Infectious bovine rhinotracheitis vaccine and preparation method thereof
A technology for rhinotracheitis and vaccine preparation, which is applied in the direction of pharmaceutical formulas, medical preparations containing active ingredients, antibody medical ingredients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Embodiment 1 bovine infectious rhinotracheitis vaccine preparation method
[0023] 1. Virus isolation: Aseptically collect the liver, spleen, heart, kidney, placenta, and lung of IBR aborted fetuses, rinse with sterilized Hanks solution several times, cut into pieces, and grind. Then add 5 times the amount of Hanks (containing 200IU / ml penicillin and 200μg / ml streptomycin) to make an emulsion, transfer it into a 10ml centrifuge tube, and freeze and thaw repeatedly three times. Centrifuge at 3000r / min for 10min, take the supernatant and filter to sterilize. Dilute 10 times, 100 times, 1000 times with Hanks solution, and store at -20°C for later use. Select the calf testicular primary cells that grow into a dense monolayer and intact form, discard the nutrient solution, wash the cells with Hanks solution for 3 times, and add the treated diseased cells according to four gradients of stock solution, 10 times, 100 times and 1000 times respectively. Feed supernatant, the am...
Embodiment 2
[0033] Embodiment 2 immune effect test
[0034] 1. Materials
[0035] 1.1 Bovine infectious rhinotracheitis oil emulsion inactivated vaccine
[0036] 1.2 Bovine infectious nasal and tracheal antibody-negative adult dairy cows
[0037] 2. Method
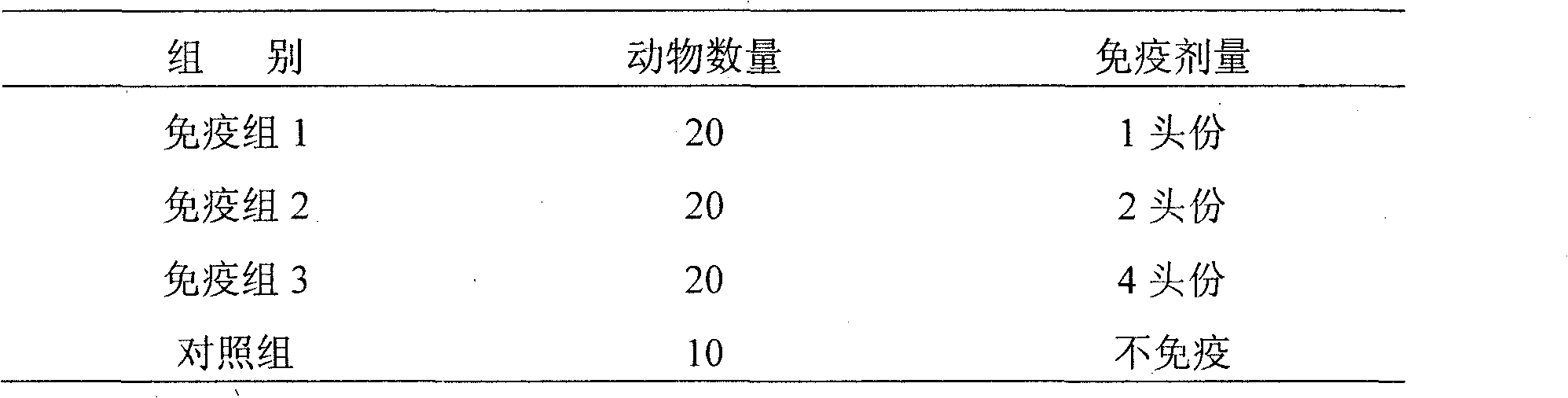
[0038] A total of 70 adult dairy cows negative for bovine infectious nasal and tracheal antibodies were selected and randomly divided into 4 groups, with 3 groups as the immunization group and 1 group as the control group. The immunization group was injected intramuscularly with 1 portion, 2 portions, and 4 portions of the bovine infectious rhinotracheitis oil emulsion inactivated vaccine prepared in Example 1 respectively; the control group was not immunized, and blood was collected to measure antibodies respectively 35 days after immunization. Grouping and immunization methods are shown in Table 1.
[0039] Table 1 Grouping and immunization methods
[0040]
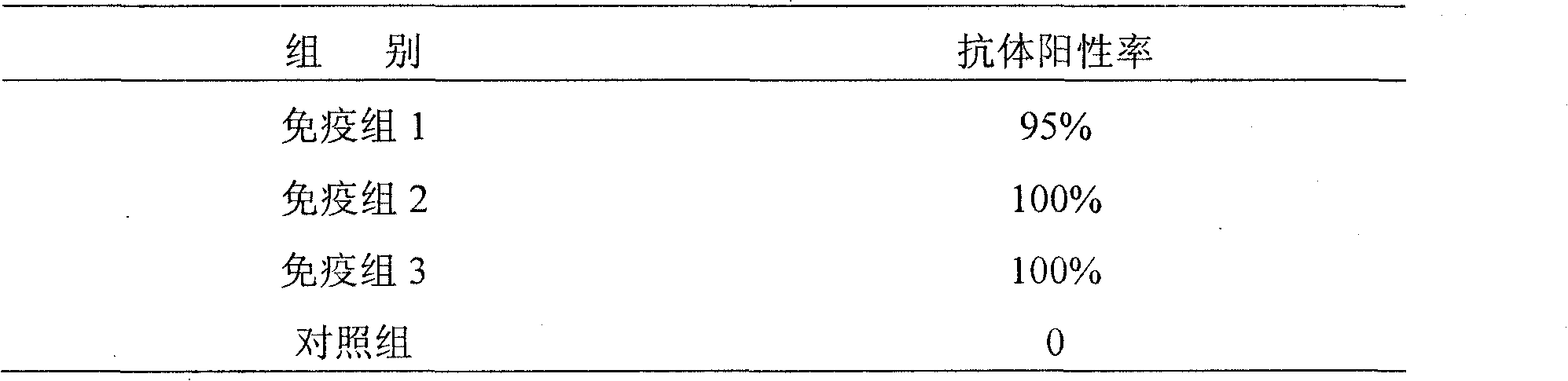
[0041] 3. Results and Analysis
[0042] 3.1 Serum test results ar...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com