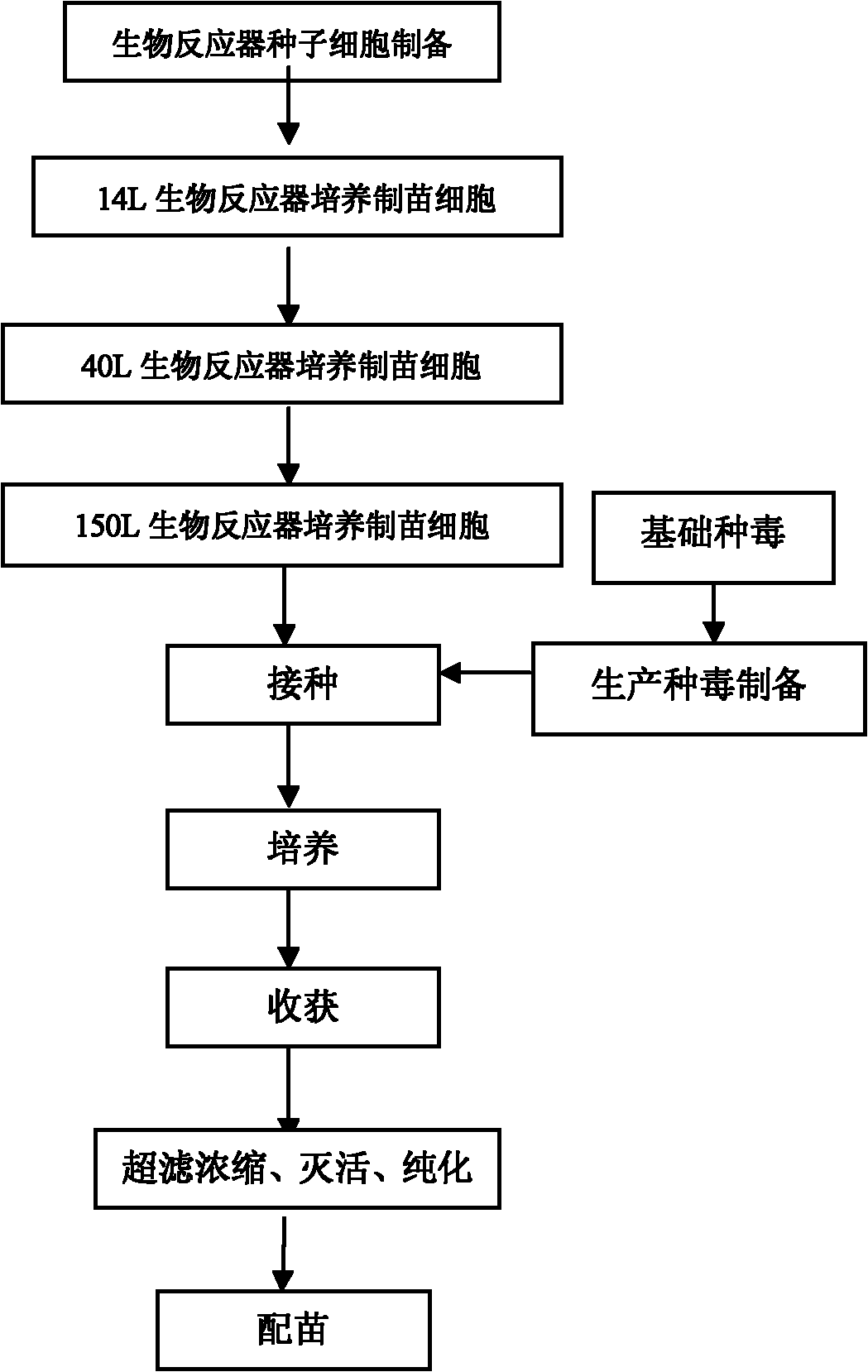
Method for industrially producing swine parvovirus vaccine by utilizing bioreactor
A bioreactor and parvovirus technology, applied in the direction of microorganism-based methods, biochemical equipment and methods, microorganisms, etc., can solve the problems of not being suitable for large-scale production of vaccines, high labor intensity in operation, and large differences between vaccine batches. Achieve the effects of saving manpower, high degree of automation control, and less production land
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Bioreactor: 14L and 40L bioreactors from NBS Company of the United States;
[0021] Microcarrier: Cytodex-1 (General Electric Healthcare Life Sciences Division);
[0022] Porcine parvovirus: HW-1 strain;
[0023] Cell growth medium: DMEM containing 8% calf serum (Beijing Qingda Tianyi Biotechnology Co., Ltd.);
[0024] Virus maintenance solution: DMEM containing 1% calf serum by volume (Beijing Qingda Tianyi Biotechnology Co., Ltd.);
[0025] Cell culture: In 14L bioreactors, add Cytodex1 at a concentration of 10g / L, after hydration, wash with pH 7.2 phosphate buffer saline PBS twice, sterilize, inoculate IBRS-2 cells, and culture ; The parameters of the culture method are: pH7.2, temperature 37°C, DO50%, stirring speed 30-100rpm; take samples regularly every day to observe the growth of the cells, count the cells, and measure the consumption of glucose. When the density of the cells reaches 1.5×10 6 / ml, start perfusion, and the perfusion speed depends on the densit...
Embodiment 2
[0030] Bioreactor: 14L and 40L bioreactors from NBS Company of the United States;
[0031] Microcarrier: Cytodex-1 (General Electric Healthcare Life Sciences Division);
[0032] Porcine parvovirus: HW-1 strain;
[0033] Cell growth medium: DMEM containing 8% calf serum (Beijing Qingda Tianyi Biotechnology Co., Ltd.);
[0034] Virus maintenance solution: DMEM containing 1% calf serum by volume (Beijing Qingda Tianyi Biotechnology Co., Ltd.);
[0035] Cell culture: In 14L bioreactors, Cytodex-1 was added at a concentration of 10g / L. After hydration, eluted twice with pH 7.2 phosphate buffered saline PBS, sterilized, and inoculated with PK-15 cells. Carry out culture; the method parameters of culture are: pH7.2, temperature 37 ℃, DO50%, stirring speed 30~100rpm; take samples regularly every day to observe the growth of the cells, perform cell counts, and determine the consumption of glucose. When the density of the cells reaches 1.5× 10 6 perml, start perfusion, the rate of p...
Embodiment 3
[0040] Bioreactor: 14L and 40L bioreactors from NBS Company of the United States;
[0041] Microcarrier: Cytodex-1 (General Electric Healthcare Life Sciences Division);
[0042] Porcine parvovirus: HW-1 strain;
[0043] Cell growth medium: DMEM / F12 containing 8% calf serum by volume;
[0044] Virus maintenance solution: DMEM / F12 containing 1% calf serum by volume;
[0045] Cell culture: In 14L bioreactors, add Cytodex1 at a concentration of 10g / L, after hydration, wash with pH7.2 phosphate buffered saline PBS twice, sterilize, inoculate ST cells, and culture; The parameters of the method are: pH7.2, temperature 37°C, DO50%, stirring speed 30-100rpm; samples are taken regularly every day to observe the growth of the cells, count the cells, and measure the consumption of glucose. When the density of the cells reaches 1-1.5×10 6 / ml, start perfusion, and the perfusion speed depends on the cell density and glucose consumption at 0.5 to 4 working volumes per day, culture for 4 d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com