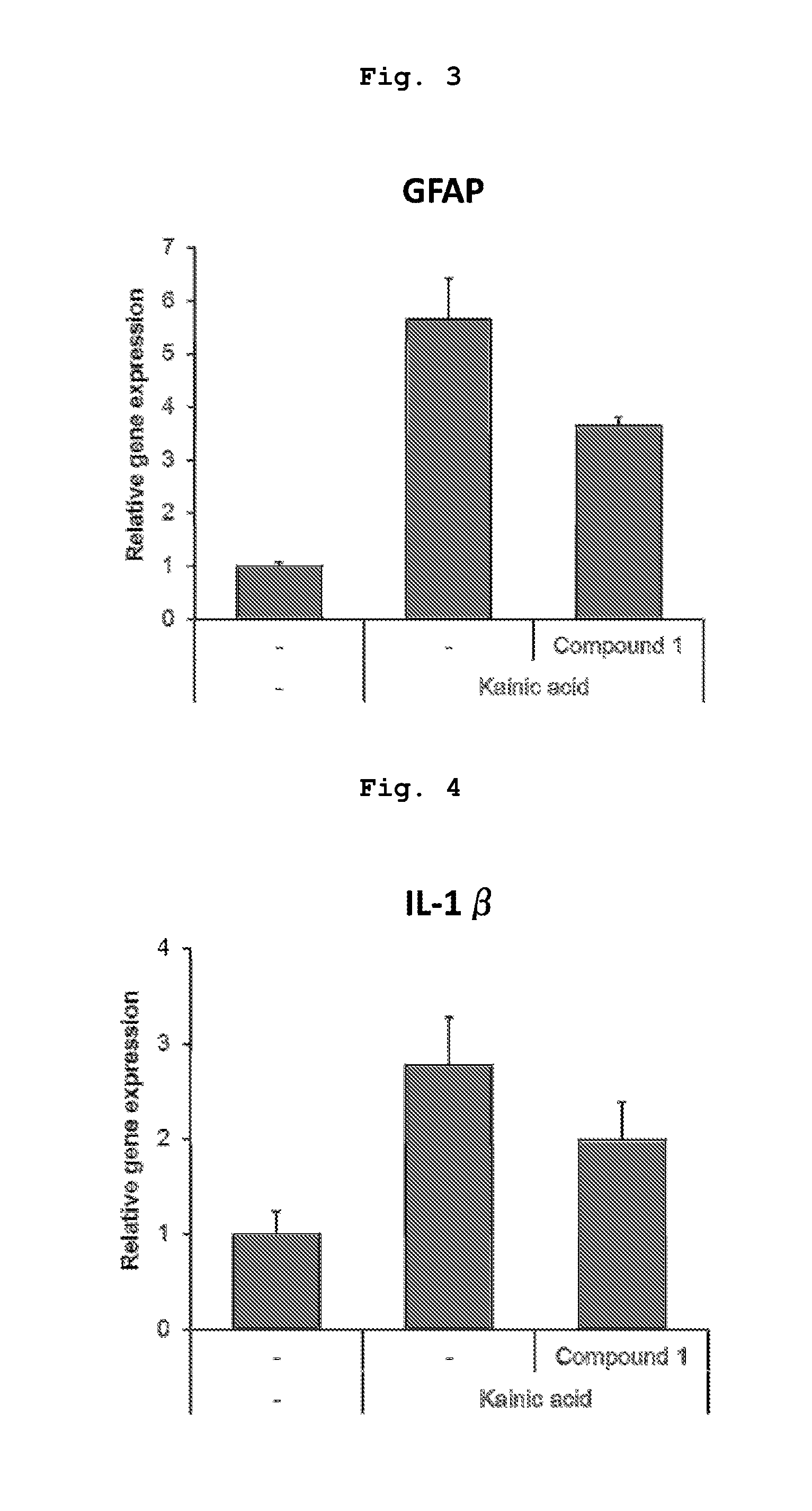
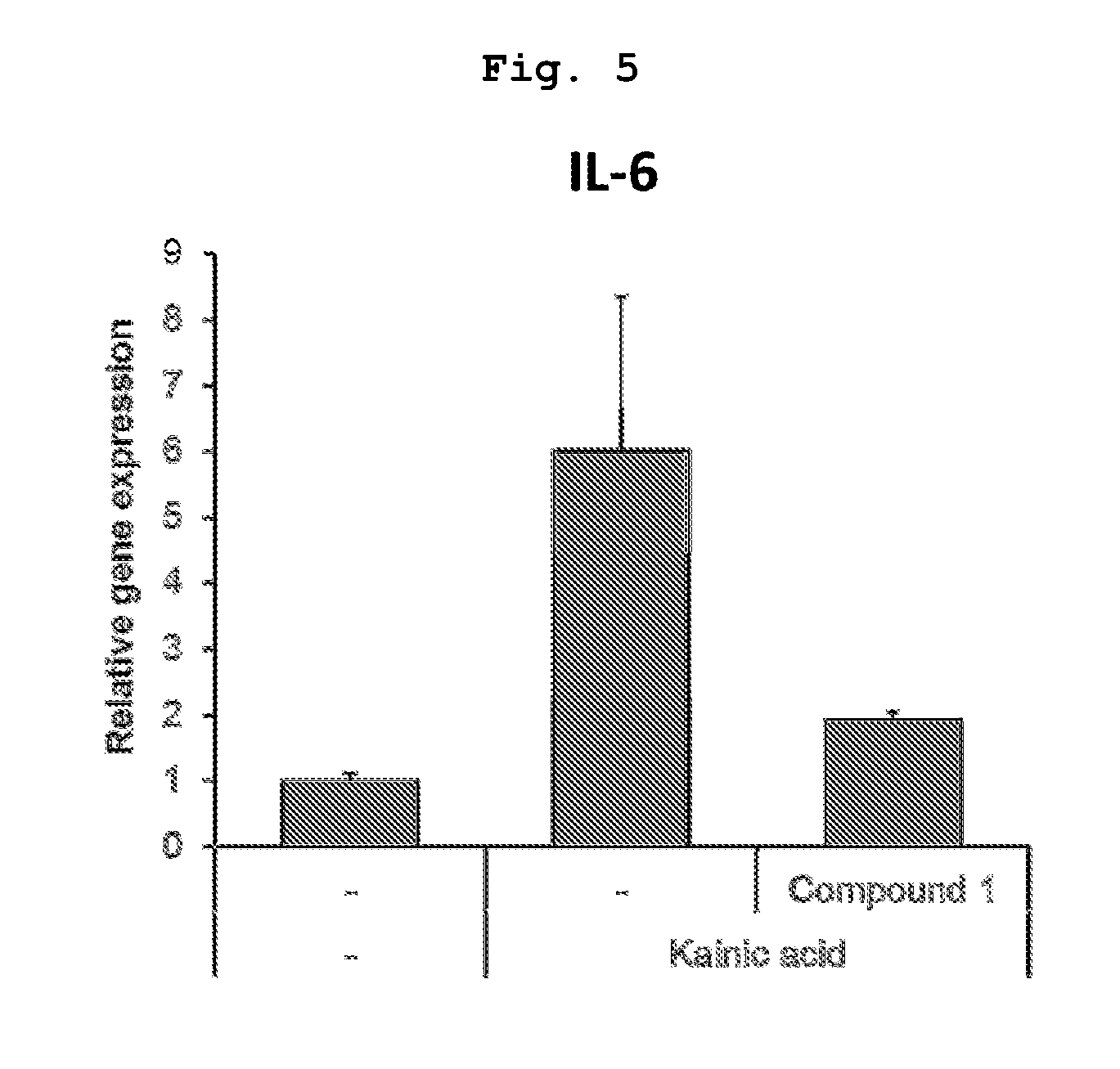
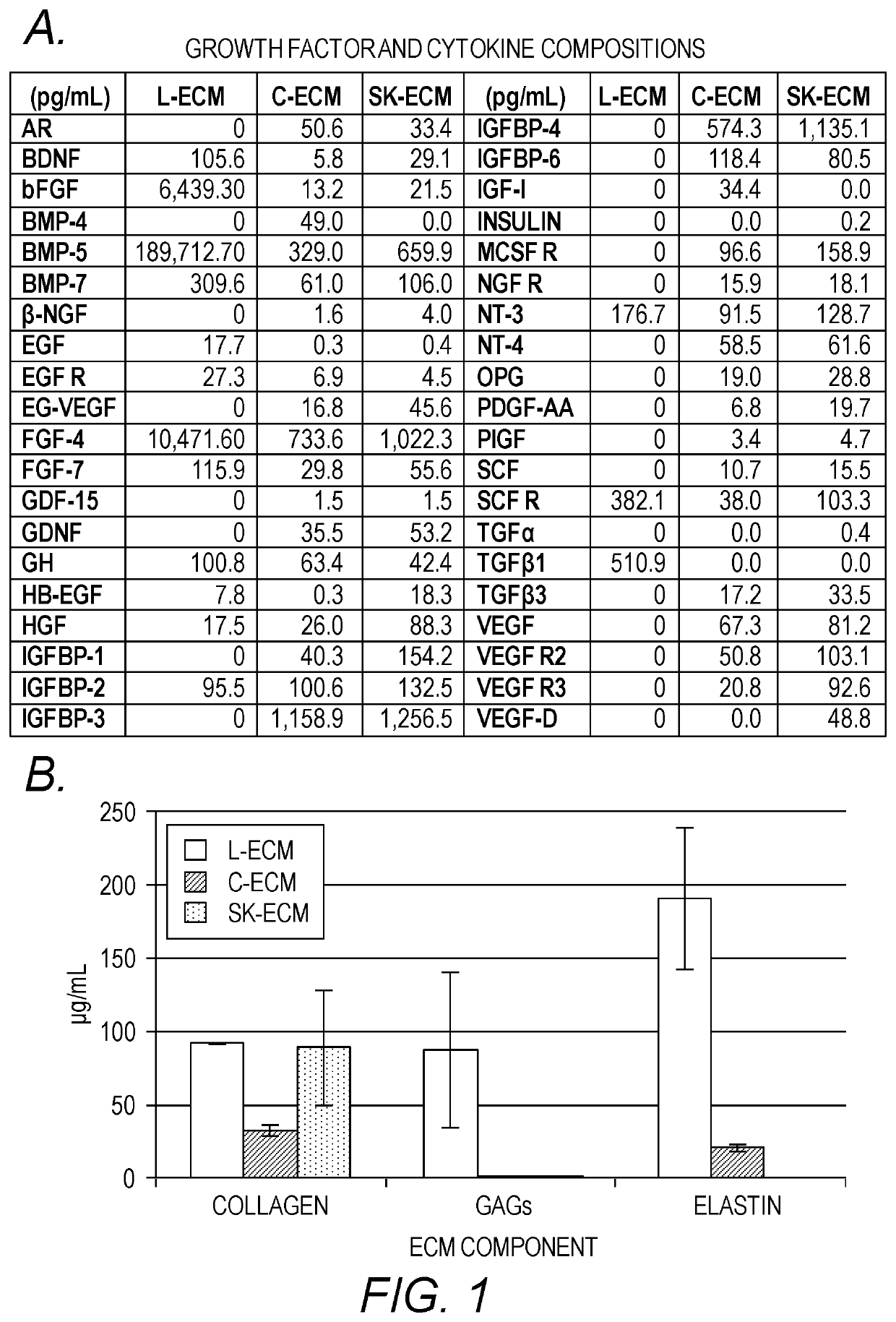
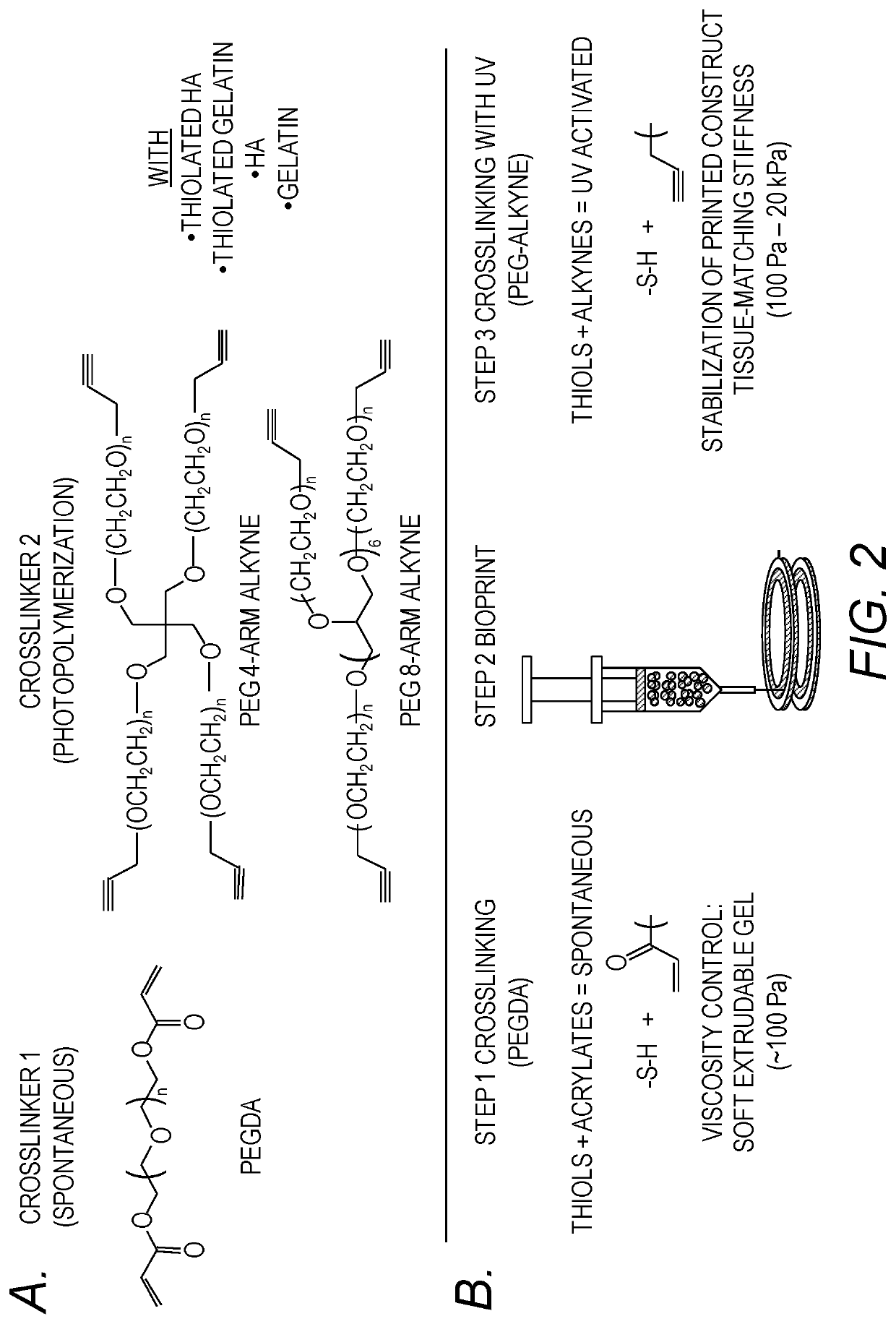
PendingUS20190345439A1Increase stiffnessImprove abilitiesDrug screeningNervous system cellsTissues typesTumor cells
An extrudable hydrogel composition useful for making a three-dimensional organ construct is described herein. Methods of using the same and products so made are also described. Also described herein is a multicellular organoid including at least two tumor cells or cell lines that are of the same tissue type, but are distinct from one another (e.g., distinct in morphology, growth rate, and / or or at least one mutation); and at least one type of non-cancerous (i.e., normal or differentiated) tissue cells, wherein the at least one type of non-cancerous tissue cells are of the same tissue type as the at least two tumor cells or cell lines. In some embodiments, the at least two tumor cells or cell lines and / or the non-cancerous tissue cells are labeled with and / or comprise a detectable compound, optionally so that each of the different cells can be distinguished from each other (e.g., optically and / or electrically distinguished).
Owner:WAKE FOREST UNIV HEALTH SCI INC
Stromal collagen in the diagnosis and characterization of breast cancer
ActiveUS7805183B2Test accurateGood informationImage enhancementRadiation pyrometryAbnormal tissue growthPresent method
The present invention provides methods and systems for evaluating biological materials for the diagnosis of disease, such as gland abnormalities, and the cancerous and precancerous conditions. Nonlinear optical microscopy techniques, such as MP microscopy and harmonic generation microscopy, are used to generate high resolution, three dimensional images of a test tissue, such as a biopsy tissue sample and tissue in whole organisms, that are analyzed, optionally in combination, to detect, identify and characterize tumor-associated collagen signatures. The presence, abundance and extent of histological features and structural motifs comprising tumor-associated collagen signatures may be directly and accurately correlated with the onset and progression of cancer, such as breast cancer. The present methods are capable of providing an accurate and selective diagnosis of cancer, and provide diagnostic information complementary to conventional diagnostic methods.
Owner:WISCONSIN ALUMNI RES FOUND
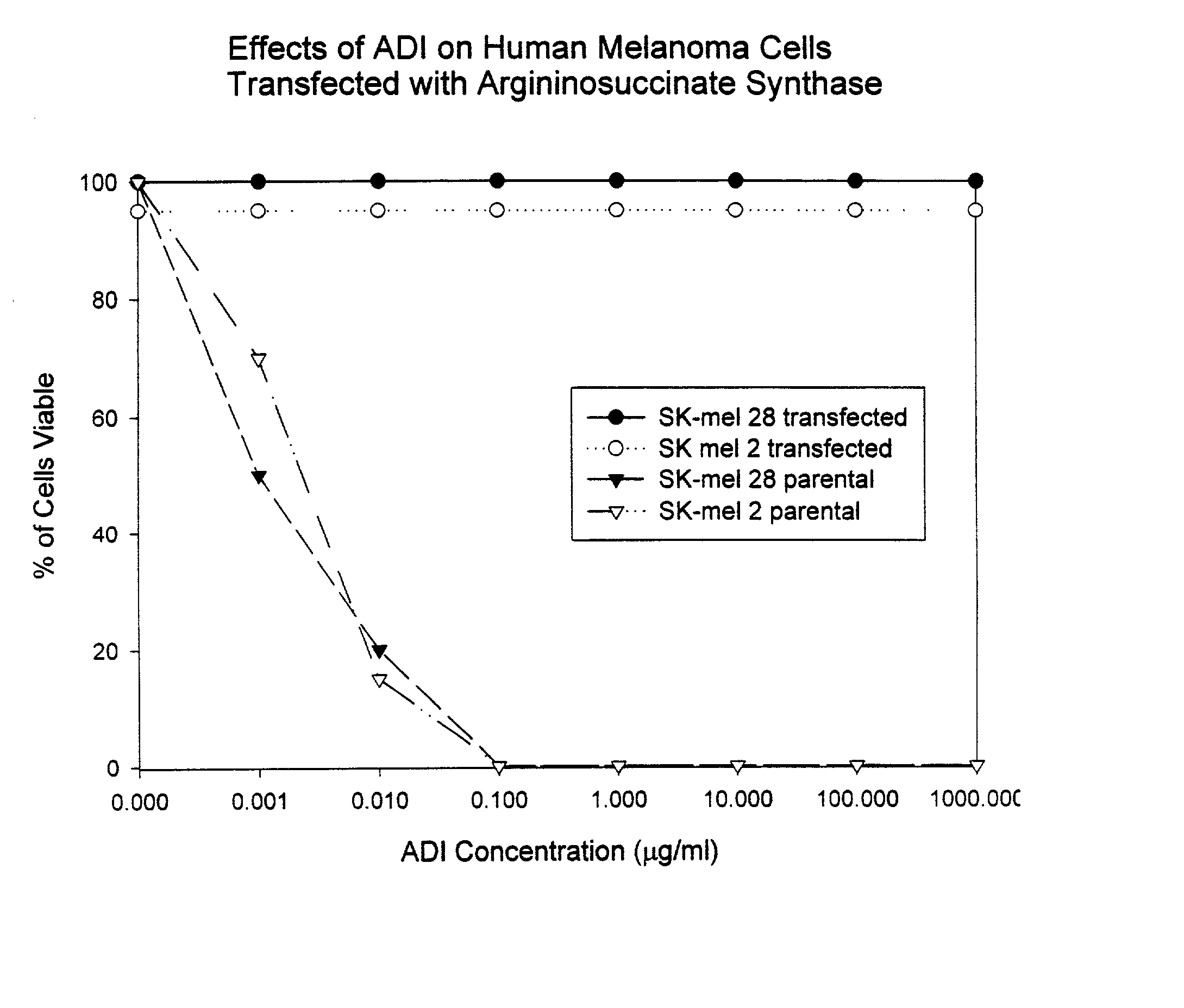
Methods for predicting sensitivity of tumors to arginine deprivation
InactiveUS20050063942A1Peptide/protein ingredientsMicrobiological testing/measurementAbnormal tissue growthUrea cycle enzymes
The present invention provides methods for determining which cancer patients are susceptible to arginine depletion therapy and methods for treating cancer. The present invention also provides methods for predicting the appropriateness of arginine deprivation therapy for a cancer patient. The methods generally comprise obtaining a tumor sample from the cancer patient and detecting the presence or absence of evidence of urea cycle enzyme expression in the tumor sample. The absence of evidence of urea cycle enzyme expression in the tumor sample is indicative of a cancer patient who is a candidate for arginine deprivation therapy, and the presence of evidence of urea cycle enzyme expression in said tumor sample is indicative of a cancer patient who is not a candidate for arginine deprivation therapy. Prior to, simultaneous with, or after testing the tumor sample, the method further comprises the steps of obtaining a non-cancerous sample from the cancer patient and detecting the presence or absence of evidence of urea cycle enzyme expression in the non-cancerous sample, wherein the absence of evidence of urea cycle enzyme expression in the non-cancerous sample and absence of evidence of urea cycle enzyme expression in the tumor sample is indicative of a cancer patient who is not a good candidate for arginine deprivation therapy, the presence of evidence of urea cycle enzyme expression in the non-cancerous sample and the absence of evidence of urea cycle enzyme expression in the tumor sample is indicative of a cancer patient who is a good candidate for arginine deprivation therapy, and the presence of evidence of urea cycle enzyme expression in the tumor sample is indicative of a cancer patient who is not a candidate for arginine deprivation therapy.
Owner:PHOENIX PHARMACOLOGICS
Brachytherapy method of treating skin tumors using a tailor-made radioactive source
ActiveUS20110201866A1Easy to controlPrevent skinAntineoplastic agentsRadiation therapyMedicineBrachytherapy
The present invention refers to a method of treating a cancerous or non-cancerous skin lesion of a subject, e.g. a human patient, by epidermal radioisotope therapy, a specialized type of brachytherapy, comprising the steps of (a) defining and marking an area of skin to be treated; (b) covering said area with a protective layer, e.g. a protective film or foil; (c) applying a tailor-made radioactive source by applying a layer of a radioactive source material on said protective layer, such that said area is covered by said material while any area not to be treated is spared; and (d) removing the radioactive source after a predetermined time period of irradiation.
Owner:ONCOBETA INT GMBH
Enzyme-based anti-cancer compositions and methods
InactiveUS20030003114A1Peptide/protein ingredientsGenetic material ingredientsCancer cellAntibody fragments
A composition for treating cancer is provided. The composition is a hexosaminidase covalently attached to a cancer cell targeting ligand and improves selectively of the hexosaminidase for tumor cells. In certain embodiments, the hexosaminidase is alternately chitinase (N-acetyl-glucosaminohydrolase), chitosanase, or N-acetyl-hexosaminidase and the targeting ligand is either a monoclonal antibody, an antibody fragment immunospecific to a tumor cell or cancer cell antigen, epidermal growth factor (EGF), fibroblast growth factor (FGF), transferrin, or folic acid. Also provided is a method for treating cancerous tumors comprising administering the composition to a patient that has a cancerous tumor.
Owner:CITY OF HOPE NAT MEDICAL CENT & BECKMAN RES INST +1
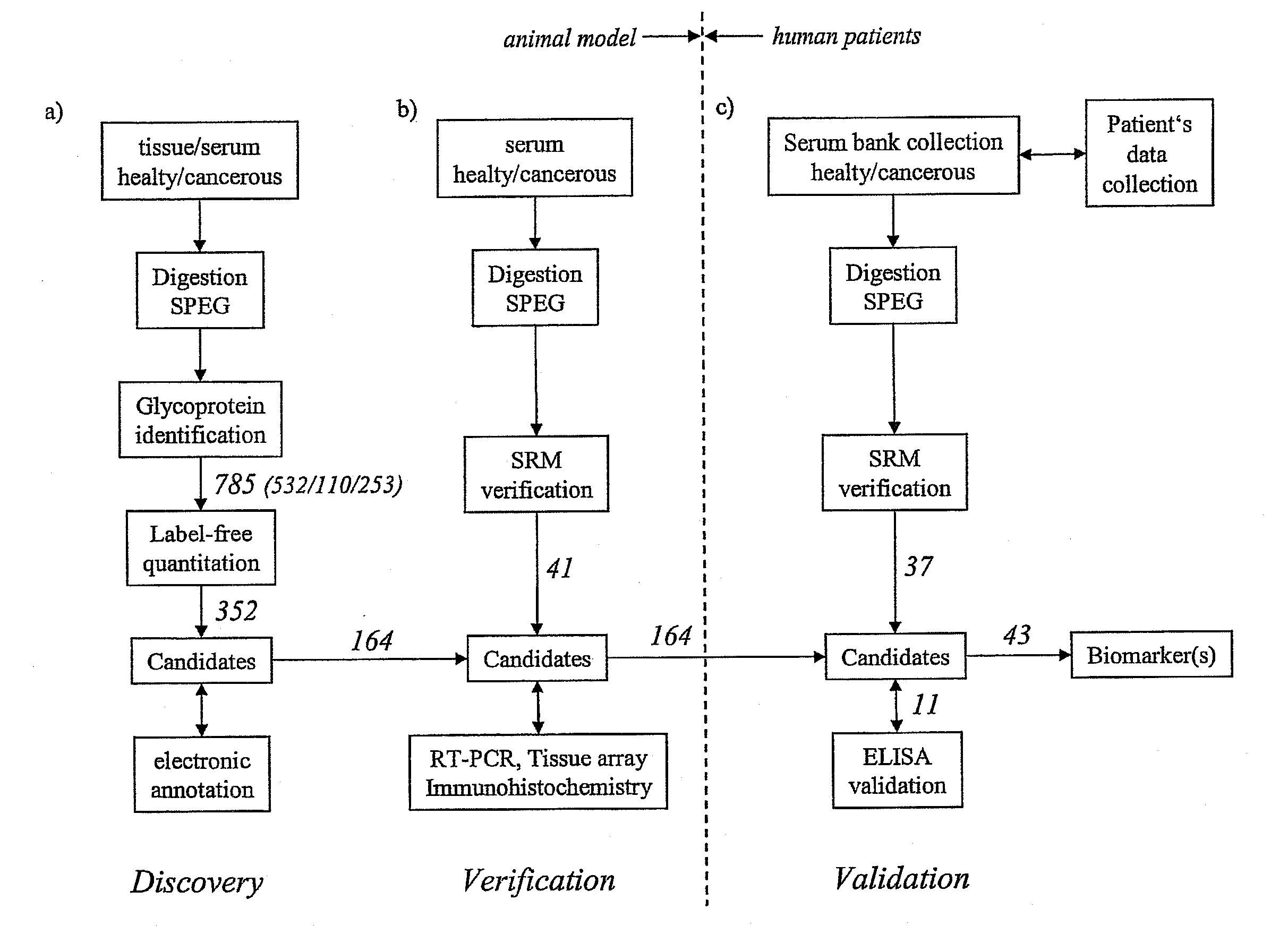
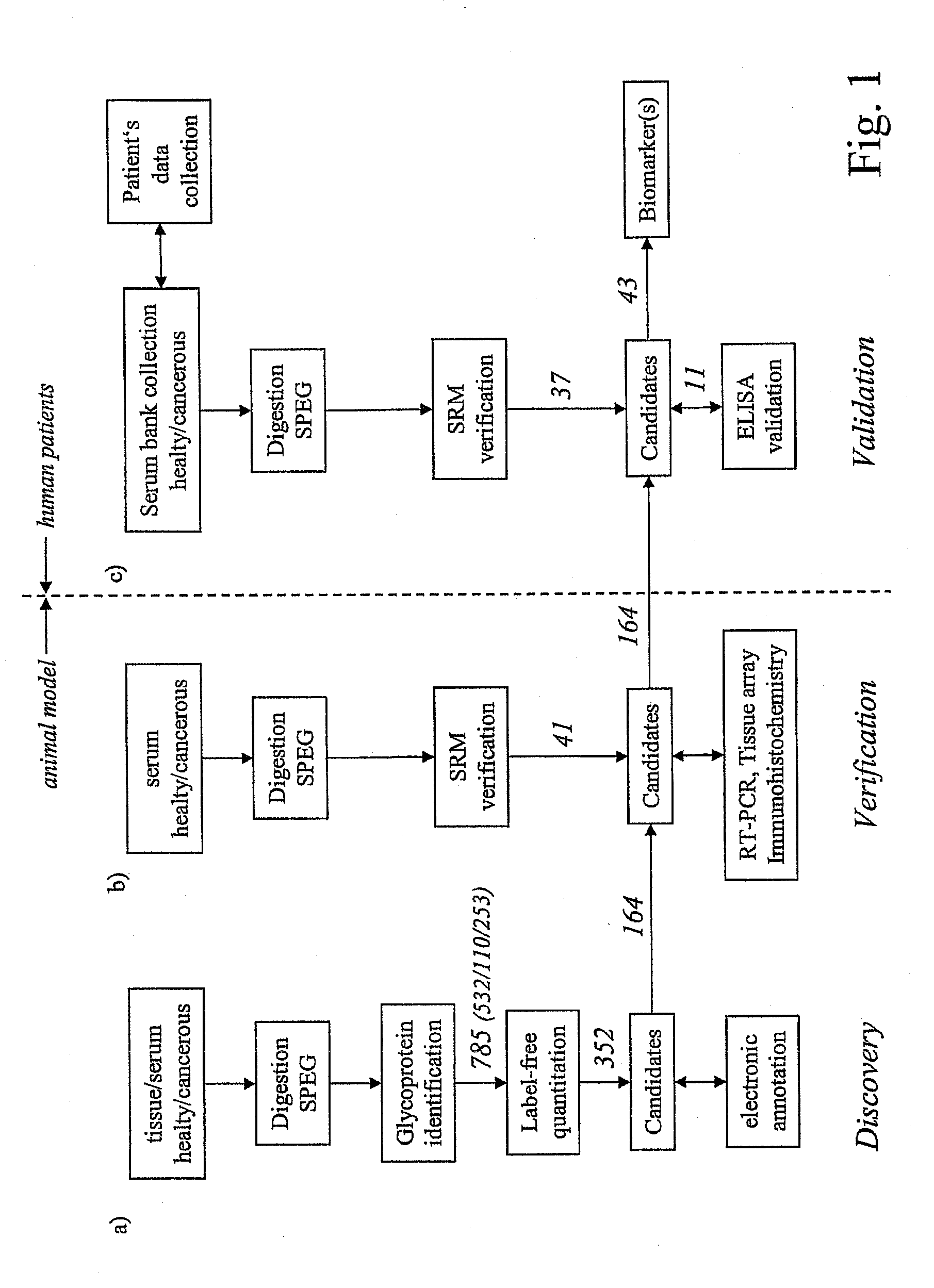
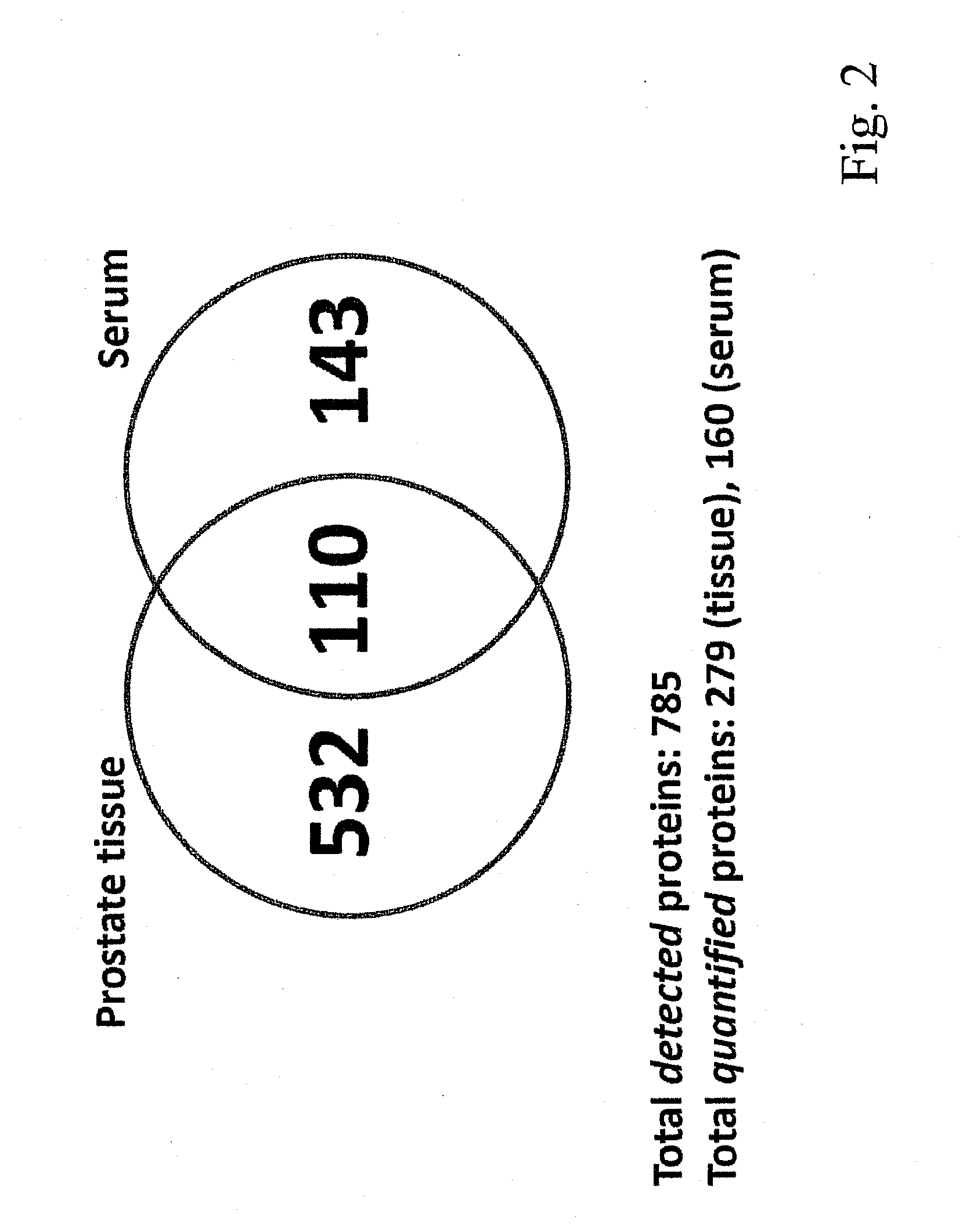
Method for biomarker and drug-target discovery for prostate cancer diagnosis and treatment as well as biomarker assays determined therewith
InactiveUS20110065605A1Improve accuracyImprove reliabilityMicrobiological testing/measurementLibrary screeningProtein proteinAntibody
The invention relates to a method for the determination of a cancer diagnostic / therapeutic biomarker assay and drug-targets including the following steps: (a) identification of potential candidate protein / peptide biomarkers and drug-targets based on the measurement of protein / peptide constituent concentrations in tissue sample proteomes as well as serum, plasma or any other derivatives of blood, or blood itself sample proteomes derived from healthy non-human mammalian individuals as well as from cancerous non-human mammalian individuals and qualitatively selecting as potential candidate protein / peptide biomarkers those which show a pronounced differential behaviour between healthy and cancerous sample proteomes; (b) optional verification of the potential candidate protein / peptide biomarkers as identified in step (a) by quantitative mass spectrometric measurement of the potential candidate protein biomarkers in serum, plasma or any other derivatives of blood, or blood itself sample proteomes derived from healthy non-human mammalian individuals as well as from cancerous non-human mammalian individuals and selecting as candidate protein / peptide biomarkers those which show a mass-spectrometrically measurable quantitative differential behaviour between healthy and cancerous sample proteomes; (c) validation of the candidate protein / peptide biomarkers as identified in step (a), or as optionally verified in step (b), by mass spectrometric measurement and / or antibody-based assays such as an Enzyme-Linked Immunosorbent Assay (ELISA) determination of the candidate protein biomarkers in serum, plasma or any other derivatives of blood, or blood itself sample proteomes derived from healthy human individuals as well as from cancerous human individuals and selecting as protein / peptide biomarkers those which show a mass-spectrometrically measurable and / or antibody-based assay detectable differential behaviour between healthy and cancerous sample proteomes; (d) application of statistical methods to uncover single or groups of protein / peptide biomarkers as validated in step (c) as signatures for the detection of patients with cancer. The invention furthermore relates to specific biomarker assays for the highly reliable diagnosis of cancer, specifically of localized or non-localized prostate cancer, using human serum, plasma or any other derivatives of blood, or blood itself.
Owner:ETH ZZURICH +1
Methods and compositions relating to HPV-associated pre-cancerous and cancerous growths, including CIN
InactiveUS7410758B2Reduce riskImproved prognosisPeptide/protein ingredientsMicrobiological testing/measurementCervical intraepithelial neoplasiaOncology
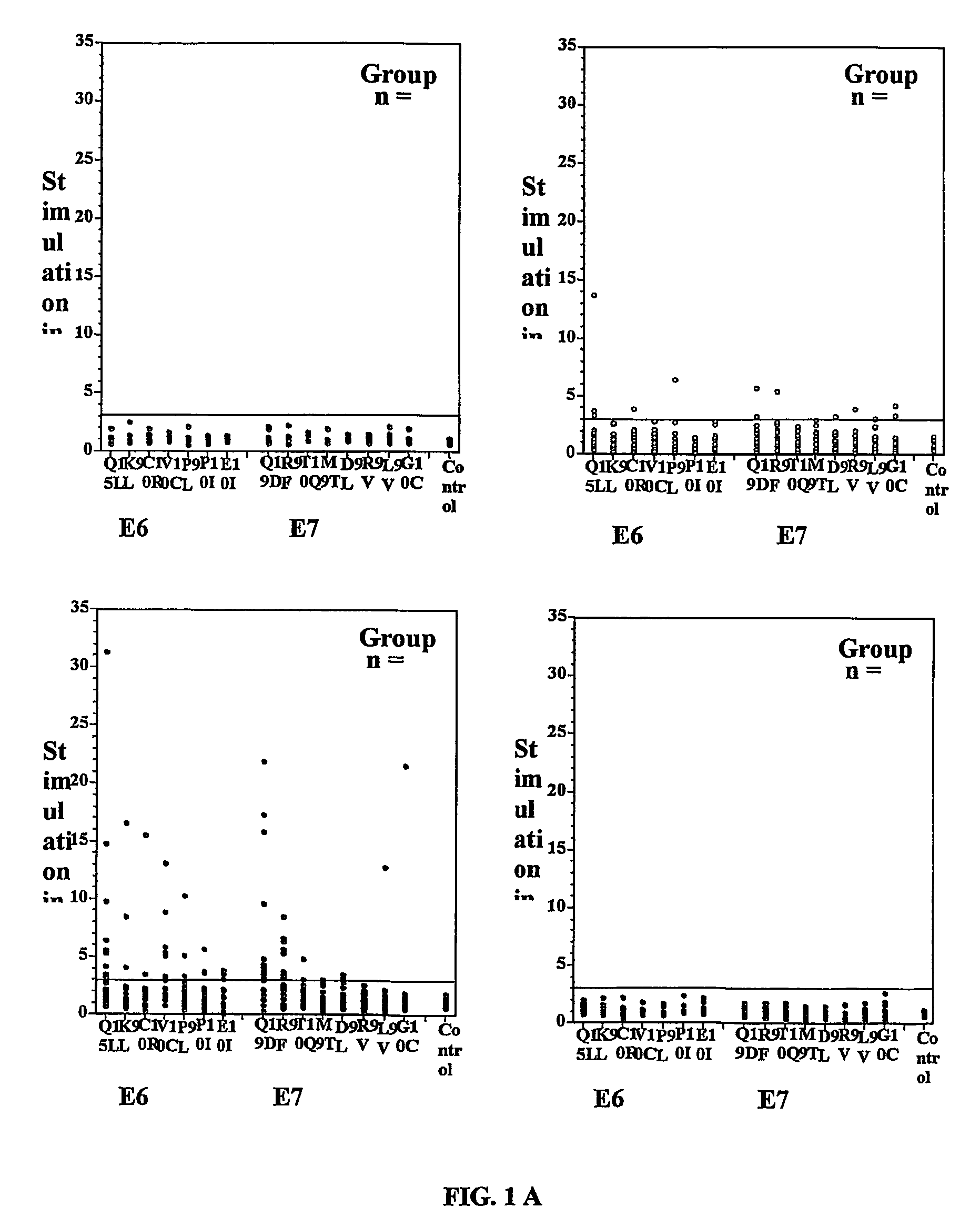
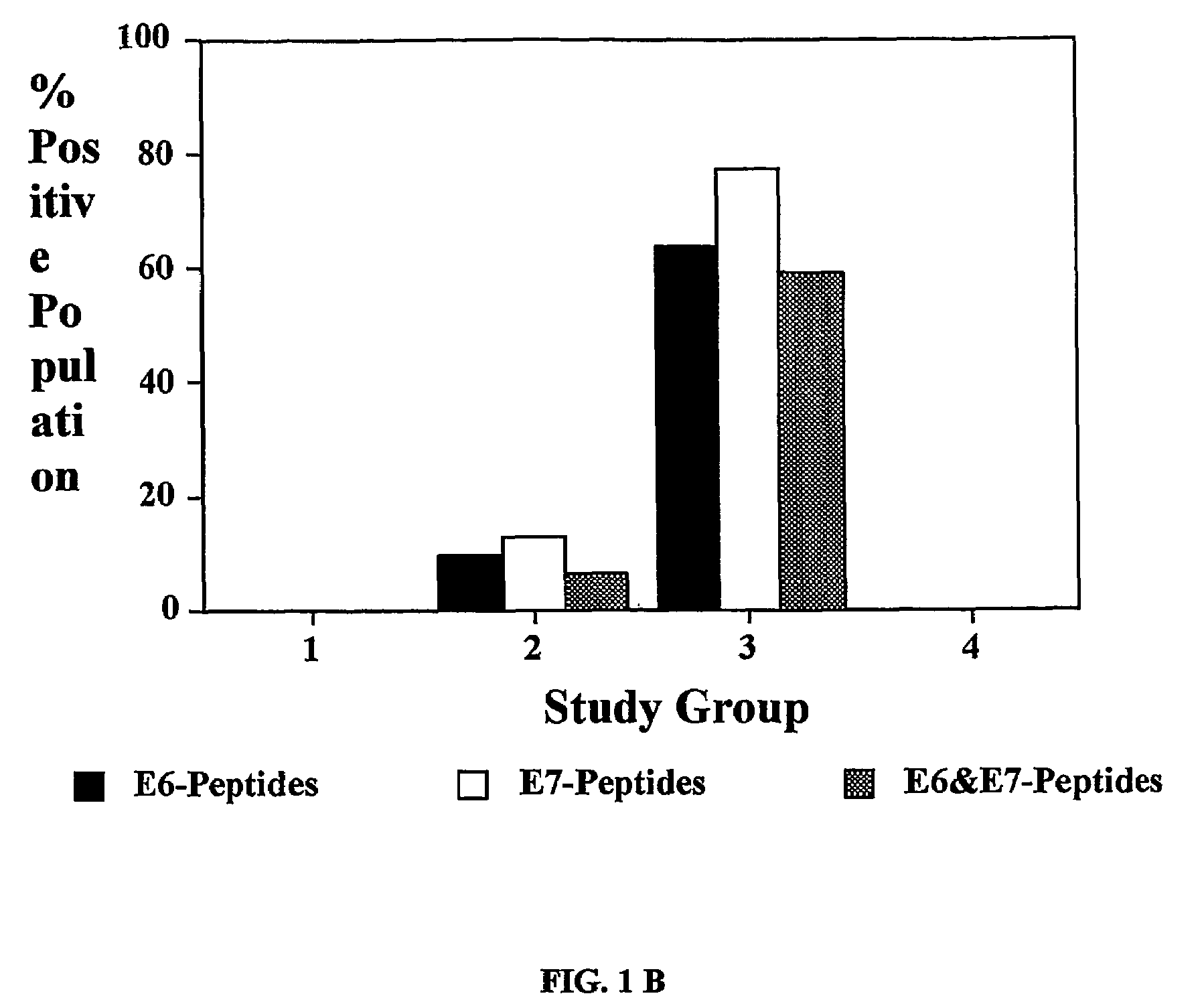
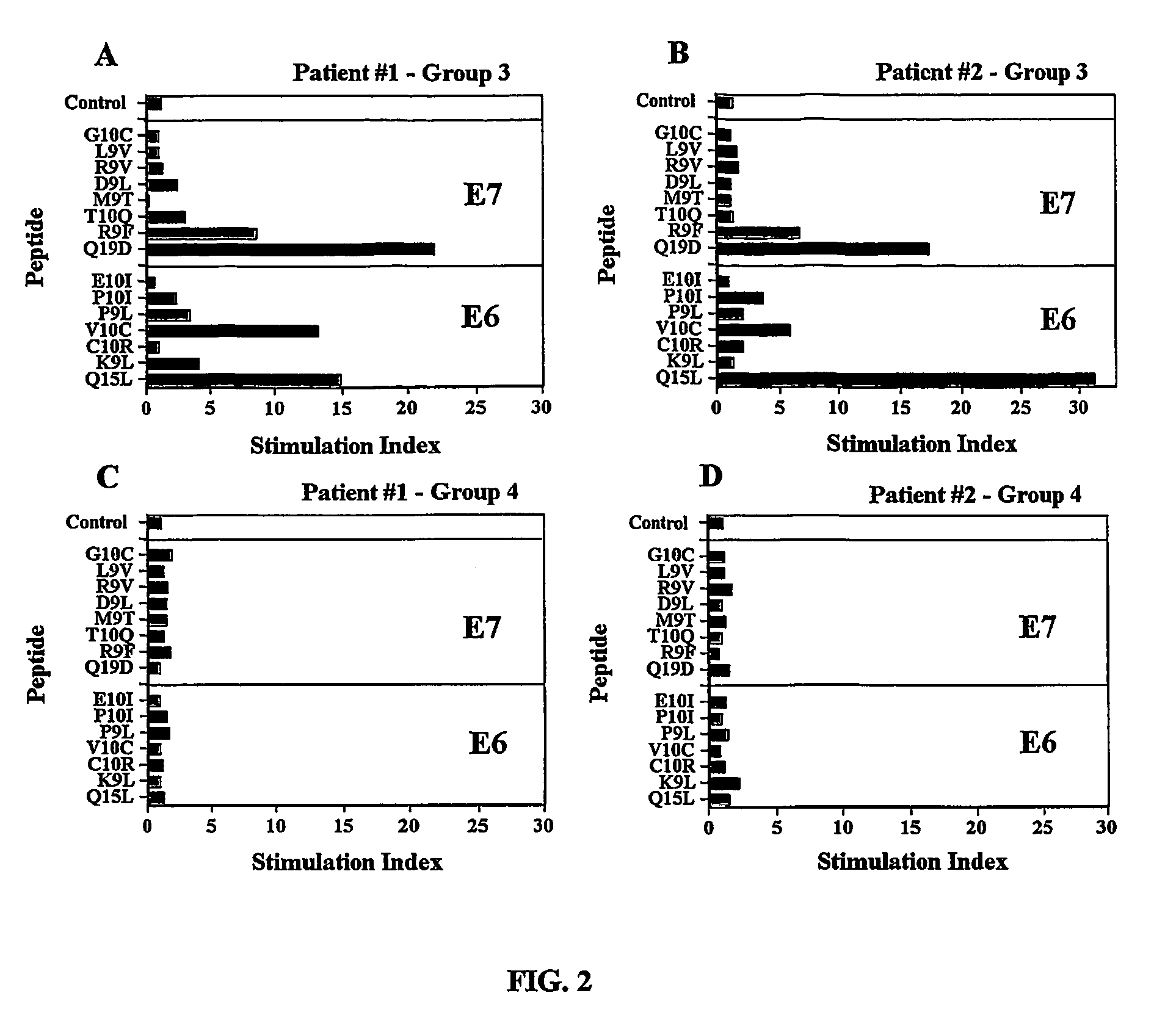
The present invention concerns the use of E6 and / or E7 peptides from human papilloma virus (HPV) to evaluate a cell-mediated response in a patient infected with HPV to determine the prognosis for that patient with respect to the development or recurrence of pre-cancerous or cancerous growths, including cervical intraepithelial neoplasia (CIN).
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Method of antigen incorporation into neisseria bacterial outer membrane vesicles and resulting vaccine formulations
InactiveUS20070166333A1Quality improvementImmune responseBacteriaDepsipeptidesEtiologyBacterial virus
Method for the insertion of protein antigens, of recombinant or synthetic origin, in outer membrane vesicles of Gram-negative bacteria without disruption of the vesicle structure, therefore maintaining the immunogenicity and immunostimulatory properties of said vesicles, and with the reported advantage that the immune response generated against the incorporated antigen is superior to the one generated when the antigen is administered alone. The resultant vaccine formulations are useful to increase protective capacity of existing vaccines and allow to extend it against different pathogens, in diseases of bacterial, viral, cancerous or other etiology. The referred formulations are applicable in the pharmaceutical industry as vaccines for therapeutic and preventive use in humans.
Owner:CENT DE ING GENETICA & BIOTECNOLOGIA
Hyperthermia, system, method, and components
ActiveUS20150190274A1Effective and safe for patientTherapeutic coolingTherapeutic heatingMedicineHigh body temperature
An IV pole mountable, therapeutic infusate processing device is incorporated into a hypothermia system to receive therapeutic fluid(s), such as normal saline, peritioneal dialysis solution, or other crystalloid solution, to heat such therapeutic fluid(s) a few degrees centigrade above normal body temperature and to direct the resulting heated infusate to and through a selected anatomical portion of a patients body to raise the temperature of that body portion so as to affect any cancerous or other tumors that may be located therein. The processing device is provided with touch screen controls and visual indicators to facilitate its proper use; while the system further includes temperature and pressure sensors to monitor the hyperthermia processing to insure patient safety.
Owner:BELMONT INSTR LLC
Loading technique for preparing radionuclide containing nanoparticles
InactiveUS20120213698A1Powder deliveryIsotope introduction to organic compoundsLiposomeDiagnostic tools
Owner:RIGSHOSPITALET +1
Compositions, methods and kits for diagnosis of lung cancer
ActiveUS20150031065A1Extend your lifeReduce morbidityBiological material analysisProteomicsHealthy subjectsBiomarker (petroleum)
The present invention provides methods for identifying biomarker proteins that exhibit differential expression in subjects with a first lung condition versus healthy subjects or subjects with a second lung condition. The present invention also provides compositions comprising these biomarker proteins and methods of using these biomarker proteins or panels thereof to diagnose, classify, and monitor various lung conditions. The methods and compositions provided herein may be used to diagnose or classify a subject as having lung cancer or a non-cancerous condition, and to distinguish between different types of cancer (e.g., malignant versus benign, SCLC versus NSCLC).
Owner:BIODESIX
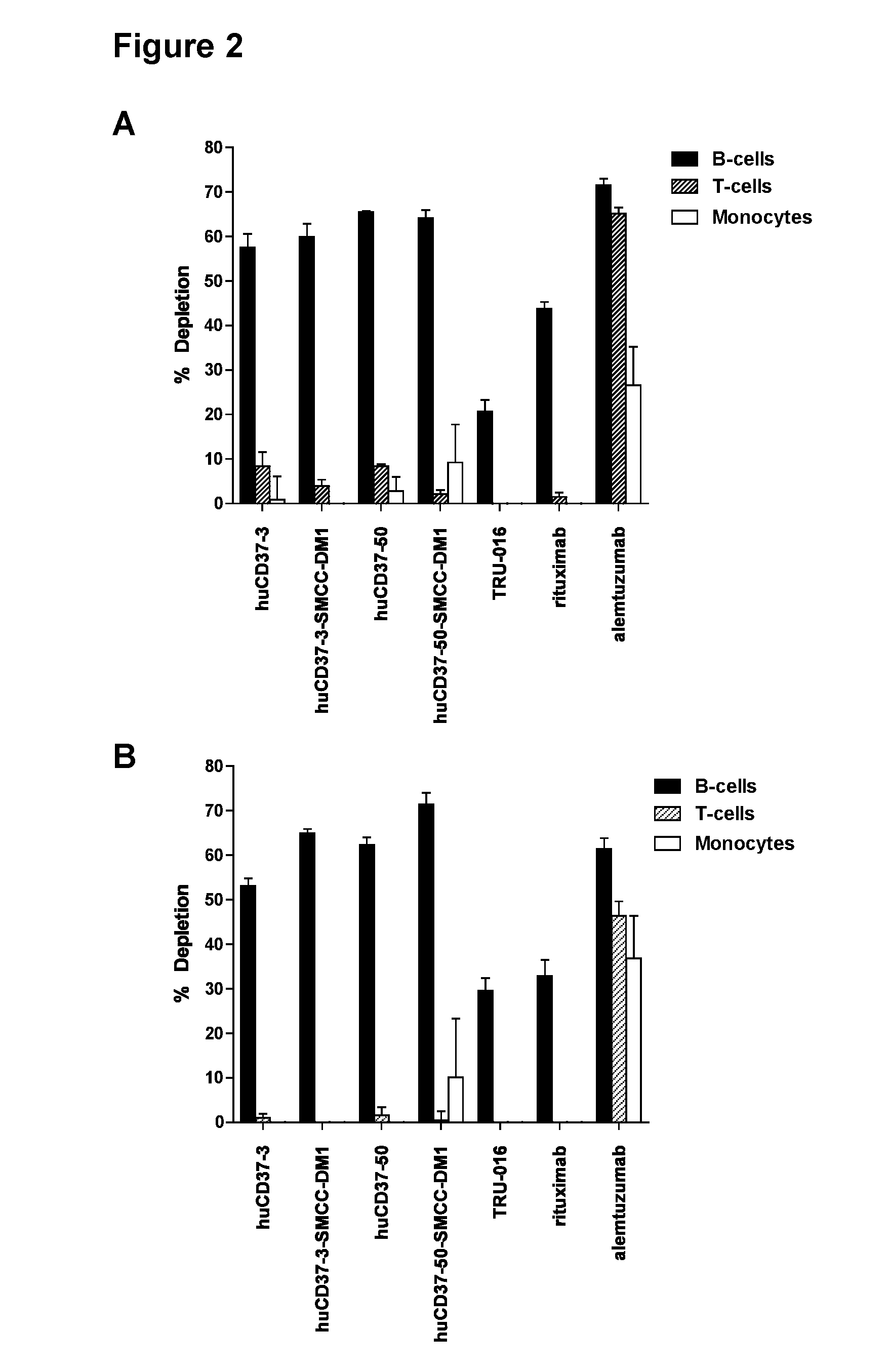
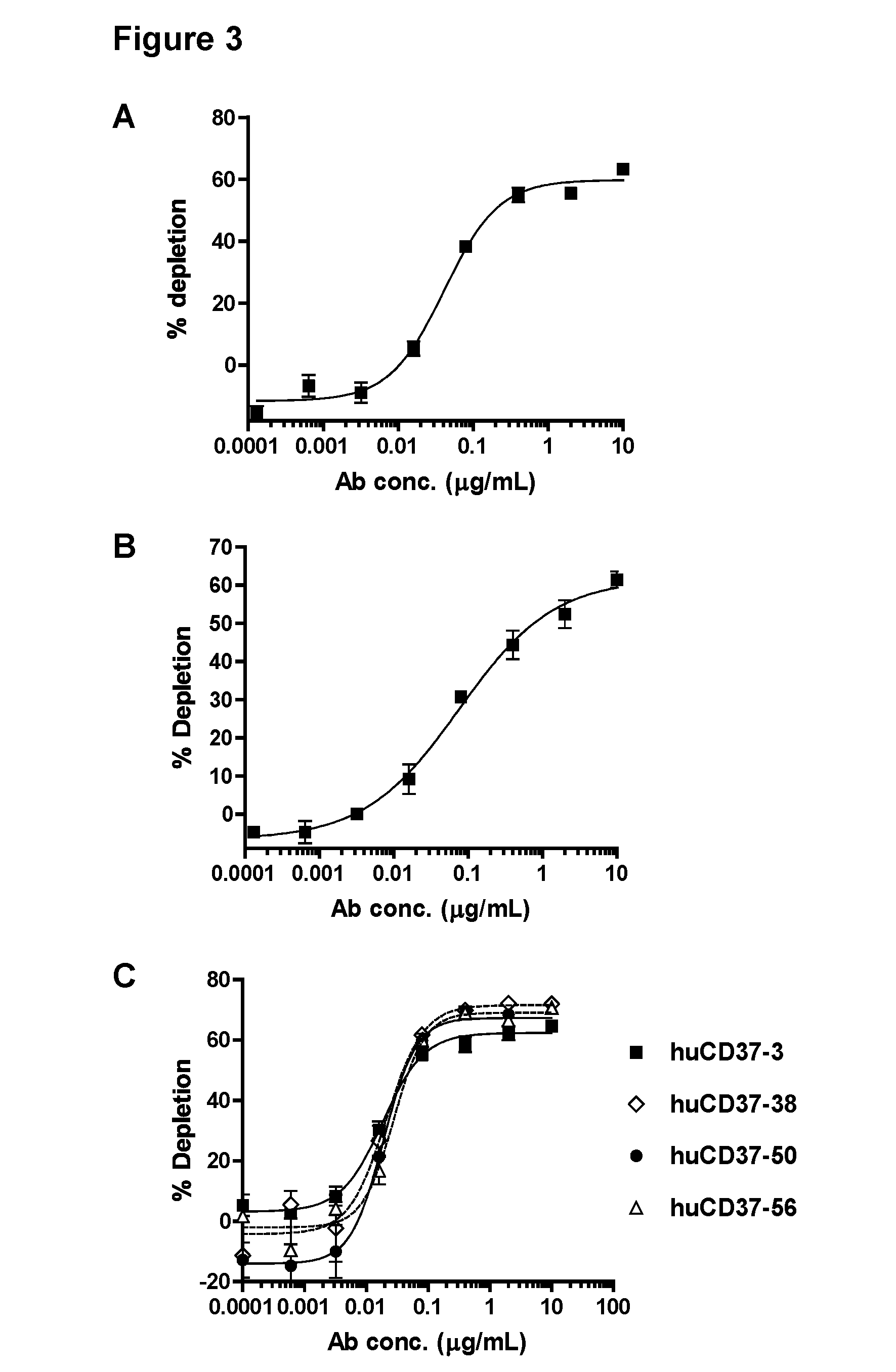
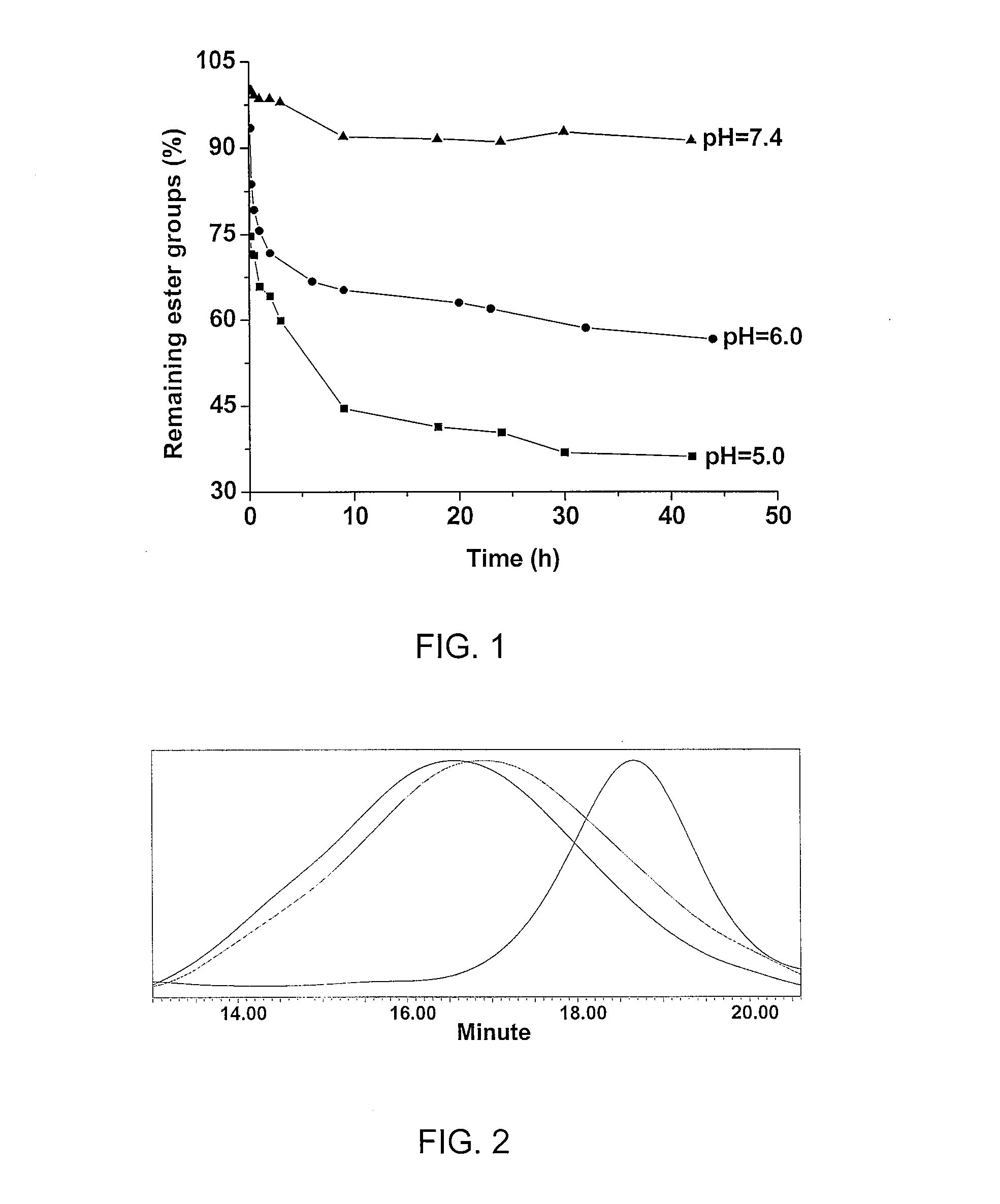
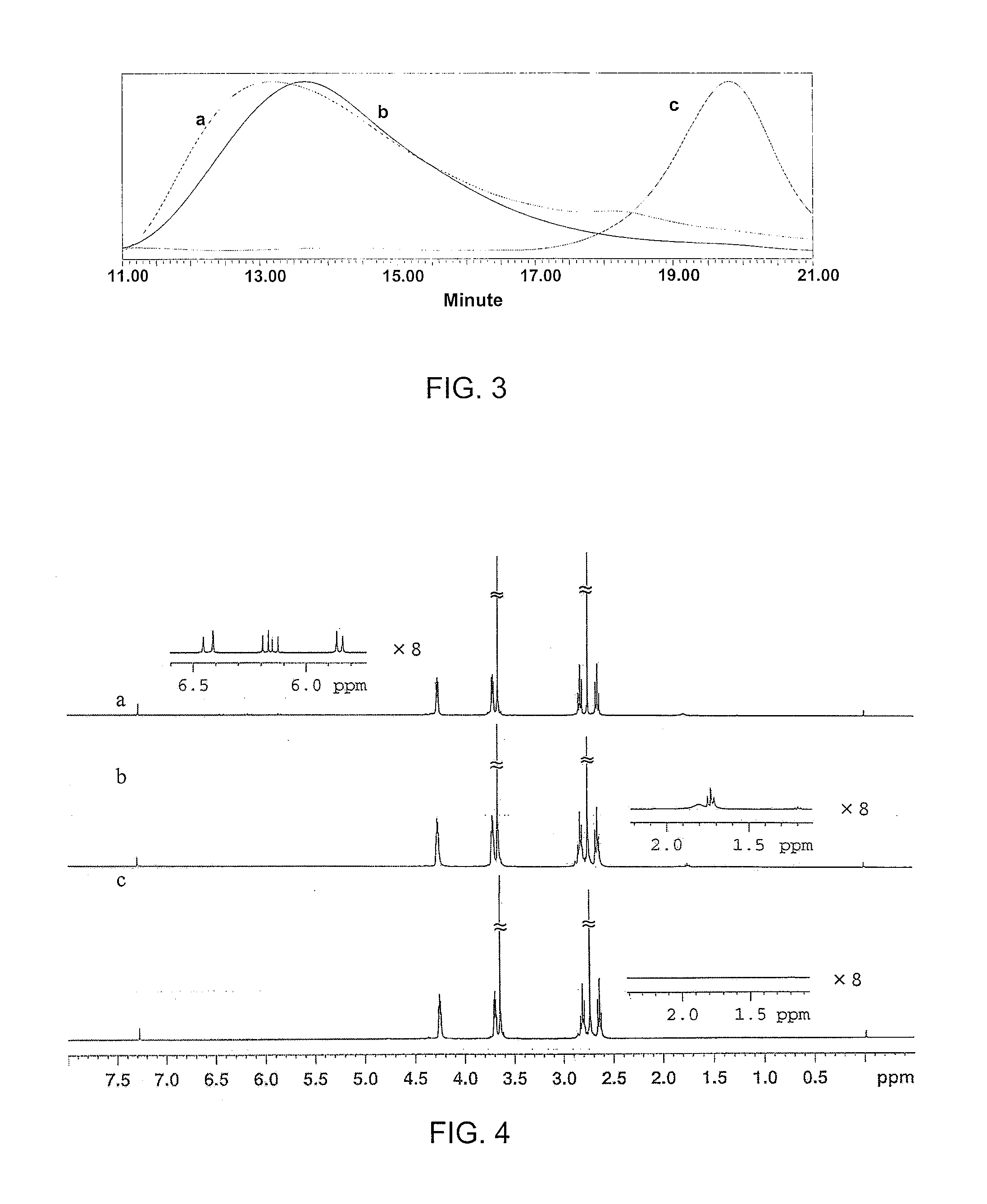
CD37-binding molecules and immunoconjugates thereof
Owner:DEBIOPHARM INTERNATIONAL SA
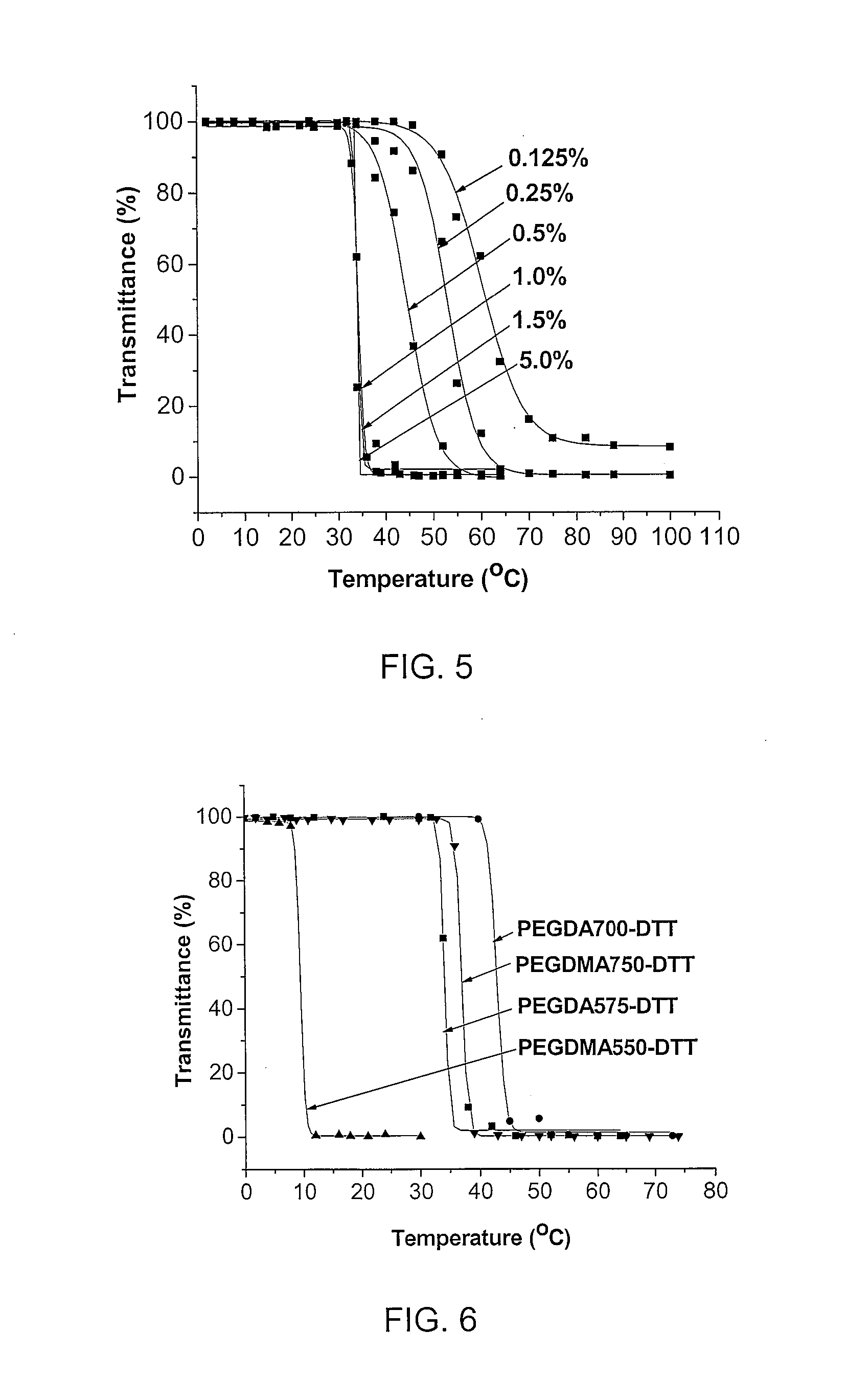
Degradable thermoresponsive poly(ethylene glycol) analogue materials
InactiveUS20120177592A1Pharmaceutical non-active ingredientsSynthetic polymeric active ingredientsCancer cellMedicine
A method of developing degradable linear poly(ethylene glycol) PEG (DPEG) with multiple functioning capacities, which can be used as drug carriers for cancer cell delivery. A DPEG may be effective in targeting cancerous tumors through an enhanced permeation and retention effect (EPR). The DPEG will then degrade in the acidic extracellular fluid of solid tumors leading to fast cellular internalizations, finally degrading in the lysosome for efficient renal clearance. These may be used in conjunction with drugs and / or targeting groups. Furthermore, DPEGs are thermoresponsive, on an as needed basis, making them useful for in vivo application.
Owner:UNIVERSITY OF WYOMING
Enclosures housing cell-coated supports for treating tumors
InactiveUS7056503B2Bioreactor/fermenter combinationsPowder deliveryAbnormal tissue growthTherapeutic protein
The present invention relates to devices, systems and methods for treating tumors. In particular, the present invention relates to enclosures housing cell-coated supports for promoting regression of tumors, such as cancerous tumors, papillomas, and warts. In preferred embodiments, the present invention provides methods of promoting tumor regression employing enclosures secreting therapeutic proteins.
Owner:RGT UNIV OF MICHIGAN
Gene therapy of tumors using non-viral delivery system
InactiveUS7157098B1Reduce lung cancer mortalityReduction in formation of tumorIn-vivo radioactive preparationsDispersion deliveryAbnormal tissue growthLipid formation
The present invention provides a pharmaceutical composition, comprising: (a) cationic lipids, wherein said lipids are a liposomal mixture of a diacyl-ethyl-phosphocholine and 1,2-diacyl-sn-glycero-3-phosphoethanolamine; and (b) a plasmid cDNA sequence encoding a protein having tumor suppressor or pro-apoptotic activity. This composition has a high gene transfection efficiency at non-toxic doses and is designed to transfect human bronchial premalignant lesions and early endo-bronchial malignancies. Also provided is a method of method of treating a cancerous or pre-cancerous condition of the respiratory tract in an individual in need of such treatment, comprising the step of administering to said individual a pharmacologically effective dose of a pharmaceutical composition, comprising: (a) cationic lipids, wherein said lipids are a liposomal mixture of a diacyl-ethyl-phosphocholine and 1,2-diacyl-sn-glycero-3-phosphoethanolamine; and (b) a plasmid cDNA sequence encoding a protein having tumor suppressor or pro-apoptotic activity.
Owner:PEREZ SOLER ROMAN +1
Heterocyclic compound
ActiveUS20200255439A1Superior MAGL inhibitory actionAgent for prophylaxis and treatmentOrganic active ingredientsNervous disorderHuntingtons choreaAmytrophic lateral sclerosis
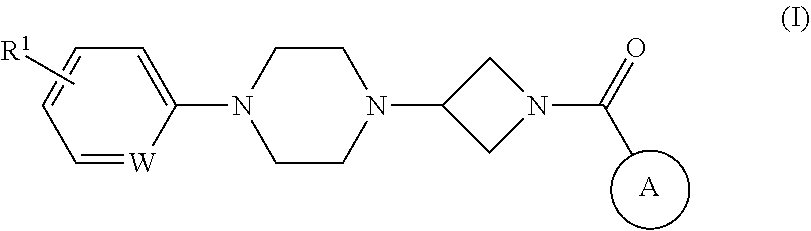
The present invention provides a compound having an MAGL inhibitory action, and expected to be useful as an agent for the prophylaxis or treatment of neurodegenerative diseases (e.g., Alzheimer's disease, Parkinson's disease, Huntington's disease, amyotrophic lateral sclerosis, traumatic brain injury, glaucoma, multiple sclerosis etc.), anxiety disorder, pains (e.g., inflammatory pain, cancerous pain, neurogenic pain etc.), epilepsy, depression and the like.The present invention relates to a compound represented by the formula (I):wherein each symbol is as defined in the description, or a salt thereof.
Owner:TAKEDA PHARMA CO LTD
PITX2 polynucleotide, polypeptide and methods of use therefor
InactiveUS7427476B2Raise the possibilityHigh sensitivityCell receptors/surface-antigens/surface-determinantsSugar derivativesT-type calcium channelCell type
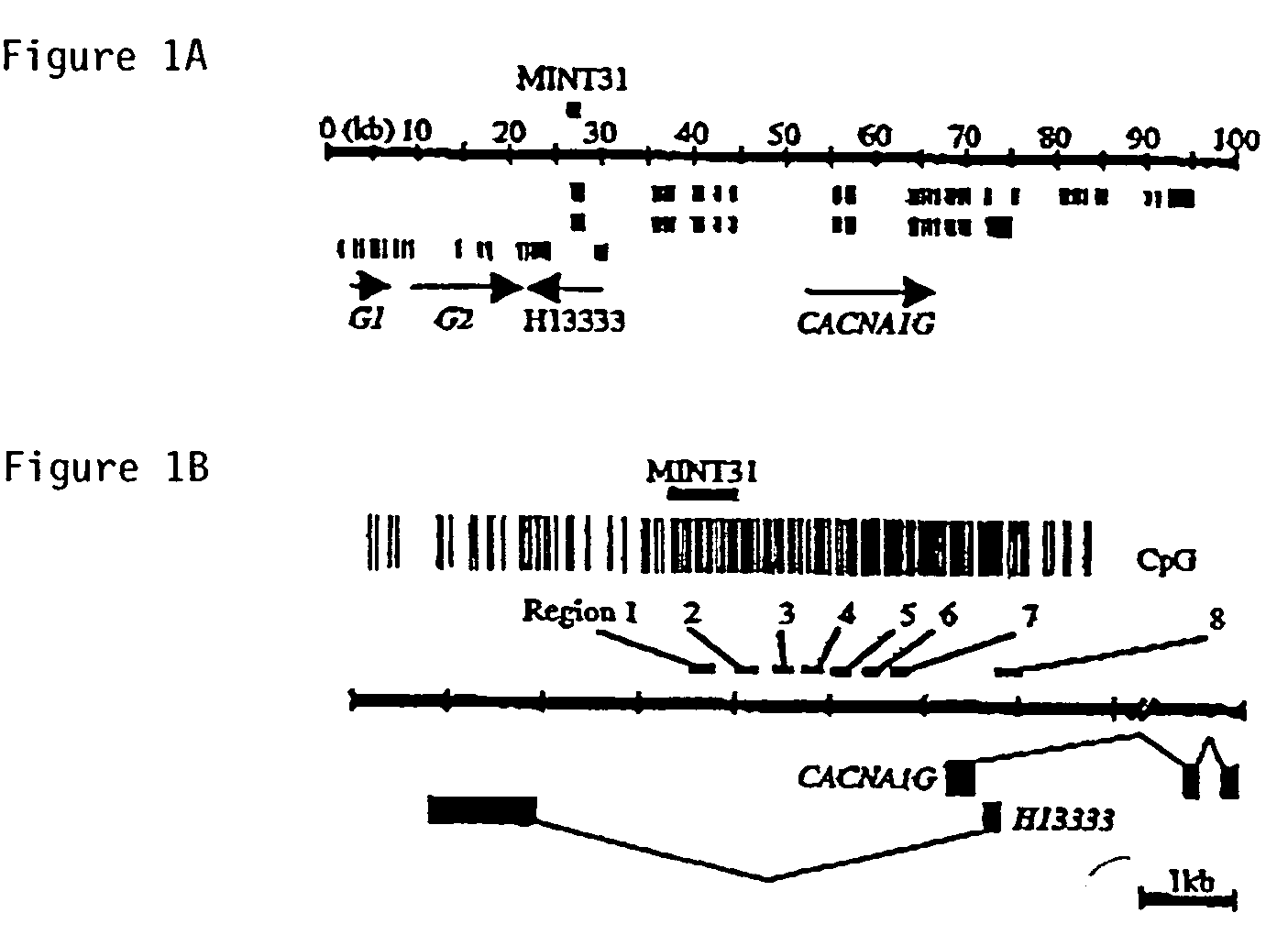
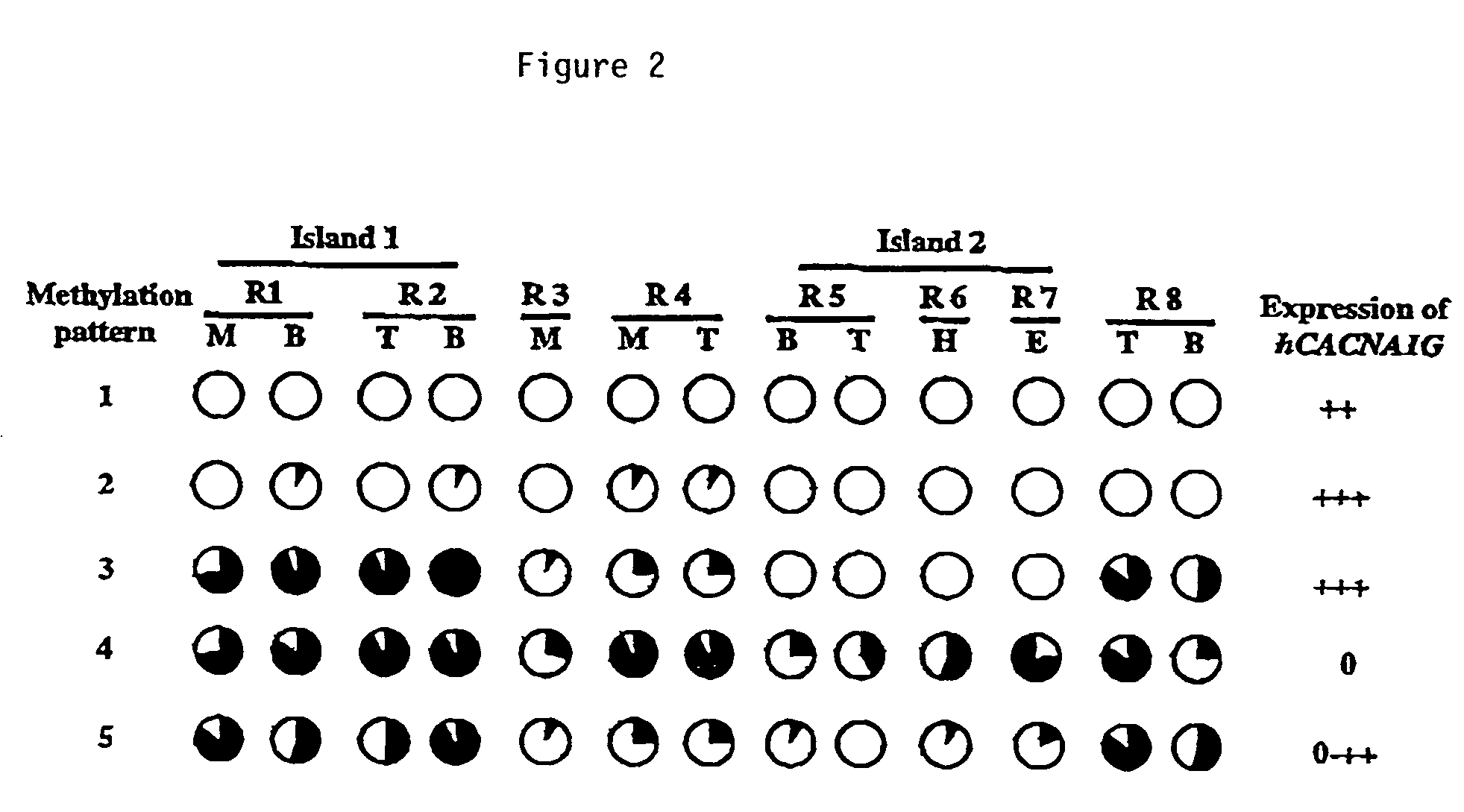
A novel T-type calcium channel (CACNA1G) is provided, as are polynucleotides encoding the same. CACNA1G has been implicated in cellular proliferative disorders. More specifically, it has been observed that the methylation state of specific regions within CpG islands associated with the CACNA1G gene correlates with a number of cancerous phenotypes involving a variety of tissue and cell types. Also provided are methods for detecting cellular proliferative disorders by determining the methylation state of genes or regulatory regions associated therewith, including CACNA1G, as well as kits containing reagents for performing invention methods.
Owner:THE JOHNS HOPKINS UNIVERSITY SCHOOL OF MEDICINE
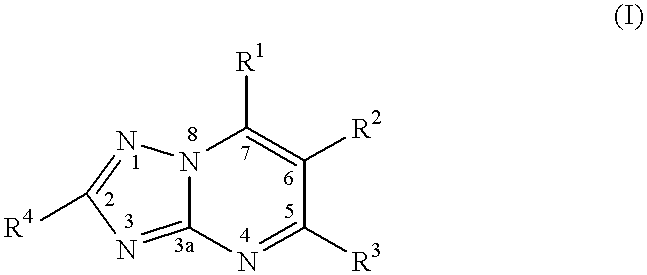
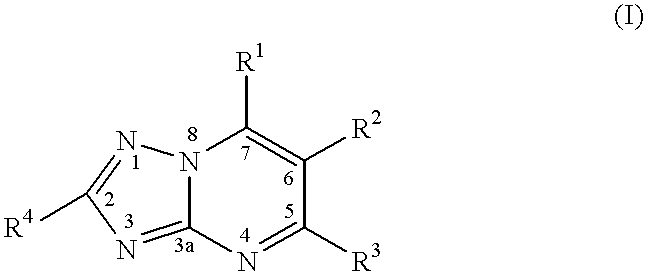
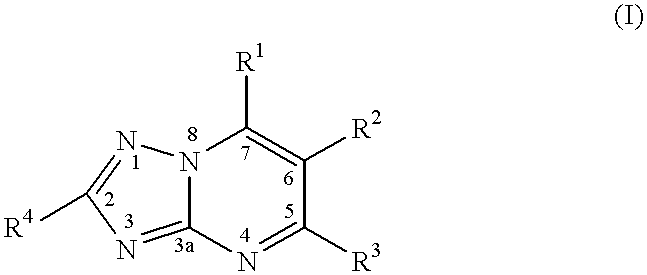
Substituted-triazolopyrimidines as anticancer agents
InactiveUS7329663B2Prevention of resistanceTreatment of resistanceBiocideOrganic chemistryDiseaseAnticarcinogen
The invention provides a method of treating or inhibiting the growth of cancerous tumor cells and associated diseases in a mammal in need thereof which comprises administering to said mammal an effective amount of a substituted triazolopyrimidine derivative or a pharmaceutically acceptable salt thereof and further provides a method of treating or inhibiting the growth of cancerous tumor cells and associated diseases in a mammal in need thereof by interacting with tubulin and microtubules and promoting microtubule polymerization which comprises administering to said mammal an effective amount of a substituted triazolopyrimidine derivative or a pharmaceutically acceptable salt thereof.
Owner:WYETH LLC
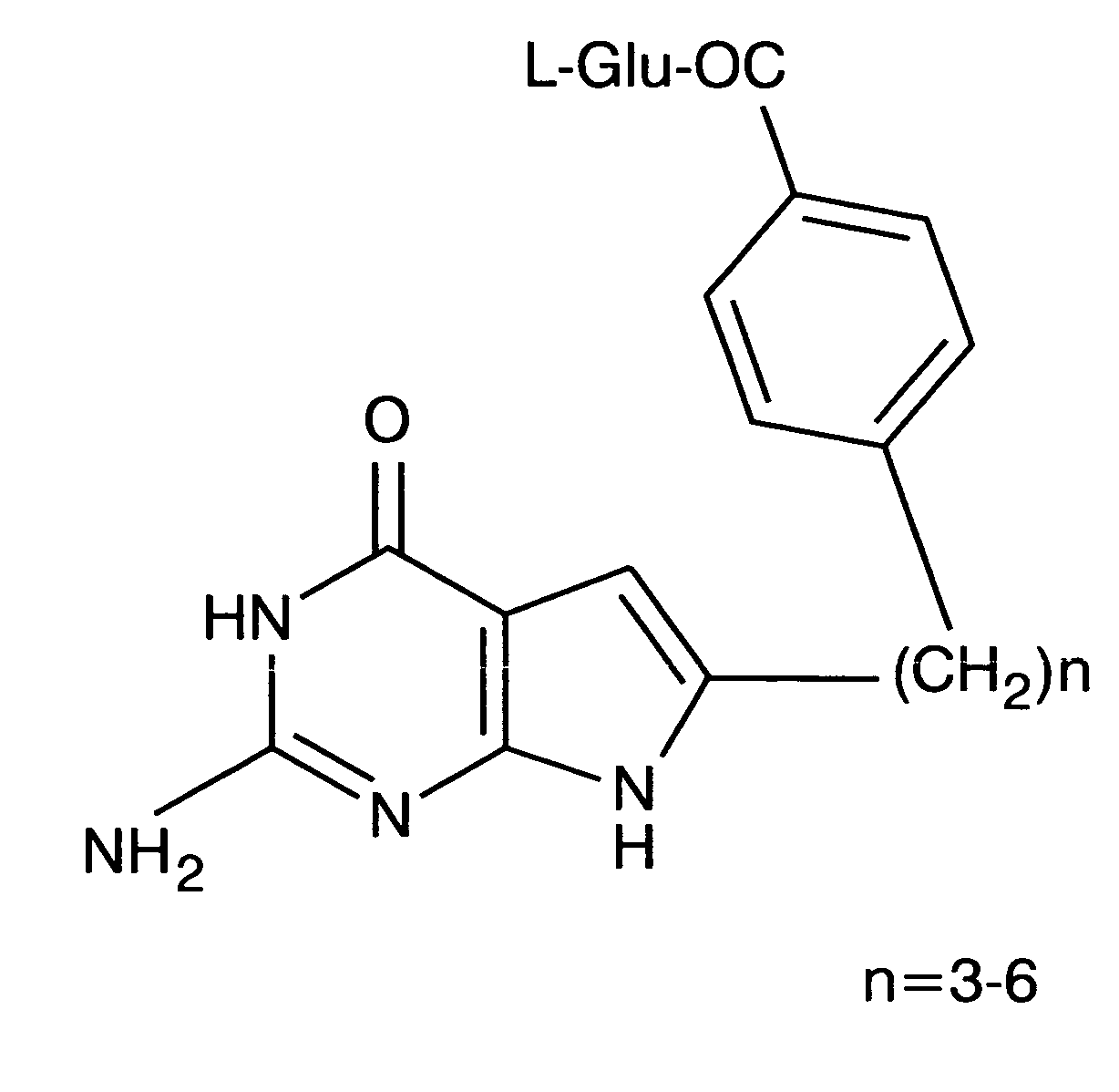
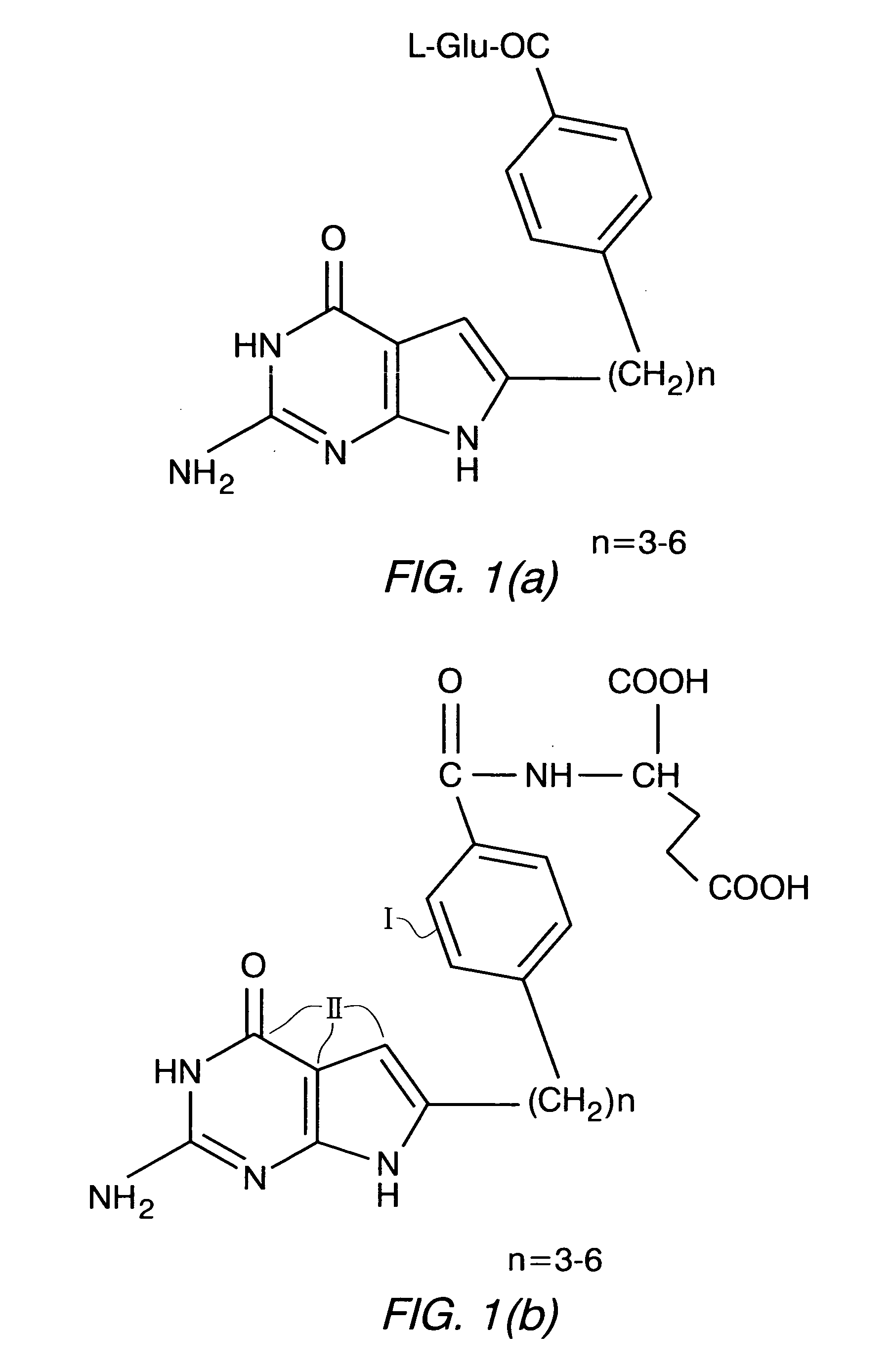
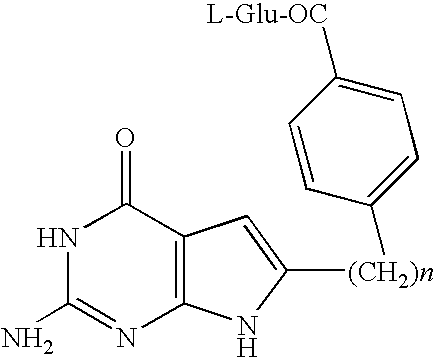
Methods of using selective chemotherapeutic agents for targeting tumor cells
A method for treating cancer tumors, particularly ovarian cancer tumors, is described, where fused cyclic pyrimidine having a cancer treating ability is selectively delivered to an FR expressing cancerous tumor.
Owner:DUQUESNE UNIVERSITY +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com