Method for producing avian influenza vaccine by using WAVE bioreactor
A technology of bioreactor and bird flu, which is applied in the direction of pharmaceutical formulations, medical preparations containing active ingredients, antibody medical ingredients, etc., can solve the problems of exogenous virus infection, high labor intensity, bacterial contamination, etc., and achieve the improvement of cultivation efficiency , the effect of improving production efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Embodiment 1 utilizes WAVE wave bioreactor to produce bird flu vaccine
[0018] 1. Cell Culture
[0019] 1.1 Microcarrier treatment: Weigh an appropriate amount of microcarrier (Cytodex-1) according to the final volume of culture, and use Ca-free 2+ , Mg 2+ -Soak overnight in PBS at room temperature, discard the PBS, and use Ca-free 2+ , Mg 2+ -Wash once with PBS, discard, and finally add Ca-free 2+ , Mg 2+ - PBS autoclaved (115 °C, 10 psi, 15 min).
[0020] 1.2 Cell recovery culture: Use a square bottle to culture MDCK cells recovered from a liquid nitrogen tank. The culture conditions include: pH value 7.2, temperature 37°C; culture for 48-72 hours, and when a good cell monolayer is formed, it is used for further passage or inoculation in Microcarrier suspension culture was carried out in a wave bioreactor; the medium used in this process was DMEM, and the serum was fetal bovine serum, and the usage amount was 10%.
[0021] 1.3 Microcarrier culture: prepare MDC...
experiment example 1
[0026] The optimization experiment of experimental example 1 microcarrier content
[0027] Cell inoculation density is closely related to the influence of microcarrier content on cell growth. The important sign is to ensure the appropriate ratio of cell density to carrier during inoculation; in order to produce high-titer virus, a higher cell density is required. At the same time, In order to culture cells faster and more efficiently, it is required that the cells have a higher cell expansion factor.
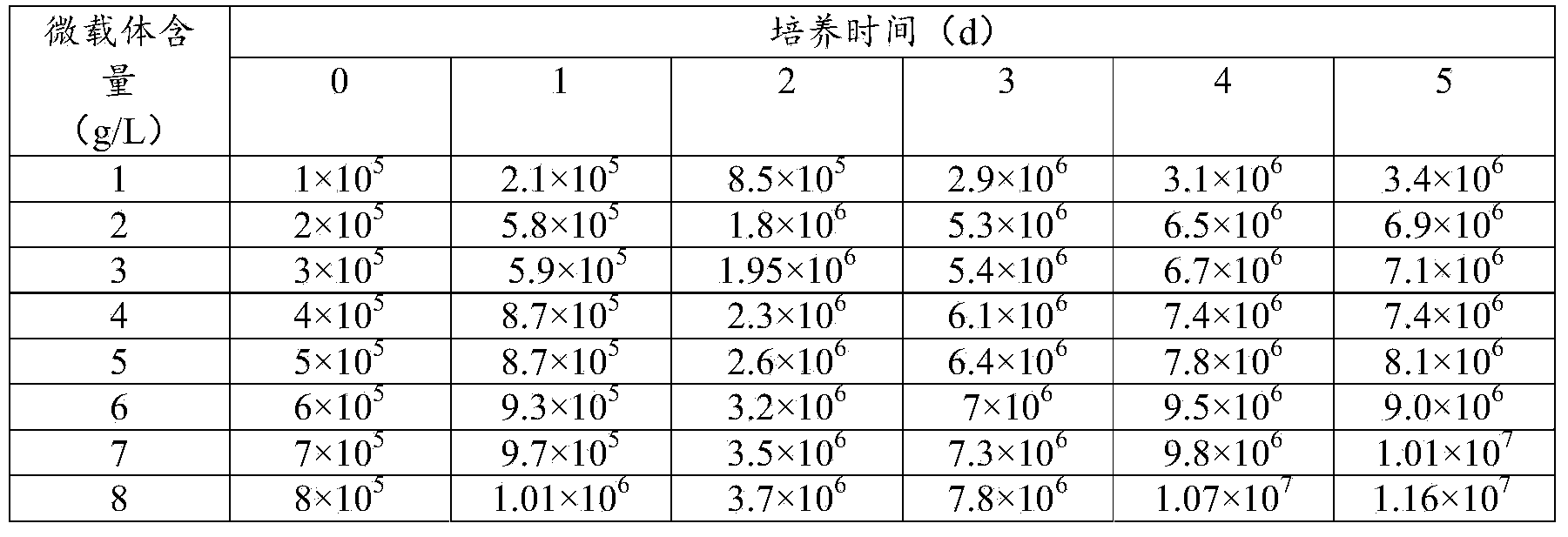
[0028] Under the premise of inoculating the same number of MDCK cells per gram of microcarrier, when the Cytodex1 content was 1g / L, 2g / L, 3g / L, 4g / L, 5g / L, 6g / L, 7g / L, 8g / L The growth of MDCK cells is used to determine the appropriate carrier dosage.
[0029]The experimental results are shown in Table 1 and Table 2. It can be seen from Table 1 and Table 2 that with the increase of the microcarrier content, the MDCK cell density will increase, but the MDCK cell expansion ratio ...
experiment example 2
[0035] Experimental example 2 Screening experiment of MDCK cell seeding density
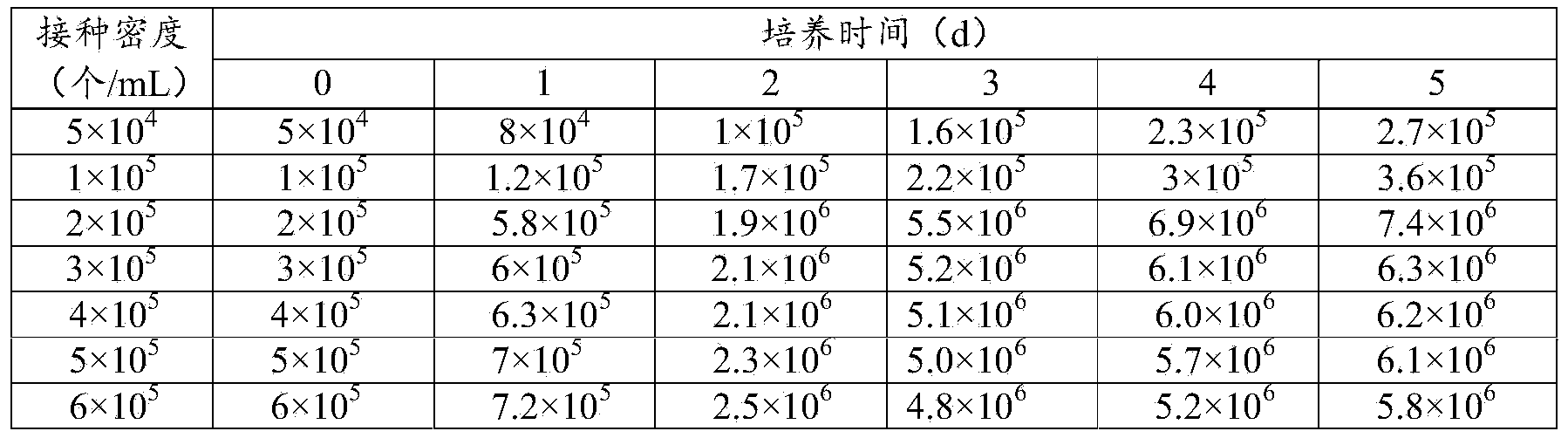
[0036] When the amount of microcarriers is 2g / L, 5×10 4 , 1×10 5 , 2×10 5 , 3×10 5 , 4×10 5 , 5×10 5 , 6×10 5 MDCK cells were inoculated at an inoculation density of 1 / mL, and samples were taken and analyzed every day. The growth of MDCK cells is shown in Table 3.
[0037] Table 3 Growth of MDCK cells at different seeding densities (unit / mL)
[0038]
[0039] It can be seen from Table 3 that when the content of microcarriers is equal, different seeding densities have a greater impact on the growth of MDCK cells. Take 5×10 4 , 1×10 5 Individual / mL inoculation, the inoculation density was low and it was still growing slowly 5 days after inoculation, and there was no obvious sign of entering the stable growth period; with 2×10 5 , 3×10 5 , 4×10 5 , 5×10 5 , 6×10 5 Cells / mL was inoculated with a high inoculation density, and MDCK cells entered a stable growth phase on the second day ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com