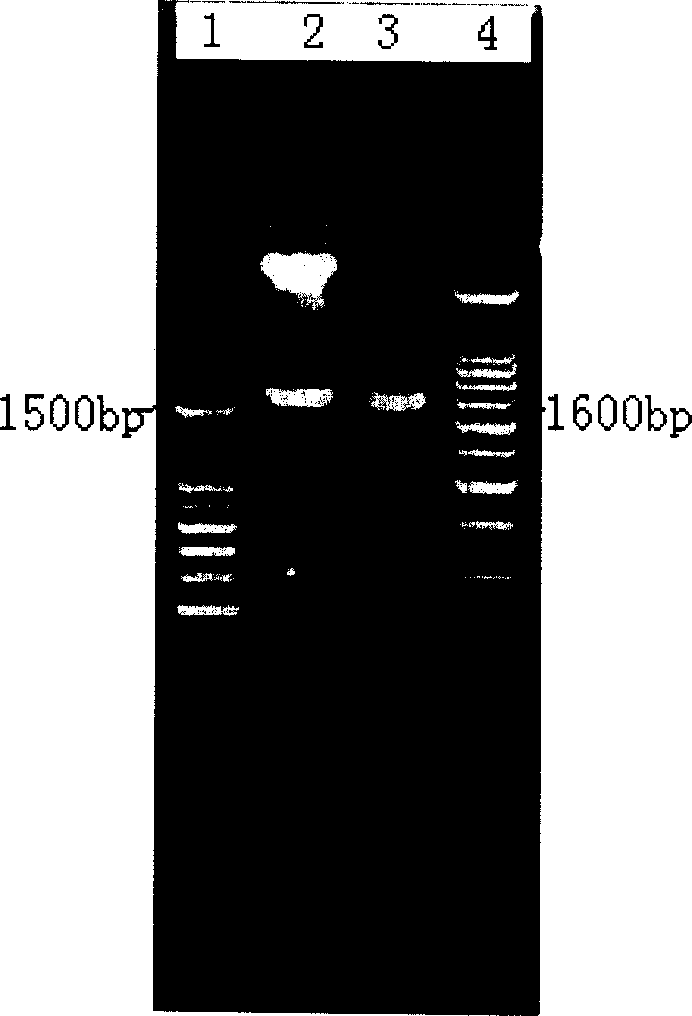
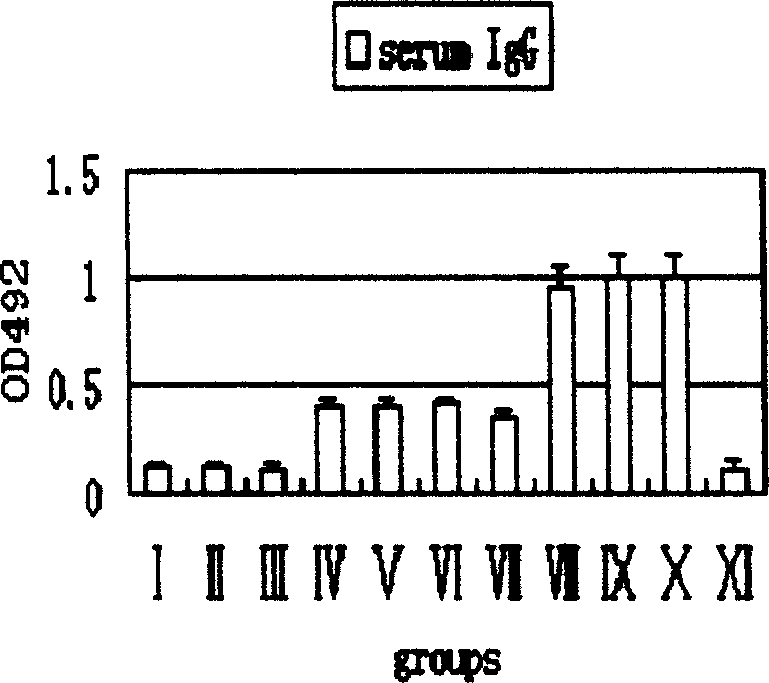Pyloric spiral bacillus antigen recombinant vaccine
A technology of Helicobacter pylori and recombinant vaccines, applied in the directions of bacterial antigen components, antibacterial drugs, etc., can solve the problems of cumbersome methods, high difficulty, and difficulty in large-scale cultivation, and achieves high expression efficiency, high yield, and easy separation and purification. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Example 1 Cloning of HpaA, UreB414, NapA encoding genes of Helicobacter pylori
[0058] 1. Helicobacter pylori was purchased from ATCC and kept in our laboratory. The strain is Helicobacter pylori 26695 (ATCC Number700392)
[0059] 2. The Hp strain taken out and stored in the liquid nitrogen tank is spread on the Hp special solid medium, at 37℃, containing 5% O 2 , 85% N 2 , 10% CO 2 Cultivate 72h under the conditions. The genome extraction kit extracts the Hp genome.
[0060] 3. The coding genes of NapA, HpaA and UreB414 were amplified separately from the Hp genome by PCR.
[0061] 1) The primer design and synthesis are as follows (underline shows the restriction site and linker base sequence)
[0062] According to the gene sequence and primer design principles published by GenBank, design corresponding primers and introduce restriction sites. Introduce the base sequence of the design linker in the middle.
[0063] HpaA P1 5’-C CCTGCTGTACCACCACCT AATTACCATCCA-3’
[0064] ...
Embodiment 2
[0092] Example 2 Obtaining the fusion gene NapA-LTB
[0093] 1. NapA and LTB gene amplification
[0094] 1) Primer design and synthesis
[0095] NapA P7 5’-GCGGG CATATG AAAACATTTGAAA-3’
[0096] NdeI
[0097] P8 5’- CGGAGGATCCTGCGG AGCTAAATGGGC-3’
[0098] linker
[0099] LTB P9 5’- GGAGGCGGAAGTGGAGGAGGTAGC GCTCCCCAGTCTAT-3
[0100] linker
[0101] P9’ 5’- CCGCAGGATCCTCCG GCTCCCCAGTCTATT-3’
[0102] linker
[0103] P10 5’- CTCGAG GTTTTCCATGCTGATTGC-3’
[0104] XhoI
[0105] Using Helicobacter pylori SS1 and wild-type toxin-producing Escherichia coli H44815 genomic DNA as templates, NapA and LTB genes were amplified with P7 and P8, P9' and P10. Bacterial genome extraction was based on the age of the day. Kit I was carried out. The PCR amplification system is: 10×10μL of magnesium ion-free amplification buffer, MgCl 2 (25mmol / L) 1...
Embodiment 3
[0110] Example 3 Obtaining the fusion gene NapA-CTB
[0111] 1. NapA and CTB gene amplification
[0112] 1) Primer design and synthesis
[0113] NapA P7 5’-GCGGG CATATG AAAACATTTGAAA-3’
[0114] NdeI
[0115] P8 5’- CGGAGGATCCTGCGG AGCTAAATGGGC-3’
[0116] linker
[0117] CTB P11’ 5’- CCGCAGGATCCTCCG ATTAAATTAAAATTTGGT-3’
[0118] linker
[0119] P12 5’- CTCGAG ATTTGCCATACTAATTGCG-3’
[0120] XhoI
[0121] P7 and P8, P11' and P12 were used to amplify the NapA and CTB genes, and the bacterial genome extraction was performed according to the day-time spin column type bacterial genomic DNA extraction kit I. The PCR amplification system is: 10×10μL of magnesium ion-free amplification buffer, MgCl 2 (25mmol / L) 10μL, dNTPs (25mmol / L each) 8μL, upstream and downstream primers each 2μL, the above NapA or CTB genome each 1μL, Ex-Taq DNA polymerase (5 units / μL) 1μL, add st...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com