Nasal spray preparation for resisting respiratory virus infection
A virus infection and anti-respiratory technology, which is applied in the direction of antiviral agents, respiratory diseases, aerosol delivery, etc., can solve the problems of clinical application limitations, achieve enhanced clinical indications, enhance the scope of anti-virus, and be convenient and simple to use Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1 Preparation of liposome-ACE2
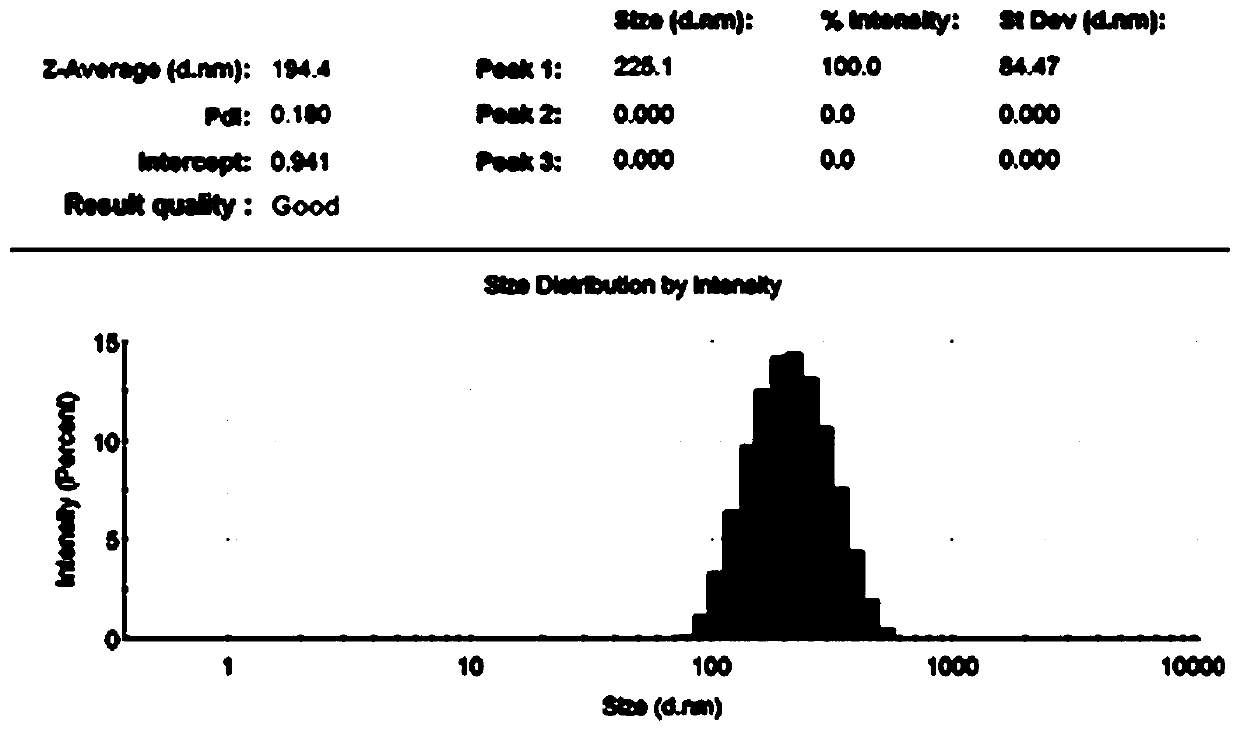
[0029] According to the liposome mixture (lipid mixture) component molar ratio is DPPC:Chol:DSPE-PEG2K:DSPE-PEG-MAL is 60:40:4:1, each component of liposome is weighed successively, and the total molar number is about 26 μmol, then add 5mL CHCl 3 dissolved, and the chloroform (CHCl 3 ), and then use a vacuum pump for 1 hour to completely remove CHCl 3 , then add 10mL of PBS solution, ultrasonic in water bath for about half an hour, then dialyze, extrude to form liposome (liposome), the ACE2 extracellular segment recombinant protein (its amino acid sequence is as SEQ ID NO) after dithiothreitol DTT treatment NO: 1) was added to the above liposome suspension, reacted at room temperature for 24 hours and dialyzed to obtain ACE2-modified liposomes (liposome-ACE2), and BCA was used to measure the concentration of recombinant protein in the extracellular segment of ACE2.
[0030] Liposome-loaded angiotensin-converting enzyme 2 (ACE2...
Embodiment 2
[0032] Preparation of nasal spray preparation for embodiment 2 anti-respiratory virus infection
[0033] 0.5 mg / mL of ACE2 extracellular segment recombinant protein (liposome-ACE2) loaded on liposomes prepared in Example 1 above, 5 mg / mL of N-acetylneuraminic acid (Neu5Ac), 0.6 mg / mL of fucoidan and Chitosan 0.4 mg / mL is dissolved in physiological saline, and the pH is adjusted to 6.5-7.0 to prepare a nasal cavity spray preparation for resisting respiratory virus infection.
Embodiment 3
[0034] Example 3 Cell Fusion Inhibition Experiment
[0035] 293T cells transfected with SARS CoV and 2019-nCoV S glycoprotein genes were pre-incubated with the anti-respiratory virus infection nasal spray prepared in Example 2 for 15 minutes at room temperature, and then mixed with 293T cells transfected with ACE2 gene in equal proportions , and incubated at 37°C for 4h. After the incubation, the cells were lysed, the β-gal activity was measured, and the inhibitory effect of nasal spray on cell fusion was calculated. In the control group, PBS was used instead of nasal spray.
[0036] The result is as Figure 4 and Figure 5 As shown, the results show that the anti-respiratory virus infection nasal spray preparation prepared by the embodiment of the present invention has a good inhibitory effect on the cell fusion mediated by ACE2 and coronavirus S protein, and compared with the PBS control group, it has the highest inhibitory rate to SARS CoV It is about 96%, and its highest i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com