Preparation of CHO cell expressed infectious bovine rhinotracheitis virus protein gD and subunit vaccine thereof and application
A subunit vaccine, rhinotracheitis virus technology, applied in vaccines, viruses, viral peptides, etc., can solve problems such as economic losses, and achieve the effects of high safety, good immunogenicity, and sufficient supply
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
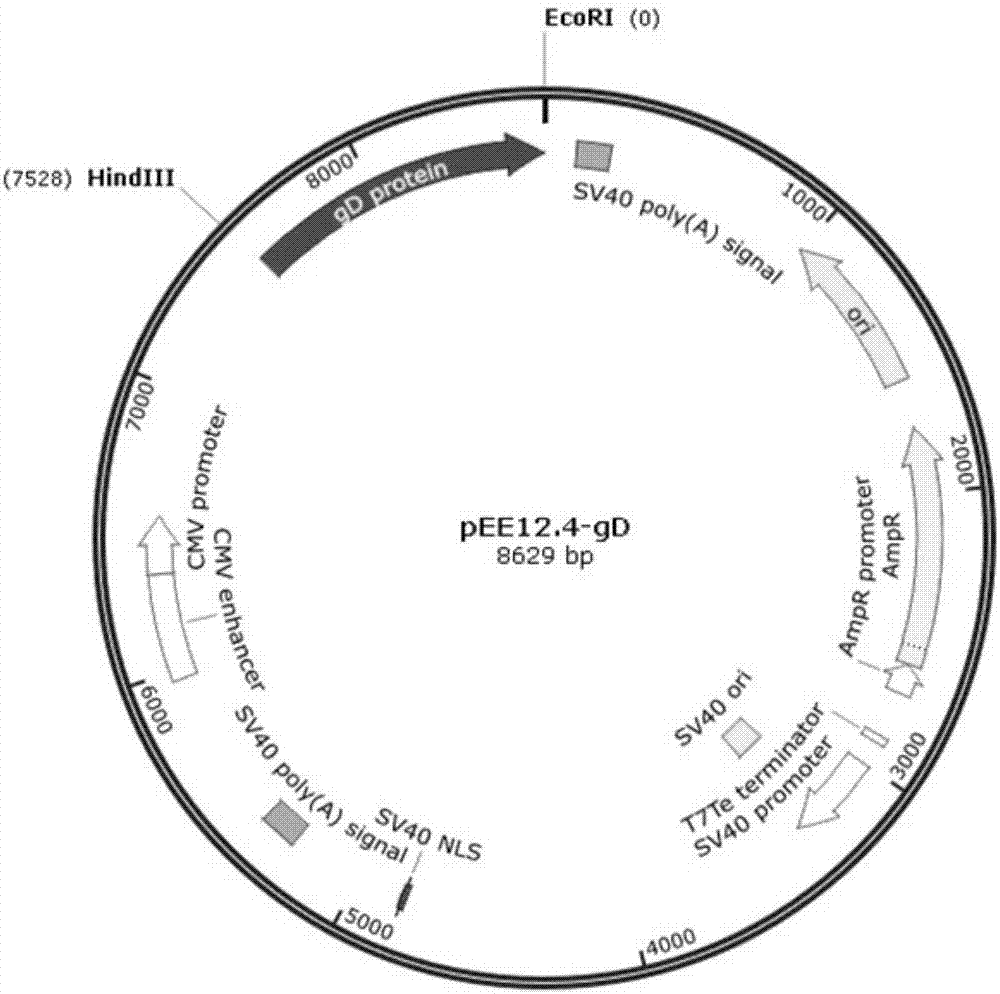
[0032] Example 1: Codon optimization of gD protein of bovine infectious rhinotracheitis virus and construction of pEE12.4-OPTI-gD recombinant plasmid
[0033] By codon-optimizing the nucleotide sequence of the gD protein of bovine infectious rhinotracheitis virus, the OPTI-gD sequence was obtained, as shown in SEQ ID NO.2, and this work was commissioned to Nanjing GenScript Biotechnology Co., Ltd.
Embodiment 2
[0034] Example 2: Construction of pEE12.4-OPTI-gD recombinant plasmid
[0035] 2.1 PCR amplification of the target fragment OPTI-gD
[0036] 2.1.1 PCR reaction
[0037] (1) Primer design and synthesis
[0038] Upstream primer: 5'-CGGAAGCTTGCCGCCACCATGCTGCCTACCCCCAGCTCCAAG-3'
[0039] Downstream primer: 5'-GGCGAATTC TTAGTGATGGTGATGGTGATGT-3'
[0040] (2) Add 50 μL of the sample system, as shown in the table below:
[0041]
[0042] PCR amplification program:
[0043]
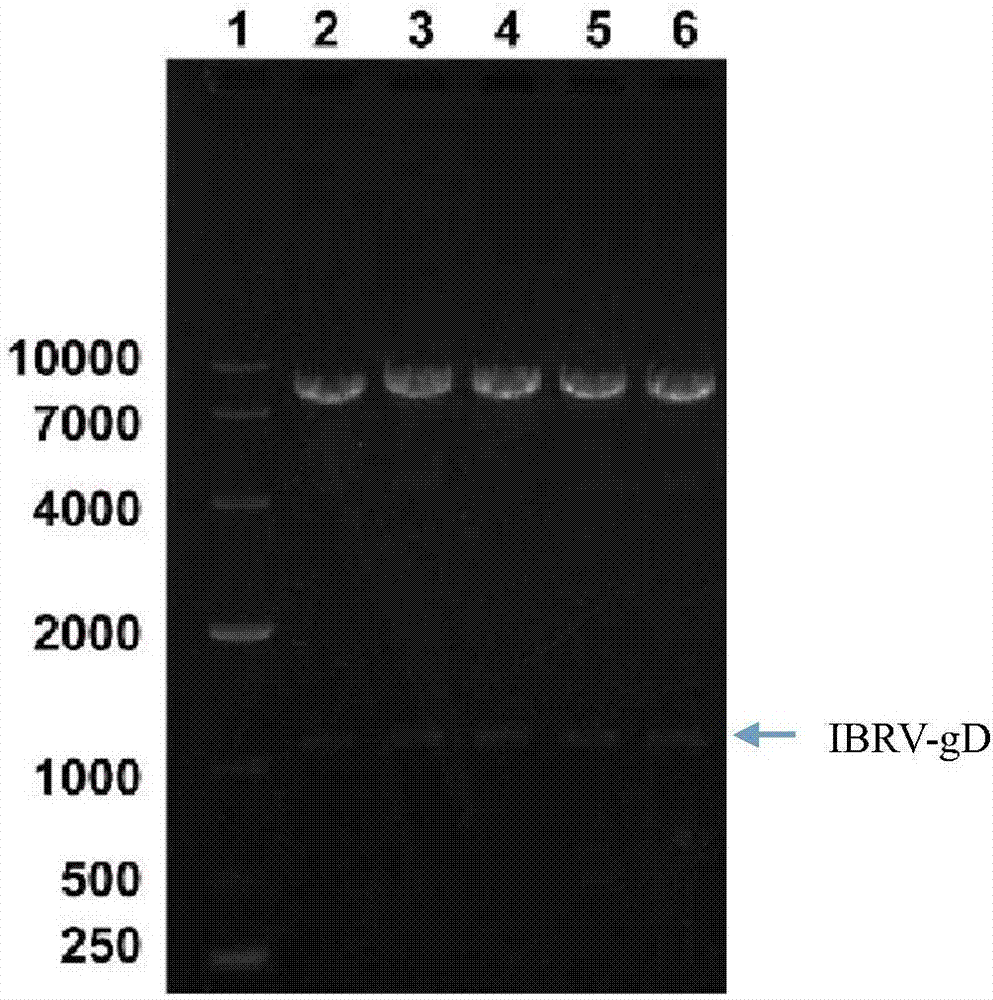
[0044] 2.1.2 Gel recovery of PCR products
[0045] (1) Mark the sample collection EP tube, adsorption column and collection tube;
[0046] (2) Take the weight of the marked empty EP tube, and record the value;
[0047] (3) Carefully cut out a single target DNA band from the agarose gel with a scalpel on a gel cutter and put it into a clean 1.5mL centrifuge tube;
[0048] (4) Add 600 μL PC buffer to the 1.5mL centrifuge tube in step (3), place in a 50°C water bath for about 5 minutes, and gently turn...
Embodiment 3
[0112] Example 3: Establishment of transfection of pEE12.4-OPTI-gD recombinant plasmid into CHO-K1 cells and monoclonal screening
[0113] 3.1 CHO-K1 cell transfection
[0114] (1) Preparation: UV sterilization in a biological safety cabinet for 30 minutes; DMEM / F12 (containing 10% serum, 1% double antibody), DMEM / F12 and PBS were placed in a 37°C water bath and preheated to 37°C.
[0115] (2) Take out the cells (10 cm cell culture dish) from the incubator at 37° C., discard the supernatant medium, wash the cells once with pre-warmed 8 mL PBS, and discard the PBS.
[0116] (3) Add 1-2mL 0.25% trypsin-EDTA to each 10cm cell culture dish, digest at room temperature for about 2 minutes, observe under the microscope that the cells shrink and become round, and appear as single cells.
[0117] (4) Add 4 mL of DMEM / F12 (containing 10% serum, 1% double antibody) to terminate the digestion reaction, and blow the cells away with a pipette.
[0118] (5) Transfer the digested cells to a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com