Antibody detection kit for infectious bovine rihinotracheitis virus and application thereof
A rhinotracheitis virus and antibody detection technology, which can be applied to measurement devices, instruments, scientific instruments, etc., can solve the problems of cross-contamination of experimental waste, complicated and complicated operations, and difficult to carry out, and achieve obvious technical improvement, high detection sensitivity, and consumption. short time effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1: Preparation of recombinant gD protein
[0035] (1) Preparation and purification of recombinant gD antigen
[0036] According to the gene information of bovine infectious rhinotracheitis virus in Genbank NC 001847, DNAStar was used to analyze the signal peptide, antigenicity and hydrophilicity of the amino acid sequence of gD protein, remove the signal peptide sequence, screen the antigenic region, and finally intercept Two pieces of protein sequence were combined in tandem to synthesize the gD gene sequence as SEQ ID No.2 sequence, and a pair of primers were designed, the upstream primer sequence was SEQ ID No.3 sequence, and the downstream sequence was SEQ ID No.4 sequence.
[0037] Using the synthetic gene as a template, carry out PCR reaction. The PCR reaction system is 50 μL. Add the following reagents to a sterile 0.2mL EP tube: template DNA 0.5μL, 10XEx TaqTN Buffer (Mg 2+ free) 5μL, dNTP Mixture (2.5mM each) 2μL, MgCl 2 (25mM) 4μL, upstream pr...
Embodiment 2
[0042] Example 2: Preparation and purification of gD protein-specific monoclonal antibody
[0043]After the prepared recombinant gD antigen was emulsified with an equal amount of Freund's adjuvant in an amount of 50 μg, several Balb / C mice were immunized by subcutaneous injection at multiple points. The adjuvant for the first immunization was complete Freund's adjuvant, and the adjuvant for subsequent immunization was incomplete Freund's adjuvant. The immunization interval was 2 weeks, and the serum titer was determined by direct ELISA after 3 immunizations. Screen the mice with high serum titer and low cross-reactivity, and perform cell fusion according to conventional methods, and screen hybridoma cell lines secreting specific monoclonal antibodies.
[0044] Screening antigens are inactivated bovine infectious rhinotracheitis virus, bovine viral diarrhea virus, bovine foot-and-mouth disease virus, etc., and recombinant gD protein antigens, and use negative serum as a refere...
Embodiment 3
[0047] Example 3: Identification of IBR-specific monoclonal antibodies
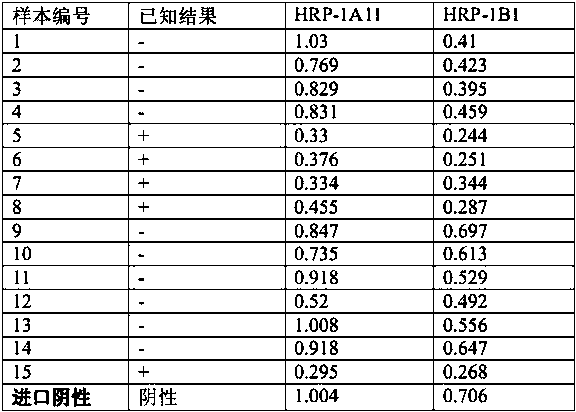
[0048] The prepared monoclonal antibodies were identified by using a commercial IgG subtype identification test kit. The results showed that 5A1-1A11 and 7C10-1B1 were IgG2a and IgG2b subtypes, respectively. The specific operations were as follows:
[0049] 1) Dilute the prepared purified monoclonal antibody with PBS solution at 1:10000, 1:15000, 1:20000, 1:25000, 1:30000, 1:35000 and 1:40000.
[0050] 2) Take the gD protein-coated plate (0.1 µg / ml), wash the plate once with 300 μl / well of 1× washing solution, and discard the washing solution. Add the corresponding diluted monoclonal antibody, 50 μl per well, make 5 replicate wells for each monoclonal antibody, shake and mix for 1 minute. After incubating at 37°C for 30 minutes, take out the reaction plate, discard the reaction solution, add 300 μl of 1× washing solution to each well, wash 3 times, and spin dry.
[0051] 3) Dilute IgG1, IgG2b and IgG3 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com