EGFR and HER2 combined polypeptide epitope vaccine
An epitope vaccine and epitope technology, applied in the field of medicine and biology, can solve the problems of single vaccine and limited effect, and achieve the effect of reducing immunogenicity and increasing safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
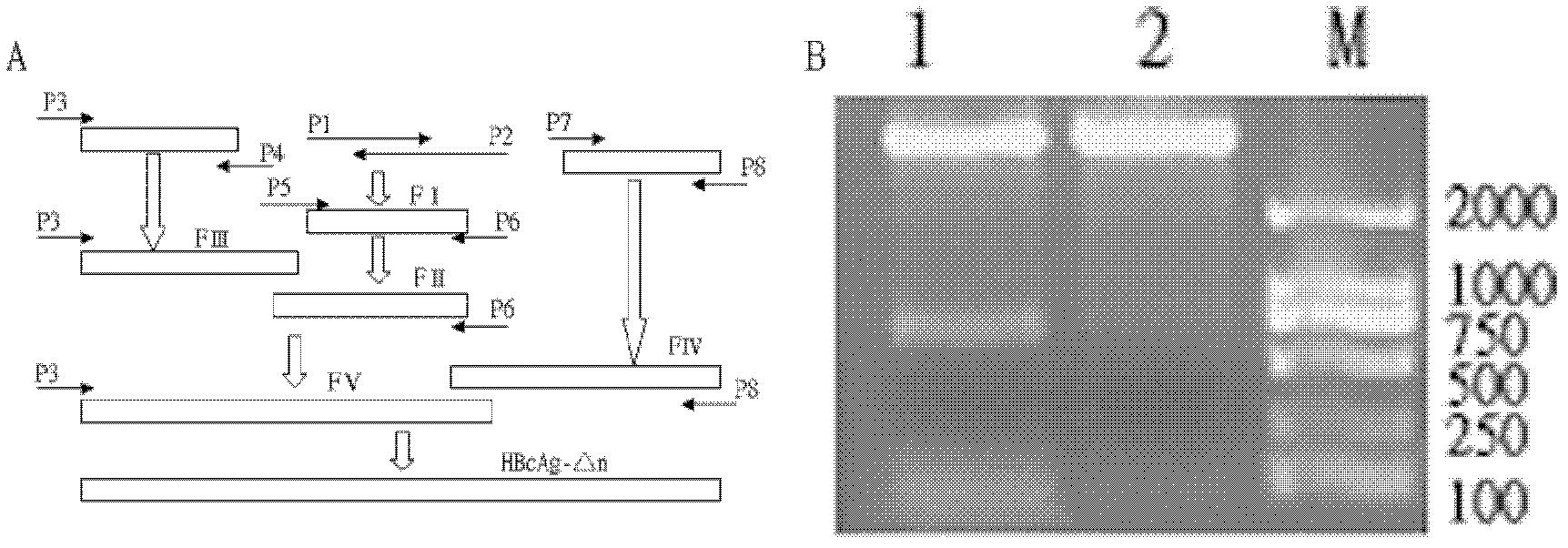
[0055] Example 1: Construction of fusion protein gene using overlapping PCR technique.
[0056] Nucleotide sequence (SEQ ID NO.9) encoding HBcAg-△n protein, before the first amino acid codon, the start codon ATG and the recognition site of restriction endonuclease are added, and the last amino acid The codon is followed by a stop codon TAA and a recognition site for a restriction enzyme.
[0057] The fragment FI (99bp) containing three B epitopes was amplified by using primers P1 and P2 as templates. The PCR reaction reagents and conditions are as follows:
[0058]
[0059] Supplement H 2 0 to 50 μl reaction conditions are as follows:
[0060]
[0061] Cool to 4°C,
[0062] After the reaction, 1.0% Agarose electrophoresis was carried out to identify and tap the gel to recover the target band.
[0063] Fragment FII (116bp) was amplified by PCR using FI as template and P5 and P6 as primers. The PCR reaction reagents and conditions are as follows:
[0064]
[0065...
Embodiment 2
[0090] Example 2: Construction of recombinant expression vector pET28a / HBcAg-△ n .
[0091]Using the expression vector pET28a, the synthesized HBcAg-△n gene (the initial end contains the NcoI restriction site, the termination end contains the HindIII restriction site), and pET28a were respectively subjected to NcoI and HindIII double enzyme digestion treatment, the enzyme digestion conditions are Incubate at 37°C for 4 hours. Then the digested products were electrophoresed on 1% agarose gel for 15 minutes at a voltage of 120 volts. The above two DNA fragments were recovered with a gel recovery kit. The process is as follows: a. Cut off the agarose gel containing the target DNA under ultraviolet light, absorb the liquid on the surface of the gel with a paper towel and chop it up. Calculate the weight of the gel (record the weight of the 1.5ml centrifuge tube in advance), and use this weight as a gel volume (eg 100mg=100μl volume). b. Add 3 gel volumes of BufferDE-A, mix wel...
Embodiment 3
[0092] Example 3: Construction of recombinant bacteria.
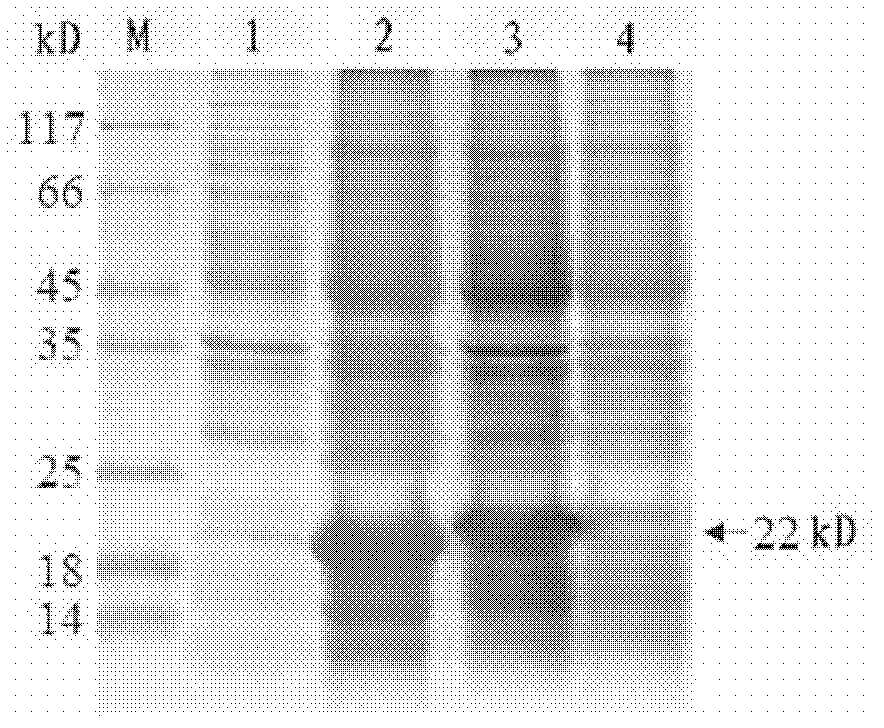
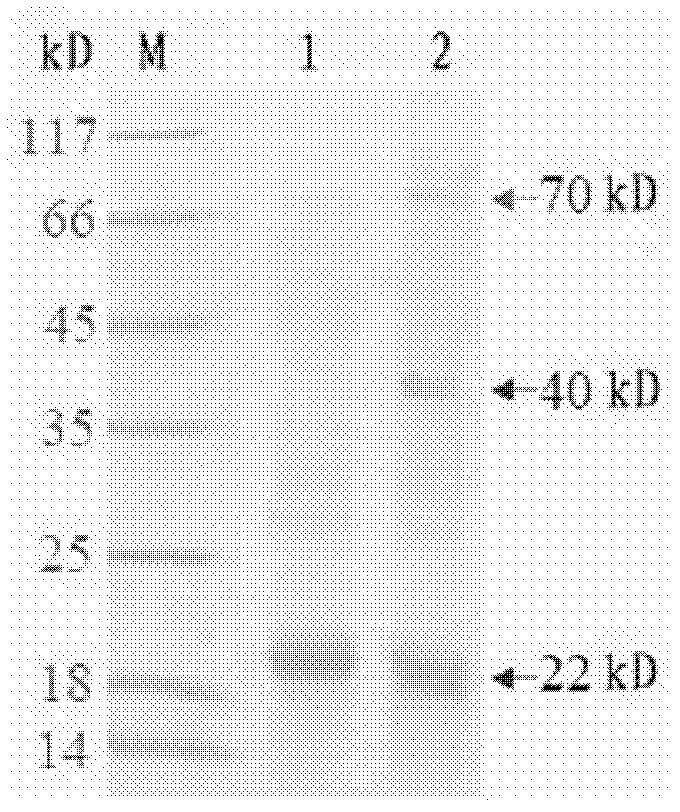
[0093] The expression vector obtained in Example 2 was transformed into host cells to form transformed cells capable of producing recombinant HBcAg-Δn fusion protein, which was then detected.
[0094] The preparation of host cells refers to the preparation of competent cells of Escherichia coli BL21 (DE3) by a conventional calcium chloride method by using molecular cloning technology. Add 20 μl of the ligation solution (composition of the ligation solution: 15 μl of DNA fragments, 2 μl of carrier fragments, 1 μl of T4 ligase, 2 μl of 10-fold DNA ligation buffer, and a total volume of 20 μl) into 200 μl of competent cells. In bacteria, place on ice for 1 hour; heat shock at 42°C for 90 sec, quickly place on ice for 5 minutes; add 800 μl 37°C preheated LB culture solution; shake at 37°C, 220 rpm for 1 hour, centrifuge and spread all over the medium containing 50 μg / ml Kan LB plates were cultured overnight at 37°C upside ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com