Preparation method and product of H9N2 subtype avian influenza inactivated vaccine
An inactivated vaccine and bird flu technology, applied in the field of bioengineering, can solve problems such as biological safety hazards and difficulty in the reproduction of bird flu virus cells, and achieve the effects of avoiding biological safety hazards, meeting the requirements of immune production, and simple and fast production methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
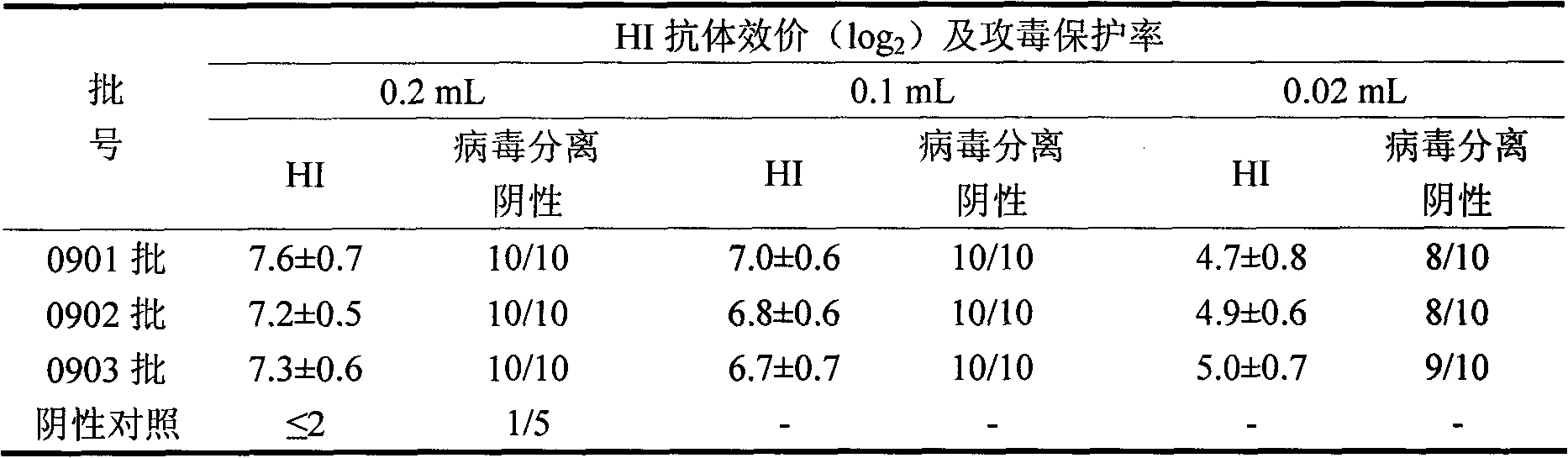
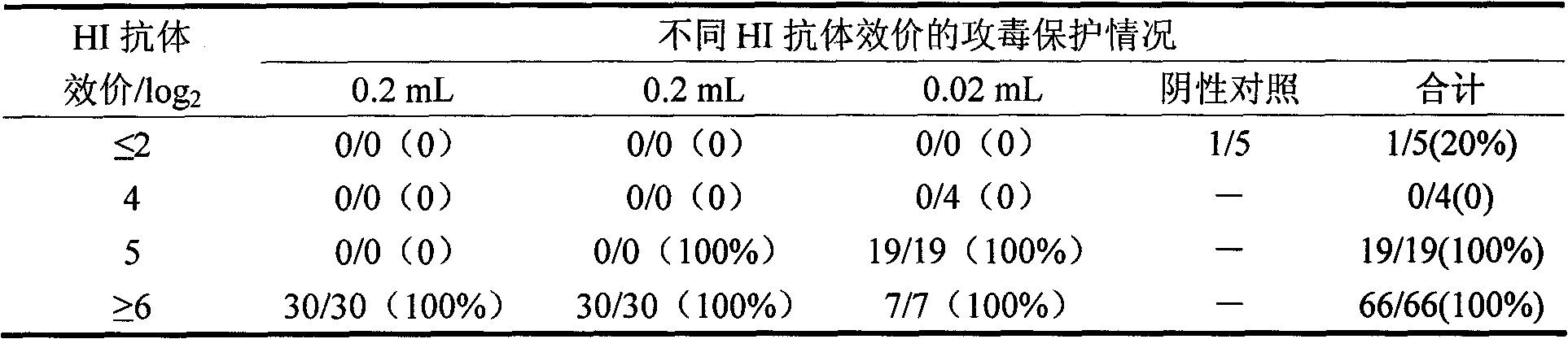
[0033] This example illustrates the screening method of the virus-adapted cell line and the final determination method of the virus-adapted cell line of the H9N2 subtype avian influenza isolate JY strain of the present invention.
[0034] 1. Screening
[0035] The H9N2 subtype avian influenza isolate JY strain described in the present invention is purchased from the Poultry Research Institute of the Chinese Academy of Agricultural Sciences, and the strain code is: type A avian influenza virus (AIV) A / Chicken / Jiangsu / JY / 99 (H9N2) strain, It is called JY strain for short.
[0036] The virus-adapted cells to be selected for screening according to the present invention are:
[0037] MDCK (canine kidney cells) was purchased from the Key Open Laboratory of Livestock and Poultry Infectious Diseases of the Ministry of Agriculture of Yangzhou University;
[0038] VERO (African green monkey kidney cells) were purchased from the School of Pharmacy, Shanghai Jiao Tong University.
[00...
Embodiment 2
[0082] This example illustrates the domestication method of the virus-adapted cell line MDCK cells determined in Example 1.
[0083] Adapt the virus to the cell line MDCK cells for carrier-free culture, change the characteristics of its adherent growth, and make it suitable for the growth environment of full suspension culture:
[0084] Digest the MDCK cells cultured by 2 to 3 passages after resuscitation with trypsin, press 2×10 5 The cells / mL density was added to the Erlenmeyer shaker flask, cultured on a shaker with F-DMEM medium containing 8-10% serum, pH 7.5-7.1, and after 40-48 hours, the cell density reached 1-2×10 6 Cells / mL was divided into flasks for passage, and the cell morphology was observed at the same time. For each passage, shake flasks with good cell morphology were selected for passage in separate flasks, cultured on a shaker according to the above method, continuous passage for 43 passages, aliquoted, and frozen in liquid nitrogen.
Embodiment 3
[0086] Methods for primary expansion and continuous culture of virus-adapted cells.
[0087] (1) Primary expansion culture:
[0088] The domesticated MDCK cells were inoculated into 5L cell culture bioreactor with 1~5×10 5 cells / mL cells, cultured in suspension until expanded to 0.3~1×10 7 cells / mL, realize primary cell expansion culture;
[0089] Wherein, the medium is: F-DMEM medium containing 6-10% serum;
[0090] The culture conditions described are: dissolved oxygen 20-60%, PH value 7.0-7.4, temperature 35-38°C, primary flow acceleration rate 6L / day, stable post-flow acceleration rate 3L / day;
[0091] (2) Continuous culture:
[0092] After the primary expansion culture is completed, add fresh medium and pump out the cell suspension in the above cell culture bioreactor to maintain the stability of the bioreactor and ensure that the cells are expanded to 0.3-1 in the residence time of the reactor. ×10 7 cells / mL, realize continuous cell culture;
[0093] Wherein, the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com