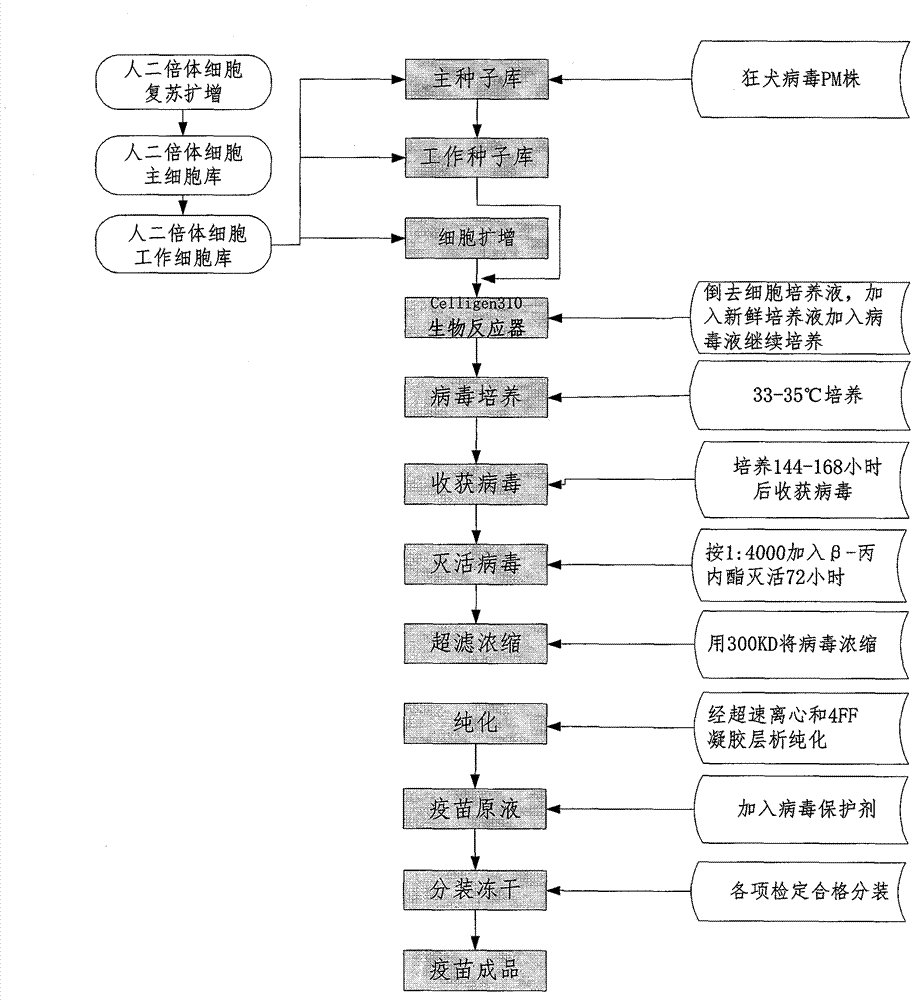
Process for preparing human diploid cell rabies vaccine through Celligen310 bioreactor
A technology of human diploid cells and bioreactors, applied in biochemical equipment and methods, microorganisms, antiviral agents, etc., can solve problems such as tumor risk, fever, and allergic reactions, and achieve maximum safety and preparation technology The effect of simple and easy industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0012] Embodiment 2: PM strain virus seed preparation
Embodiment 2
[0013] The PM strain of rabies virus was purchased from the Wistar Institute in the United States. According to the requirements of vaccine preparation, firstly, a PM-adapted virus strain adapted to human diploid cells is established.
[0014] The cells passaged by the above method are inoculated with the PM strain, the MOI is 0.01-0.001, the pH is adjusted to 7.6-7.8, and then cultured at 33°C-35°C. Harvest the virus after 96-120 hours, take a sample, and check the virus titer with the mouse inoculation method, and its lgLD 50 / mL is ≥ 7.0.
[0015] Identification test
[0016] The specificity of the virus species was identified by the mouse brain neutralization test. Make 10-fold serial dilution of the virus seed, mix the appropriate dilution of the virus solution with the same amount of rabies virus-specific immune serum, and set up a normal serum control group at the same time, and inoculate 11-13g mice at each dilution of the test group and the control group Inoculate...
Embodiment 3
[0024] When the cells were confluent, the cell surface was washed with phosphate buffered saline (PBS). Then replace it with a new culture medium, and adjust the pH of the culture medium to 7.6-7.8.
[0025] Example 4: Inoculation of virus
PUM
| Property | Measurement | Unit |
|---|---|---|
| Titer | aaaaa | aaaaa |
| Titer | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com