Influenza virus subunit vaccine and preparation method thereof
A subunit vaccine and influenza virus technology, applied in the new influenza virus subunit vaccine and the field of preparation thereof, can solve problems such as intractable induration, itching, etc., and achieve the effects of easy and rapid preparation and reduction of redness and swelling.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
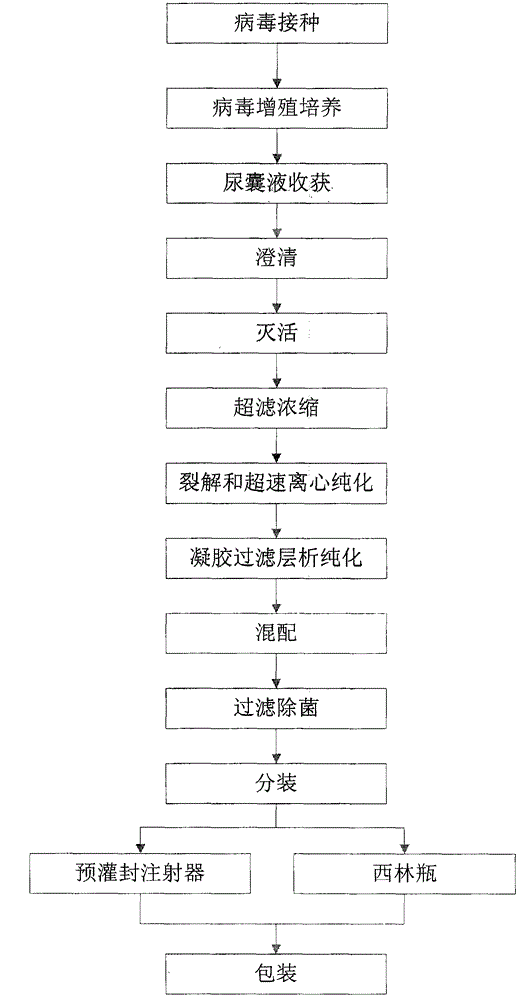
[0030] The invention relates to an influenza virus subunit vaccine, wherein each dose of the influenza virus subunit vaccine contains three influenza hemagglutinins of type A1 (H1N1), type A3 (H3N2) and type B (B) with a content of more than 80%.
[0031] Further, the influenza virus subunit vaccine does not contain adjuvants such as CpG ODN adjuvant or aluminum hydroxide, and the vaccine does not contain preservatives such as thimerosal.
[0032] (1) Virus inoculation: Inoculate A1 (H1N1), A3 (H3N2) and B (B) influenza virus working seeds into the allantoic cavity of 9-11 day-old healthy chicken embryos respectively to obtain chicken embryos after inoculation. ;
[0033] (2) Virus proliferation culture: transfer the inoculated chicken embryos to a 33-35° C. incubator for 48-72 hours to propagate influenza virus;
[0034] (3) Harvesting of allantoic fluid: screen live chicken embryos, place cold embryos at 2-8°C for 10-24 hours, and harvest allantoic fluid;
[0035] (4) Clar...
Embodiment 2
[0051] This embodiment is a preferred solution based on Example 1. This embodiment is a method for producing a monovalent stock solution of a subunit vaccine of A1 (H1N1) type influenza virus, which comprises the following steps in turn:
[0052] (1) Virus inoculation: Inoculate the A1 (H1N1) type influenza virus working seeds in the allantoic cavity of 10-day-old healthy chicken embryos;
[0053] (2) Virus propagation and cultivation: transfer the inoculated chicken embryos to a 34° C. incubator for 48 hours to propagate the influenza virus;
[0054] (3) Harvesting of allantoic fluid: screen live chicken embryos, place cold embryos at 2-8°C for 16 hours, and harvest allantoic fluid;
[0055] (4) Clarification: remove most impurities such as chicken embryo red blood cells by centrifugation.
[0056] (5) Inactivation: add formaldehyde with a final concentration not higher than 200 μg / ml to the monovalent virus harvest solution, and inactivate at 2-8°C for 80 hours;
[0057] (...
Embodiment 3
[0061] This embodiment is a preferred solution based on Example 1. This embodiment is a method for producing a monovalent stock solution of a subunit vaccine of A3 (H3N2) type influenza virus, which comprises the following steps in turn:
[0062] (1) Virus inoculation: Inoculate the A3 (H3N2) type influenza virus working seeds in the allantoic cavity of 10-day-old healthy chicken embryos;
[0063] (2) Virus propagation and cultivation: transfer the inoculated chicken embryos to a 34° C. incubator for 48 hours to propagate the influenza virus;
[0064] (3) Harvesting of allantoic fluid: screen live chicken embryos, place cold embryos at 2-8°C for 16 hours, and harvest allantoic fluid;
[0065] (4) Clarification: remove most impurities such as chicken embryo red blood cells by centrifugation.
[0066] (5) Inactivation: add formaldehyde with a final concentration not higher than 200 μg / ml to the monovalent virus harvest solution, and inactivate at 2-8°C for 100 hours;
[0067] ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com