High molecular anticarcinogenic prodrug, its preparing method and use
A polymer and anticancer drug technology, applied in the field of medicine and chemical industry
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
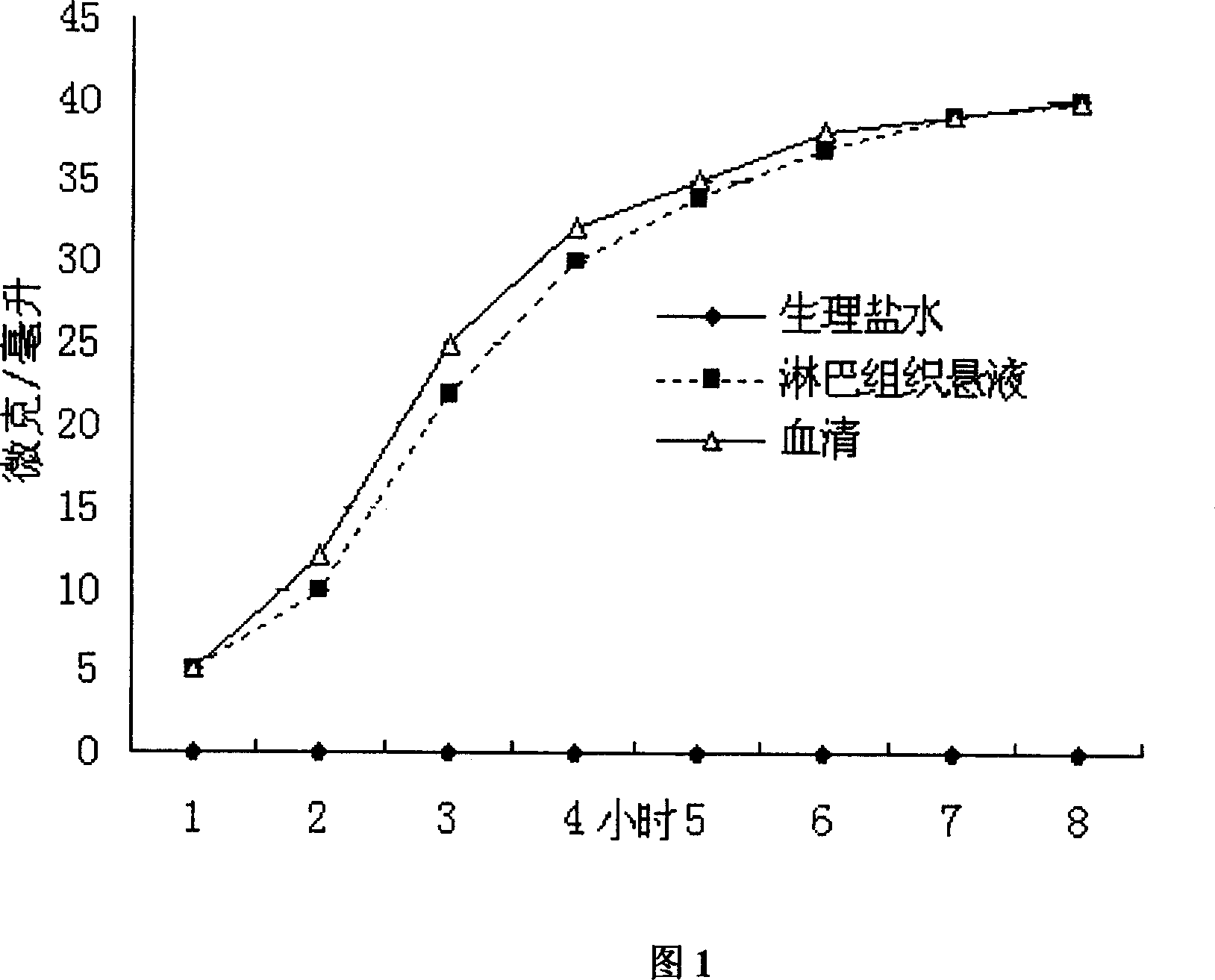
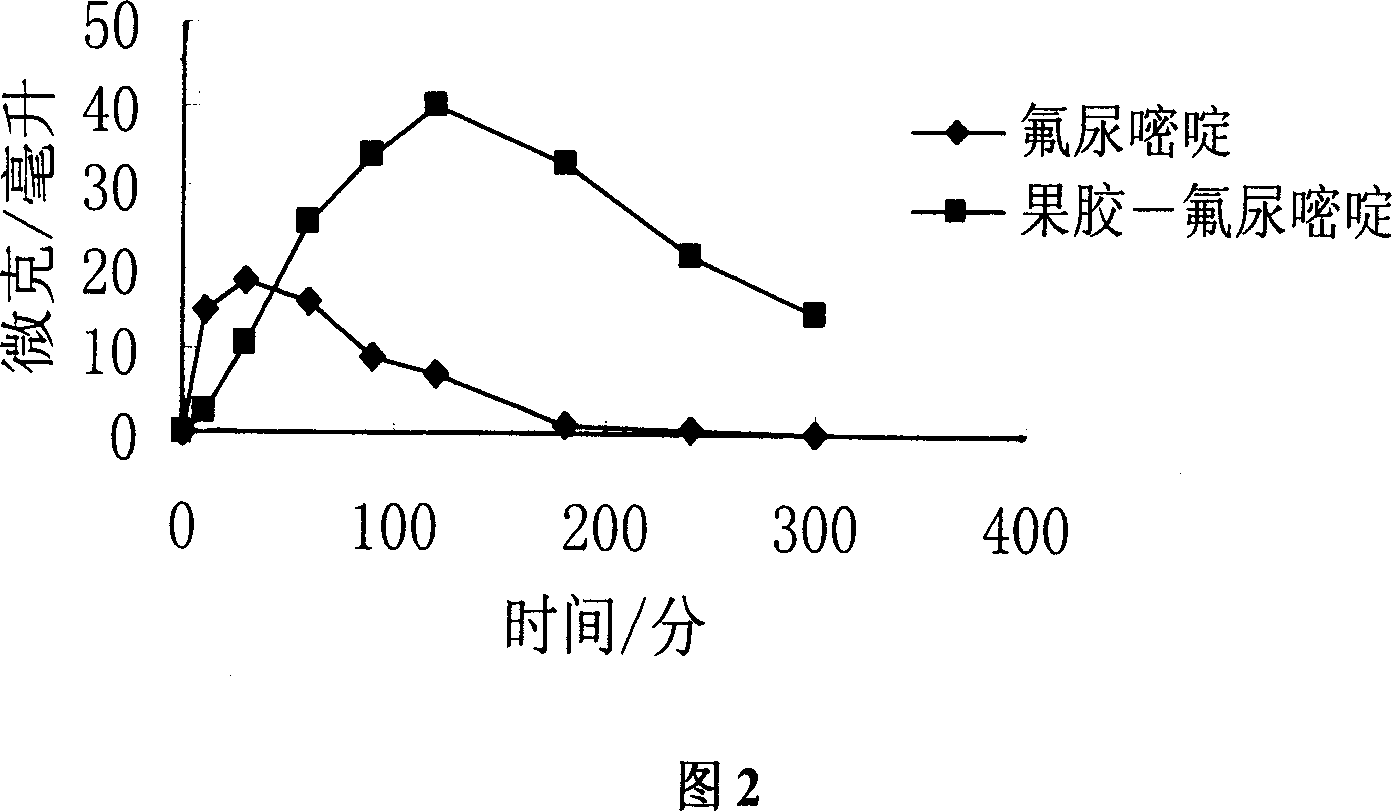
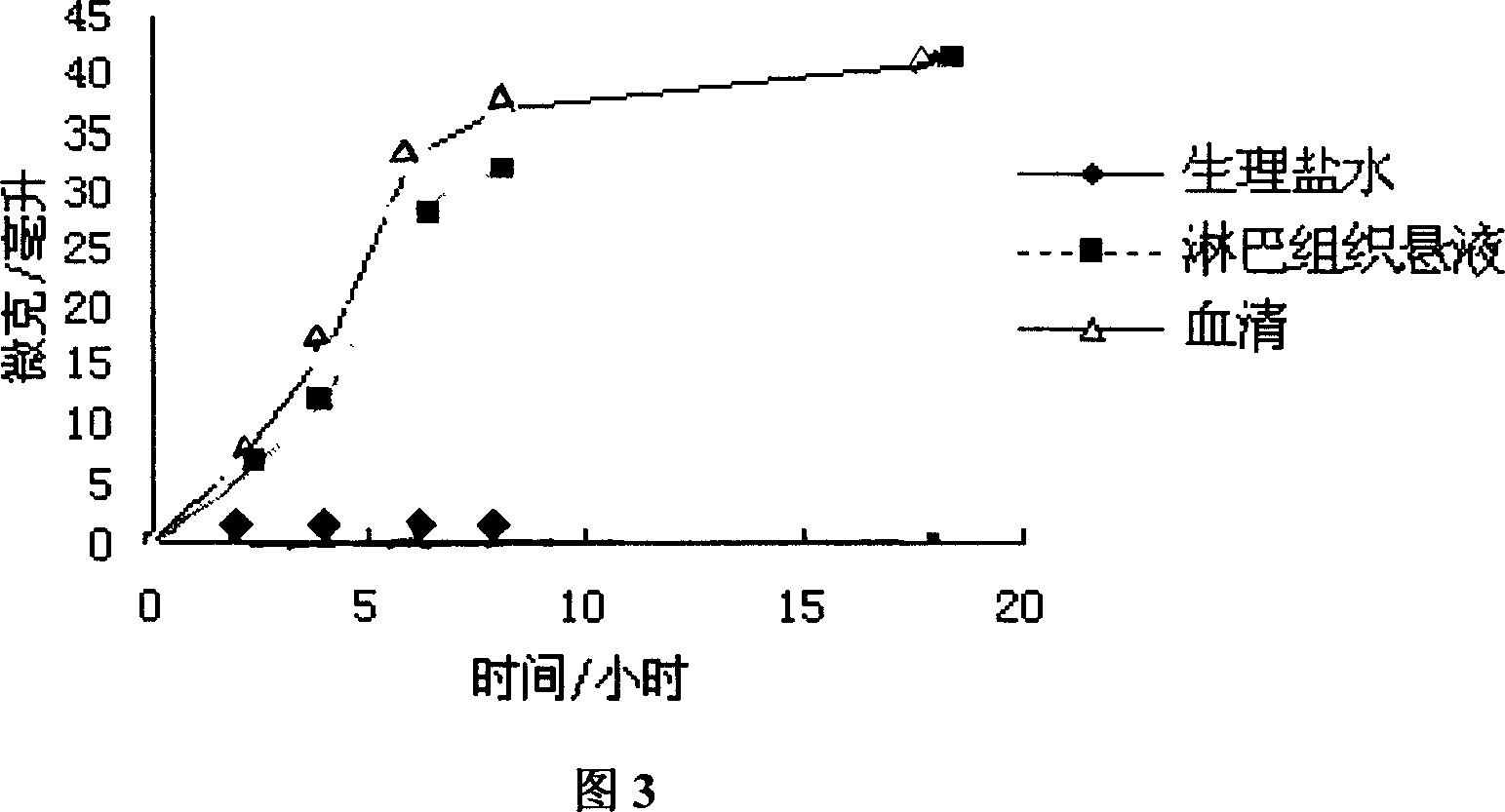
Image
Examples
Embodiment 1
[0084] Example 1: Synthesis of 5-fluorouracil-pectin prodrugs:
[0085] During its preparation, dissolve 15mmol of 5-fluorouracil in a mixed solvent of 30ml of anhydrous pyridine and 50ml of anhydrous sulfolane, control the temperature at 15°C, stir slowly and dropwise add 20ml of anhydrous toluene solution containing 1.4ml (about 16mmol) of oxalyl chloride, dropwise After the addition, control the temperature at 50°C, stir for 4 hours, weigh 1g of pectin and add it to the reaction bottle, control the temperature at 50°C, and stir for 48 hours. After the reaction, add acetone to precipitate, centrifuge, dissolve the precipitate in secondary water, and use The dialysis bag was dialyzed and vacuum-dried at 60°C to obtain the water-soluble 5-fluorouracil-pectin prodrug. Its synthesis process is as follows:
[0086]
[0087] Molecular structure of pectin
[0088]
Embodiment 2
[0089] Embodiment 2: the synthesis of alzyme-pectin prodrug:
[0090] In the preparation process, dissolve 0.2mmol azin in a mixed solvent of 20ml sulfolane and 10ml anhydrous pyridine, control the temperature at 15°C, and slowly add 10ml anhydrous toluene solution containing 0.11ml (about 1.2mmol) oxalyl chloride dropwise with stirring in the dark After the dropwise addition, continue to stir for 4 hours, weigh 0.3g of pectin and add it to the reaction bottle, control the temperature at 30°C, and stir for 48 hours. Dialysis, vacuum drying, to obtain water-soluble alzin-pectin prodrug. Its synthesis process is as follows
[0091]
[0092] (Note: R stands for R 1 represent )
Embodiment 3
[0093] Embodiment 3: daunorubicin-pectin prodrug:
[0094] In the preparation process, dissolve 0.2mmol daunorubicin in a mixed solvent of 20ml sulfolane and 10ml anhydrous pyridine, control the temperature at 15°C, and slowly add 0.1ml (about 1.15mmol) oxalyl chloride containing 10ml anhydrous Toluene solution, after the dropwise addition, continue to stir for 4 hours, weigh 0.3g pectin into the reaction bottle, control the temperature at 30°C, and stir for 48 hours. After the reaction is completed, add acetone to precipitate, centrifuge, and dissolve the precipitate in secondary water. The dialysis bag was dialyzed and vacuum-dried to obtain the water-soluble daunorubicin-pectin prodrug. Its synthesis process is as follows:
[0095]
[0096] (Note: R stands for R 1 represent )
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com