Epitope vaccine for resisting subgroup J avian leukosis virus infection as well as preparation method and application of epitope vaccine
A technology of avian leukosis virus and epitope vaccines, applied in the field of immunology, can solve the problems of long purification cycle, severe situation in the prevention and control of subgroup J avian leukemia, virulence recovery, etc., to achieve immune failure avoidance, efficient and safe vaccine protection, specific strong effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1 Screening and synthesis of ALV-J multiple epitope genes
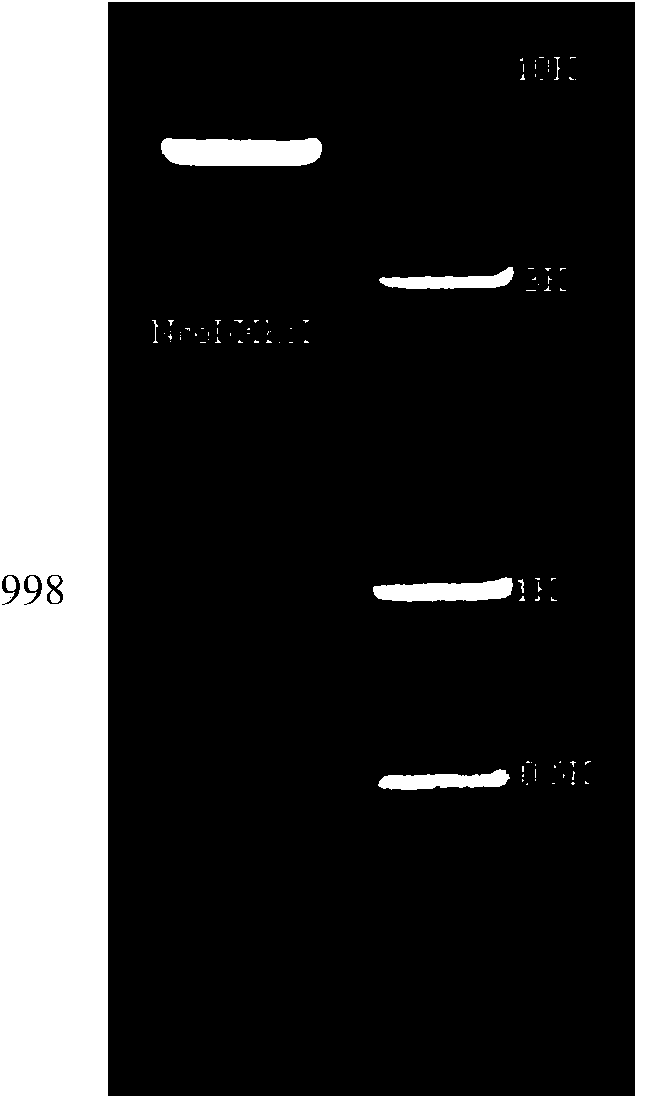
[0041] Search for the ALV-J envelope gene sequence published on NCBI, use bioinformatics methods to analyze the 22 envelope epitope, and screen out the epitope gene sequence representing the epitope of the epidemic strains in China in recent years, using encoding glycine, The serine codons are connected in series to obtain a recombinant envelope gene sequence of 998 bp, whose gene sequence is shown in SEQ ID NO.1. The 5'end of the gene sequence contains an Nco Ⅰ restriction site, and the 3'end contains an Xho Ⅰ restriction site.
[0042] The above-mentioned gene sequence was synthesized by Shanghai Shenggong Biological Engineering Co., Ltd., and the synthetic product cENV was further connected to the pUC57 vector using conventional techniques.
[0043] The synthesized product is sequenced, and its gene sequence is shown in SEQ ID NO.1;
[0044] The pET30a and the above-mentioned pUC-57 cloning vector linked to...
Embodiment 2
[0048] Example 2 Expression and purification of recombinant envelope protein
[0049] After sequencing, the positive BL21 bacteria containing the recombinant plasmid were inoculated into LB liquid medium containing kanamycin (final concentration 10μg / ml) (commercially available conventional medium containing 1% (w / v) Tryptone, 0.5 %(W / v) Yeast Extract, 1%(w / v) NaCl, 0.1mg / ml Kanamycin), shake at 37°C (200rpm), culture to OD600 = 1.0, add IPTG at a concentration of 500mMol to the entire medium IPTG The final concentration was 1mmol / L, and the culture was continued at 37°C for 4h. At the same time, the uninduced recombinant plasmid was transformed into BL21 bacteria as a control.
[0050] SDS-PAGE analysis shows that the detection steps are as follows: collect the cultured engineered bacteria solution, centrifuge at 5000 rpm for 5 minutes, discard the supernatant, and resuspend the pellet with standard PBS to obtain a resuspension; follow the ratio of 100ml initial medium to 3ml stan...
Embodiment 3
[0073] Example 3 Activity detection of recombinant protein
[0074] The purified His-cENV protein was used to coat the ELISA plate, and the biological activity of the protein was detected by enzyme-linked immunosorbent assay. Gp85 protein immunized mouse positive serum, HR1 polypeptide, HR2 polypeptide immunized chicken positive serum and IDEXX detection kit test positive chicken serum as positive control, healthy SPF chicken serum as negative control, HRP-labeled goat anti-mouse IgG, goat anti-chicken IgG enzyme-labeled secondary antibody is used according to the instructions. The results showed that the OD ratio of the positive control and the negative control was greater than 2.1, indicating that the protein has good biological activity and antigenicity.
[0075] The specific steps of ELISA are as follows:
[0076] 1) Coating: Take the purified recombinant protein, dilute it with physiological saline to the optimal concentration, and coat the reaction plate with 100μl / well at 4°...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com