Methods and reagents for vaccination which generate a CD8 T cell immune response
a technology reagents, which is applied in the field of vaccines, can solve the problems of inability to generate high levels of cd8 t cells by immunization, impede the development of vaccines against several diseases, and no means of vaccinating against malaria infection, and achieve good boosting effect to a primed ctl response
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0083] Materials and Methods
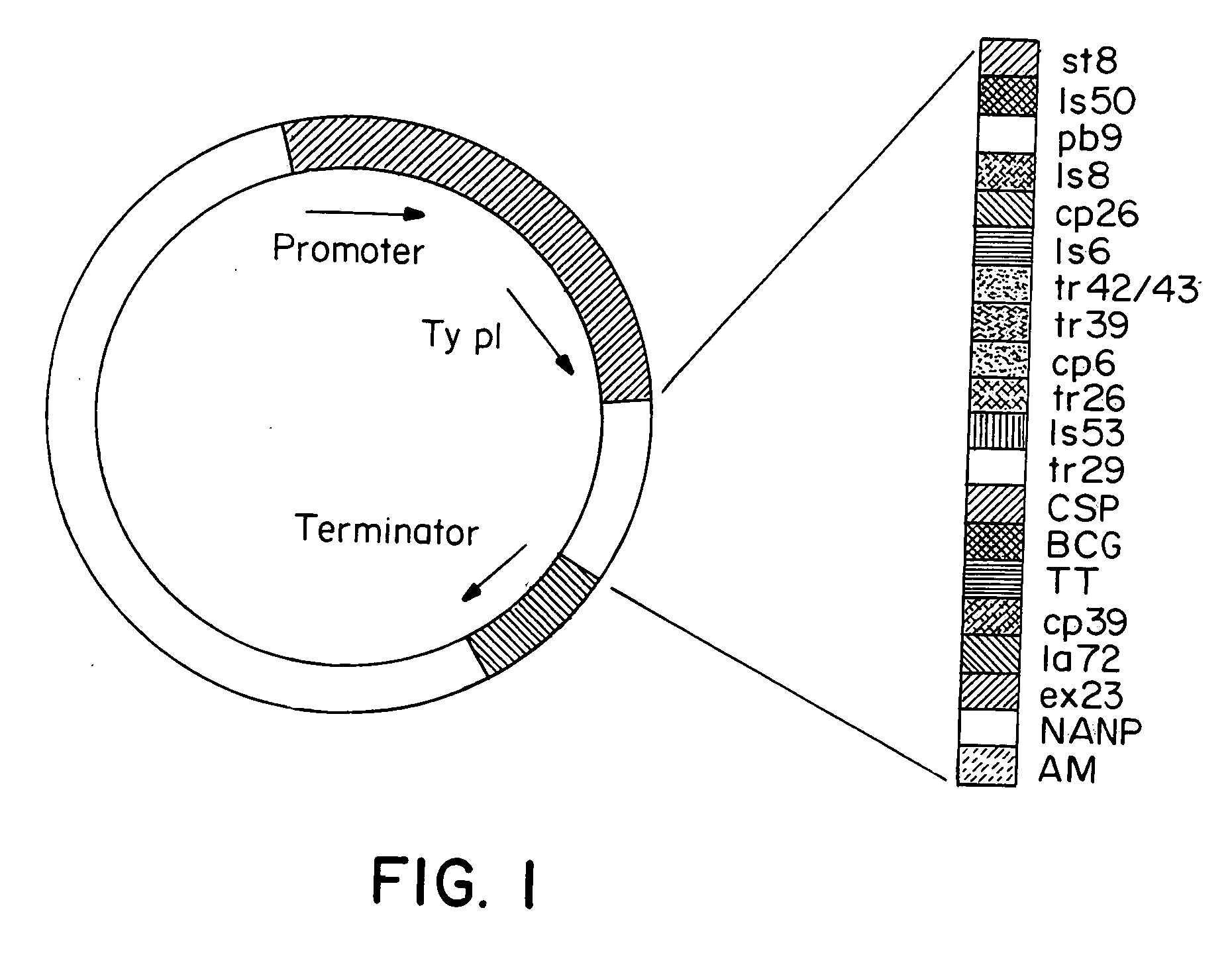
[0084] Generation of the Epitope Strings
[0085] The malaria epitope string was made up of a series of cassettes each encoding three epitopes as shown in Table 1, with restriction enzyme sites at each end of the cassette. Each cassette was constructed from four synthetic oligonucleotides which were annealed together, ligated into a cloning vector and then sequenced to check that no errors had been introduced. Individual cassettes were then joined together as required. The BamHI site at the 3' end of cassette C was fused to the Bg1II site at the 5' end of cassette A, destroying both restriction enzyme sites and encoding a two amino acid spacer (GS) between the two cassettes. Cassettes B, D and H were then joined to the string in the same manner. A longer string containing CABDHFE was also constructed in the same way.
5TABLE 1 CTL Epitopes of the Malaria (M) String Amino acid HLA Cassette Epitope Sequence DNA sequence Type restriction A Ls8 KPNDKSLY AAGCCGAACG...
example 2
[0131] Immunogenicity Studies in Mice
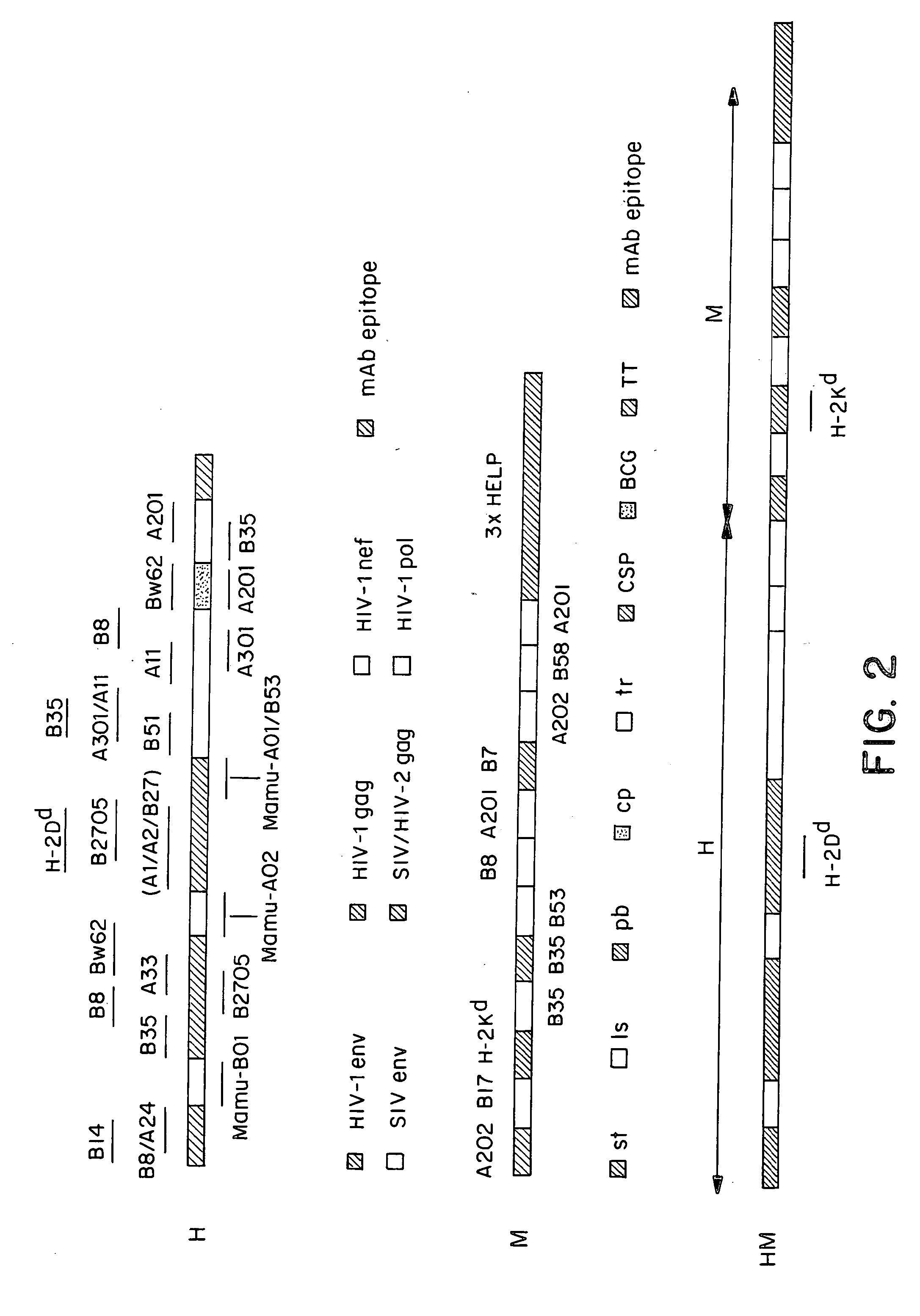
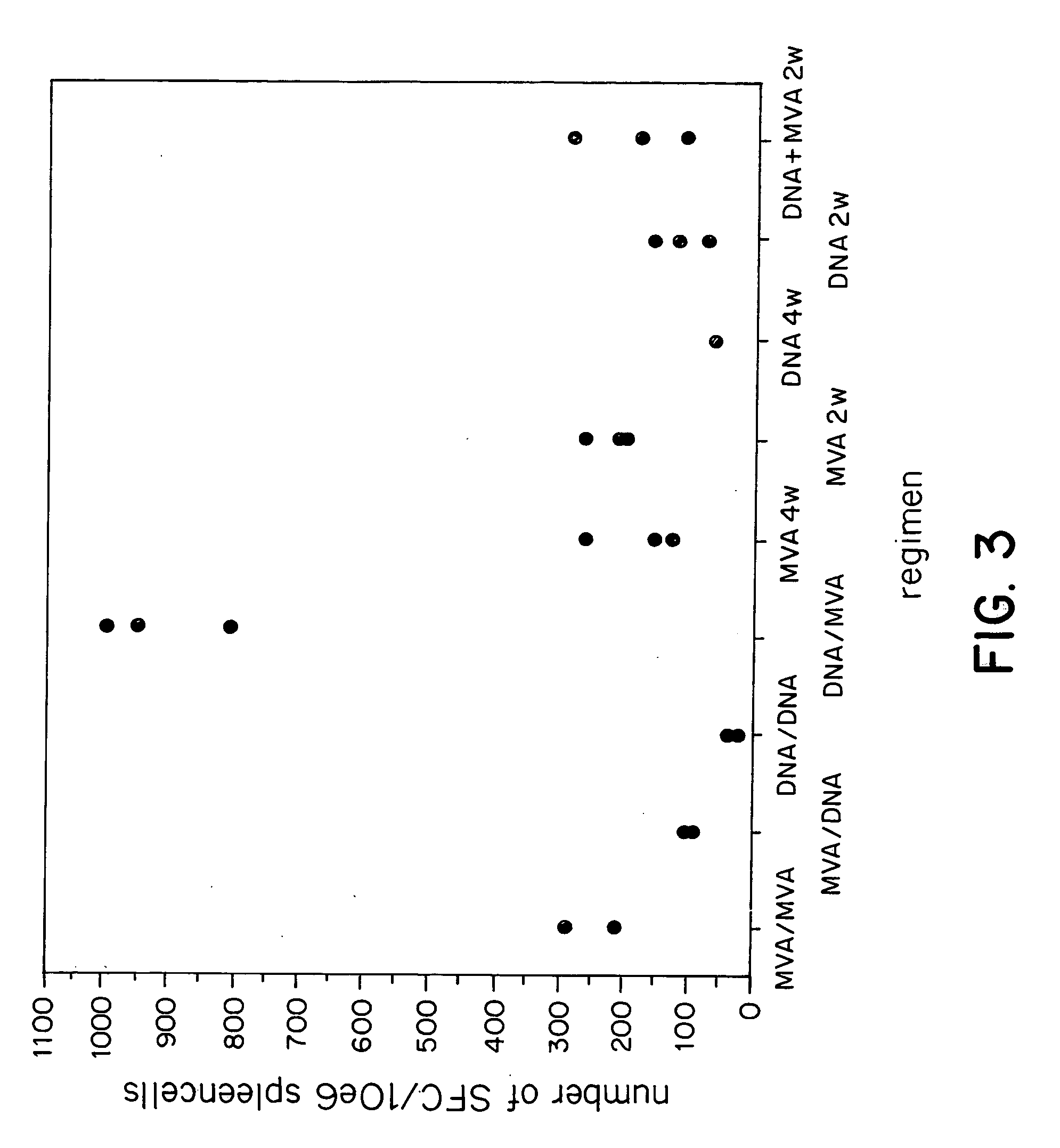
[0132] Previous studies of the induction of CTL against epitopes in the circumsporozoite (CS) protein of Plasmodium berghei and Plasmodium yoelii have shown variable levels of CTL induction with different delivery systems. Partial protection has been reported with plasmid DNA (Sedegah et al. 1994), influenza virus boosted by replicating vaccinia virus (Li et al. 1991), adenovirus (Rodrigues et al 1997) and particle delivery systems (Schodel et al. 1994). Immunisation of mice intramuscularly with 50 micrograms of a plasmid encoding the CS protein produced moderate levels of CD8+cells and CTL activity in the spleens of these mice after a single injection (FIGS. 3, 4A-4D).
[0133] For comparison groups of BALB / c mice (n=5) were injected intravenously with 10.sup.6 ffu / pfu of recombinant vaccinia viruses of different strains (WR, NYVAC and MVA) all expressing P. berghei CSP. The frequencies of peptide-specific CD8+T cells were measured 10 days later in...
example 3
[0167] Malaria Challenge Studies in Mice
[0168] To assess the protective efficacy of the induced levels of CD8+T cell response immunised BALB / c or C57BL / 6 mice were challenged by intravenous injection with 2000 or 200 P. berghei sporozoites. This leads to infection of liver cells by the sporozoites. However, in the presence of a sufficiently strong T lymphocyte response against the intrahepatic parasite no viable parasite will leave the liver and no blood-stage parasites will be detectable. Blood films from challenged mice were therefore assessed for parasites by microscopy 5-12 days following challenge.
[0169] BALB / c mice immunised twice with a mixture of two plasmid DNAs encoding the CS protein and the TRAP antigen, respectively, of P. berghei were not protected against sporozoite challenge. Mice immunised twice with a mixture of recombinant MVA viruses encoding the same two antigens were not protected against sporozoite challenge. Mice immunised first with the two recombinant MVAs ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| time | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com