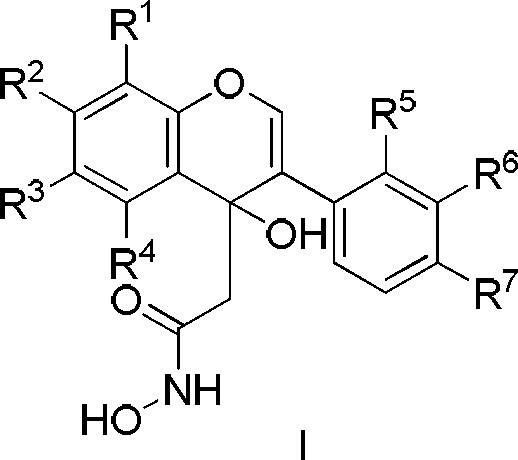
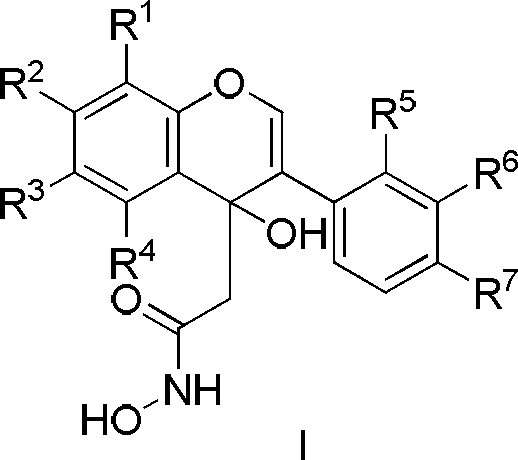
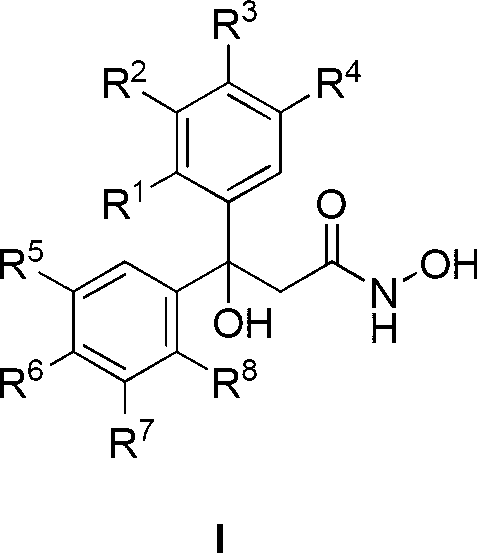
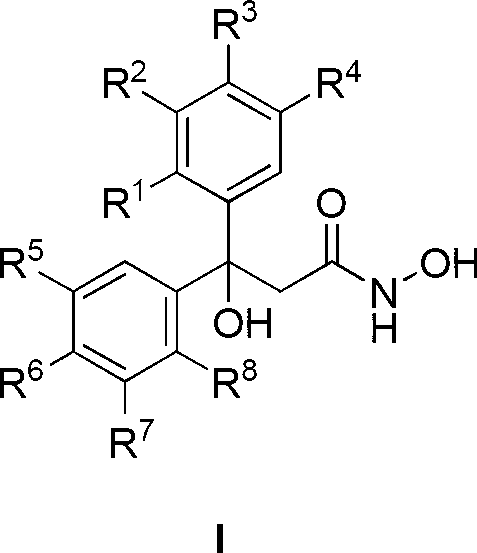
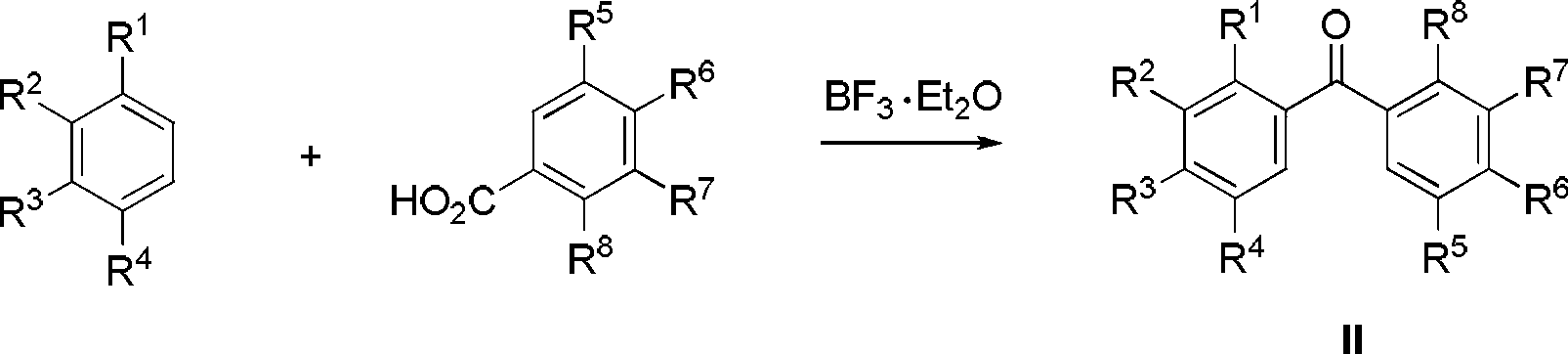
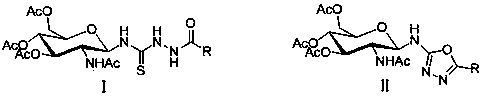
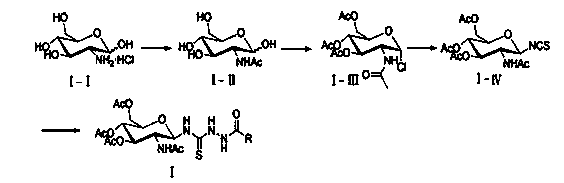
Patents
Literature
71 results about "Urocaninase" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
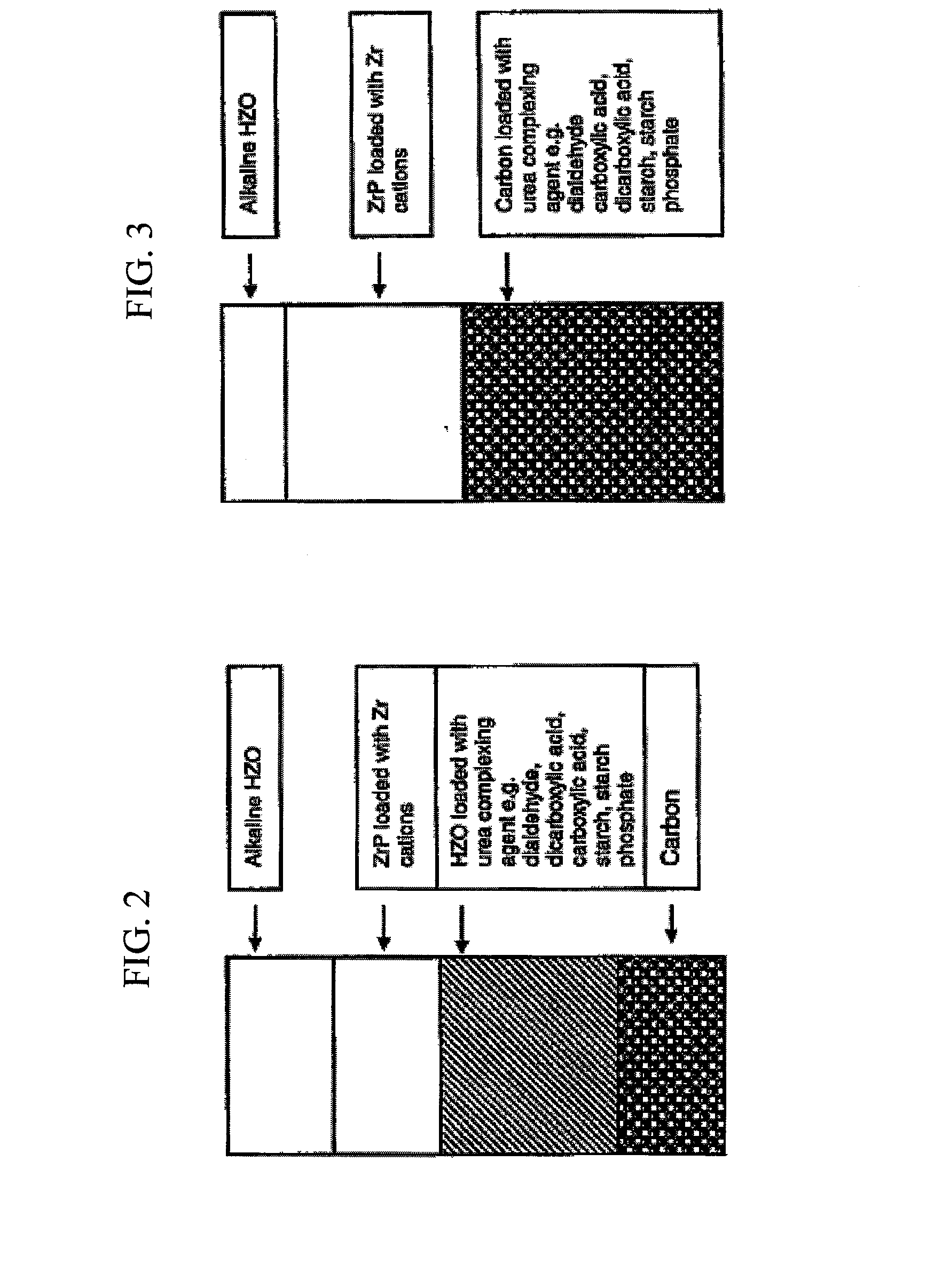
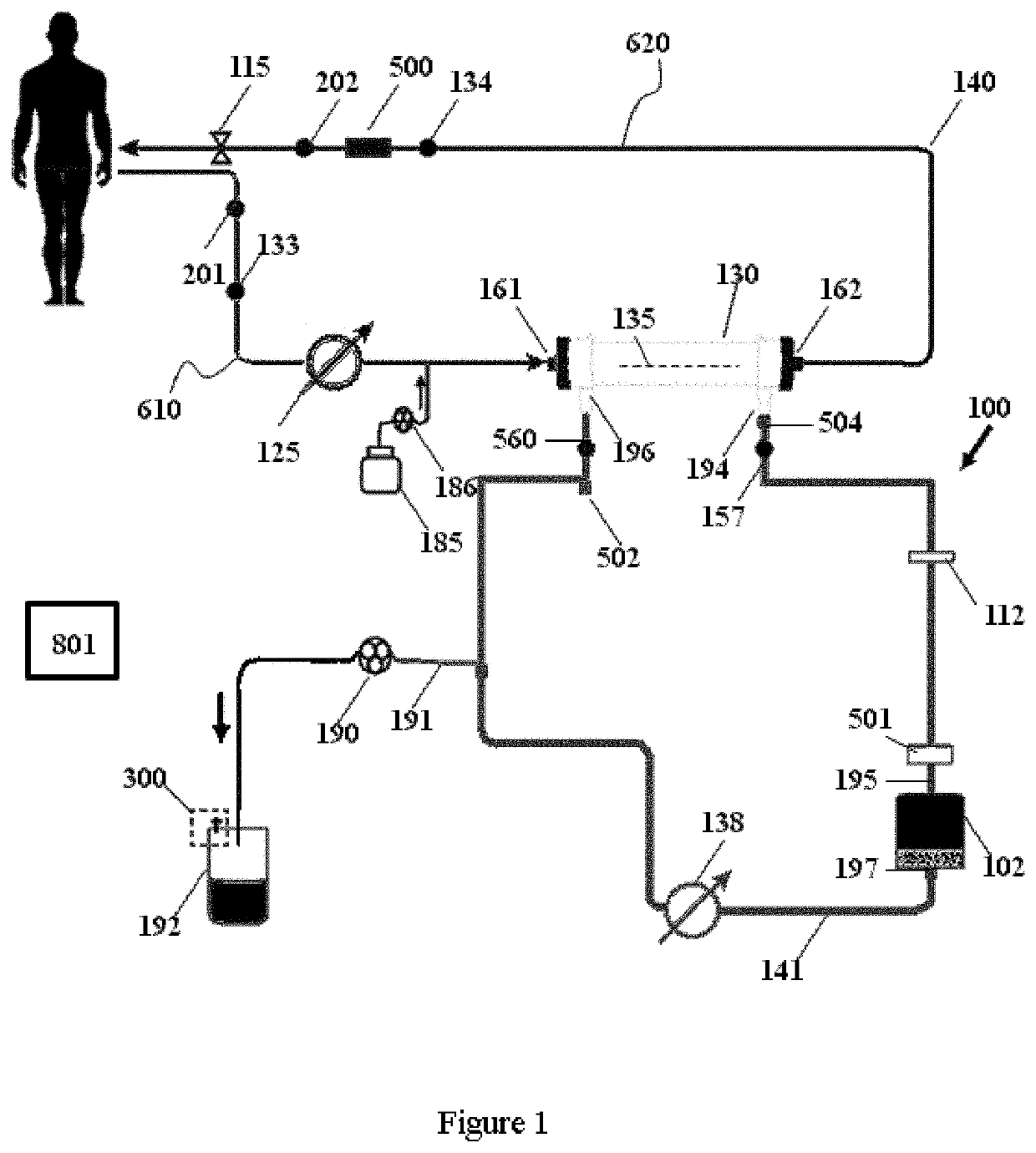
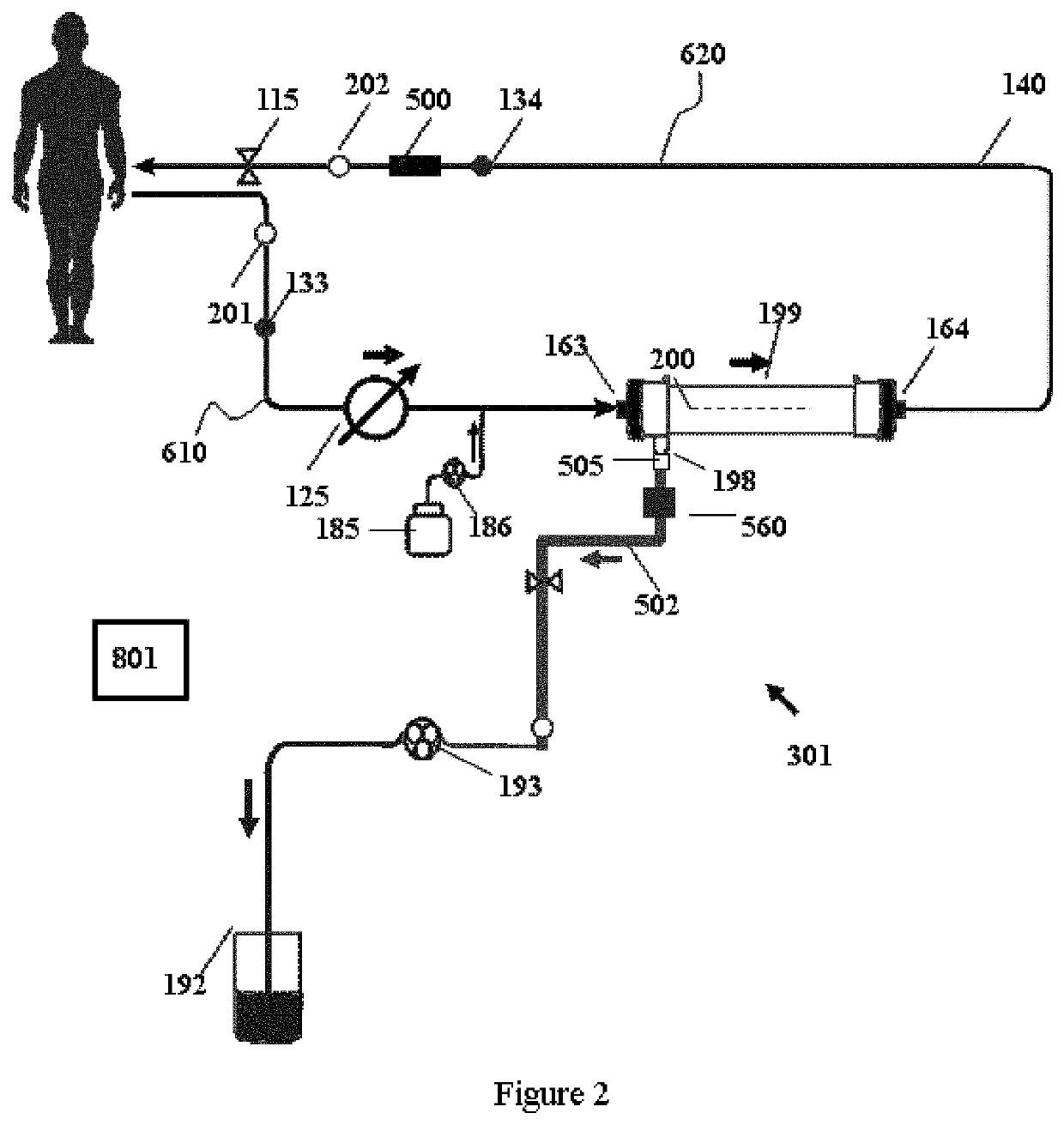
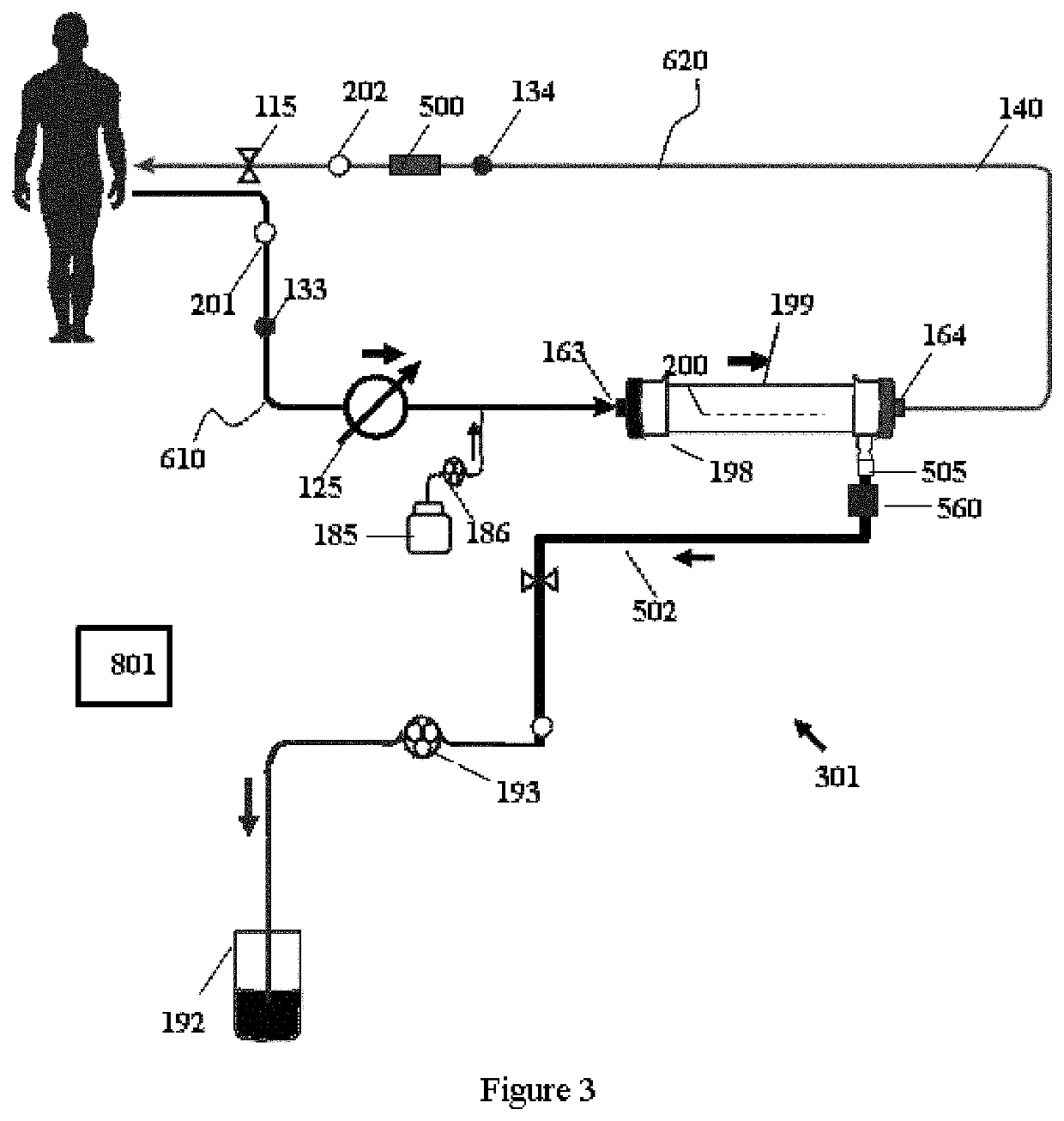
Modular hemodialysis system
ActiveUS20130213890A1Mechanical/radiation/invasive therapiesSolvent extractionDialysis membranesActivated carbon
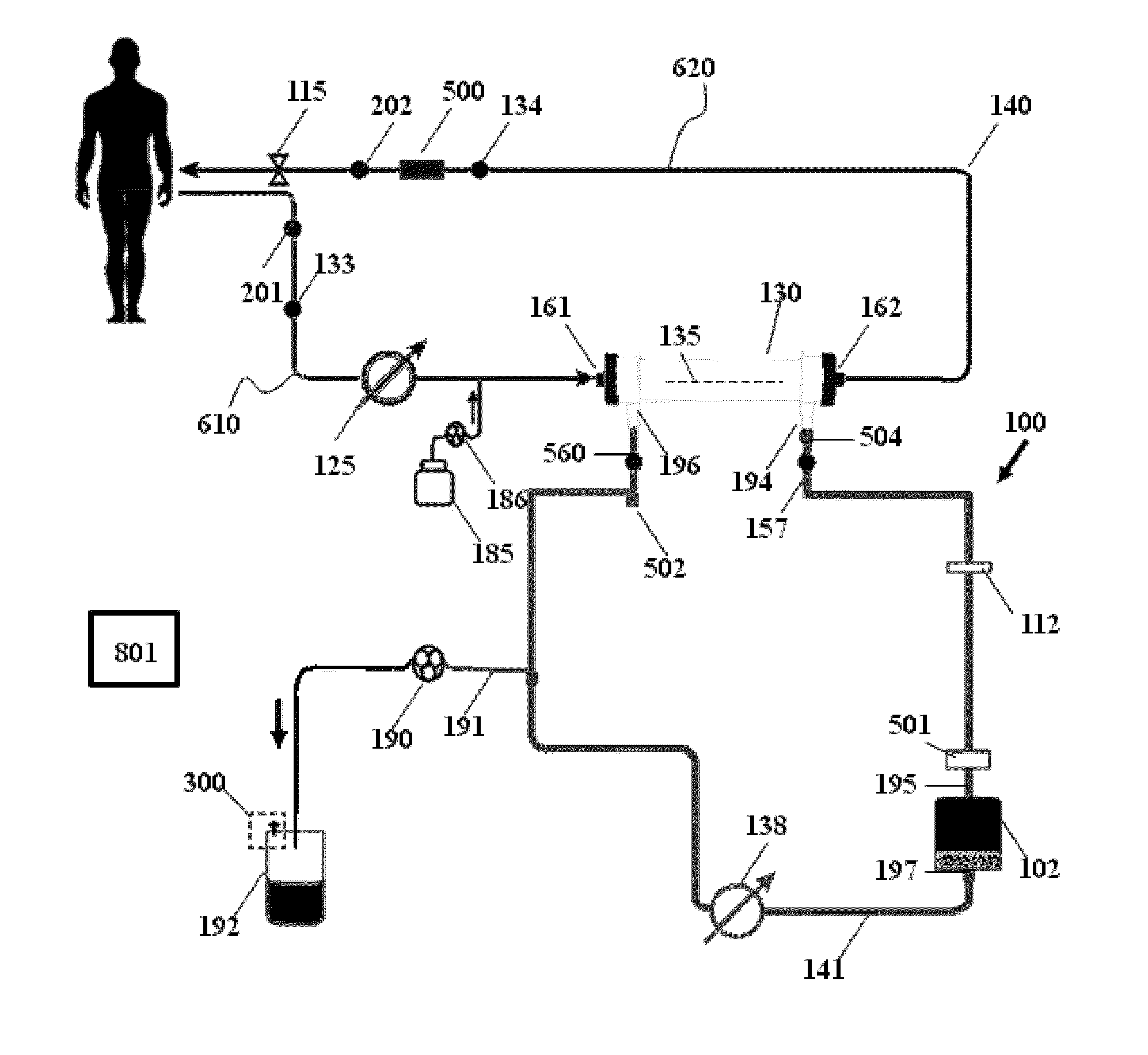
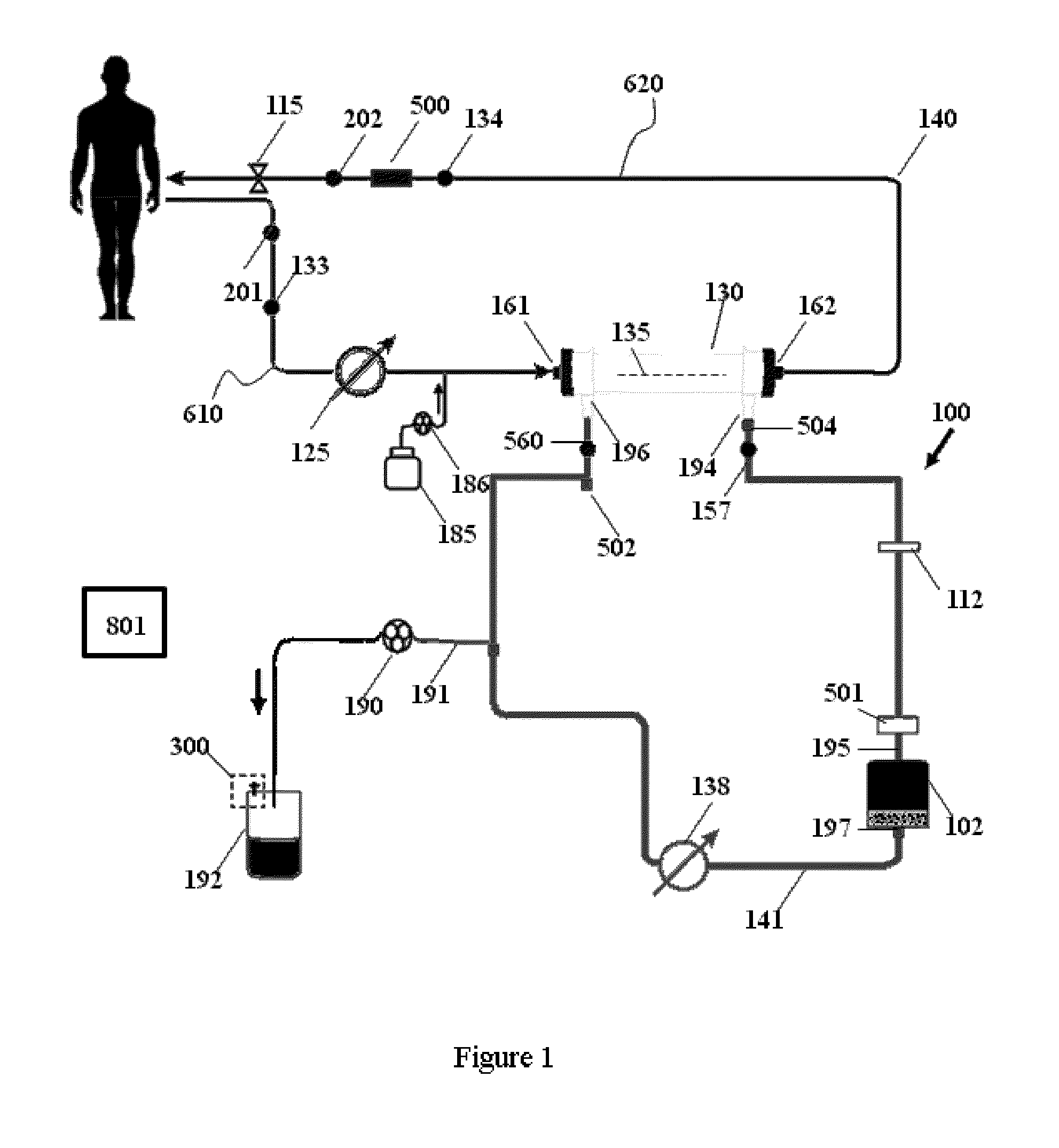
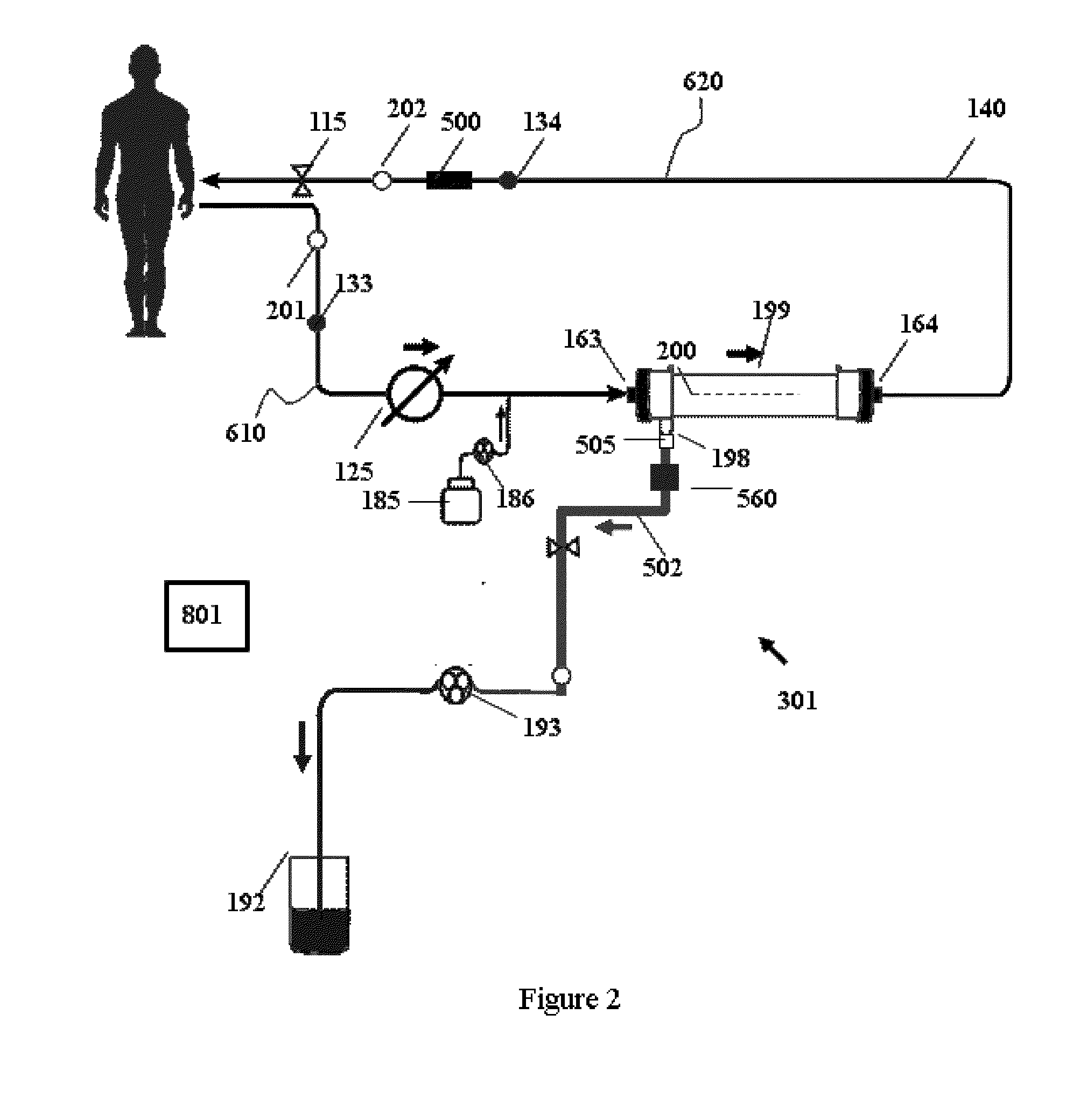
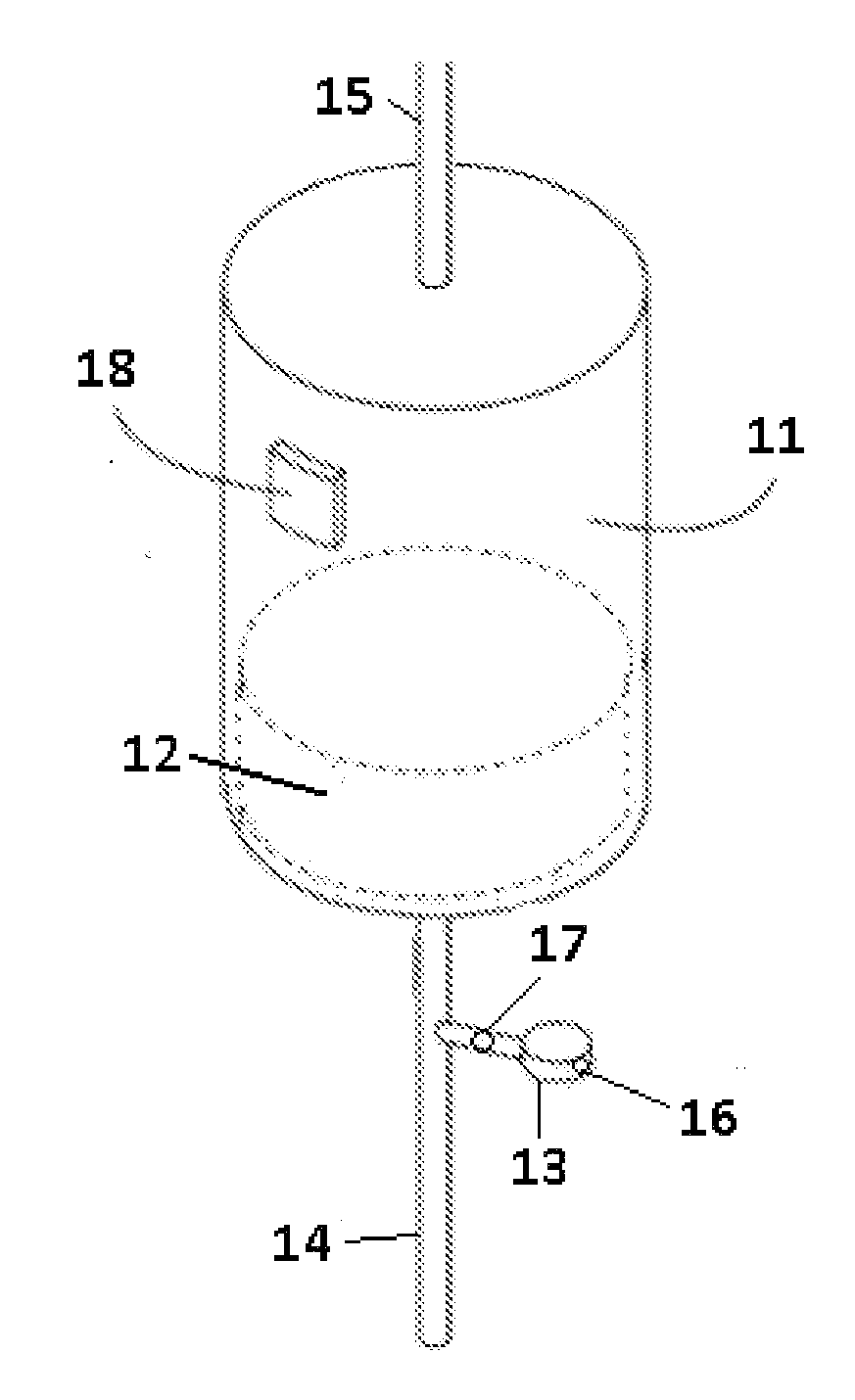
Apparatuses, systems, and methods for the performance of kidney replacement therapy having or using a dialyzer, control components, sorbent cartridge, and fluid reservoirs configured to be of a weight and size suitable to be worn or carried by an individual requiring treatment are disclosed. The system has a controlled compliance dialysis circuit, where a control pump controls the bi-directional movement of fluid across a dialysis membrane. A first sorbent cartridge is provided for use in a portable treatment module having activated carbon and zirconium oxide. The system also provides for the monitoring of an inlet and outlet conductivity of a sorbent cartridge containing urease to provide a facility to quantify or monitor the removal of urea by a detachable urea removal module.
Owner:MOZARC MEDICAL US LLC
Replenisihing urease in dialysis systems using a urease introducer
An apparatus and method for replenishing urease in a sorbent cartridge for use in sorbent dialysis. The sorbent cartridge is configured to allow an amount of urease to be added to the sorbent cartridge. A urease solution can be injected into the sorbent cartridge to replenish the urease containing module, or solid urease can be added to the sorbent cartridge. The sorbent module can also comprise other, rechargeable, sorbent materials for removing toxins other than urea from spent dialysate.
Owner:MOZARC MEDICAL US LLC
Materials For Removal Of Toxins In Sorbent Dialysis And Methods And Systems Using Same
ActiveUS20140336568A1Size reduction requirementsSemi-permeable membranesOther chemical processesUrocaninaseCross linker
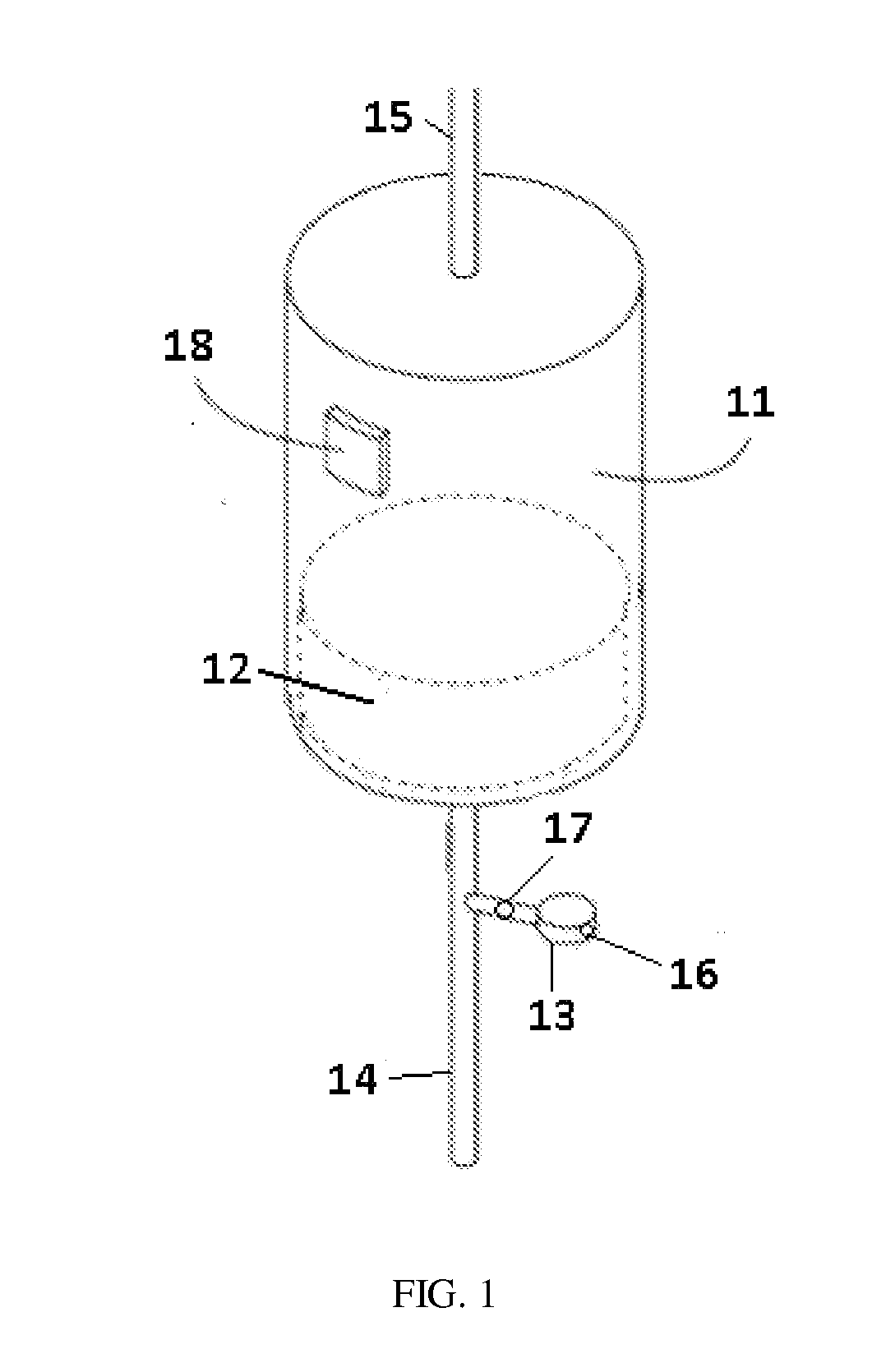
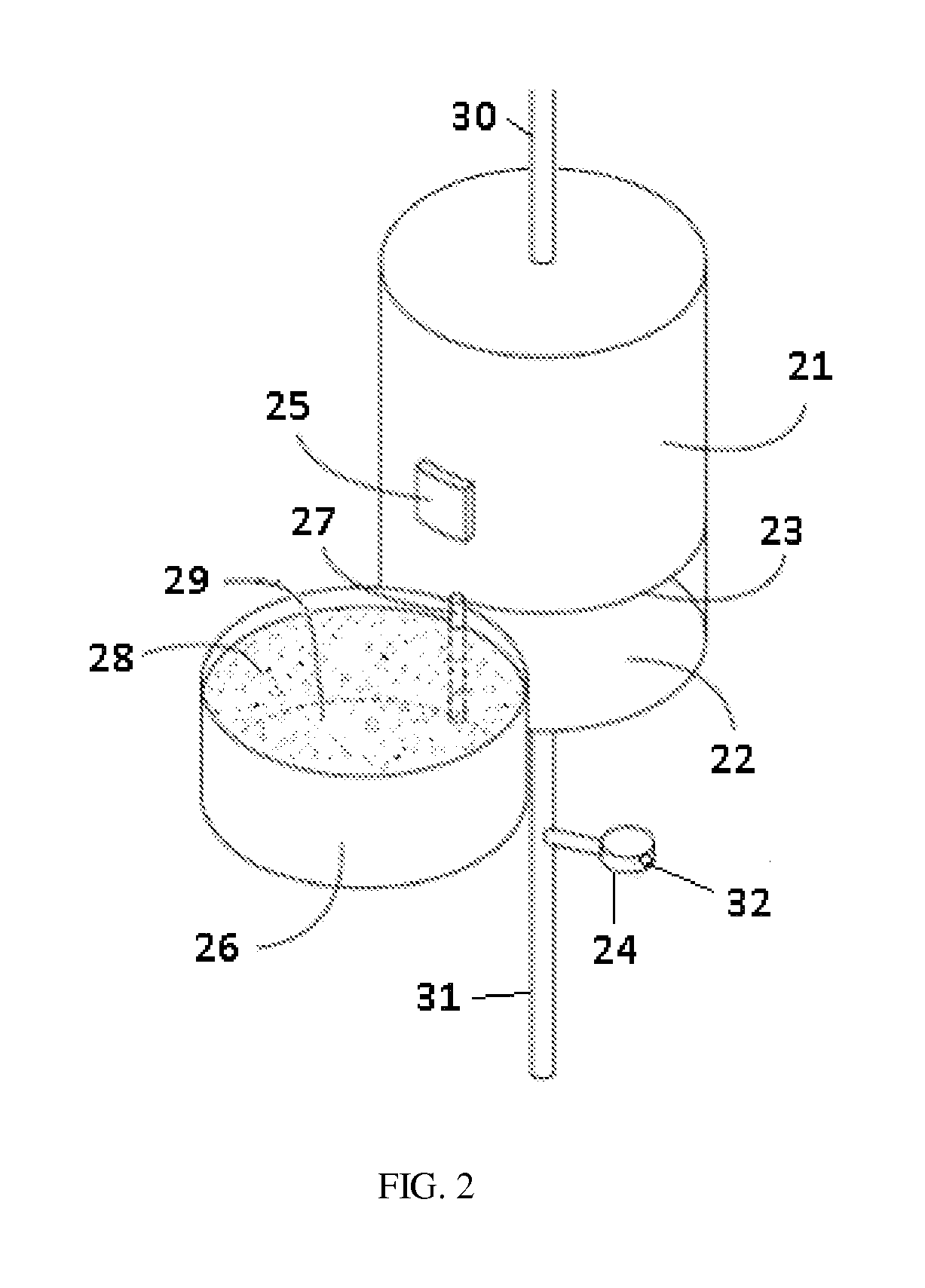
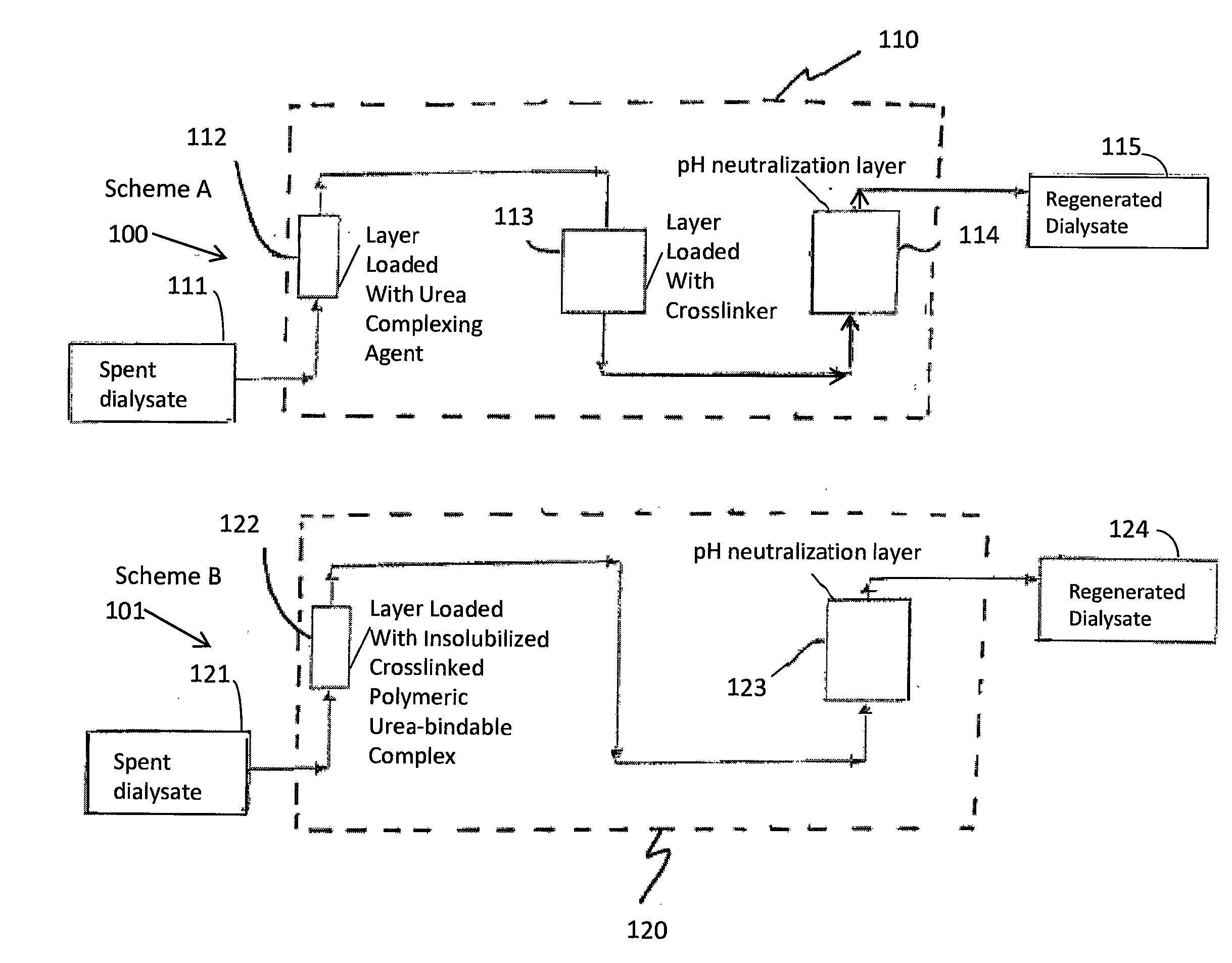
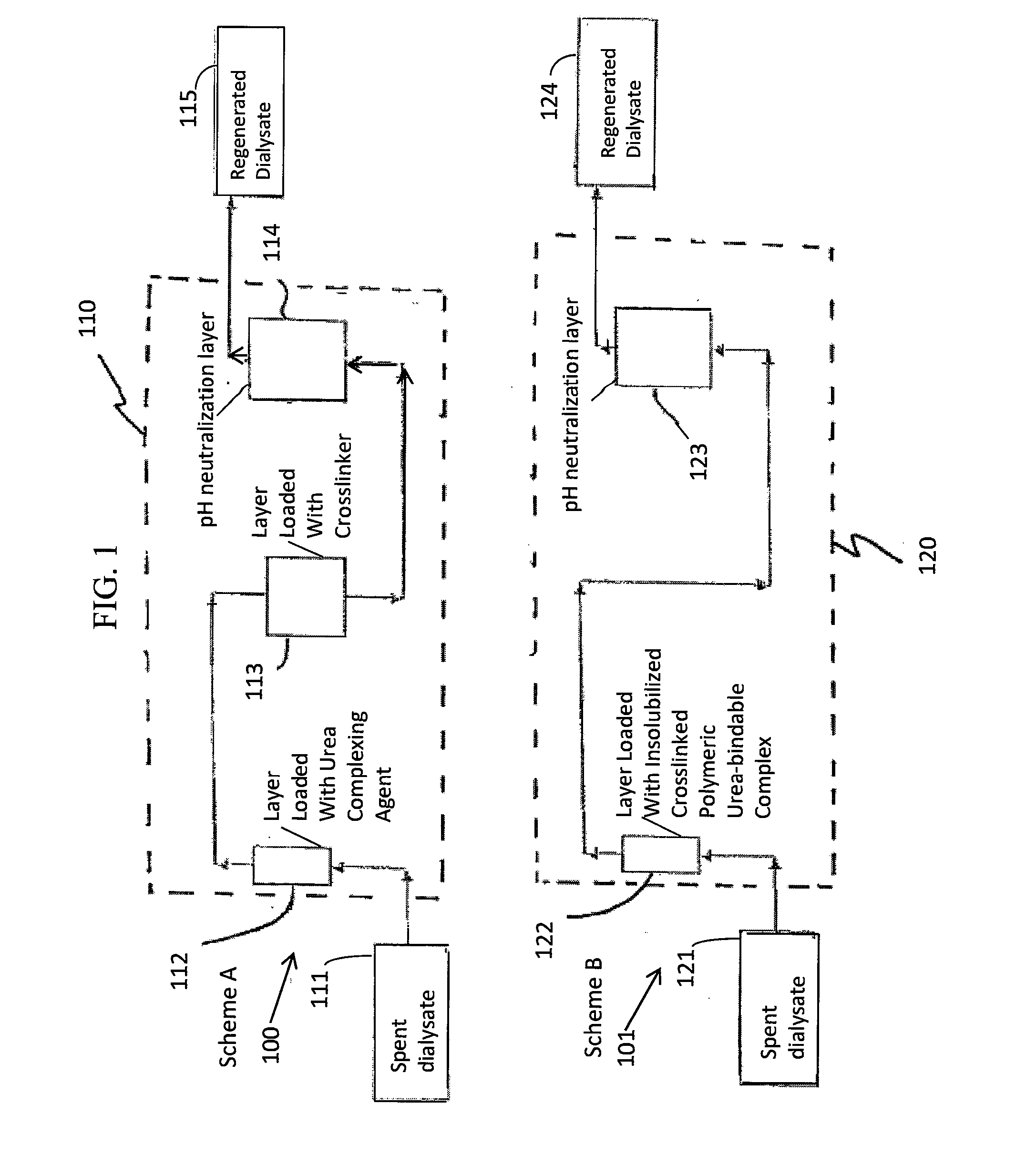
A sorbent dialysis cartridge is provided for removal of uremic toxins from dialysate wherein the sorbent cartridges can use non-enzymatic urea-binding materials in place of urease. The cartridge can have a first sorbent layer loaded with a polymerizable urea complexing agent and a second sorbent layer loaded with a crosslinker. The crosslinker can be crosslinkable with a soluble urea complex reaction product of the polymerizable urea complexing agent and urea when passing through the first sorbent layer to form a crosslinked polymeric urea complex which is attachable to the second sorbent layer. In another option, a sorbent layer can be used which has an insolubilized crosslinked polymeric urea-bindable complex attached thereto, wherein the crosslinked polymeric urea-bindable complex can be a reaction product of a crosslinker and polymerizable urea complexing agent. Methods and sorbent dialysis systems using the cartridge, and methods of making the sorbent material, are provided.
Owner:FRESENIUS MEDICAL CARE HLDG INC
Replenishing urease in dialysis systems using urease pouches
InactiveUS20150367060A1Simple designReduce cost per sessionOther blood circulation devicesIon-exchanger regenerationUrease enzymeUrocaninase
An apparatus and method for replenishing urease in a sorbent cartridge for use in sorbent dialysis using urease pouches. The sorbent cartridge is configured to allow insertion of a urease pouch or injection of a urease solution into the sorbent cartridge containing a urease pouch. The sorbent module can also comprise other, rechargeable, sorbent materials for removing toxins other than urea from spent dialysate.
Owner:MOZARC MEDICAL US LLC
Recombinant helicobacter pylori protein vaccine and preparation method thereof
InactiveCN107298716ARetain the infrastructureRetain activityAntibacterial agentsBacterial antigen ingredientsAdjuvantVaccine antigen
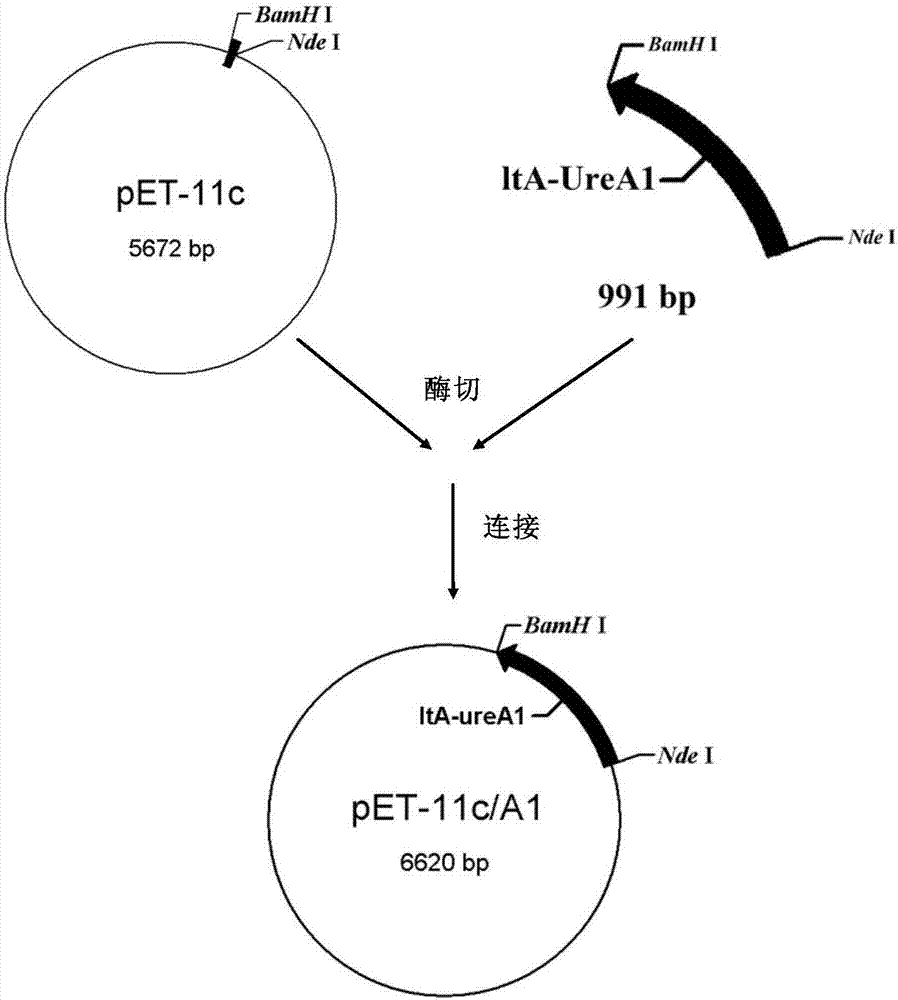
The invention discloses a recombinant helicobacter pylori protein vaccine and a preparation method thereof. The active ingredient recombinant fusion protein of the vaccine consists of recombinant LTAl-Ureal protein and LTB protein, the amino acid sequence of the recombinant LTAl-Ureal protein is shown as Seq ID No.1, the amino acid sequence of the LTB protein is shown as Seq ID No.2. Epitope-containing gene segments of Hp urease A subunit is inserted in an LTA subunit-encoded gene, a toxic part-containing segment is replaced to prepare a recombinant plasmid so as to express and obtain recombinant fusion protein polymer as a vaccine antigen, the fusion antigen and LTB protein pentamer are combined to form a hexamer structure, so that not only can the structure basis and activity of an LT mucosa adjuvant be remained, but also the toxicity can be removed, the immune response of organism muscosa can be effectively induced through immunity of mucosa path, to generate specific IgA antibody. The recombinant helicobacter pylori protein vaccine provides a vaccine manner for preventing and treating infection of helicobacter pylori.
Owner:成都亿妙生物科技有限公司
Urease epitope fusion peptide liposome bacterin for preventing the helicobacter pylori infecting
InactiveCN101062015AEffective in inducing an immune responsePromote wound healingAntibacterial agentsBacterial antigen ingredientsPylorusAdjuvant
The invention discloses an urea enzyme epitope fuse peptiolipid plastid vaccine to against pylorus bolt bacteria infection, which is characterized by the following: choosing fuse peptide of pylorus bolt bacteria urea enzyme B subunit and stick film adjuvant cholera morbus toxin B subunit as immunogen; coating the immunogen with liposome; producing the liposome vaccine. This liposome vaccine can evoke organism to generate special immune response and inhibit planting of pylorus bolt bacteria in stomach.
Owner:CHINA PHARM UNIV
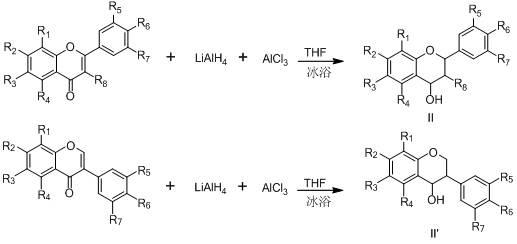
Flavane (isoflavane) urease inhibitor and synthesis and use thereof
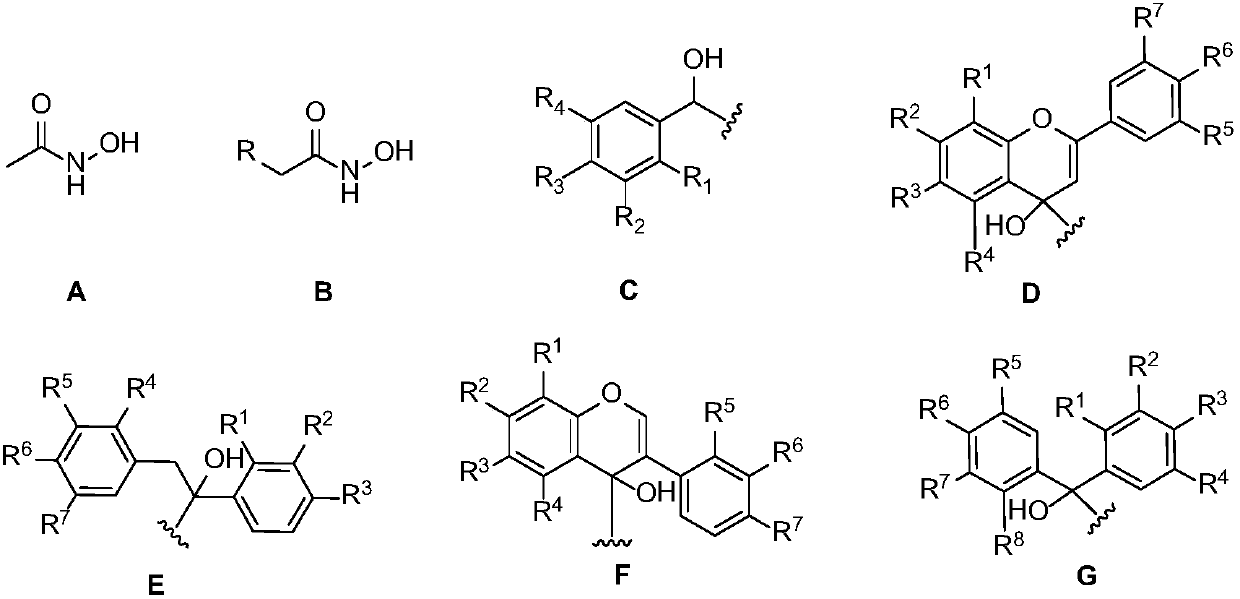
The invention provides a type of flavane (isoflavane) compounds, which have the following structural general formula; the compound has a good inhibitory action on urease, so the compound can be used for preparing medicines for preventing gastritis, gastric ulcer and lithangiuria, and the like; and the invention discloses a preparation method thereof.
Owner:JISHOU UNIVERSITY
Preparation technique and application of non-odor soybean
InactiveCN102907627ANo beany smellEliminate anti-nutritional factorsFood preparationBiotechnologySodium bicarbonate
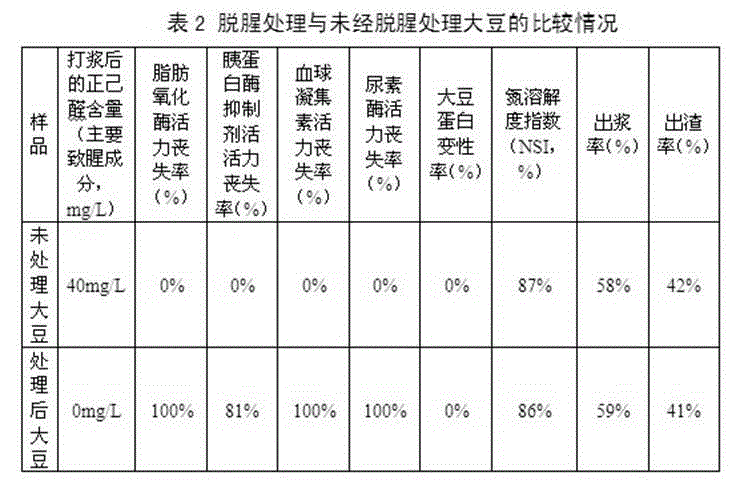
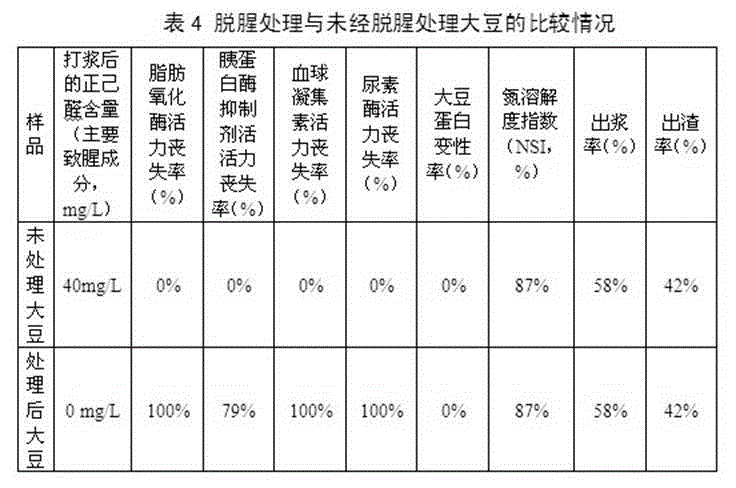
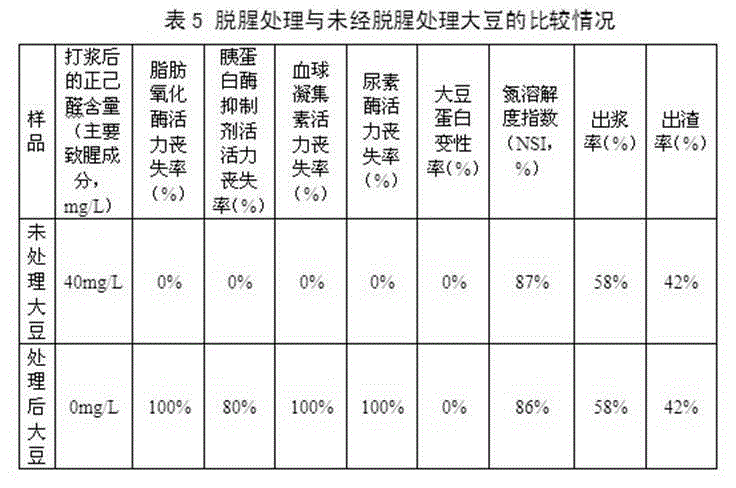
The invention relates to preparation technique and application of non-odor soybean. The preparation technique of non-odor soybean comprises cleaning, alkali treatment, microwave treatment, drainage and drying treatment and package, wherein the aqueous alkali comprises one or mixture of a plurality of sodium carbonate, sodium bicarbonate, potassium carbonate, potassium bicarbonate, sodium hydroxide and potassium hydroxide. The preparation technique combines the chemical treatment mode with the physical treatment mode to remove the beany flavor, the activity of the lipoxidase of the treated soybean is completely lost, and bean products prepared by the non-odor soybean do not generate n-hexylaldehyde or any beany flavor; besides, the preparation technique provided by the invention can eliminate the antinutritional factors in the soybean, the activity of the hemagglutinin and the urease of the treated soybean is completely lost, and the activity loss rate of the trypsin inhibitor is larger than 75%; and meanwhile, the nutritive value of the soybean is not being damaged at all.
Owner:桂仕林
Quantitative determination and drug allergy determination kits for helicobacter pylori viable bacteria and determination method
InactiveCN103757088ASolve the deficiency of only qualitative detection of Helicobacter pyloriSimple methodMicrobiological testing/measurementUrocaninaseDrug allergy
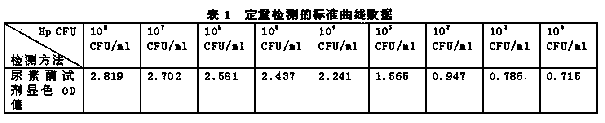
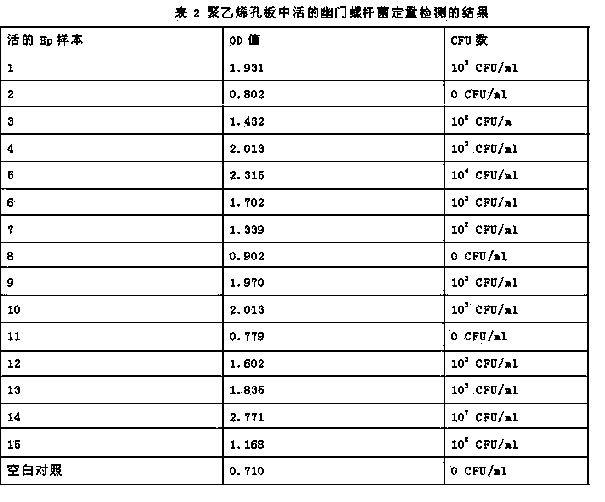
The invention discloses quantitative determination and drug allergy determination kits for helicobacter pylori viable bacteria and a determination method. The method adopts viable helicobacter pylori as a sample to be detected; the property that the viable helicobacter pylori can generate urease is used for rapidly and accurately reading the quantity of the helicobacter pylori by a helicobacter pylori colony counting standard method (CFU / ml, namely the bacterium individual quantity in each ml of bacterium liquid and a colony quantity standard curve); the detection result is accurate and sensitive and has high reliability; the counting difficulty of existing helicobacter pylori clinic microbiological identification, caused by difficult culture and complicated operation, is solved; the drug allergy determination kit and a preparation method, which are expanded by the invention, can be used for carrying out various drug allergy tests at the same time; clinicians can rapidly and conveniently screen suitable anti-helicobacter pylori medicines so that the time and the labor are saved and the cost is saved, so as to provide a beneficial technical solution for clinical scientific treatment.
Owner:SICHUAN VACCINE TECH
Pyloric spiral bacillus antigen recombinant vaccine
InactiveCN1899610AHigh yieldHigh yield for high purityAntibacterial agentsBacterial antigen ingredientsAntigenAdjuvant
The present invention discloses recombinant vaccine based on the Helicobacter pylori neutral granulocyte activated protein A (NapA) antigen and its preparation process and application in inducing the protecting immune reaction resisting Helicobacter pylori infection. The vaccine consists of independent Helicobacter pylori NapA as the basic active component, or the fusion protein comprising Helicobacter pylori NapA, adhesion HpaA and urase B subunit active segment (UreB414), and one or several kinds of pharmaceutically acceptable adjuvant and excipient.
Owner:ARMY MEDICAL UNIV
Flavanol (isobutene flavanol) urease inhibitor and synthesis and application thereof
InactiveCN102503922AStrong inhibitory activityOrganic active ingredientsOrganic chemistryFlavanololGastritis
The invention discloses a type of flavanol (isobutene flavanol) compounds. The structural formulae of the compounds are shown in the specifications. The compounds have good inhibiting actions on urease, and can be used for preparing medicaments for resisting gastritis, gastric ulcer, lithangiuria and the like. The invention further discloses a preparation method of the compounds.
Owner:JISHOU UNIVERSITY
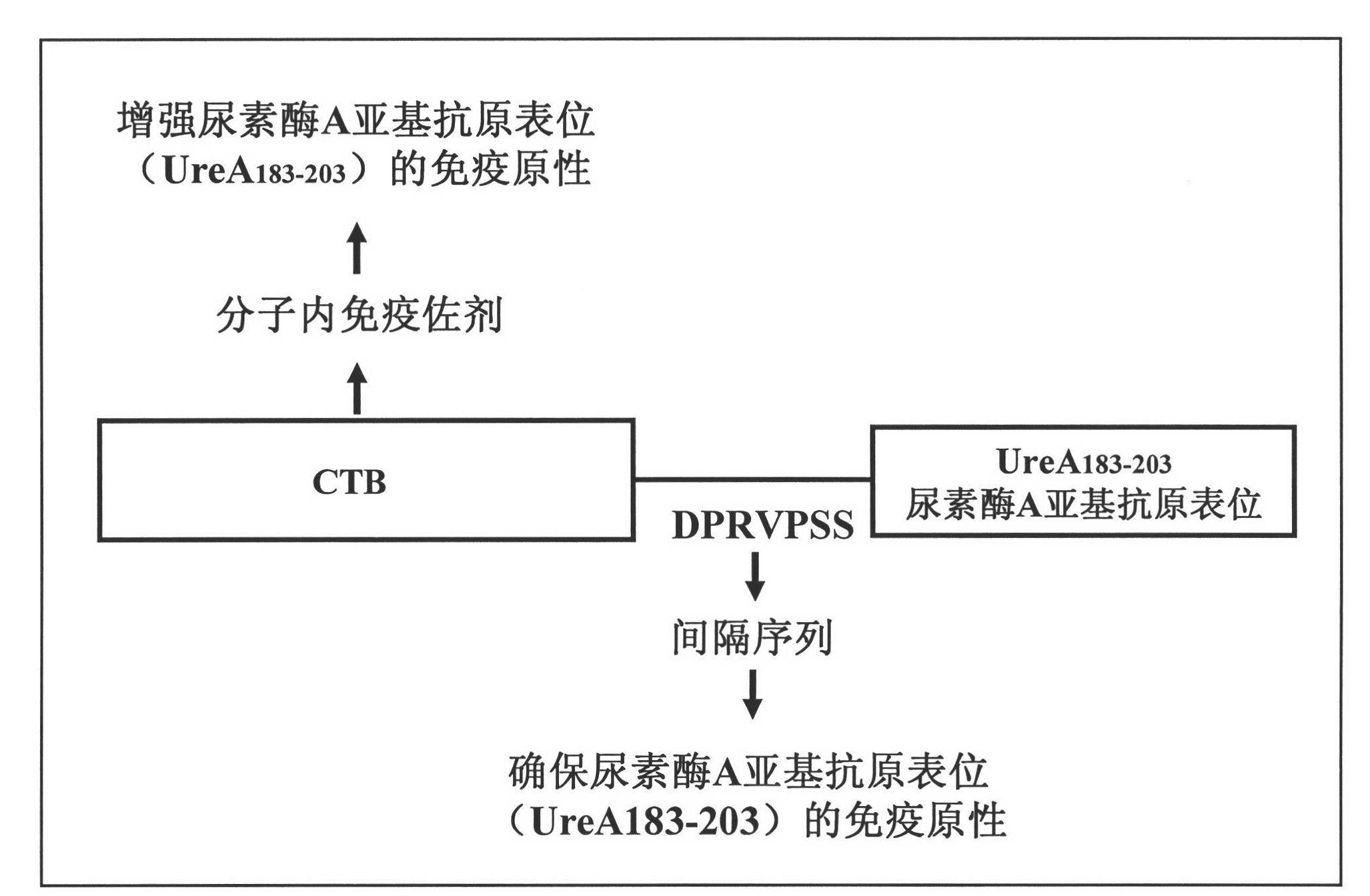
Helicobacter pylori epitope vaccine, design method thereof, preparation method thereof and application thereof
InactiveCN102151332AAntibacterial agentsAntibody medical ingredientsEscherichia coliSpecific antibody
The invention provides a helicobacter pylori epitope vaccine. The active constituent of the helicobacter pylori epitope vaccine is a polypeptide and mainly consists of a cholera toxin B subunit and a B cell antigen epitope from a urease A subunit. A preparation method of the helicobacter pylori epitope vaccine comprises the following steps of: synthesizing the nucleotide sequence of the B cell antigen epitope from the urease A subunit by a PCR (polymerase chain reaction) technology, coupling the nucleotide sequence with the gene sequence of the cholera toxin B subunit to form into a fusion gene, and expressing the fusion gene in the escherichia coli by an expression vector to obtain the fusion protein of the epitope vaccine by means of protein purification. The helicobacter pylori epitope vaccine can be used for inducing the human body to generate an epitope specific antibody which is higher in titer to the urease. In the biologic medical field, the epitope vaccine can be used for preventing and curing the relevant diseases caused by the helicobacter pylori infection, thereby being great in economic benefit and social benefit.
Owner:CHINA PHARM UNIV
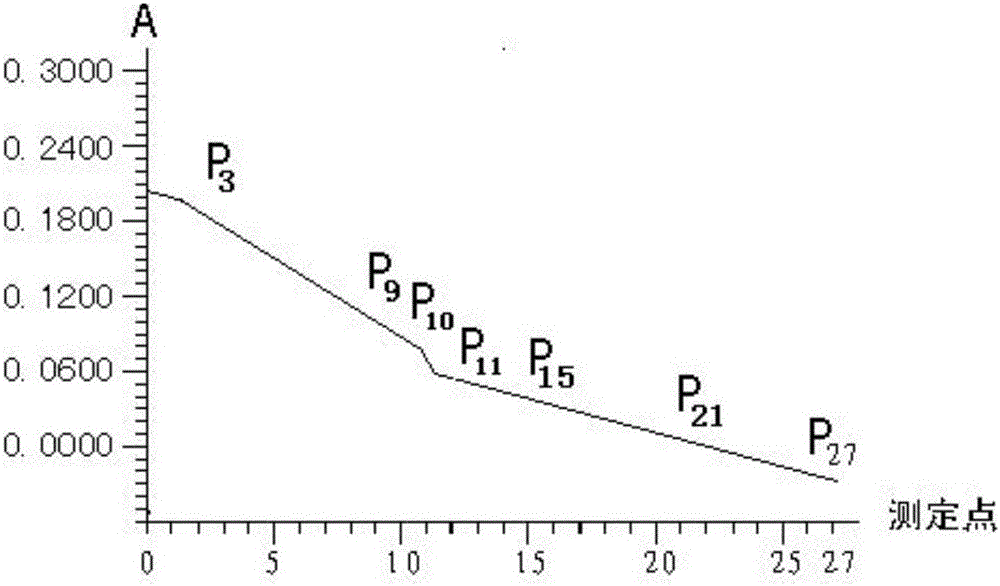
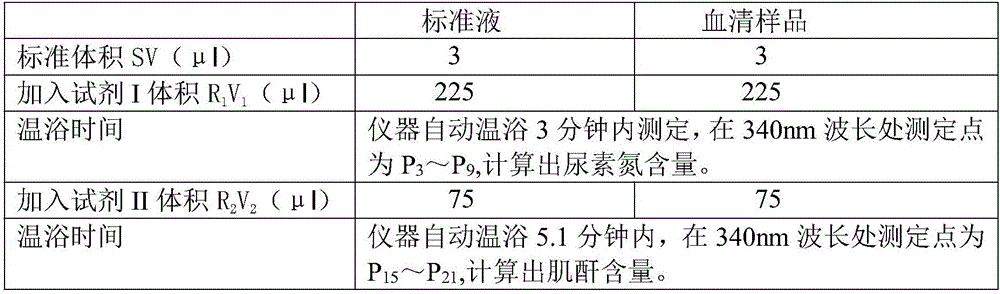
Method for simultaneous determination of double items of urea nitrogen and creatinine in serum
The invention discloses a method for simultaneous determination of double items of urea nitrogen and creatinine in serum, and belongs to the method for testing a material through testing color changes of reaction results by using visible light; the technical scheme comprises that a reagent II only comprises effective components of creatinine amidohydrolase and creatine amidinohydrolase; a reagent I contains effective components of urease, glutamate dehydrogenase, alpha-ketoglutarate and NADH. The determination method comprises the steps: firstly, carrying out 37 DEG C warm bath of serum with the reagent I for 3-5 minutes; carrying out a reaction of urea in the serum with the reagent I to generate NAD+; adding the reagent II, carrying out 37 DEG C warm bath for 4-7 minutes, hydrolyzing creatinine with the creatinine amidohydrolase to generate creatine; making the creatine generate urea under the action of the creatine amidinohydrolase, and making the urea and the reagent I generate NAD+ under the action of urease; at the wavelength of 340 nm, comparing the reaction speed with that of a standard treated by the same way, determining the change of the first-step reaction NADH, namely the content of urea nitrogen in the serum, and determining the change of the second-step reaction NADH, namely the content of the creatinine in the serum.
Owner:TIANJIN BAODI HOSPITAL
Solvent systems of n-alkyl thiophosphoric triamides and methods of use in agricultural applications
ActiveCN104136398ALimit the scope of protectionOrganic compound preparationGroup 5/15 element organic compoundsUrocaninaseUrease Inhibitors
Owner:RHODIA OPERATIONS SAS +1
Method for producing organic-inorganic hybrid membrane by enzyme induction
ActiveCN107441946AImprove throughputImprove hydrophilicityMembranesUltrafiltrationCationic polyelectrolytesUrease
A method for or producing an organic-inorganic hybrid membrane by enzyme induction belongs to the technical field of membrane separation and comprises steps: firstly, assembling a plurality of layers of cationic polyelectrolyte and anionic polyelectrolyte alternately on the surface of a treated membrane to obtain a membrane 1; secondly assembling a plurality of layers of cationic polyelectrolyte, calcium salt and anionic polyelectrolyte and urease alternately on the surface of the membrane 1 to obtain a membrane 2; and finally soaking the membrane 2 in a urea solution, catalyzing urea in the urea solution by the urease in the membrane 2 to generate carbon dioxide, and then reacting with calcium salt on the surface of the membrane 2 to obtain calcium carbonate particles. The obtained calcium carbonate particles can effectively enhance the hydrophilic performance of the surface of the membrane, so that the membrane flux is increased.
Owner:BEIJING UNIV OF TECH
Urease inhibitor genistein hydroxamic acid compound and synthesis and application thereof
ActiveCN102993152AStrong inhibitory activityHigh activityOrganic active ingredientsOrganic chemistryGastritisHydroxamic acid
A genistein hydroxamic acid compound has the following structural general formula. The genistein hydroxamic acid compound has good inhibitional effect on urease and can be used for preparing drugs for treating gastritis, gastric ulcer, lithangiuria and the like. A manufacturing method of the genistein hydroxamic acid compound is disclosed.
Owner:杭州圣捷菲科技有限公司
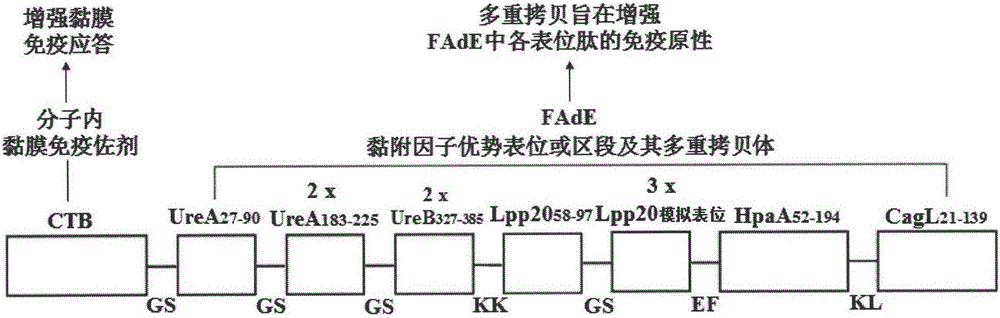
Helicobacter pylori tetravalent adhesion multi-epitope vaccine and preparation method thereof
ActiveCN105126093ABiological toxicity avoidanceProtect against immunopathological damageAntibacterial agentsPeptide preparation methodsEscherichia coliDisease
The invention provides a multi-epitope vaccine for a helicobacter pylori tetravalent adhesion, wherein the activity of the multi-epitope vaccine is presented as a polypeptide which mainly consists of urease A and B subunits, superior Th and B cell epitopes or fragments of three outer membrane proteins (Lpp20, HpaA and CagL) as well as cholera toxin B subunit. According to the invention, an artificial gene is synthesized by virtue of gene synthesis technology, wherein the synthesized artificial gene consists of urease A and B subunits, and superior Th and B cell epitopes or fragments of three outer membrane proteins (Lpp20, HpaA and CagL), and the artificial gene is coupled with gene sequence of the cholera toxin B subunit, so as to form a fusion gene. The fusion gene is expressed by escherichia coli, and upon protein purification, the tetravalent adhesion multi-epitope vaccine is obtained. The vaccine can be used for inducing a body to generate T cellullar immunologic response and specific antibody humoral immune response in accordance with the urease A and B subunits and the three outer membrane proteins (Lpp20, HpaA and CagL); and the vaccine is suitable for preventing and controlling helicobacter pylori infection related diseases.
Owner:NINGXIA MEDICAL UNIV
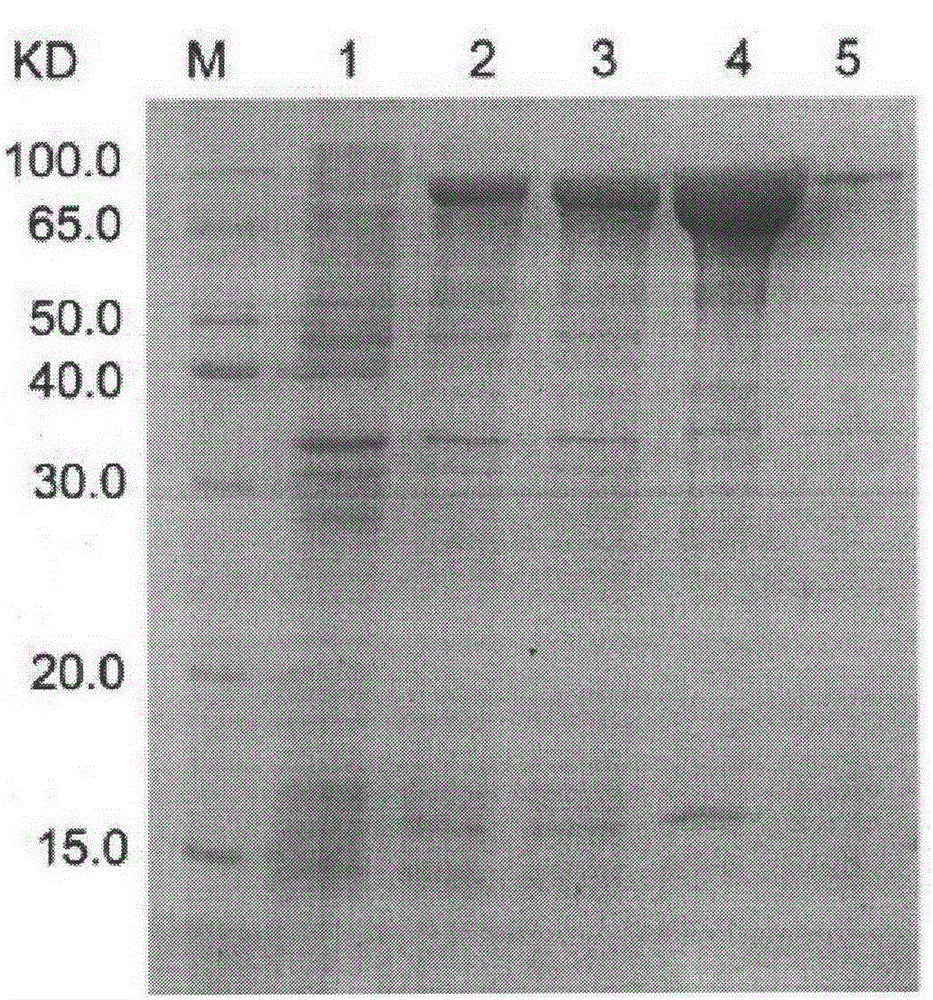
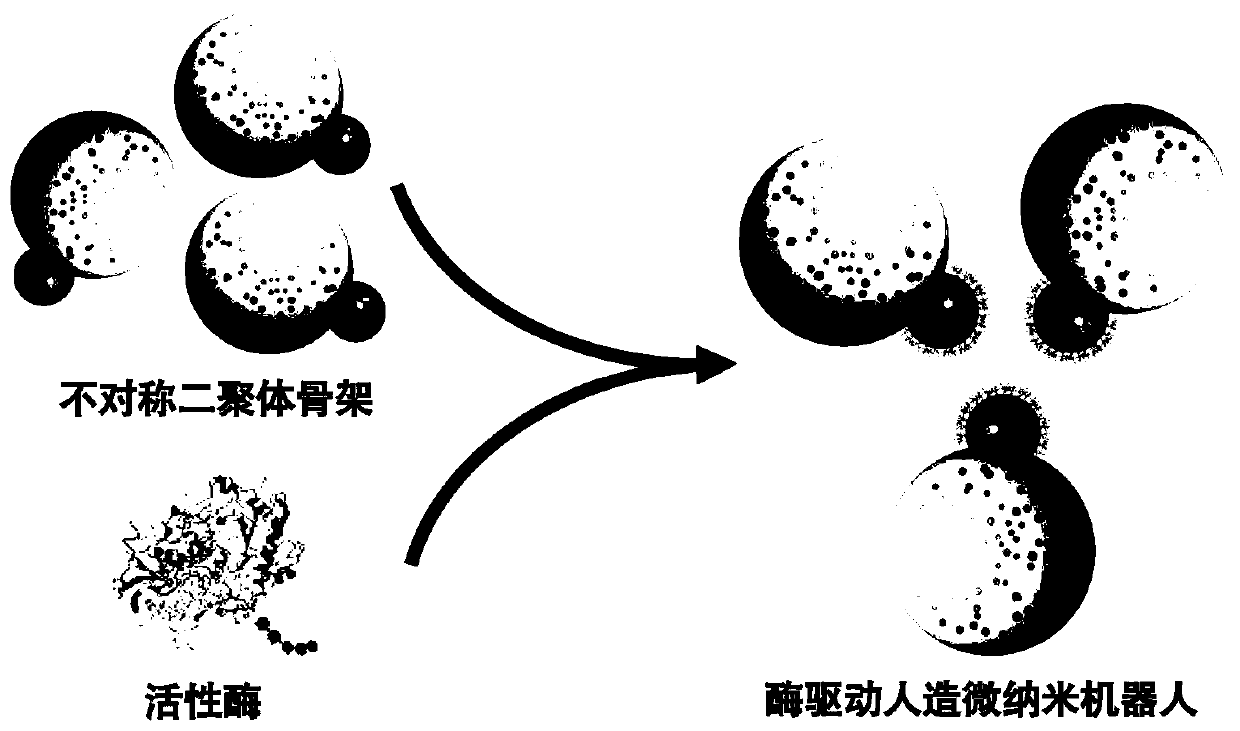
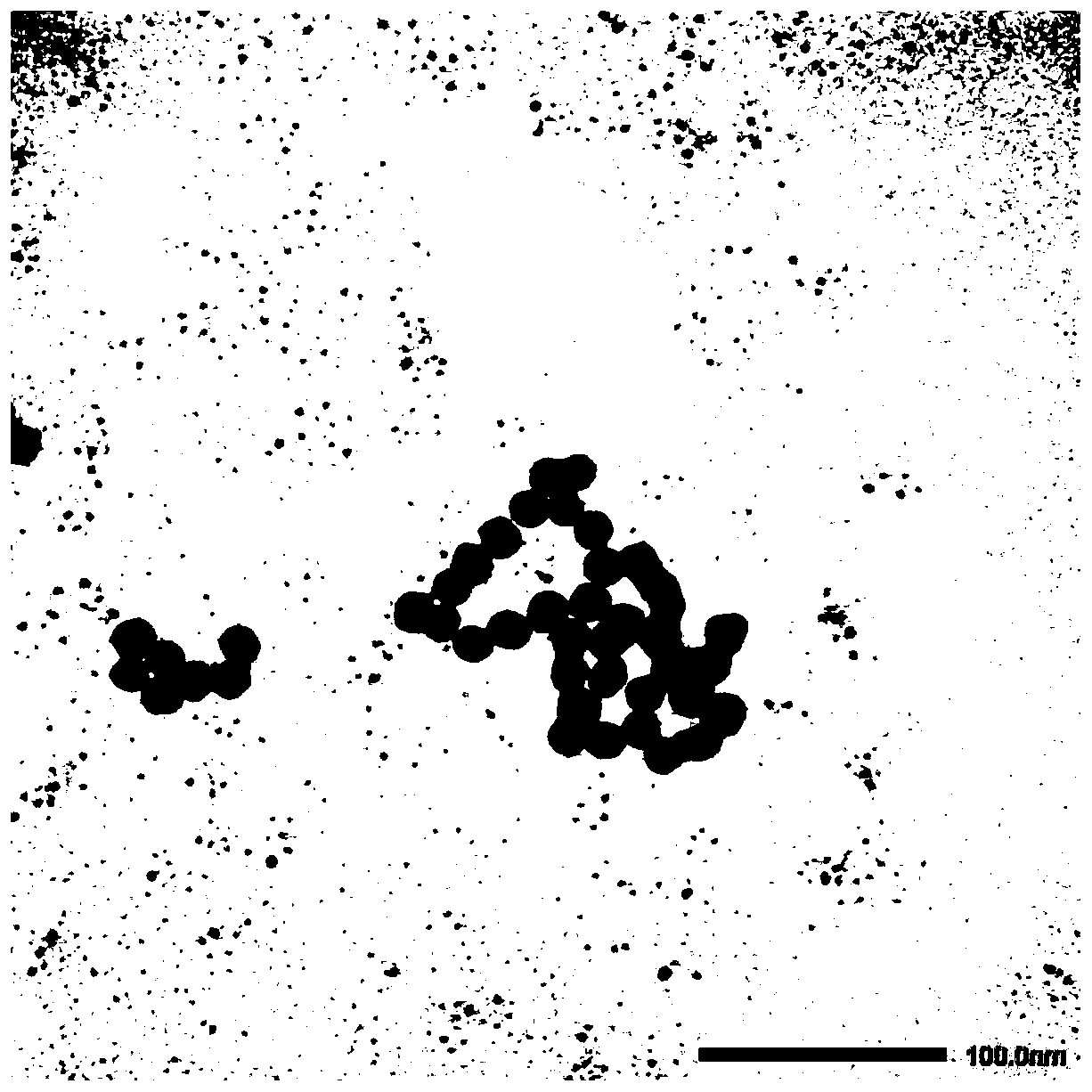
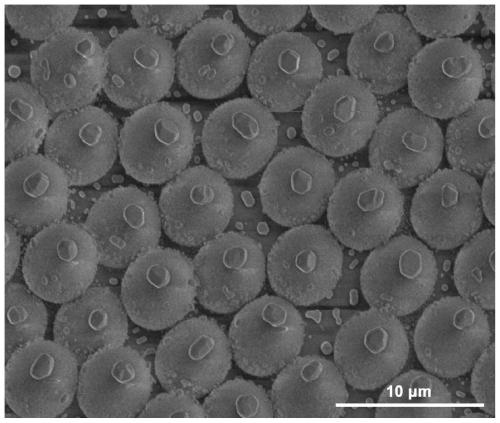
Artificial micro-nano robot and preparation method thereof
PendingCN111575267AAchieve targeted movementGood repeatabilityHydrolasesVacuum evaporation coatingActive enzymeUrocaninase
The invention discloses an artificial micro-nano robot and a preparation method thereof, and relates to the field of micro-nano robots. By taking active enzymes such as magnetic micro-nano particles,silicon dioxide microspheres, nano-gold particles, glucose oxidase, catalase and urease as materials, the artificial micro-nano robot with an asymmetric dimer structure is prepared by fusing the enzymes with the catalytic activity, the magnetic micro-nano particles, the silicon dioxide microspheres and the nano-gold particles together by utilizing a vacuum plasma sputtering technology and a chemical modification method. The method is high in repeatability and simple in preparation process. The prepared artificial micro-nano robot can rapidly move by decomposing biological media such as glucosein blood and urea in the bladder without generating any substance having toxic and side effects on the body, or by applying an exogenous magnetic field, targeted movement of the artificial micro-nanorobot in the movement direction is achieved, and the artificial micro-nano robot is applied to the biomedical fields of drug targeting delivery, tumor treatment and the like.
Owner:LULIANG UNIV
Diaryl propionyl hydroxamic compound and preparation method and use thereof
The invention relates to a diaryl propionyl hydroxamic compound, which has the following general formula of structure shown in the specification. The compound has relatively good inhibitory action on urease, and can be used to prepare drugs for resisting gastritis, gastric ulcer, lithangiuria and the like. The invention discloses a preparation method of the compound.
Owner:杨秀菊
1,3,4-oxadiazole derivative containing glucosamine fragment as well as synthetic method and use of derivative
InactiveCN104177459ACheap and easy to getLow toxicityOrganic active ingredientsSugar derivativesUrocaninaseChemical compound
The invention discloses a 1,3,4-oxadiazole derivative containing a glucosamine fragment as well as a synthetic method of the 1,3,4-oxadiazole derivative containing the glucosamine fragment and a use of the derivative. The 1,3,4-oxadiazole derivative containing the glucosamine fragment is a novel compound, and has relatively high inhibitory activity on urease. The synthetic method disclosed by the invention is a convenient, high-yield and efficient method; paratoluensulfonyl chloride is taken as a cyclization reagent, the operation is simple and safe, the environmental pollution is small, the scope of application is wide and the yield is high.
Owner:HUAIHAI INST OF TECH
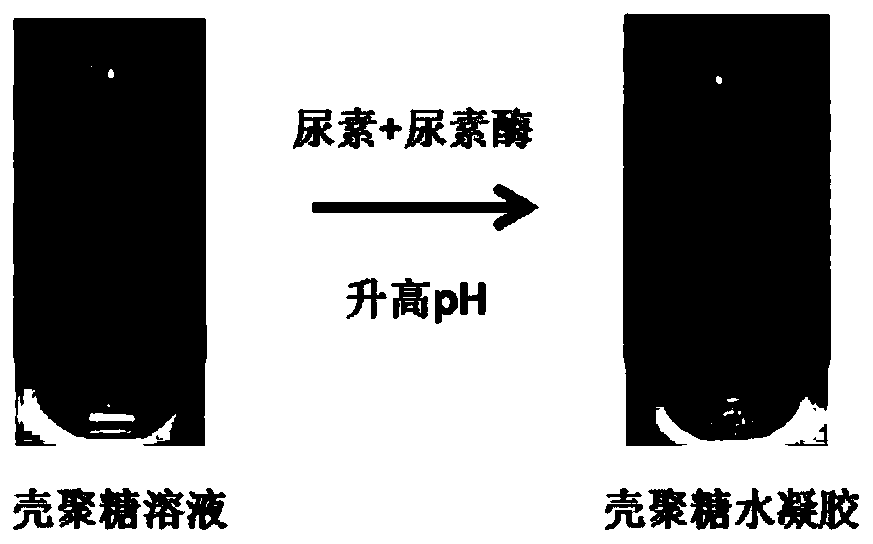
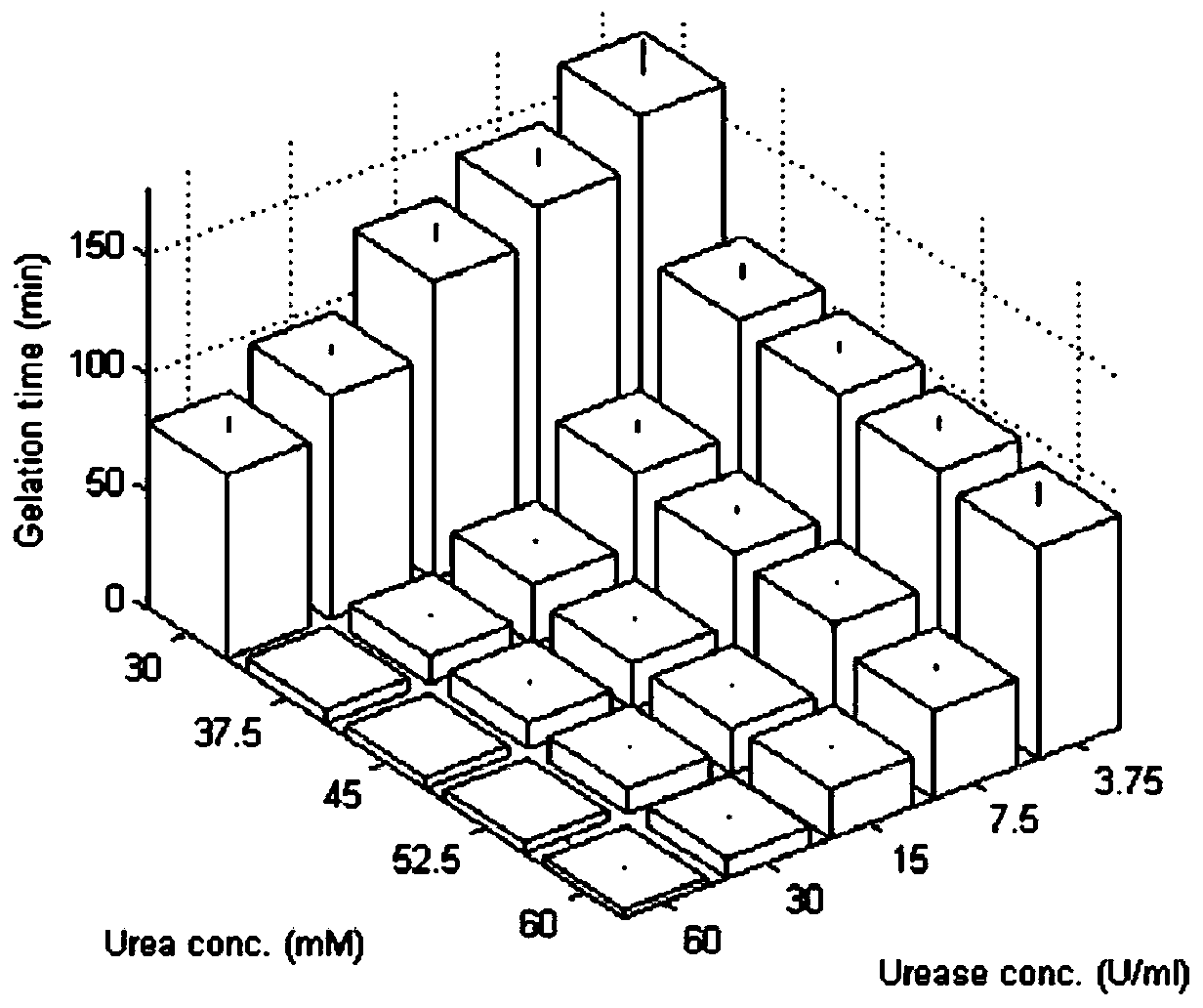
Preparation method of chitosan hydrogel
The invention relates to a preparation method of chitosan hydrogel. The preparation method comprises the following steps: adding urea and urease into a chitosan solution, and increasing the pH value of the chitosan solution to gelate the chitosan solution, so that the chitosan hydrogel is obtained. Compared with the prior art, the method provided by the invention is simple and is easy for manufacturing, and the gelation time of the chitosan enzymatic physical-crosslinking hydrogel can be accurately regulated and controlled by adjusting concentrations of urea and urease.
Owner:TONGJI UNIV
Preparation method of reagent for quantitatively determining Helicobacter pylori antigen in excrement
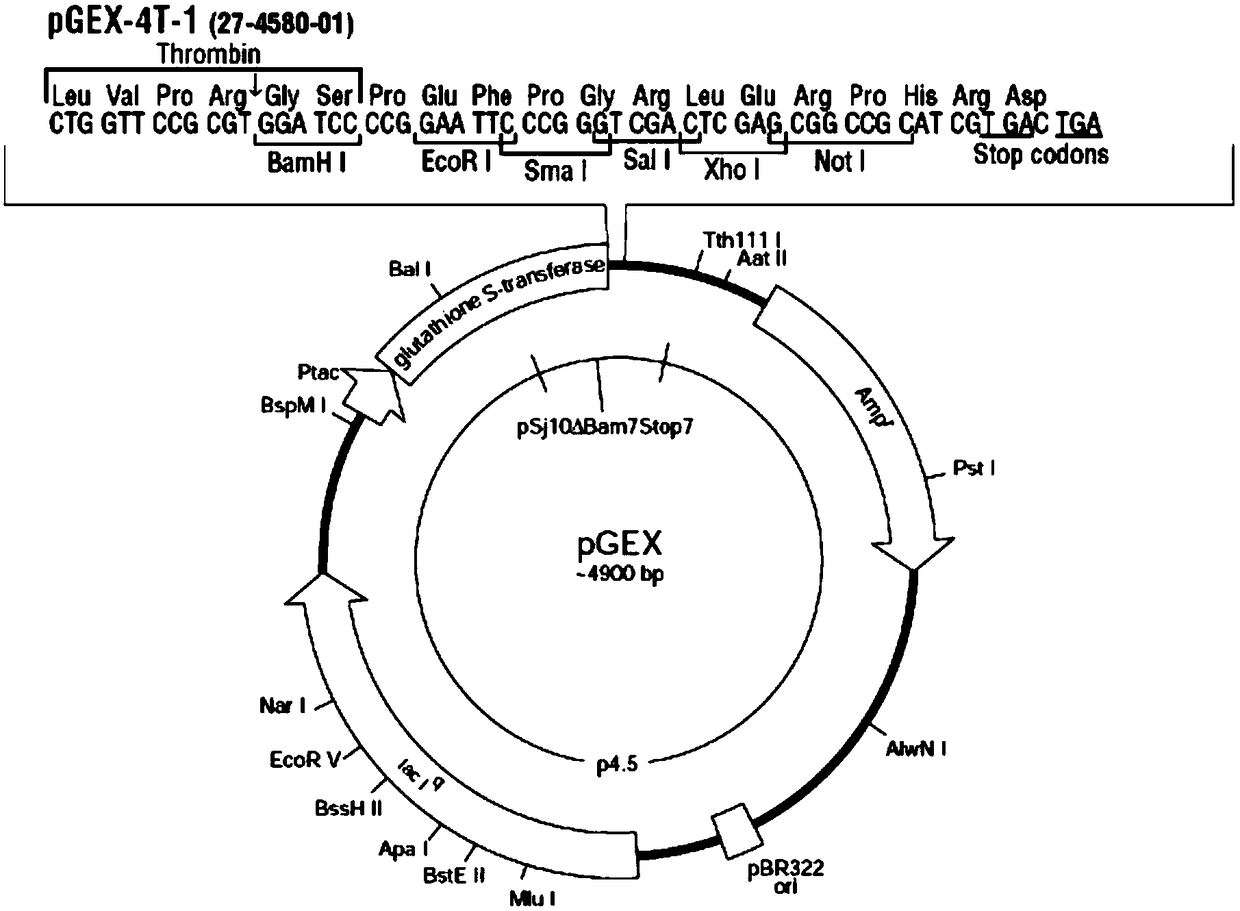
The invention discloses a preparation method of a reagent for quantitatively determining a Helicobacter pylori antigen in excrement. The method comprises following steps of preparing an R2 reagent: S1, extracting Helicobacter pylori antigen (urease) protein, cloning a urease gene into a pGEX-4T-1 plasmid, fermenting and expressing by escherichia coli, and purifying by affinity chromatography to obtain a recombinant Helicobacter pylori antigen (urease); S2, preparing a polyclonal antibody of an anti-Helicobacter pylori antigen (urease); S3, preparing latex particles coated with the polyclonal antibody of the anti-Helicobacter pylori antigen (urease); and S4, dispersing in buffer solution to obtain the R2 reagent. The method is a latex enhanced immunoturbidimetry method, the prepared reagentis solution capable of directly detecting Helicobacter pylori through excrement, detection can be completed by only a excrement sample, and therefore sampling is convenient. The human body is free ofpotential safety hazards, detection efficiency is high, stability is good, specificity is high, and probability of interference is low.
Owner:SHENZHEN HONGMED INFAGEN CO LTD
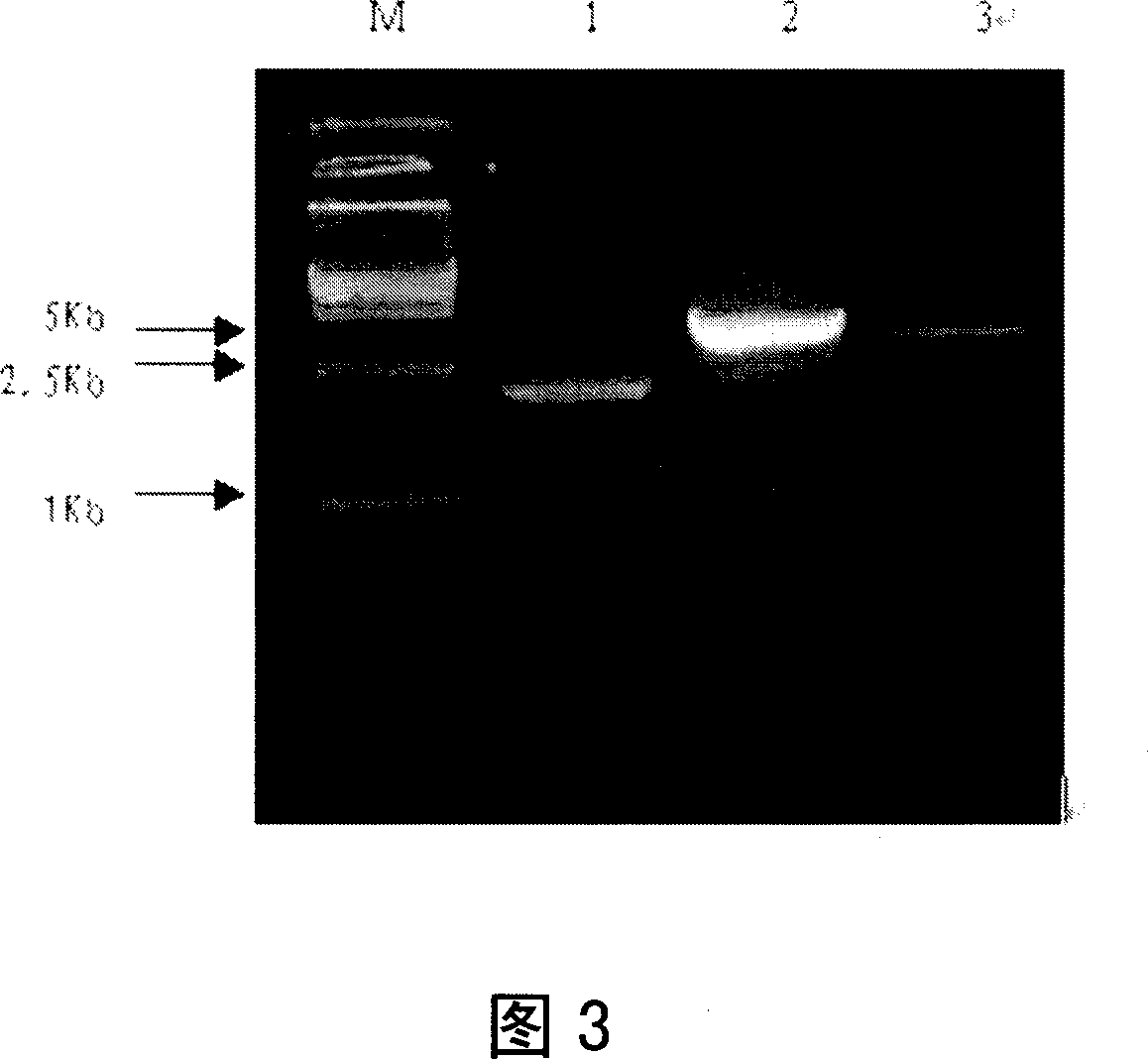
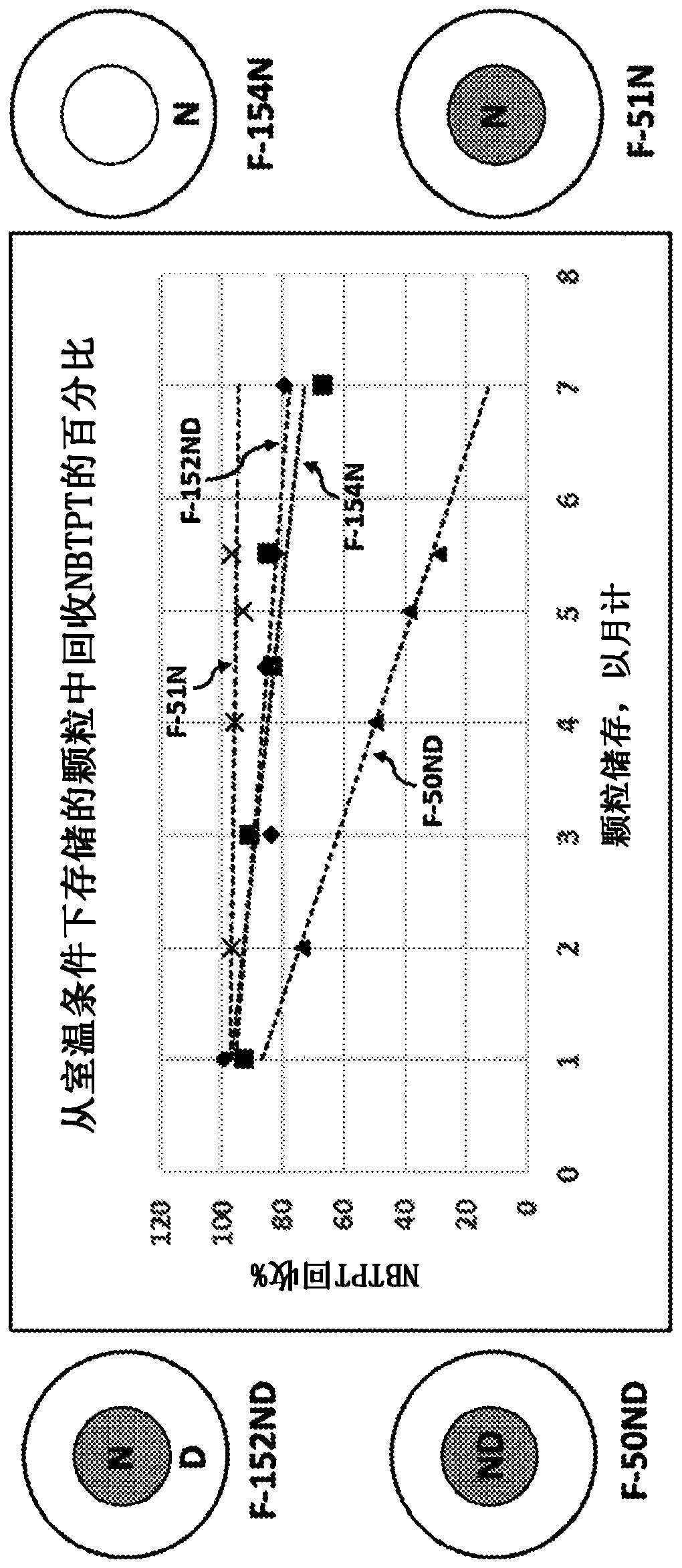
Extruded fertilizer granules with urease and/or nitrification inhibitors
ActiveUS20200172447A1Increase nitrificationImprove stabilitySolid/semi-solid fertilisersUrea compound fertilisersUrocaninaseUrease enzyme
Fertilizers with urease inhibitors and / or nitrification inhibitors are described. The fertilizer can include an extruded granule containing urea, a polymeric binder, and a nitrification inhibitor and / or an urease inhibitor.
Owner:SABIC GLOBAL TECH BV
Rapid detection reagent for helicobacter pylori
PendingCN114277086ARapid secretionHigh activityMicrobiological testing/measurementMicroorganism based processesBiotechnologySodium Chloride / Urea
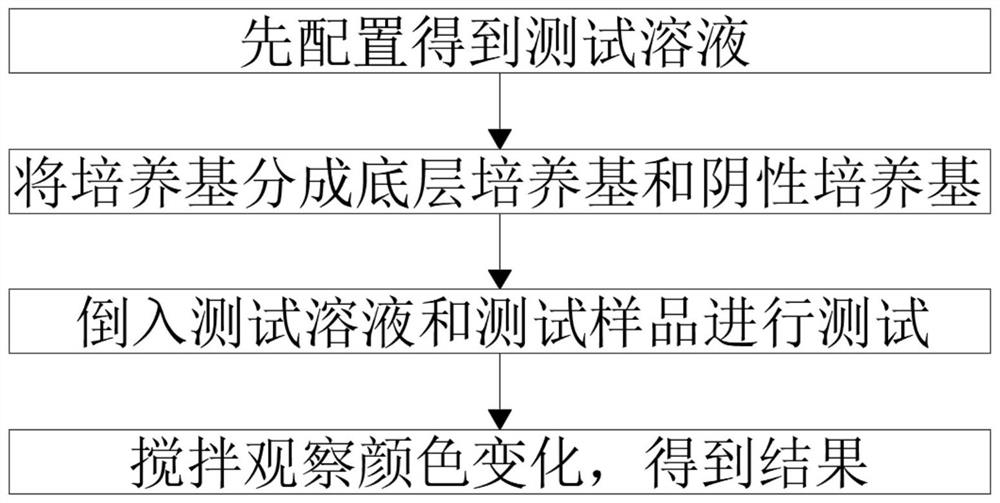
The invention belongs to the field of detection, particularly relates to a rapid helicobacter pylori detection reagent, and provides the following scheme aiming at the problems of low test speed and poor test accuracy in the prior art: the detection reagent comprises sodium chloride, urea, an indicator, an ethanol solution, an absorbent, an additive and a culture medium, the absorbent comprises bischofite, plant ash and salt, the additive comprises vinegar, disodium hydrogen phosphate and sodium hydroxide, and the helicobacter pylori is rapidly cultured by adopting an improved urease method and an international common ultra-rapid culture medium culture test, so that the helicobacter pylori rapidly secretes high-activity urease to decompose a substrate urea to generate ammonia gas, and the ammonia gas is converted into the high-purity helicobacter pylori. When ammonia is dissolved in water, the PH value is increased, the color of the indicator is changed, the helicobacter pylori in saliva, tartar or gastric mucosa tissue is indirectly dyed, whether the helicobacter pylori exists or not is judged, the testing speed is high, and the accuracy is high.
Owner:南京康容健康科技有限公司
Phenol hydroxamic acid urease inhibitors and preparation method and application thereof
The invention provides a kind of phenol hydroxamic acid compounds. The general formula of the compounds is shown in the description. The compounds have a good inhibition function on urease and can beused for preparing medicine resisting gastritis, gastric ulcer, urinary calculi and the like. The invention discloses a preparation method of the phenol hydroxamic acid compounds.
Owner:JISHOU UNIVERSITY
Saliva streptococcus 57.I urase gene and its urase
This invention relates to a urase gene of streptococcus salivarius, concretely, concerning about a 57.I urase gene of streptococcus salivarius and its urase. A 57.I urase gene of streptococcus salivarius , whose nucleotide sequence is what is described in SEQ ID NO.1. Nickel ion bonding in the gene and nucleotide sequence of transit-related gene MQO are what are described in SEQ ID NO.2. The advantage of this invention is that gaining the cloning of urase gene of streptococcus salivarius U35248 by the method of segment cloning and gradually enzyme-cutting connection ,and its object gene length proceeds to 8Kb, increasing Nickel ion bonding of the gene and transit-related gene MQO compared with existing technology , therefore, expressing ureolytic activity without adding exogenous NiCl2.Attained cloning can be used for future replacement therapy in anti-caries research building without adding exogenous NiCl2,more suitable for clinical application of production alkali effect germ.
Owner:SHANGHAI NINTH PEOPLES HOSPITAL SHANGHAI JIAO TONG UNIV SCHOOL OF MEDICINE
Enhanced efficiency fertilizer with urease inhibitor and nitrification inhibitor separated within the same particle
ActiveCN110809569AAgriculture gas emission reductionUrea compound fertilisersUrocaninaseUrease Inhibitors
Fertilizer particles with urease inhibitors and nitrification inhibitors are described herein. The fertilizer particles can include a core particle comprising a urease inhibitor and an outer layer comprising a nitrification inhibitor.
Owner:SABIC GLOBAL TECH BV
Creatinine content determination method and creatinine diagnosis kit
InactiveCN1766641AStrong specificityLess susceptible to interferenceMaterial analysis by observing effect on chemical indicatorColor/spectral properties measurementsPhosphoenolpyruvate carboxylaseCreatinine rise
The invention relates to a method for measuring the creatinine content and a creatinine diagnosis agent box in the field of medical testing technology. The agent box comprises: buffer solution, reducing coenzyme, phosphoenolpyruvate, creatinine enzyme, atinase enzyme, urease, phosphoenolpyruvate carboxylase, malate dehydrogenase and stabilizer. It mixes the sample and the agent with a certain volume ratio to do enzyme coupling reaction; then it dispositions the end resting material on the chemical analyzer to detect the speed of the main wavelength light absorption to measure the content of the creatinine.
Owner:王尔中
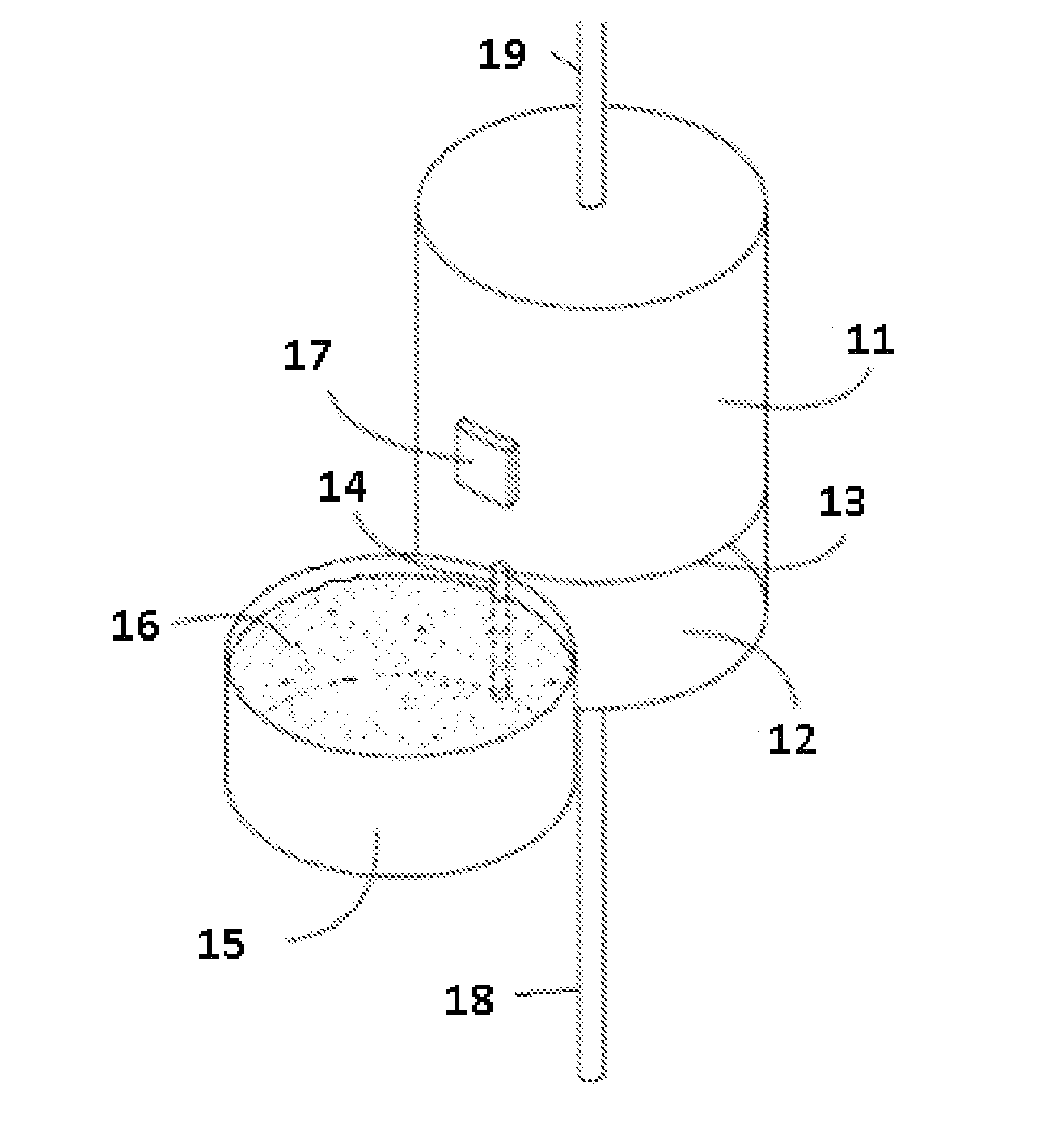
Modular hemodialysis system
ActiveUS10857277B2Mechanical/radiation/invasive therapiesOther blood circulation devicesDialysis membranesActivated carbon
Apparatuses, systems, and methods for the performance of kidney replacement therapy having or using a dialyzer, control components, sorbent cartridge, and fluid reservoirs configured to be of a weight and size suitable to be worn or carried by an individual requiring treatment are disclosed. The system has a controlled compliance dialysis circuit, where a control pump controls the bi-directional movement of fluid across a dialysis membrane. A first sorbent cartridge is provided for use in a portable treatment module having activated carbon and zirconium oxide. The system also provides for the monitoring of an inlet and outlet conductivity of a sorbent cartridge containing urease to provide a facility to quantify or monitor the removal of urea by a detachable urea removal module.
Owner:MOZARC MEDICAL US LLC
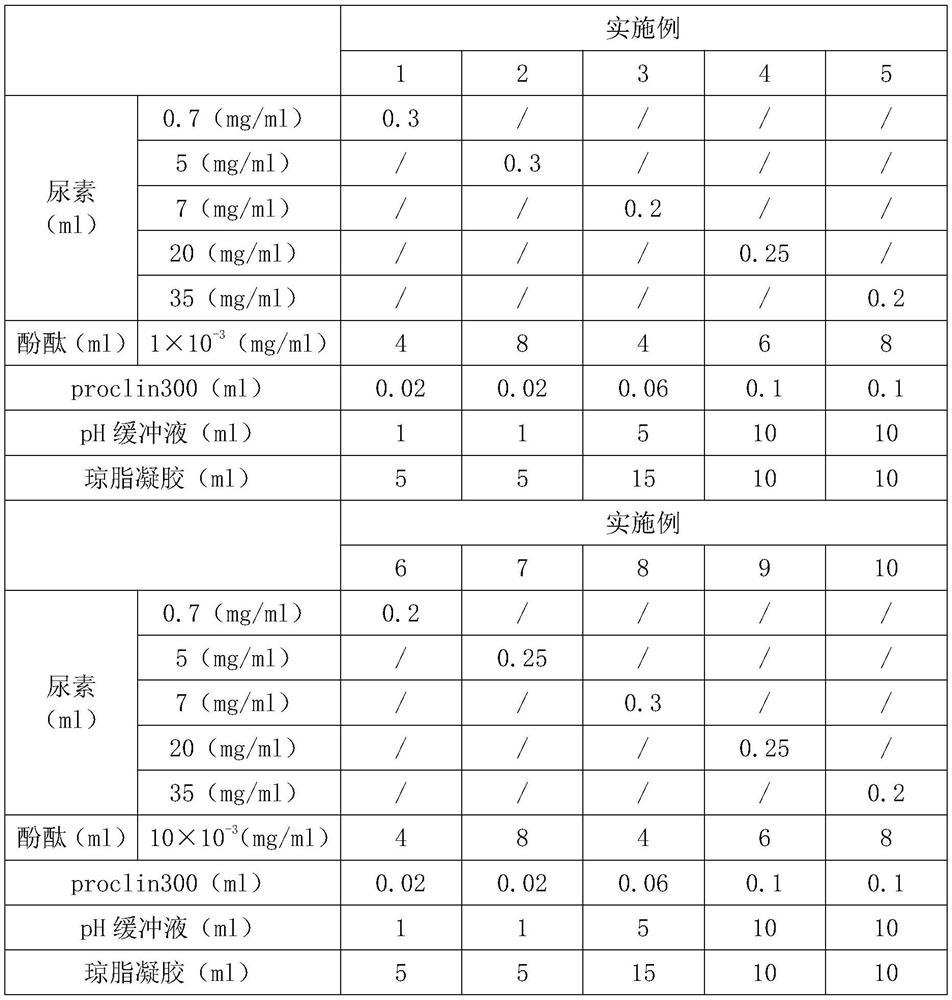
Gel reagent for rapid screening of urease activity, kit and rapid screening method
InactiveCN113092459AIntuitive and fast detectionSimple and fast operationMaterial analysis by observing effect on chemical indicatorUrocaninaseUrocanase activity
The invention discloses a gel reagent for rapid screening of urease activity, a kit and a rapid screening method, and belongs to the field of medical detection. The gel reagent for rapid screening of urease activity comprises 0.2-0.3 part by volume of urea; 4-8 parts by volume of phenol red; 0.02-0.1 part by volume of a proclin300; 2-10 parts by volume of a pH buffer solution; and 5-10 parts by volume of agar gel, whereinthe concentration of urea is greater than or equal to 7mg / ml, and the concentration of phenol red is greater than or equal to 30 * 10 <-3 > mg / ml. According to the detection method, a reaction product of urease and urea in saliva or a biopsy specimen is utilized to trigger corresponding index change so as to measure the urease activity. The detection steps: are as follows collecting a proper amount of samples by using an applicator device, pushing the whole samples from tweezers to a position below the surface of gel in the kit by using the applicator device, exposing the samples in the gel as many as possible, standing for 2-3 minutes, observing the color change of the gel by a visual method, determining that urease is positive if the gel becomes red, and determining that urease is negative if the color is not changed.
Owner:许奕鹏
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com