Diaryl propionyl hydroxamic compound and preparation method and use thereof
A technology of diaryl propionyl hydroxamic acid and diaryl propionyl hydroxamic acid, which is applied in the field of preparation of anti-gastritis and gastric ulcer drugs, and can solve problems such as instability, low activity, and hindering application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
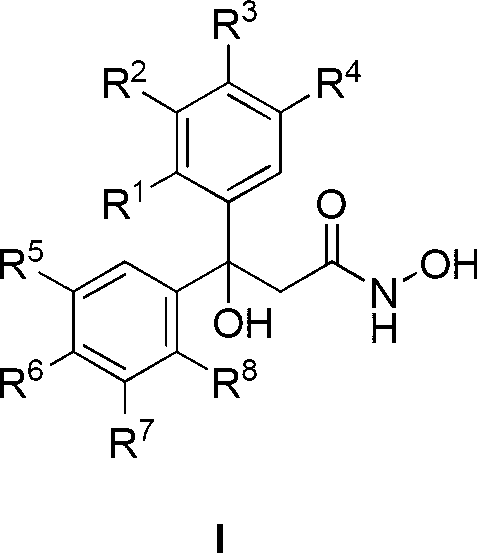
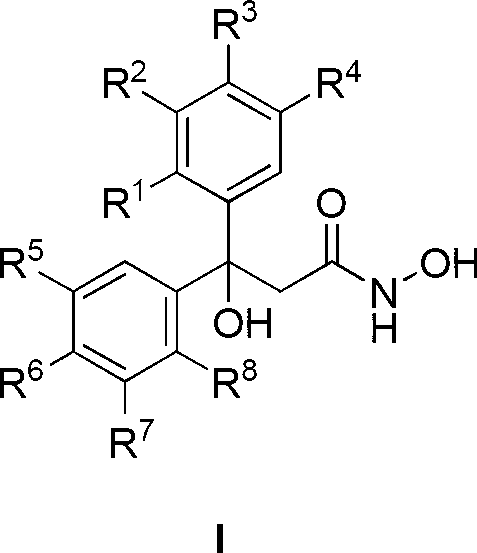
[0038] Example 1: Preparation of 3-(3-hydroxyphenyl)-3-(2,3,4-trihydroxyphenyl)-3-hydroxypropionylhydroxamic acid (50)
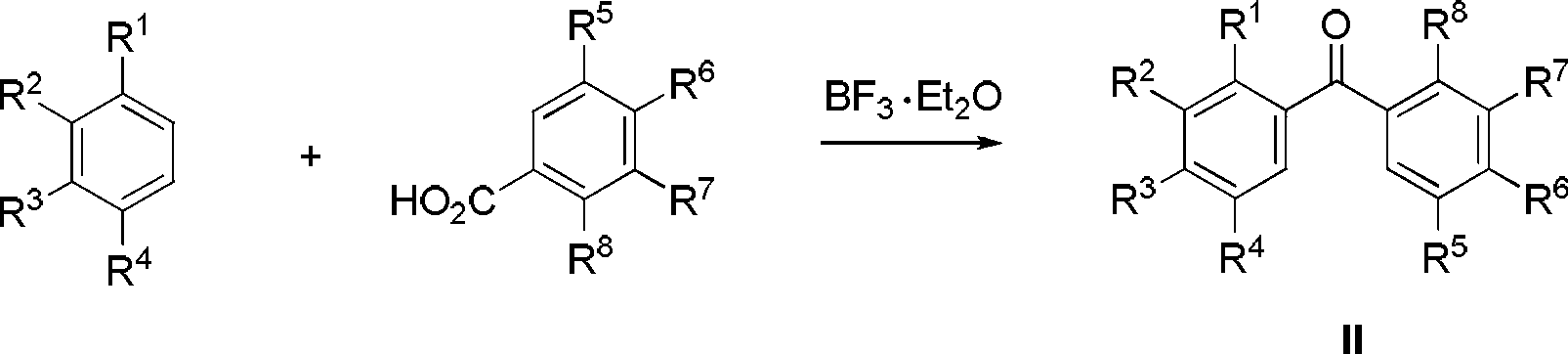
[0039] Add 2.52g pyrogallic acid to 20ml BF 3 ·Et 2 O, then add 3.31g of m-hydroxybenzoic acid, heat up to 85°C and react for 15h. After the reaction is complete, pour it into the aqueous solution of AcONa, filter with suction, wash, and EtOH-H 2 O recrystallized to obtain 3.78 g of 3-hydroxyphenyl-2,3,4-trihydroxyphenyl ketone, yield 77%;
[0040] 2.46g of 3-hydroxyphenyl-2,3,4-trihydroxyphenyl ketone, 6.5g of Zn powder, 4.82g of NH 4 Cl and 4.45mL ethyl bromoacetate were ground together until uniform. After standing at room temperature for 18 h, pour saturated NH 4 Cl solution, AcOEt extraction, anhydrous MgSO 4 Dry, evaporate the solvent, purify with silica gel column, eluent volume ratio: AcOEt:petroleum ether=1:2, get 3-hydroxyl-3-(3-hydroxyphenyl)-3-(2,3,4- Trihydroxyphenyl) ethyl propionate, 2.17g, productive rate 65%;
[0041] Dissolve 1.67g o...
Embodiment 2
[0043] According to the method similar to Example 1, using different substituted forms of benzoic acid and benzene as raw materials, the diaryl propionylhydroxamic acid series compounds 1-86 listed in Table 1 were synthesized.
[0044] Each R group of diaryl propionyl hydroxamic acid series compound in the general formula I of table 1
[0045] serial number
[0046] serial number
[0047] serial number
[0048] serial number
[0049] Note: The initial raw materials were purchased from aldrich company
Embodiment 3
[0050] Embodiment 3: the inhibitory enzyme activity of compound
[0051] Add 25 μL of Jack bean urease (4U) and 25 μL (1 mM) of the test compound solution to the 96-well plate, incubate at 37 °C for 2 h, then add 55 μL of phosphate buffer containing 100 mM urea and 100 mM, and incubate at 30 °C. Incubate at ℃ for 15 min, add 45 μL of phenol reagent (mixed solution containing 1% phenol and 0.005% sodium nitroprusside) and 70 μL alkali reagent (mixed solution of NaOCl containing 0.5% NaOH and 0.1% active chlorine), at room temperature After standing for 50 minutes, measure the OD value at 630nm with a microplate reader, and the percentage inhibition rate is calculated according to the following formula:
[0052]
[0053] All experiments were performed in solutions at pH 8.2 (0.01M K 2 HPO 4 , 1mM EDTA, 0.01M LiCl), the level of activity is measured by the half-inhibition rate IC 50 to indicate that the IC 50 The smaller the value, the higher the activity of the compound. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com