Helicobacter pylori tetravalent adhesion multi-epitope vaccine and preparation method thereof
A technology of Helicobacter pylori and adhesion factor, which is applied in the field of biomedicine, can solve the problems of vaccine difficulties, poor patient compliance, and increased Hp drug resistance, and achieves prevention of immune pathological injury, biological toxicity, and high immune specificity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
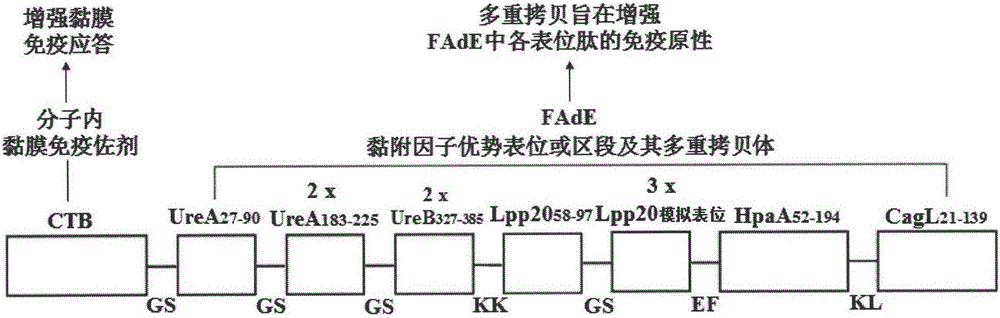
[0053] Example 1: Molecular structure design of Helicobacter pylori tetravalent adhesion factor multi-epitope vaccine CFAdE
[0054] According to "the body's immune protection mechanism against Hp" and "the immunological properties of key adhesion factor epitopes or segments such as Hp urease A and B double subunits, outer membrane protein Lpp20, HpaA and CagL", through biological information UreA 27-90 、UreA 183-225 , UreB 327-385 , Lpp20 58-97 , Lpp20 mimotope (SWPLYSDASGLG), HpaA 52-194 , CagL 21-139 The isoantigen epitopes or segments and the intramolecular mucosal immune adjuvant CTB were used in the construction of the Helicobacter pylori tetravalent adhesion factor multi-epitope vaccine CFAdE. Then, through the construction theory of the epitope vaccine and the analysis of bioinformatics, the connection sequence, spacer sequence and antigen epitope copy number of the selected antigenic epitope or segment are analyzed and determined, and finally a scientifically rea...
example 2
[0056] Example 2: Construction of recombinant expression vector pCzn1-CFAdE (containing fusion gene CFAdE)
[0057] (1) Gene synthesis of adhesion factor polyepitope peptide FAdE nucleotide sequence
[0058] The amino acid sequence of the previously designed adhesion factor multi-epitope peptide FAdE was transformed into the corresponding nucleotide sequence according to the codon preference principle of Escherichia coli, and Zhongding Biotechnology Company was entrusted to carry out gene synthesis.
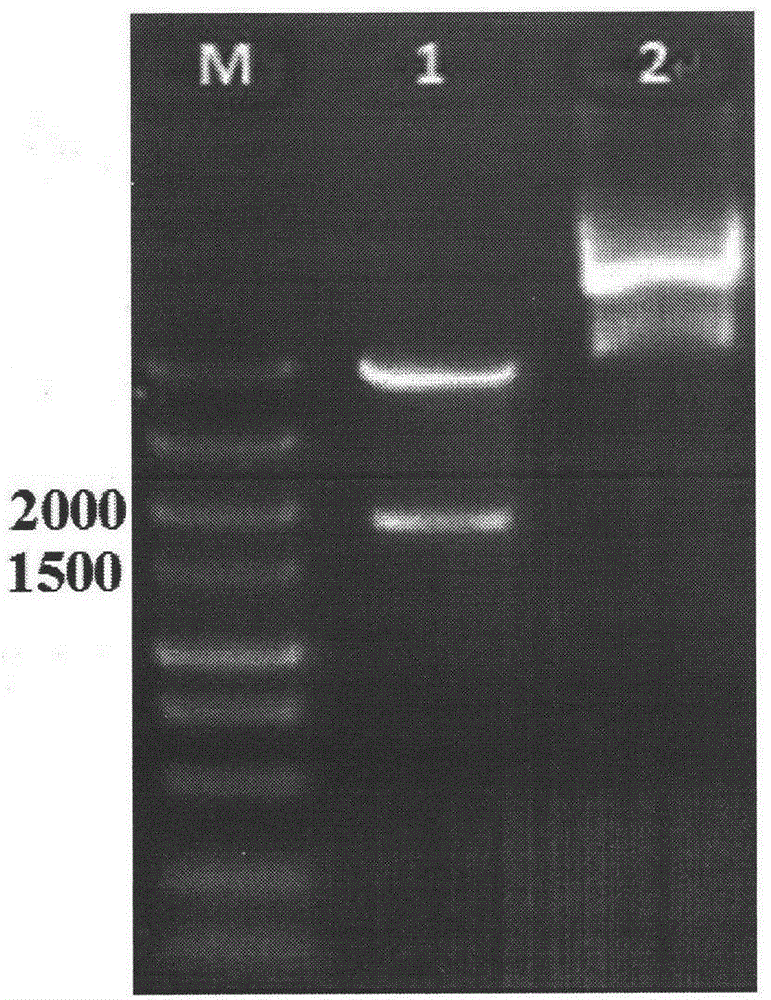
[0059] (3) Connection of adhesion factor multi-epitope peptide FAdE gene and pCzn1-C expression vector
[0060] The synthesized adhesion factor multi-epitope peptide FAdE gene and pCzn1-C (containing CTB gene) were double digested with BamHI / Xbal respectively, and the reaction system was as follows:
[0061]
[0062] 37°C enzyme digestion reaction for 2h, then electrophoresis with 1% agarose gel, and observe the electrophoresis results. The adhesion factor multi-epitope pept...
example 3
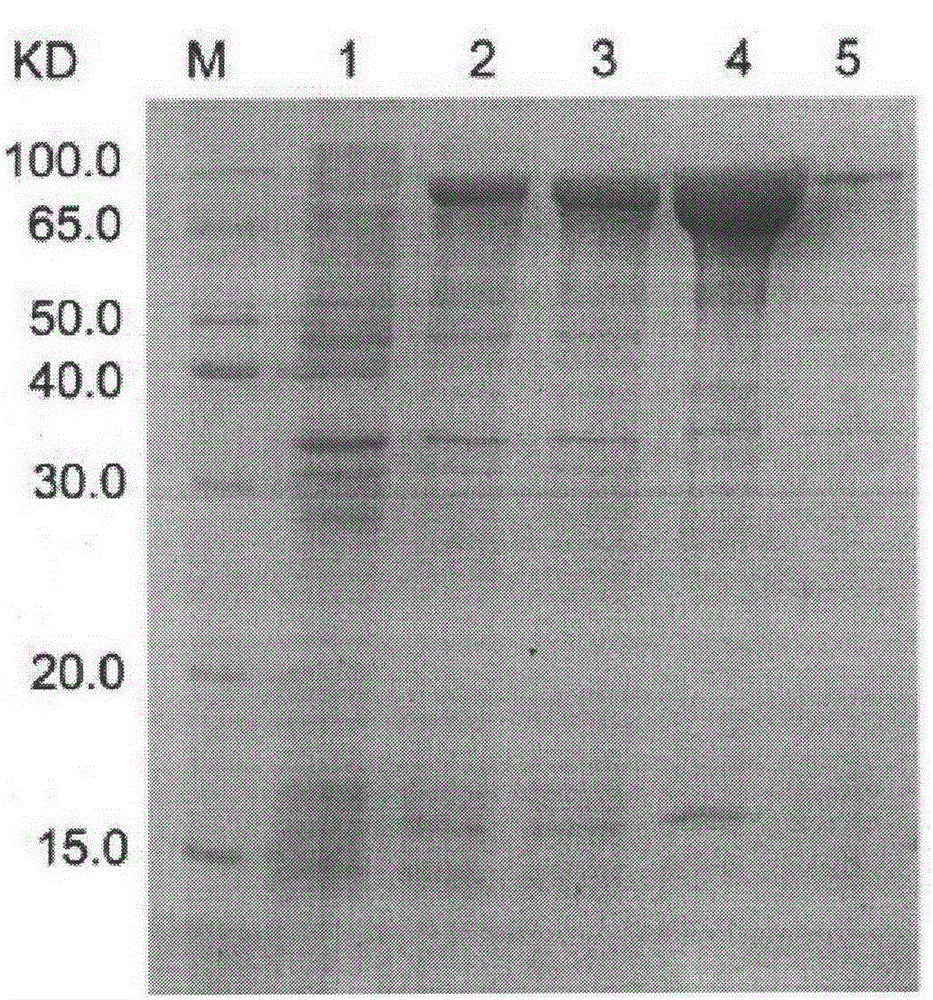
[0066] Example 3: Prokaryotic expression of recombinant protein CFAdE
[0067] Transform the correct recombinant expression plasmid pCzn1-CFAdE into the ArcticExpress strain. On the pre-prepared LB plate containing 50 μg / mL Amp, inoculate the loop-streaked genetically engineered strain ArcticExpress / pCzn1-CFAdE, place it upside down in a 37°C incubator, and after cultivating overnight for 12-16 hours, pick a single colony and inoculate it on a plate containing 50 μg In a test tube of 3ml LB culture solution containing AMP / mlAMP, shake overnight at 37°C at 220rpm; inoculate at 1:100 the next day in 30ml LB culture solution containing 50μg / mlAMP, shake at 37°C at 220rpm until the cell OD600 is 0.6-0.8 (about 2h ). Take out 1ml of the culture, centrifuge at 10000g for 2min at room temperature, discard the supernatant, and resuspend the bacterial pellet with 100μl of 1× loading buffer. Add IPTG to the remaining culture to a final concentration of 0.5 mM, shake at 220 rpm at 37°C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com