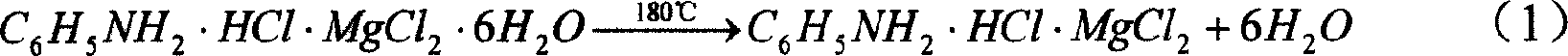Patents
Literature
147 results about "Bischofite" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
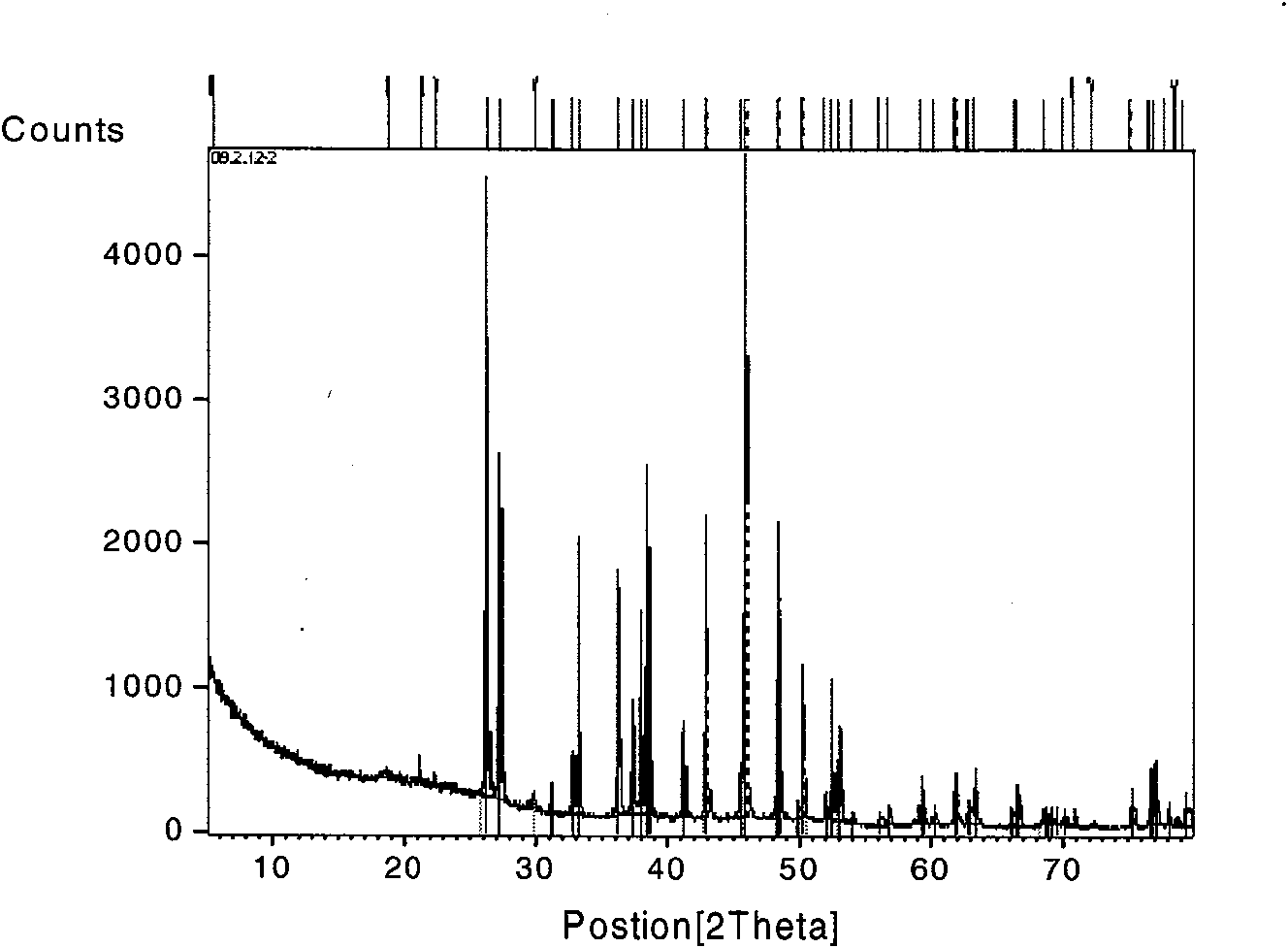
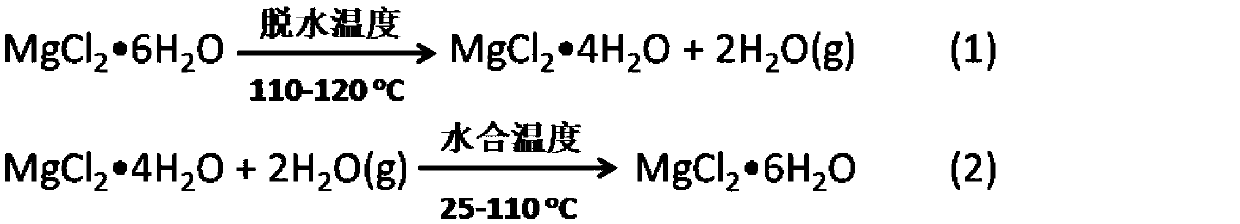
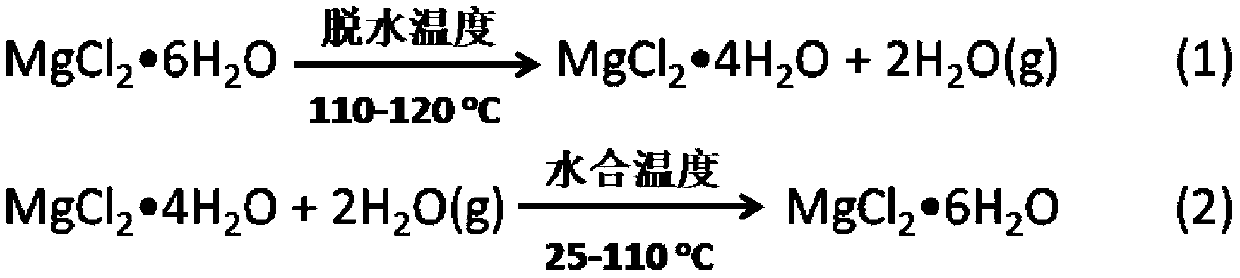
Bischofite (bischofit) is a hydrous magnesium chloride mineral with formula MgCl₂·6H₂O. It belongs to halides and is a sea salt concentrate. Bischofite is ecologically pure natural magnesium poly-mineral with a unique composition. It contains many macro- and micro-elements vital for human health, in much higher concentrations than can be found in sea or ocean salt. The main bischofit compound is magnesium chloride (up to 350 g/L), moreover, it contains about 70 other elements as impurities, including potassium, sodium, bromine, boron, calcium, silicon, molybdenum, silver, zinc, iron and copper.
Salt lake brine treatment method for separating lithium from high-magnesium-lithium-ratio salt lake brine
ActiveCN103074502ATake advantage ofHigh recovery rateProcess efficiency improvementReverse osmosisEvaporation
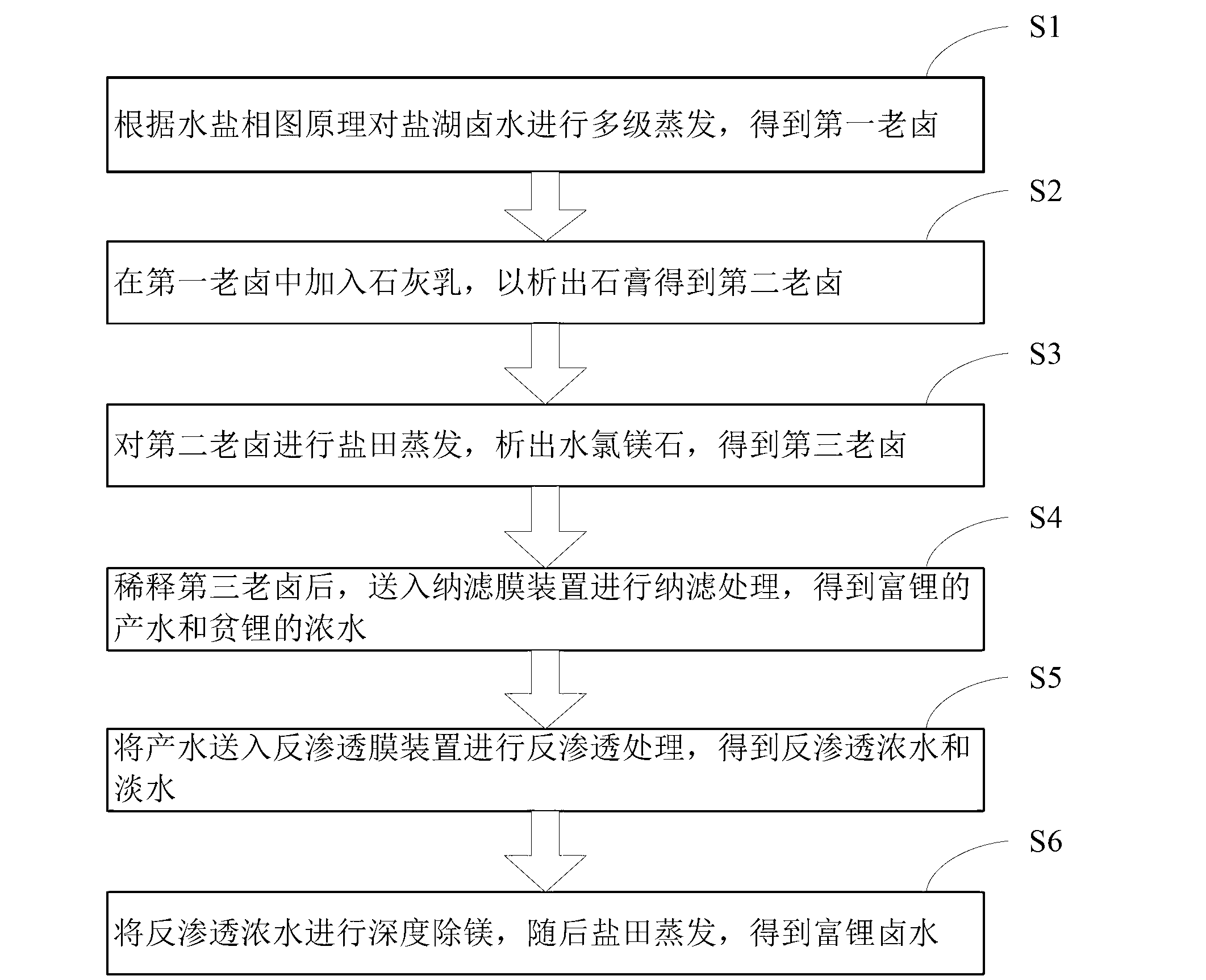
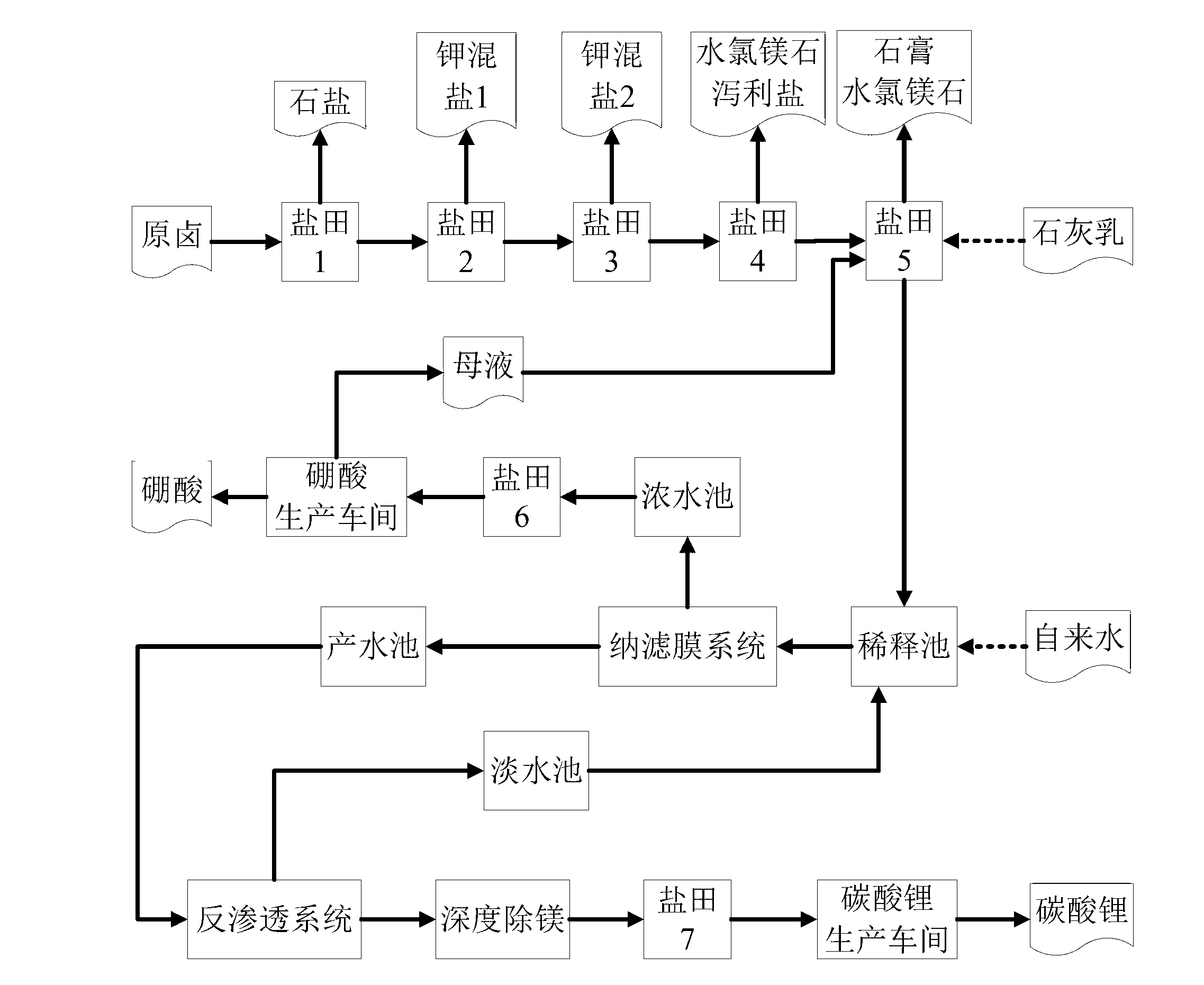
The invention discloses a salt lake brine treatment method for separating lithium from high-magnesium-lithium-ratio salt lake brine. The treatment method comprises the steps that S1, the salt lake brine is subjected to multistage salt pan evaporation to form first old brine; S2, sulphur removal is conducted: lime milk is added to the first old brine for separating out gypsum, and second old brine is obtained; S3, the second old brine is subjected to salt pan evaporation, bischofite is separated out, and third old brine is obtained; S4, the third old brine is diluted, and sent to a nanofiltration membrane device for nanofiltration treatment, and contributing water rich in lithium and thick water poor in lithium are obtained; and S5, the contributing water in Step S4 is sent to a reverse osmosis membrane device for reverse osmosis treatment, and reverse osmosis thick water and fresh water are obtained. The method combines a salt pan technology with a membrane system, makes full use of solar energy, and reduces energy consumption; a technological process is simple; equipment is easy to configure, mount and transfer; popularization and an application are very easy.
Owner:QINGHAI INST OF SALT LAKES OF CHINESE ACAD OF SCI +1
Method of preparing high purity magnesiun sand using salt lake bischofite as raw material
InactiveCN1618998ANo pollution in the processLow free ammonia concentrationMagnesiaHigh concentrationSalt lake
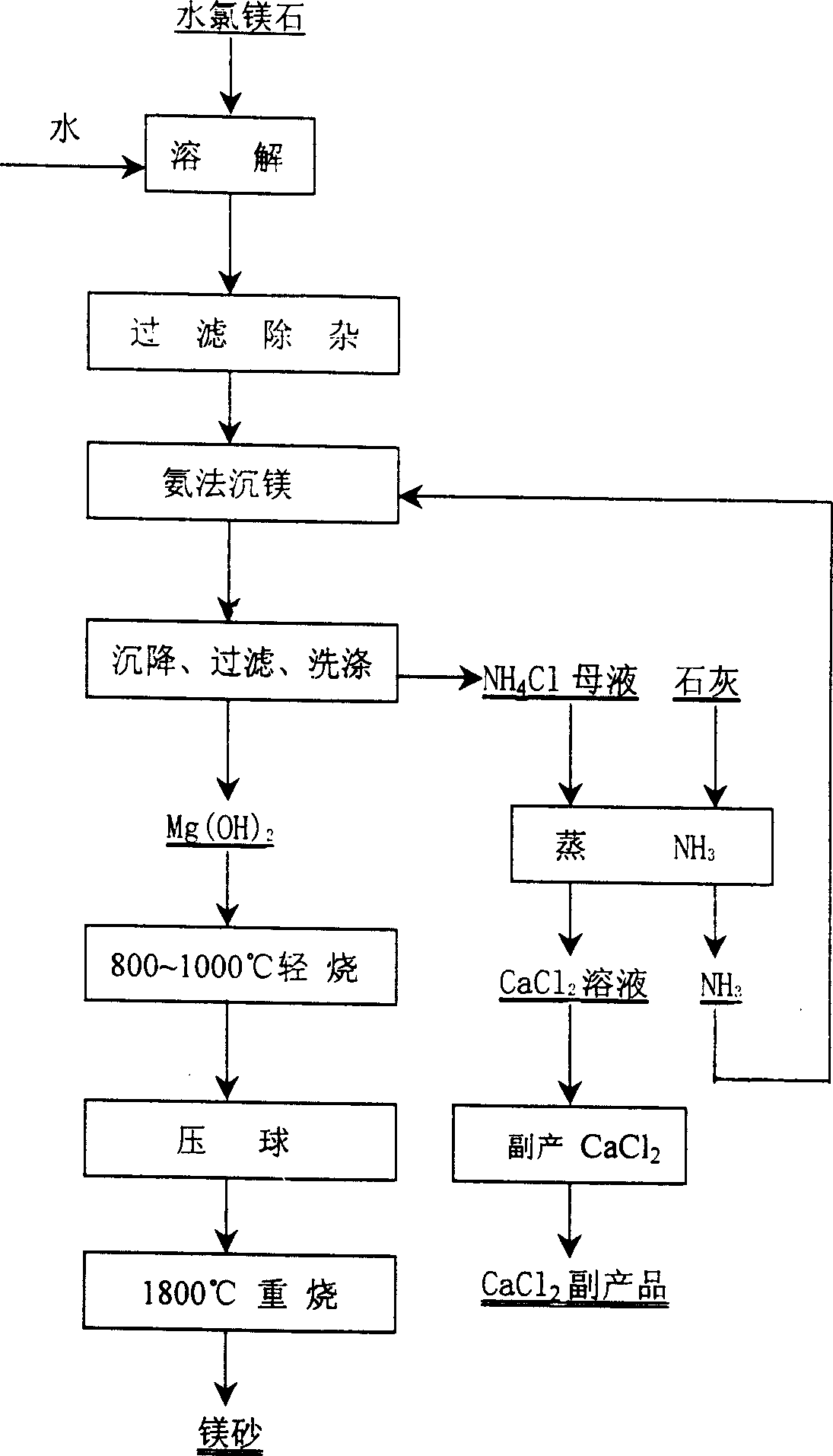
A process for preparing high-purity magnesium sand from the bischofite of salt lake includes such steps as preparing high-concentration saline from bischofite, depositing magnesium by ammonia method including adding crystal seeds, controlled reacting, depositing particles of magnesium hydroxide, filtering, washing, drying and two-step calcining to obtain target product containing MgO (more than 99.95%).
Owner:青海西部镁业有限公司
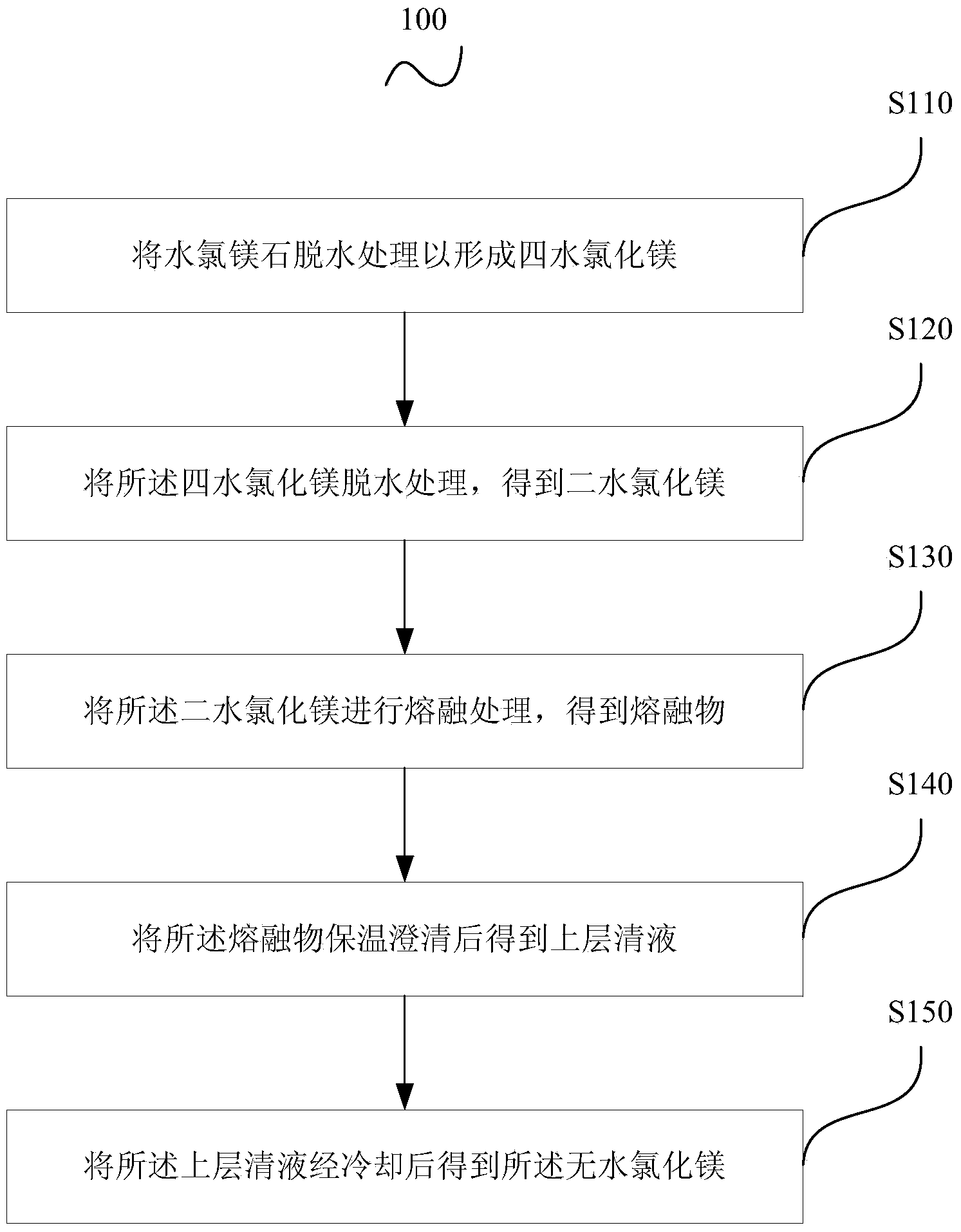
Method for preparing anhydrous magnesium chloride from bischofite
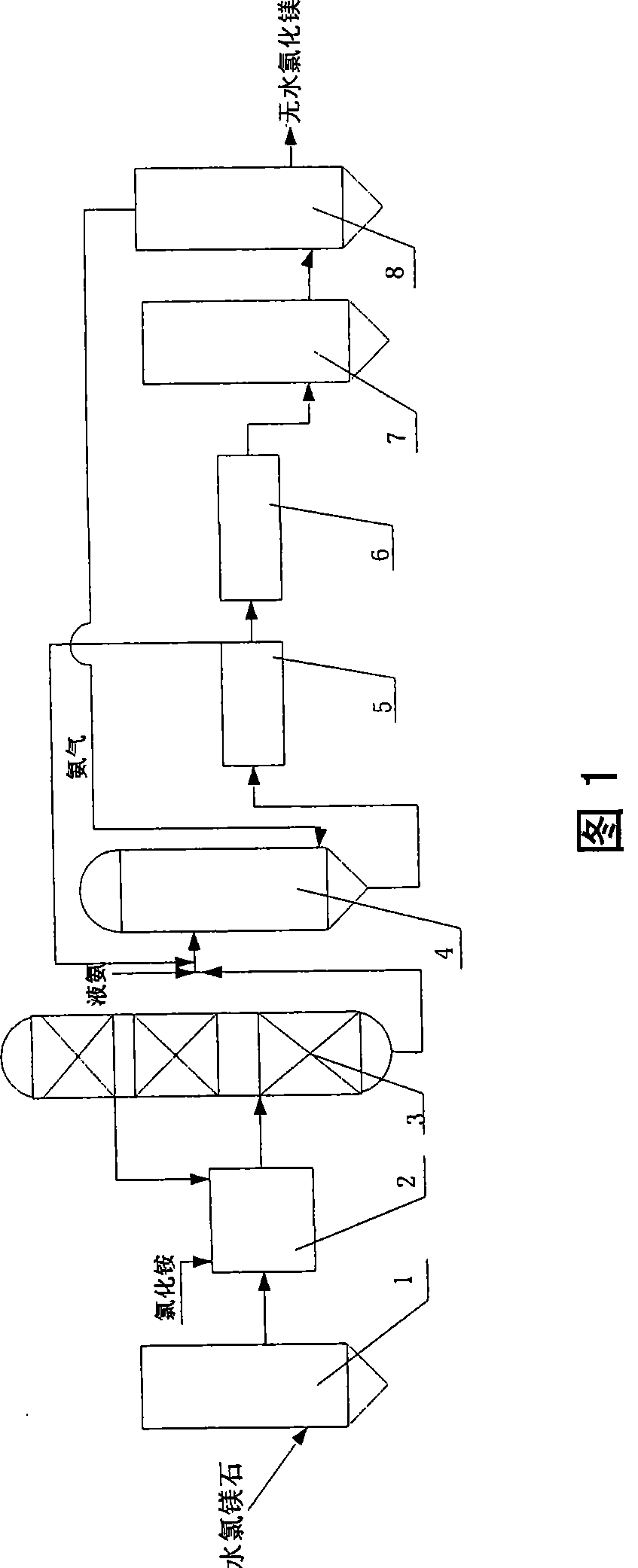
The invention relates to a method for preparing anhydrous magnesium chloride from bischofite, which uses the bischofite as a raw material for preparing the anhydrous magnesium chloride. The method comprises the following technical processes: (1) firstly, drying the bischofite for dewatering most crystallization water; (2) dissolving the dried magnesium chloride in glycol for preparing a glycol solution of the magnesium chloride and using a vacuum distillation method for dewatering water in the glycol solution of the magnesium chloride; (3) reacting the dewatered glycol solution of the magnesium chloride with ammonia for crystallizing so as to generate magnesium chloride hexammoniate crystals; (4) washing, filtering and drying for obtaining the magnesium chloride hexammoniate crystals; and (5) heating, decomposing and deaminating the dried magnesium chloride hexammoniate crystals for preparing the anhydrous magnesium chloride. In each process, ammonia gas, detergent and a crystallization mother liquor are recycled. The process has the characteristics of convenient operation, easy large-scale production, low energy consumption, low product cost and no pollution to environment and the prepared anhydrous magnesium chloride can be used as a raw material for producing magnesium metal by an electrolysis method.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
Method for preparing calcium carbonate crystal whisker with controllable shape
InactiveCN101684570AHigh purityImprove qualityCalcium/strontium/barium carbonatesPolycrystalline material growthWhiskersChloride
Owner:成都市蜀阳硼业化工有限公司
Method for preparing metal magnesium by silicothermic process
InactiveCN101698907AAvoiding the Difficulties of Preparing Anhydrous Magnesium ChlorideLow equipment requirementsPotassiumPidgeon process
The invention provides a new method for preparing metal magnesium by a silicothermic process and by using bischofite from the seawater salt making or salt lake brine salt and potassium chloride making as a raw material. The method comprises the steps of: reacting lime and solution of magnesium chloride to generate a calcium-magnesium mixed hydroxide; calcining the calcium-magnesium mixed hydroxide to obtain a calcium-magnesium mixed oxide; finely grinding the mixture of calcium-magnesium mixed oxide, silicon iron and fluorite in a ball mill, pressing the ground mixture into a walnut shape or a sheet shape on a forming press, and reducing the walnut-shape or sheet-shape mixture under vacuum in a magnesium smelting reduction pot to obtain the magnesium crystalline; and finally refining the magnesium crystalline, casting the refined magnesium crystalline into ingot, and performing surface treatment to obtain a magnesium metal ingot. The method overcomes the defect that a great amount of carbon dioxide greenhouse gas is exhausted in the Pidgeon process for calcining dolomite, avoids the problem of preparing anhydrous magnesium chloride by an electrolytic process, overcomes the defect that the direct calcining of bischofite gives corrosive and toxichydrogen chloride gas, has low requirements on equipment and is clean, environmentally-friendly and safe in production. The method can also be applied to the field of alkaline waste liquor treatment and comprehensive utilization.
Owner:QINGHAI INST OF SALT LAKES OF CHINESE ACAD OF SCI
Novel magnesium oxychloride cement mixed by water
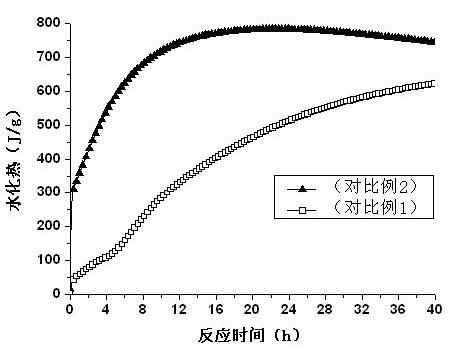
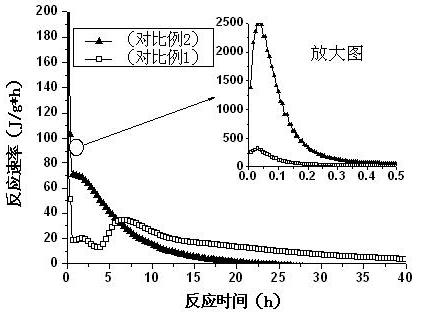
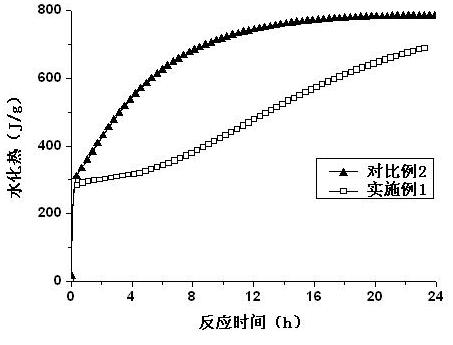
The invention relates to novel magnesium oxychloride cement mixed by water, which is obtained by mixing and grinding 50-100 parts of a part of pyrolysis product of bischofite, 0.5-5 parts of organic acid, 0-50 parts of active mixed materials and 0-5 parts of monocalcium phosphate into 180 meshes. The novel magnesium oxychloride cement has the characteristics of low solution heat, low hydration heat and high strength.
Owner:QINGHAI INST OF SALT LAKES OF CHINESE ACAD OF SCI
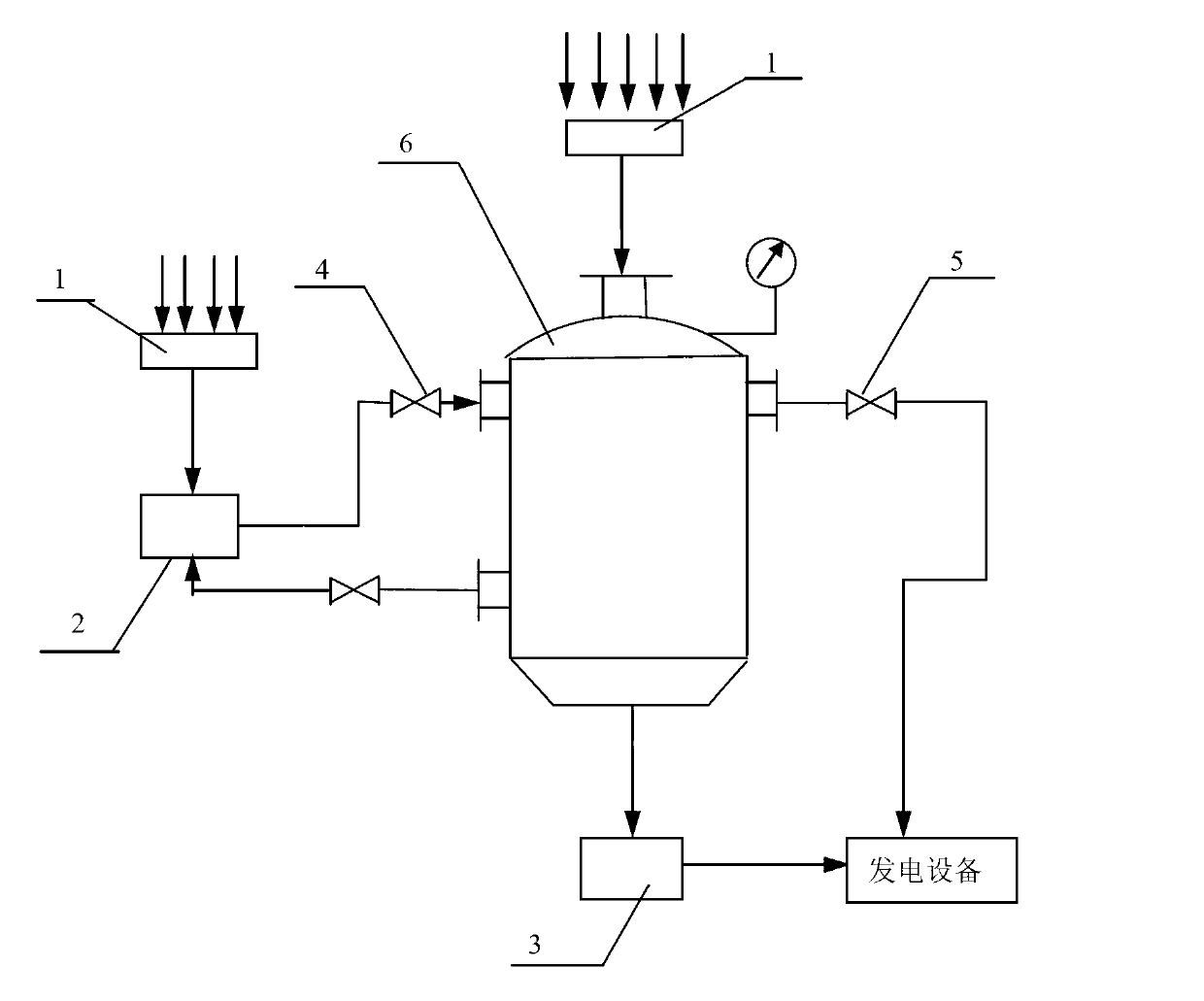
Method for converting and storing solar energy with low-hydration magnesium chloride as energy storage working medium
ActiveCN103134213AAbundant salt lake magnesium resourcesAbundant resourcesFrom solar energySolar heat devicesThermal energyHydration reaction
The invention aims at providing a method for converting and storing solar energy with low-hydration magnesium chloride as an energy storage working medium and relates to effective utilization of magnesium in salt lakes and storage and conversion of solar energy. The method particularly includes: in the periods when solar radiation is sufficient in the daytime, the solar radiation is received through a flat solar thermal collector, heat is transferred to a one-time dehydration fluidized bed loaded with bischofite (MgCl2 6H2O), the temperature of the bed is increased to the dehydration temperature, then the MgCl2 6H2O loses the crystal water to form the low-hydration magnesium chloride (MgCl2 4H2O), conversion from the solar energy to chemical energy is achieved, and water vapor generated by the reaction is delivered to a steam turbine set to generate electricity; and in the nighttime or when the solar radiation is insufficient, the water vapor is enabled to enter the dehydration fluidized bed where the low-hydration magnesium chloride is stored, under the condition that the temperature and the partial pressure of the water vapor are controlled, the low-hydration magnesium chloride is enabled to be subjected to hydration reaction, the number of crystal water molecules is increased to form MgCl2 6H2O again, absorption (hydration) heat is released, the conversion from chemical energy to thermal energy is achieved, and the released heat is transferred to a heat preservation water tank so as to generate water vapor to drive the steam turbine set to generate electricity.
Owner:BEIJING UNIV OF CHEM TECH
Preparation method of high length-diameter ratio calcium carbonate whiskers
InactiveCN101935865ALarge aspect ratioIncrease productionCalcium/strontium/barium carbonatesPolycrystalline material growthDiameter ratioWhiskers
The invention relates to a preparation method of high length-diameter ratio calcium carbonate whiskers. In the method, quicklime is used as a raw material, bischofite is used as a crystal type control agent, the calcium carbonate whiskers are prepared through the carbonation of carbon dioxide, and then mother liquid is recycled to prepare the calcium carbonate whiskers, so the multi-level growth of a high length-diameter ratio calcium carbonate whisker product is finished. The preparation method has a simple, low-cost and easily-available raw material, and increases the length-diameter ratio of the whiskers through the multi-level growth of the whiskers, thereby not only increasing the yield of the whiskers and reducing the cost, but also being capable of realizing scale industrial production.
Owner:QINGHAI INST OF SALT LAKES OF CHINESE ACAD OF SCI +1
Method for producing potash magnesium sulphate fertilizer
ActiveCN101602617ALow firing temperatureEasy to separateMagnesium fertilisersSolid waste disposalMagnesium saltQuenching
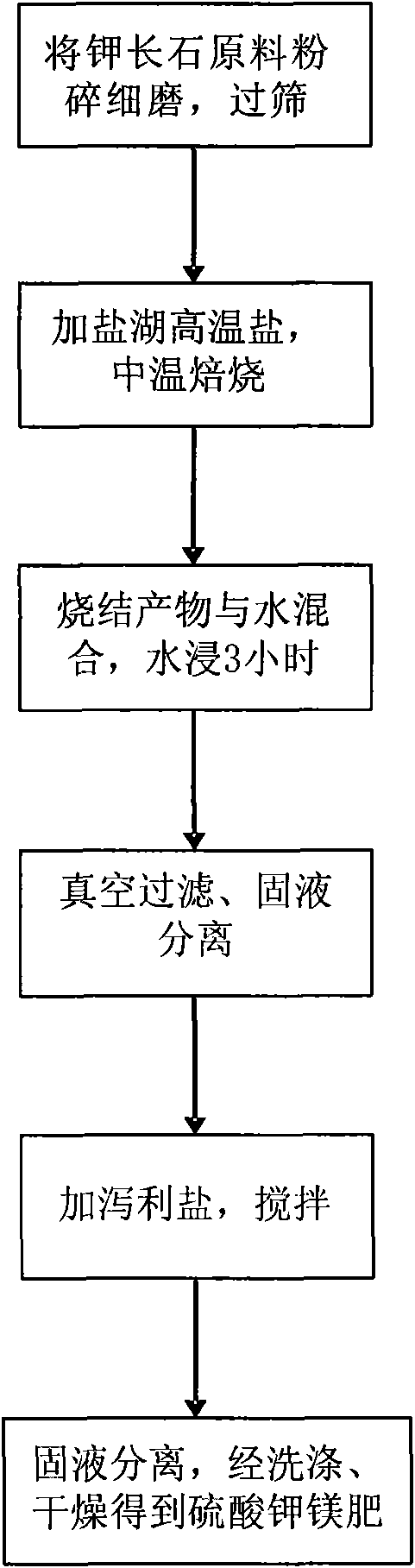
The invention discloses a method for producing potash magnesium sulphate fertilizer, comprising the following steps: a, crushing and grinding natural potash feldspar used as a raw material, screening the crushed raw material with a sieve of 150 meshes; b, adding proper amount of salt lake high-temperature salt of salt lake kalium extracting waste taking bischofite as a main component, baking for 2 to 3 hours at middle temperature under HCl protective atmosphere or sealed condition; c, after water quenching and solution leaching, adding salt lake kalium extracting waste epsomite into the solution obtained by filtrating, and evaporating the solution to concentrate and crystallize so as to obtain potash magnesium sulphate fertilizer. Compared with the prior art, the invention has advantages of taking magnesium salt lake kalium extracting waste as additive, having low baking temperature and simple separation process, recycling potassium chloride, potassium sulfate, picromerite and kainite left in the salt lake waste, being more feasible in economics, so that the potash magnesium sulphate fertilizer prepared by the method provides a plurality of nutrient elements for crops.
Owner:SHANGHAI YARET IND GROUP
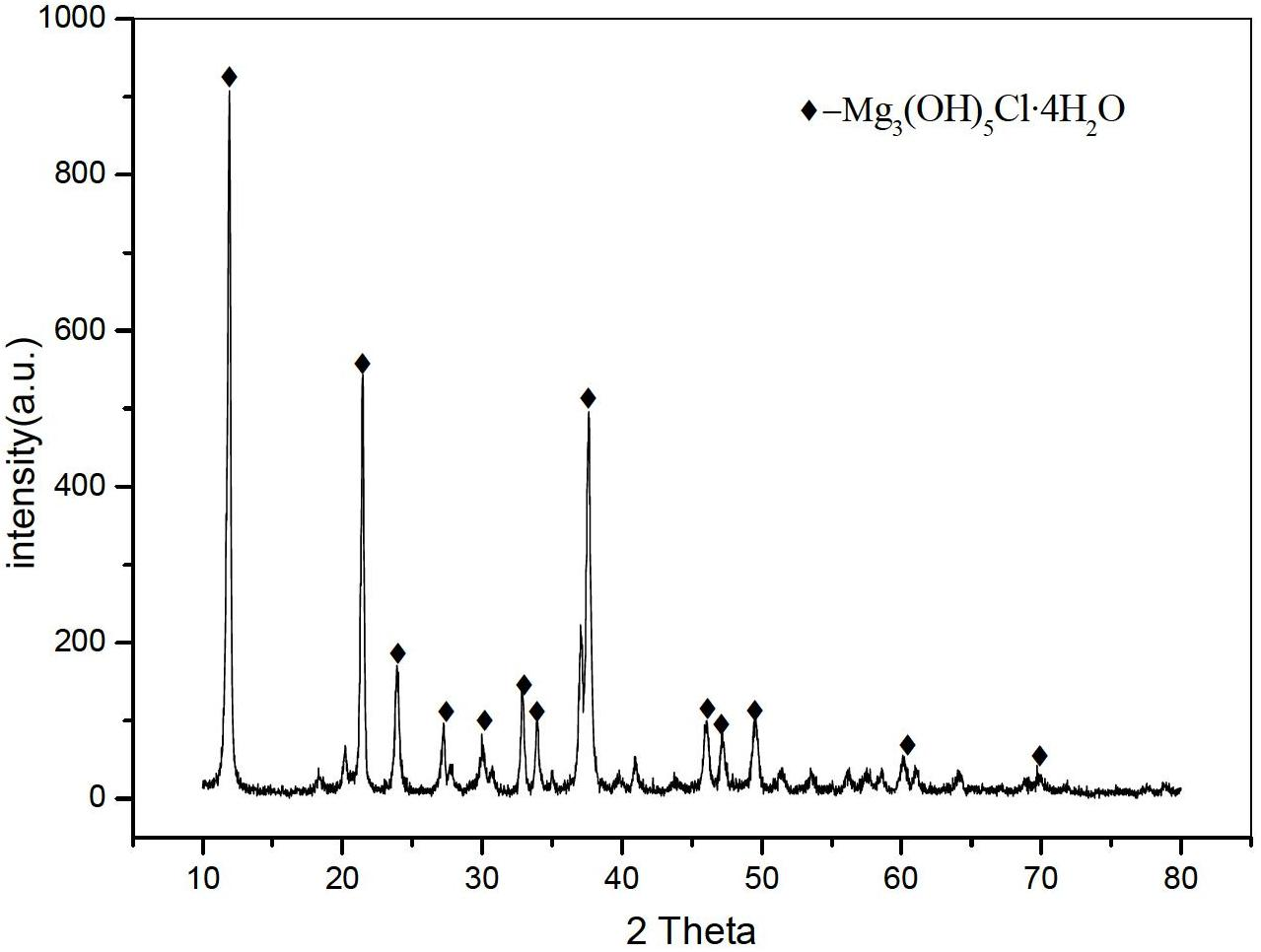
Method for preparing basic magnesium chloride and magnesium oxide by pyrolyzing bischofite
InactiveCN101624198AReduce moisture contentHigh recovery rateMagnesium chloridesMagnesiaPhysical chemistryChloride
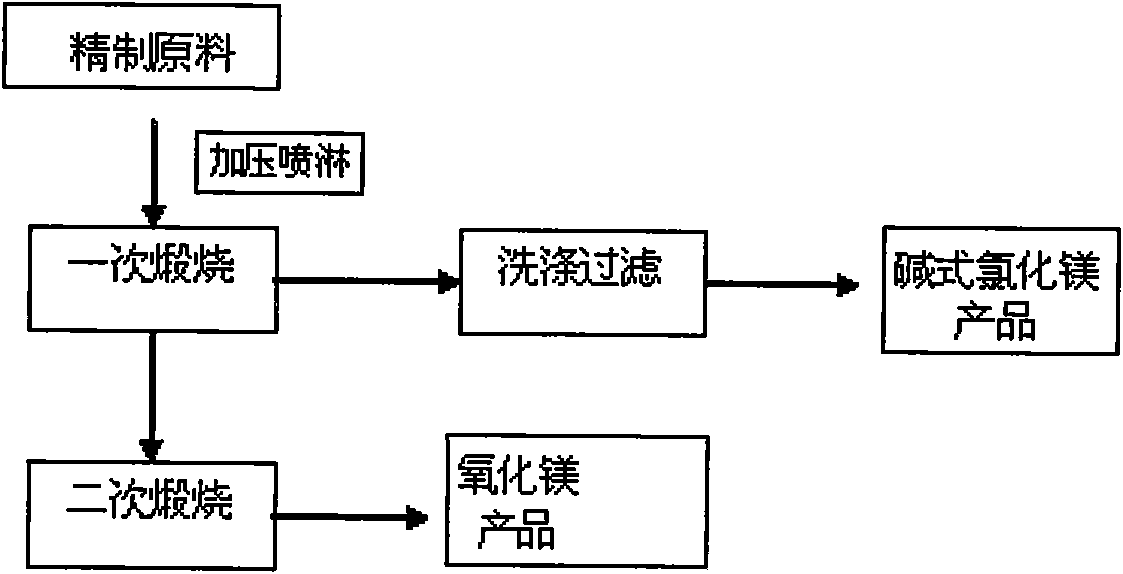
The invention relates to a method for preparing basic magnesium chloride and magnesium oxide by pyrolyzing bischofite. The method is characterized by adopting section calcining, recovering HCl in a section manner, particularly pressurizing and spraying the hot solution of refined bischofite under the temperature of 130 to 150 DEG C to a reaction kettle, carrying out primary calcining under the 250 to 300 DEG C after removing parts of crystal water with the calcining time of 1 to 3 hours, and obtaining the product of basic magnesium chloride (MgOHCl); absorbing the tail gas with water to obtain hydrochloric acid; the washing the calcining product which contains little amount of undecomposed magnesium chloride hydrate to obtain the basic magnesium chloride (MgOHCl); carrying out secondary calcining on the obtained MgOHCl under a temperature of 450 to 500 DEG C with the calcining time for 1 to 3 hours to obtain MgO, and cooling and recovering the produced HCl to obtain hydrochloric acid. The method has the advantages of low calcining temperature, little equipment corrosion, little energy consumption, high HCl recovery rate, simple technique, high product purity and low cost.
Owner:EAST CHINA UNIV OF SCI & TECH
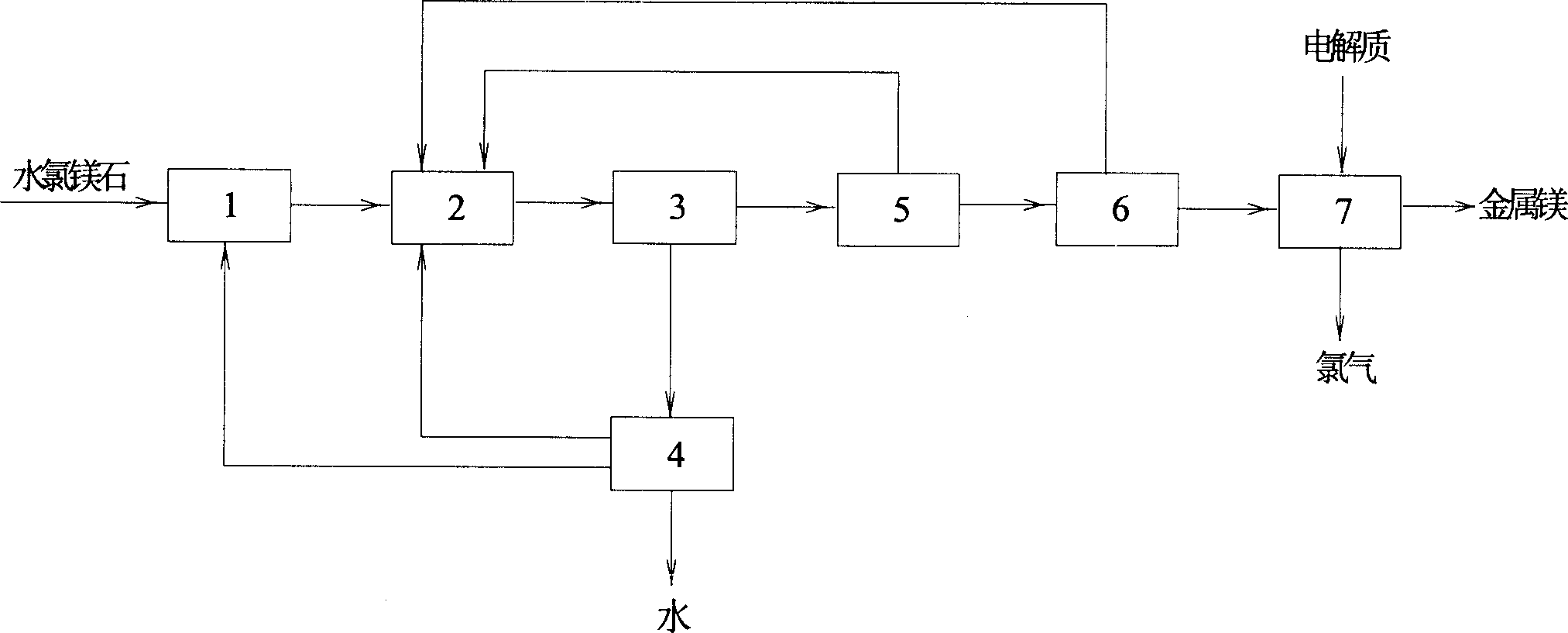
Bischofite dehydration-electrolysis method for refining magnesian
InactiveCN1736872AReduce recycling costsReduce manufacturing costMagnesium chloridesSolubilityRare earth
The invention relates to method to dewater to bischofite and electrolyse to obtain magnesium. Wherein, using improved reaction crystal coupling dewater technique to prepare anhydrous magnesium chloride with magnesia of 1-2% more cheaper, adding new electrolyte system with fluorides (such as, MgF2, LiF, NaF and CaF2) and rare-earth chloridate (such as, NdCl3, LaCl3) to increase effectively the solubility of MgO in complex electrolyte system and make the said anhydrous magnesium chloride fit to the require raw material, eliminating the negative effect of MgO in the process. This method can increase the current efficiency, makes the MgO impurity achieve 3%, and decease dewatering cost.
Owner:EAST CHINA UNIV OF SCI & TECH
Method for preparing ultra-fine high dispersing magnesium hydrate flame retardant from saline lake bittern or bischofite
The invention provides a method for preparing an ultrafine high-dispersion magnesium hydroxide flame retardant by taking salt lake brine or bischofite as a raw material, which comprises the following steps that: the salt lake brine or the bischofite is prepared into a solution with 3 to 4mol / L magnesium chloride, 3 to 14mol / L ammonia water is quickly added into the solution at a temperature of between 25 and 95 DEG C to perform a precipitation reaction, and then an ultrafine high-dispersion magnesium hydroxide flame retardant product can be obtained through washing, filtration and drying. The method has simple process flow and low cost, and can produce the magnesium hydroxide flame retardant with good dispersivity, evenly distributed particle size, and average grain diameter of between 0.5 and 1mu m without adding a dispersing agent and a flocculating agent during the reaction.
Owner:QINGHAI INST OF SALT LAKES OF CHINESE ACAD OF SCI
Method for preparing rod-like magnesium hydroxide from salt lake brine
InactiveCN102674409ALarge particlesImprove filtering effectCalcium/strontium/barium chloridesMagnesium hydroxidePotassiumSodium hydroxide
Owner:CENT SOUTH UNIV
Process for preparing calcined dolomite from magnesium chloride of chloride type by-product of potassium-extracting from salt lake
The invention provides a method for preparing a composed calcined dolomite by taking the byproduct of brine ( a saturated liquor which is mainly magnesium chloride ) after potassium extraction or the bischofite separated out from the brine by naturally evaporating as the materials to react with excess lime to prepare the floccule mixture of magnesium hydrate and calcium hydroxide; then the mixture is calcined and dehydrated under the temperature of 400 to 1200 DEG C after the mixture is deposited, filtered, washed, dried and cracked. The materials of the invention are easily obtained and cheap; the production process is simply and easily controlled; no carbon dioxide is discharged; besides, the invention basically has no corrosion to a device. The mol ratio of MgO to CaO in the composed calcined dolomite is within the range of 1:0.8 to 1:1.2; the activity of water is 25 to 36 percent; the composed calcined dolomite is applicable for the areas rich in the resource of magnesium chloride to use a Pidgeon process to produce the metal of magnesium.
Owner:QINGHAI INST OF SALT LAKES OF CHINESE ACAD OF SCI
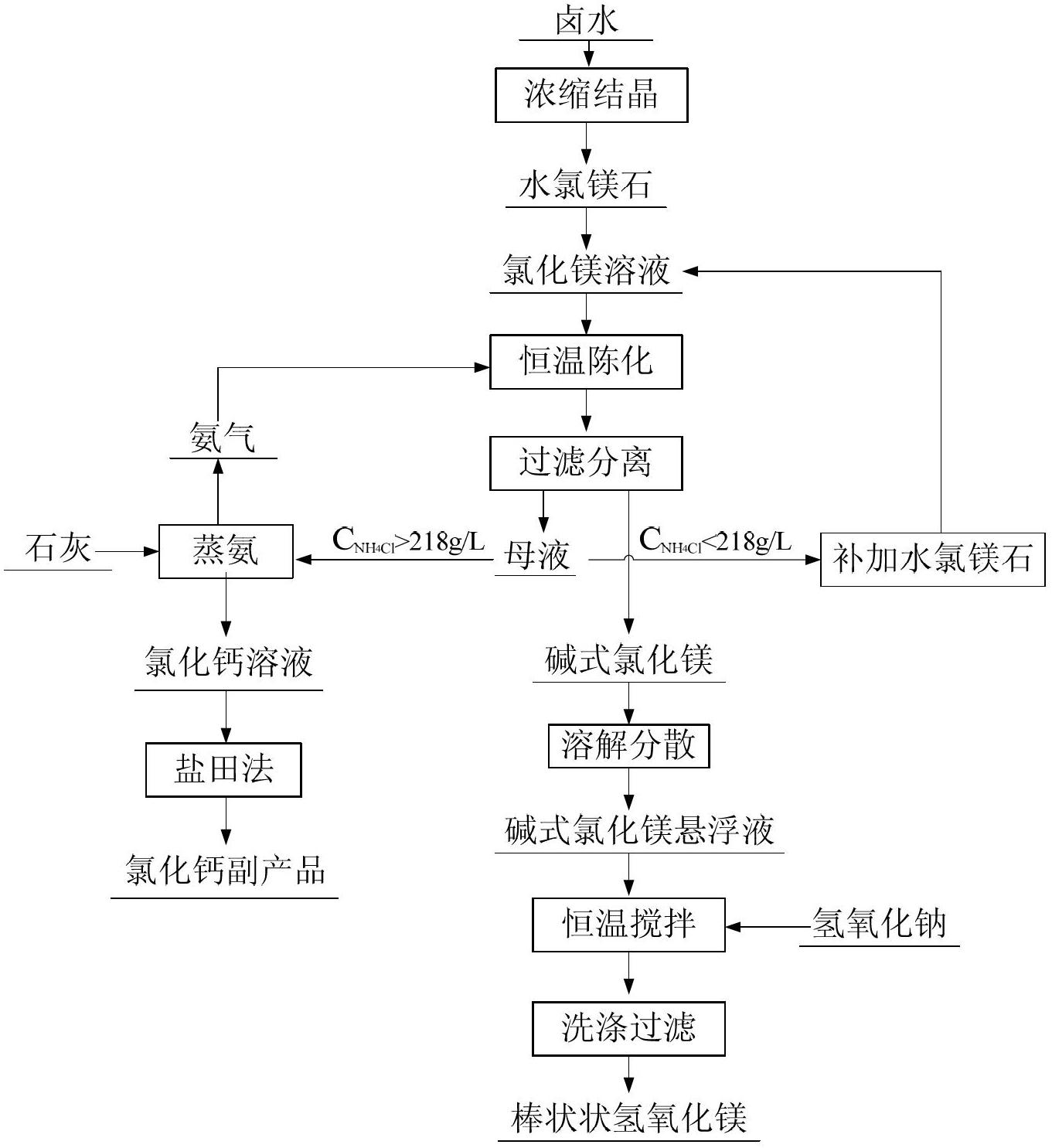
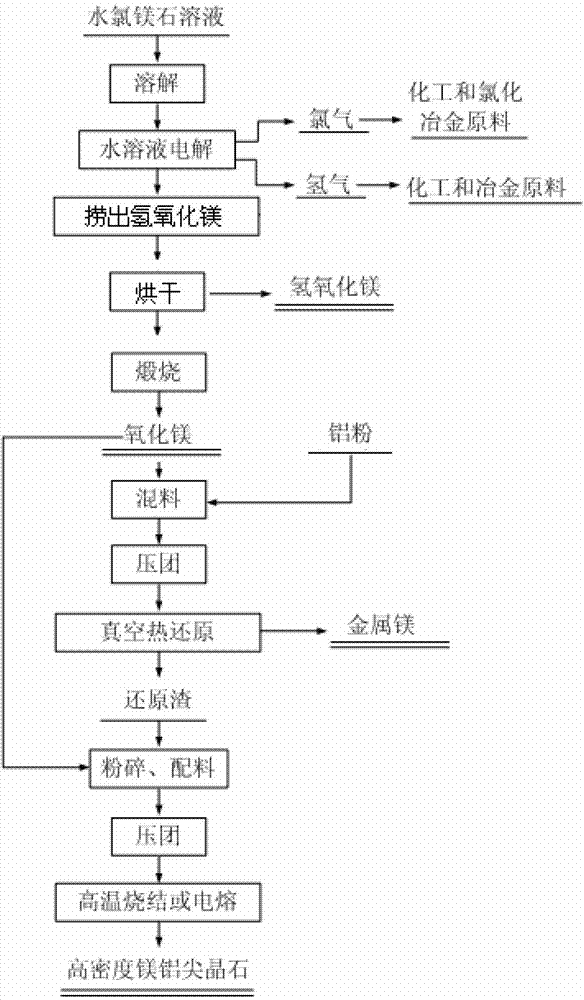
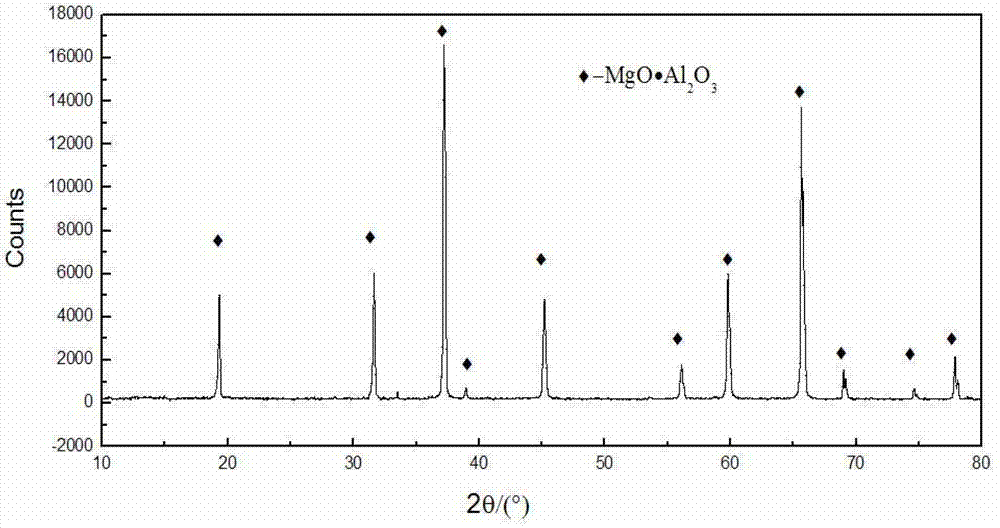
Method for preparing magnesium hydroxide, magnesium and magnesium aluminate spinel by bischofite
InactiveCN102817041AHigh priceLow impurity contentElectrolysis componentsProcess efficiency improvementChemical industryElectrolysis
The invention discloses a method for preparing magnesium hydroxide, magnesium and magnesium aluminate spinel by bischofite and belongs to the field of magnesium metallurgy and chemical industry. The method comprises the steps of: taking bischofite as a raw material to produce magnesium hydroxide, hydrogen gas and chlorine gas through electrolysis, and then taking magnesium oxide, which is obtained by calcining magnesium hydroxide, as a raw material to produce magnesium metal and magnesium aluminate spinel through vacuum thermal reduction. The by-product, i.e., bischofite, during extraction of potassium salt and lithium salt in west salt lakes and in the salt manufacturing industry of the eastern coastal regions, is utilized for producing the magnesium hydroxide, magnesium oxide and magnesium aluminate spinel, therefore, the purpose of changing waste into valuable is realized. Compared with the traditional electrolysis technology of fused magnesium chloride, the method has the characteristics of low energy consumption, low cost, small environment pollution and the like.
Owner:NORTHEASTERN UNIV
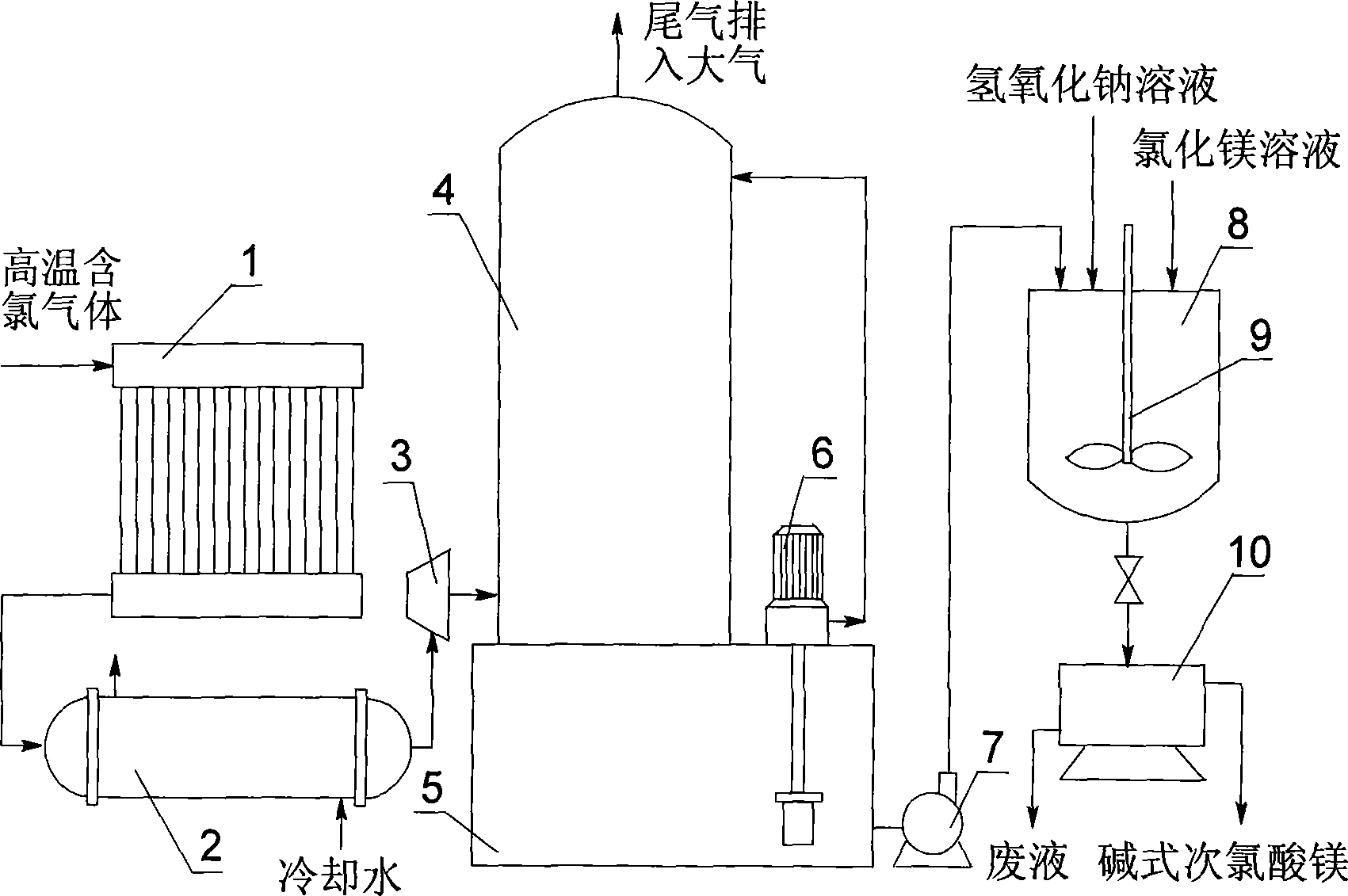
Method and device for preparing basic magnesium hypochlorite by using waste chlorine
The invention discloses a method for preparing basic magnesium hypochlorite by using waste chlorine. Firstly, chlorine is absorbed by a solution of sodium hydroxide to form a solution of sodium hypochlorite; and secondly, the solution of sodium hypochlorite is reacted with a solution of magnesium chloride to form solid basic magnesium hypochlorite. In the basic magnesium hypochlorite product prepared by the method, the effective chlorine content is 38 to 42 percent, the magnesium content is 28 to 32 percent and the effective chlorine yield is 85 to 95 percent. The method uses chlorine-containing gases produced in the production processes of electrolytic industry or other industries and makes full use of the cheap but high-quality bischofite resources in salt lake areas. The prepared basic magnesium hypochlorite is stable in property and mild in oxidation and can avoid the adverse impact of pinhole damages when used on textiles. The waste liquid produced in the preparation process mainly contains sodium chloride and magnesium chloride and can be directly discharged into salt lakes after simple treatment without adverse impact on the resource structure of the salt lakes.
Owner:QINGHAI INST OF SALT LAKES OF CHINESE ACAD OF SCI
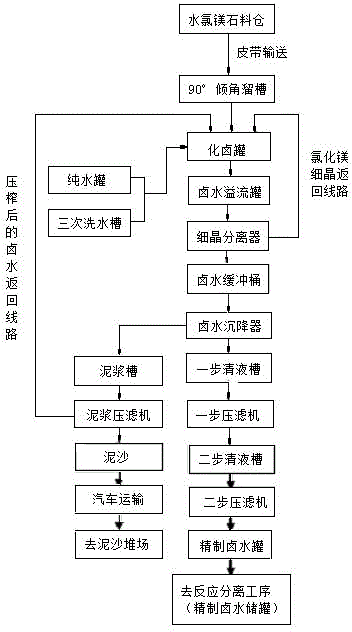
Method of continuous dissolution of salt lake bischofite to prepare high-concentration magnesium chloride solution
ActiveCN104030327AFast dissolutionIncrease the speed of saltMagnesium chloridesHigh concentrationSalt lake
The invention relates to the technical field of salt chemistry, particularly relates to a method of continuous dissolution of salt lake bischofite to prepare a high-concentration magnesium chloride solution, to a production device for achieving a process of large-scale bischofite dissolution to prepare refined brine, and provides a industrial demonstration effect route for developing magnesium series oxides in the Qinghai salt lake in the future. The method includes steps of: unloading bischofite, conveying and loading the material by conveying belts, feeding, dissolving into brine, allowing crude brine to overflow, performing magnesium chloride fine grain separation, buffering the brine, thickening and depositing the brine, obtaining a first-step clear liquid, subjecting the first-step clear liquid to first-step filter pressing, obtaining a second-step clear liquid, subjecting the second-step clear liquid to second-step filter pressing, squeezing and filtering the bottom material of a thickening machine, conveying mud cake, subjecting the brine to accident handling, and conveying and storing the refined brine so as to obtain the brine with technical indexes meeting requirements of ammonia gas combined reactions. The concentration of Mg<2+> in the refined brine is 115+ / -3 g / L. The refined brine is clear, transparent and free of solid suspension impurities.
Owner:青海西部镁业有限公司
Method for preparing magnesia for magnesium cement by using bischofite and dolomite
The invention relates to a method for preparing magnesia for magnesium cement by using bischofite and dolomite. The method comprises the following steps of: (1) grinding dolomite calcined at the temperature of 900-1,000 DEG C into dolomite powder, and digesting the dolomite powder into caustic dolomite lime milk with a calcium chloride solution; (2) preparing a magnesium chloride solution, dissolving bischofite with water into a magnesium chloride solution of which the specific weight is 1.19-1.23 g / cm<3>; (3) reacting the magnesium chloride solution obtained in the step (2) with the caustic dolomite lime milk obtained in the step (1) while stirring in a constant-temperature water bath to generate a magnesium hydrate precipitate; (4) washing the magnesium hydrate precipitate with water for multiple times, and performing suction filtration to obtain a filter cake; and (5) drying the filter cake, calcining at the temperature of 500-600 DEG C for 2-4 hours to obtain a sinter, naturally cooling the sinter, smashing and packaging to obtain magnesia for magnesium cement. The method has the advantages of simple process, good product quality, low cost and easiness for realizing industrial production.
Owner:QINGHAI INST OF SALT LAKES OF CHINESE ACAD OF SCI
Process for preparing anhydrous magnesium chloride by dewatering bischofite
InactiveCN1944261ASimple structureEasy to manufactureMagnesium chloridesFluidized bedMagnesium chloride hexahydrate
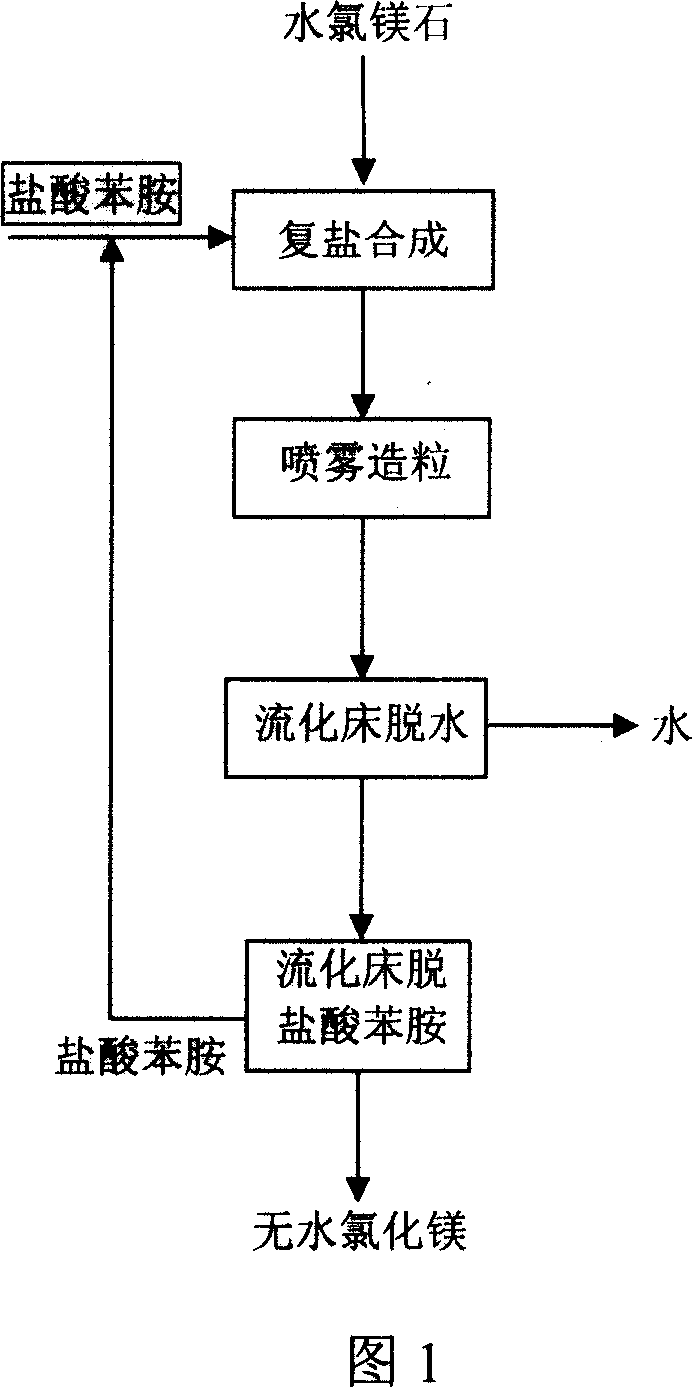
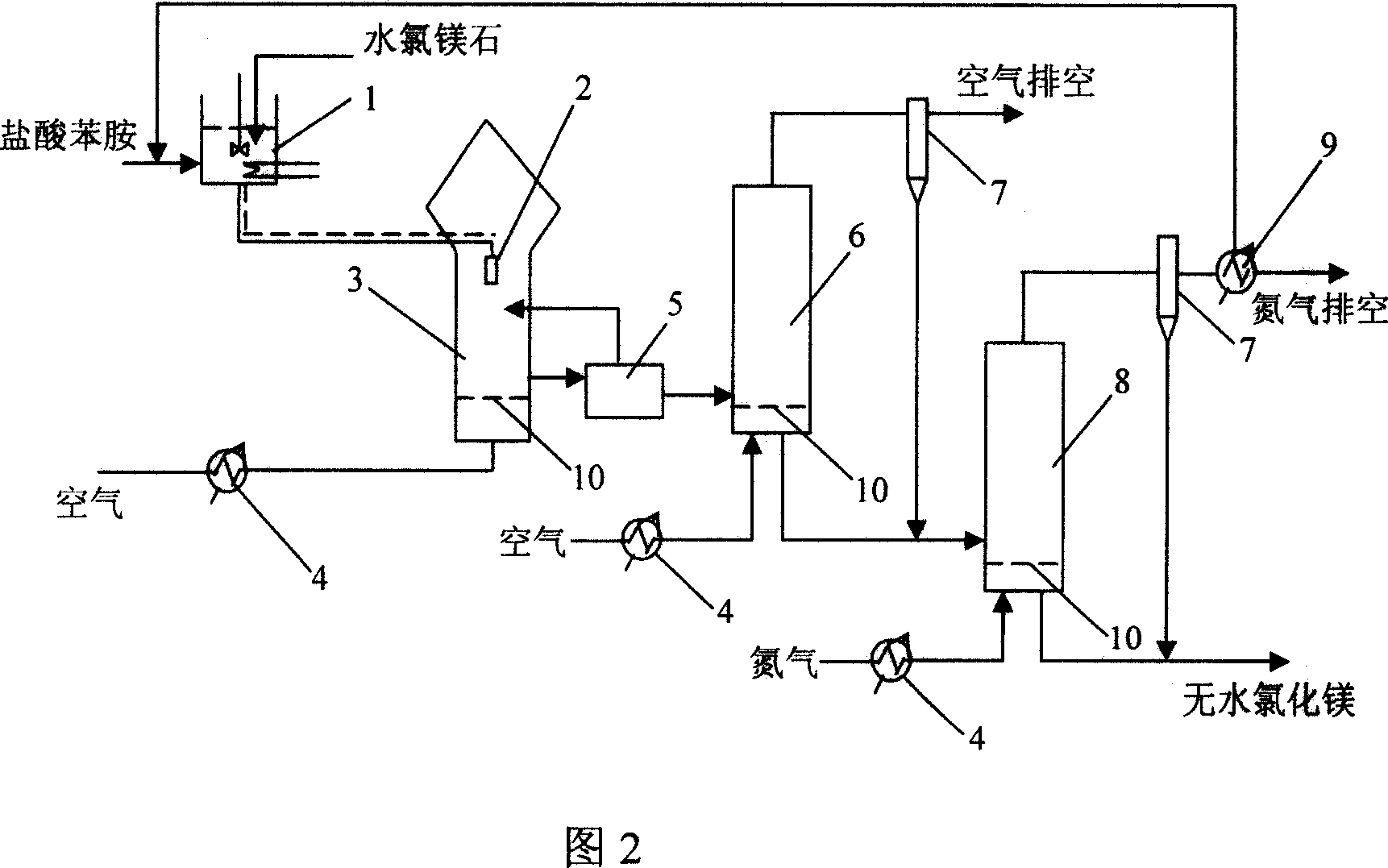
The present invention relates to chemical and metallurgical technology, and is especially process of preparing anhydrous magnesium chloride through dewatering bischofite. The process includes the first reaction of aniline hydrochloride and bischofite to produce aniline hydrochloride-magnesium chloride hexahydrate complex salt, the subsequent spray drying and pelletizing the complex salt, and final eliminating crystallized water and aniline hydrochloride in a fluidized bed to obtain the anhydrous magnesium chloride product. The present invention has the advantages of low power consumption, simple technological process, easy industrial amplification, environment friendship, stable product quality, etc.
Owner:TSINGHUA UNIV
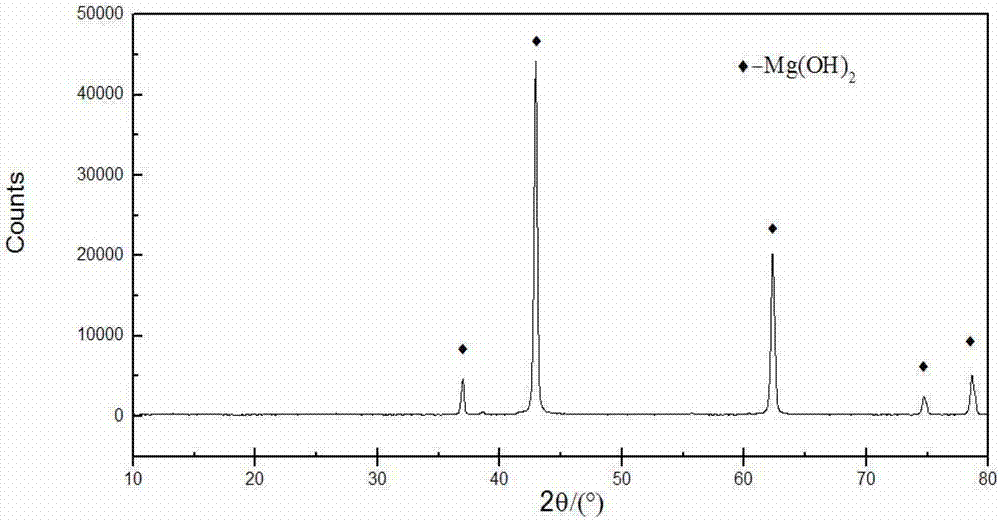
Technology for producing and preparing high-purity lamellar magnesium hydroxide
InactiveCN101700899ASolve the difficult technical problems of filtrationReduce manufacturing costMagnesium hydroxideHydrogenThermal insulation
The invention provides a technology for producing and preparing high-purity lamellar magnesium hydroxide, which can solve the problem that the magnesium hydroxide is difficult to filter, so that the purity is difficult to improve. The basic technology comprises the following steps: taking bischofite or magnesium chloride as a main raw material, taking hydrogen sodium as a main precipitating agent, adding crystal seeds before the reaction, carrying out liquid phase precipitation reaction at 15-100 DEG C, simultaneously adding a crystal accelerator, and sequentially carrying out thermal insulation aging, filtering, washing and drying after the reaction is finished, thereby obtaining the lamellar magnesium hydroxide with the purity of more than 99%. The technology can solve the problem that the magnesium hydroxide is difficult to filter and has the advantages of simple technology, low cost and high utilization rate of magnesium.
Owner:钟辉
Continuous preparation method of high-purity magnesium hydroxide
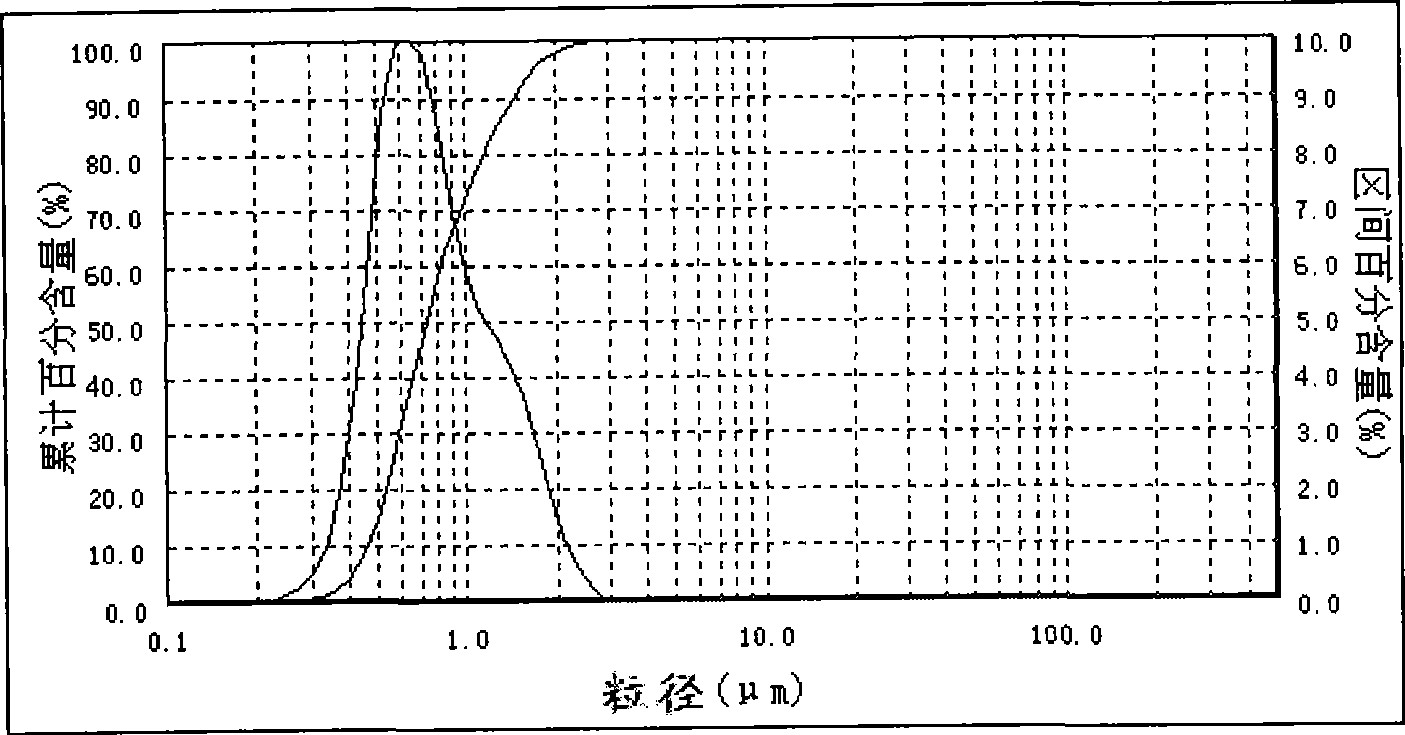
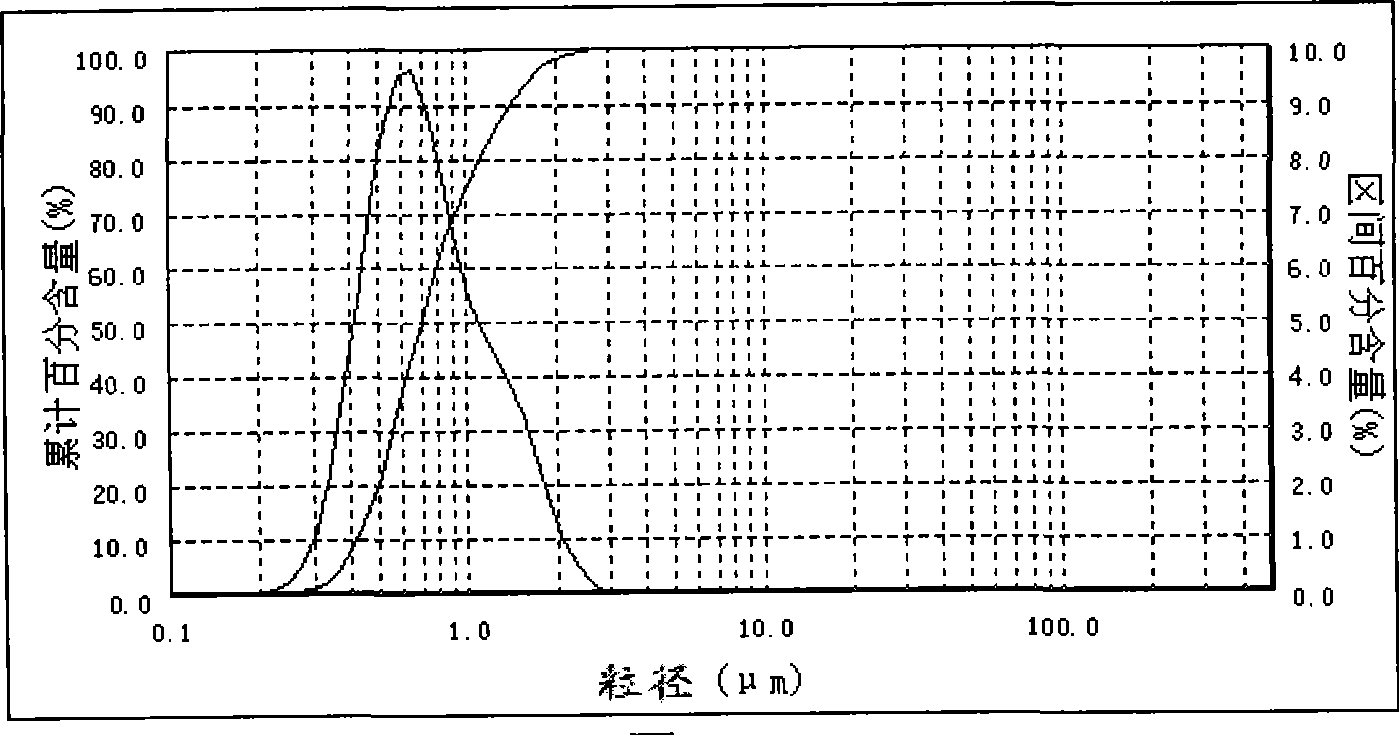
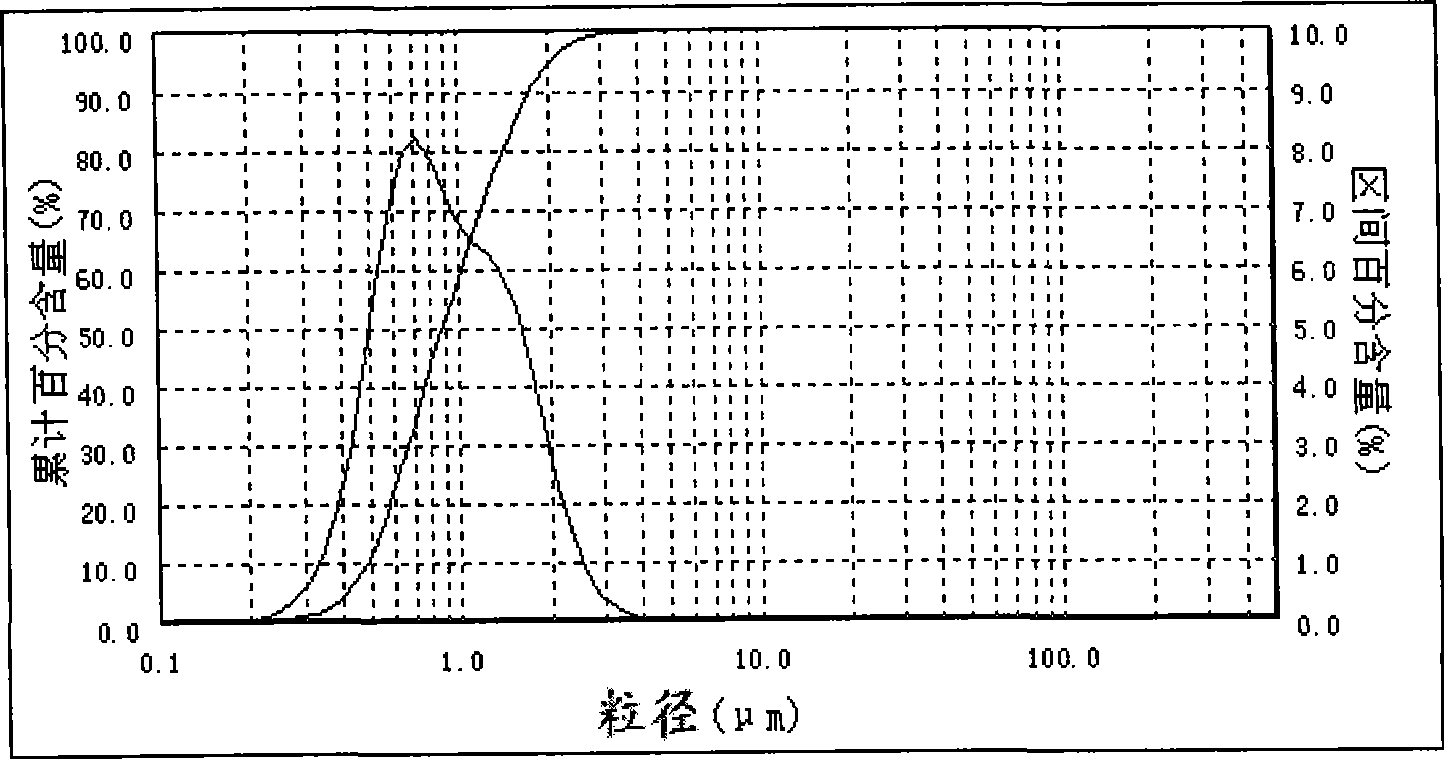
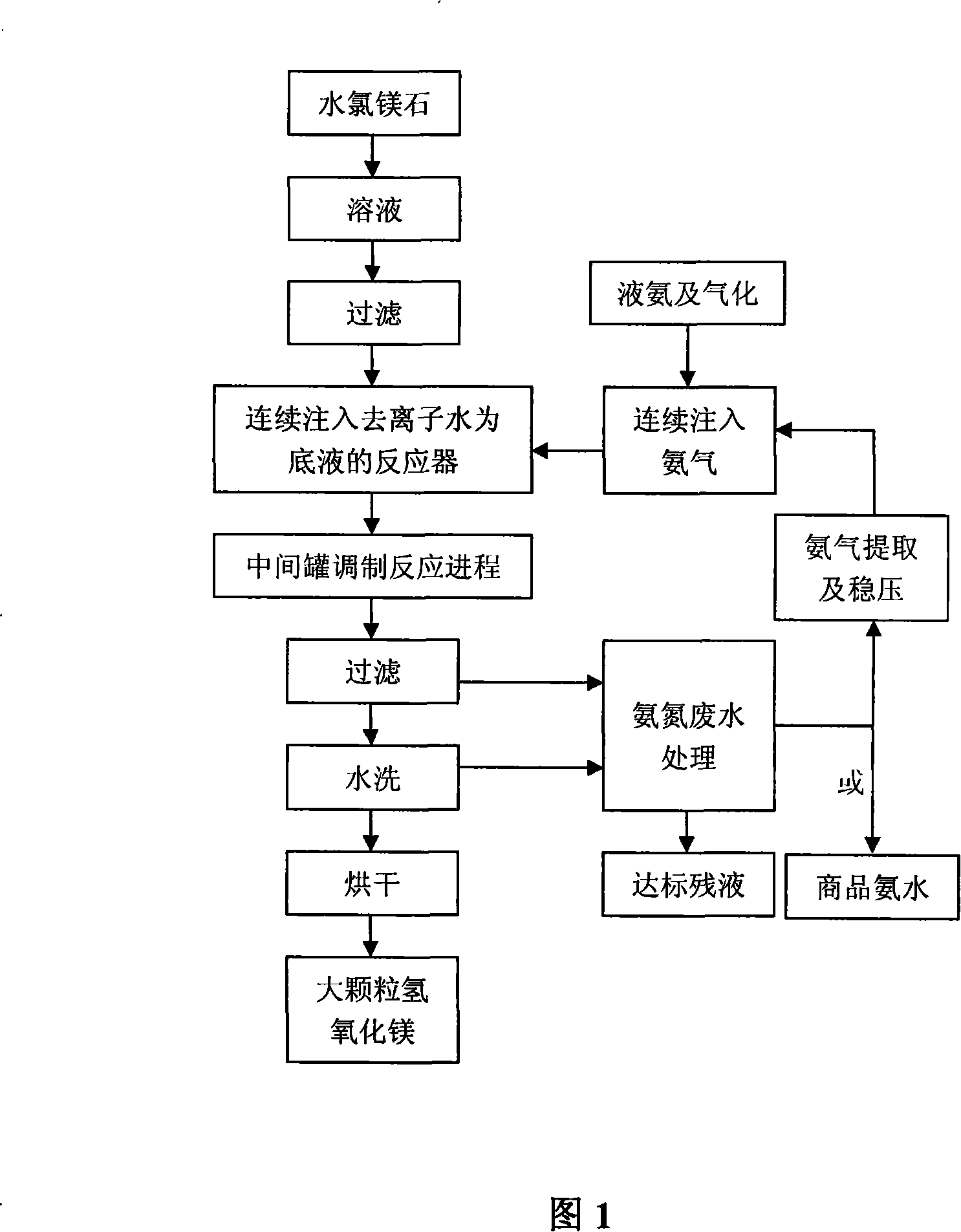
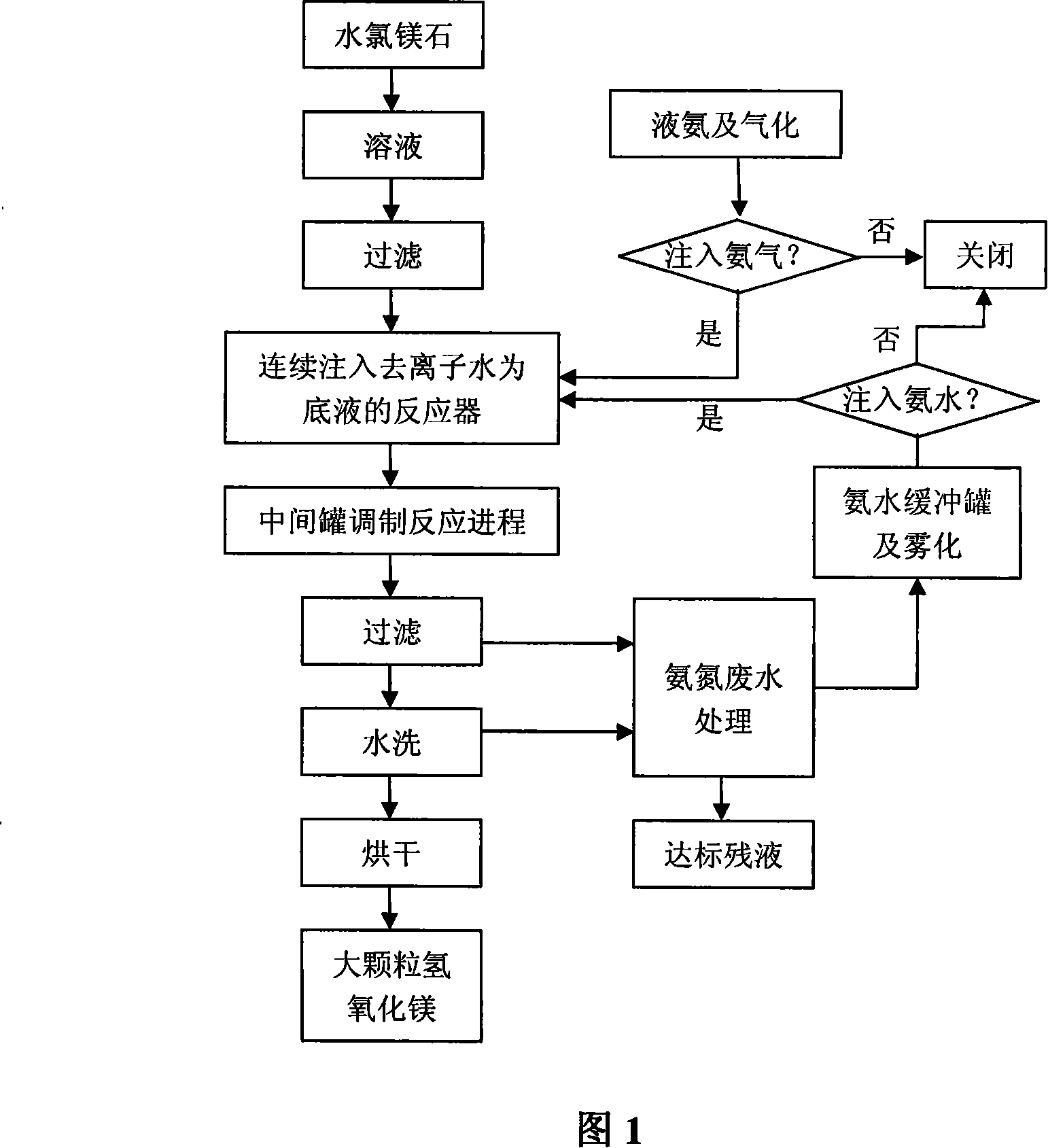
The invention provides a method for continuously preparing highly pure magnesium hydroxide which relates to a method for continuously producing highly pure magnesium hydroxide with bischofite as raw material and ammonia as precipitant. The method is characterized in that: a treated bischofite is prepared into a solution with the concentration of 0.5 mol / L-4 mol / L, the bischofite solution and ammonia gas are added continuously after the reaction begins and the chemical reaction for depositing the magnesium hydroxide is carried out continuously. Characteristics of the reaction vessel utilization ratio, the productive capacity of unit volume, technical reliability and stability and so on of the method are greatly improved compared with intermittent reaction technology. The base liquid does not need to be replaced or heated repeatedly, thus energy-saving effect is distinct and labor productivity is high. The method can be used for obtaining magnesium hydroxide with the granularity of 10 microns-100 microns and the purity of 99 percent-99.999 percent. The ammonia nitrogen treatment of the tail solution adopts a commercial method that meets environmental protection requirement. The product produced by the invention has large granule size, high purity, more specifications, low cost, high equipment utilization rate and low one-time investment and has no pollution to the environment.
Owner:DALIAN MARITIME UNIVERSITY +1
Method of producing metal magnesium by bischofite drying calicing to make magnesium oxide to vacuum thermo reduction
A process for preparing metal Mg from bischofite includes removing impurities, spray drying at 150-450 deg.C, calcining at 550-1200 deg.C to obtain MgO, proportionally adding calcium compound, ferrosilicon or silicoaluminium as reducer and fluorite as catalyst, mixing, sphericizing and vacuum reducing at 1150-1200 deg.C for 6-10 hr.
Owner:青海中信国安科技发展有限公司
Method for preparing calcined dolomite for silicothermic process magnesium production by aid of carbide slag and bischofite
The invention relates to a method for preparing calcined dolomite for silicothermic process magnesium production by the aid of carbide slag and bischofite. The method includes the steps: firstly, placing magnesium chloride and the carbide slag into water, performing stirring reaction for 0.5-3h at the normal temperature and generating mixed slurry of calcium hydroxide and magnesium hydroxide after sufficient reaction; secondly, performing solid-liquid separation for the slurry to obtain sediment; thirdly, washing the solid sediment, drying the solid sediment to reach constant weight and obtaining dried solid powder; and finally, calcining the dried solid powder for 0.5-3h at the temperature of 600-1000 DEG C to obtain the calcined dolomite. The carbide slag serving as raw materials is turned from harm into good and turned from waste into wealth, and the whole technical process is simple, easily controlled, non-corrosive to equipment and applicable to metallic magnesium production by the aid of a Pidgeon process for regions with high carbide slag byproduct yield or rich magnesium chloride resources.
Owner:QINGHAI INST OF SALT LAKES OF CHINESE ACAD OF SCI
Method for duplex deposition of high-purity magnesium hydroxide by liquid ammonia-ammonia
InactiveCN101224902AAchieve recyclingContinuous productionMagnesium hydroxideDecompositionGranularity
The invention provides a method for depositing highly pure magnesium hydroxide with a liquid ammonia-ammonia twofold-gang and relates to a method for continuously producing the highly pure magnesium hydroxide with a bischofite as raw material and ammonia and ammonia water as a precipitant. The treated bischofite is prepared into a solution with the concentration of 0.5 mol / L-4 mol / L, the precipitant is supplied by ammonia gas at the early stage of the reaction, then the precipitant is provided by the ammonia water reclaimed from the decomposition of the reaction product, ammonium chloride, the ammonia gas supplements the rest amount and the reaction process can be carried out continuously or intermittently. The method can be used for producing the magnesium hydroxide with the granularity of 10 microns-100 microns and the purity of 99 percent-99.999 percent. The product, ammonium chloride, adopts alkaline splitting and vacuum ejection flow to reclaim the ammonia gas, prepare the ammonia water for recycling utilization. The product produced by the invention has large granule size, high purity, more specifications, low cost, high equipment utilization rate, high reliability and stability of the technique, low one-time investment and has no pollution to the environment.
Owner:DALIAN MARITIME UNIVERSITY +1
Method for producing magnesium using bischofite as raw material
InactiveCN1663913AUniform particle sizeAvoid the dehydration processProcess efficiency improvementMagnesiaSolubilityMetallic materials
A method of preparing magnesium metal with bischofite, pertains to the field of metallic material. The invention is characterized in that it takes the bischofite as stock and prepare magnesium hydroxide with ammonia process under the following conditions: at the temperature between 45~55 DEG C for 20~30 minutes, with the proportion between magnesium and ammonia 1:1.2~1.5:2.2 and with the concentration of magnesium chloride about 35~55 g / L, calcinating the magnesium hydroxide under the temperature of 900~1000 DEG C for 3-4hours, getting the magnesia with the purity of more than 99.5%,in the molten salt electrolyte system of lanthanum chloride and magnesium chloride, the proportion between lanthanum chloride and magnesium chloride in moles is 10~30%, the solubility of magnesia is 5~10 mass%, thaw temperature is 700~750 DEG C, the tank voltage 4.5~6.5 volt, the current efficiency comes to 85~90%, the consumption of the direct current of magnesium is 11~12.5kWh per kilogram and the purity of magnesium is above 95%. The method avoids the dehydration process of bischofite and is characterized by the energy-saving and consumption-deducing, the highly effective and cleaning of electrolytic process, particularly for its not emitting chlorine and not polluting the environment.
Owner:UNIV OF SCI & TECH BEIJING
Method of preparing low sodium carnallite by blending halogen
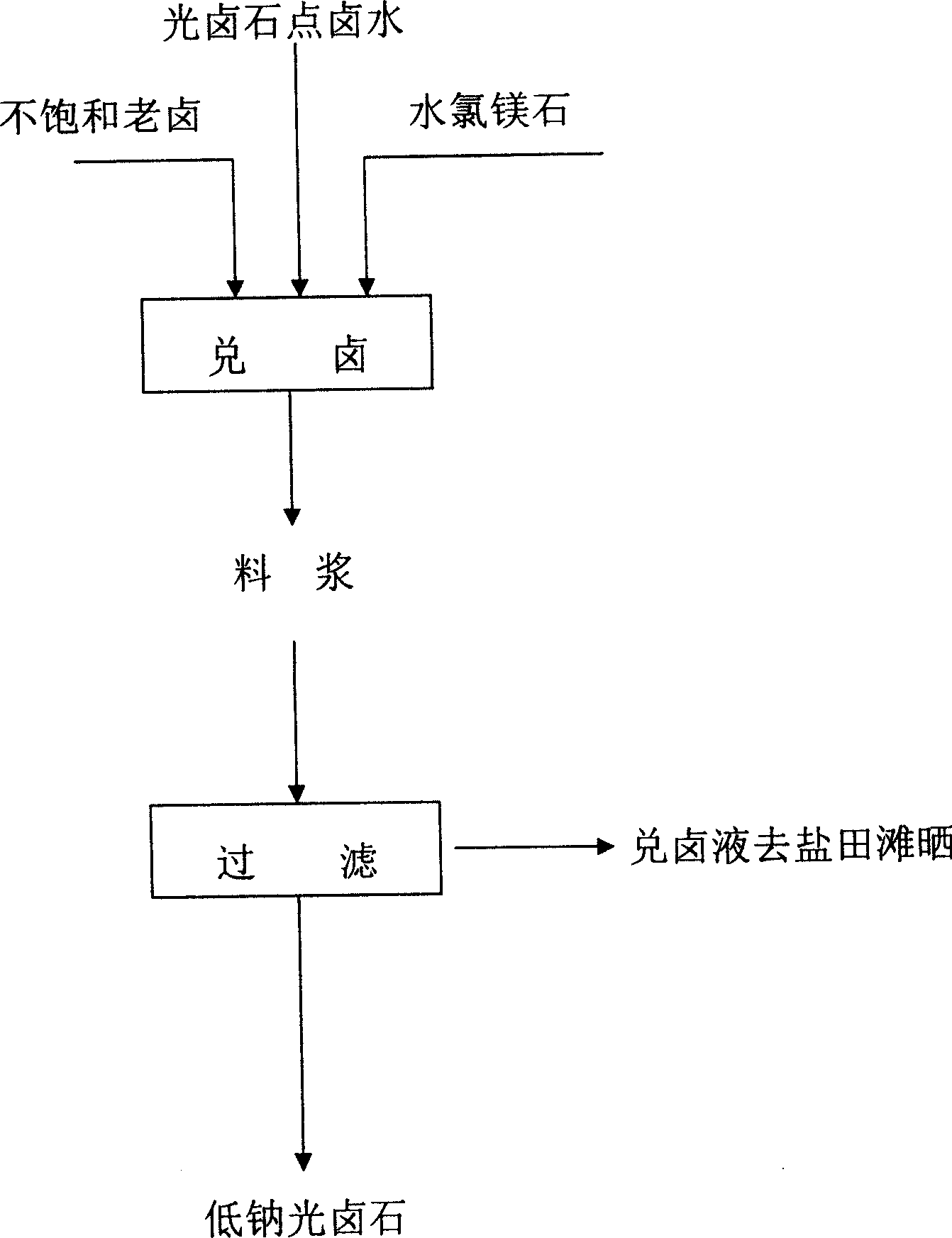
ActiveCN1751999ALow purity requirementAchieve reuseMagnesium chloridesAlkali metal chloridesHalogenCarnallite
A process for preparing low-Na carnallite by removing halogen includes such steps as sunning in salt field for natural evaporating to obtain bischofite, mixing it with unsaturated brine, stirring until the content of Mg ions is not less than 8%, adding carnallite, dehalogenating-crystallizing reaction, and filtering.
Owner:BLUESTAR LEHIGH ENG INST CO LTD
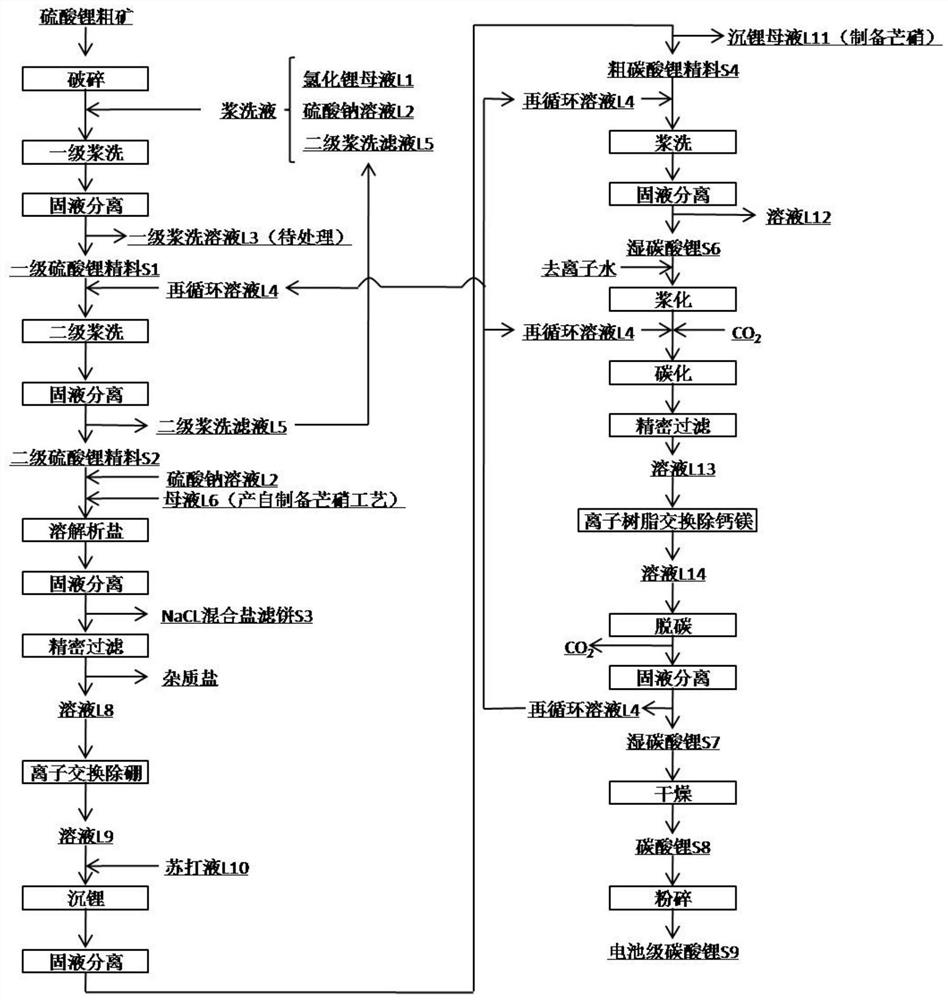
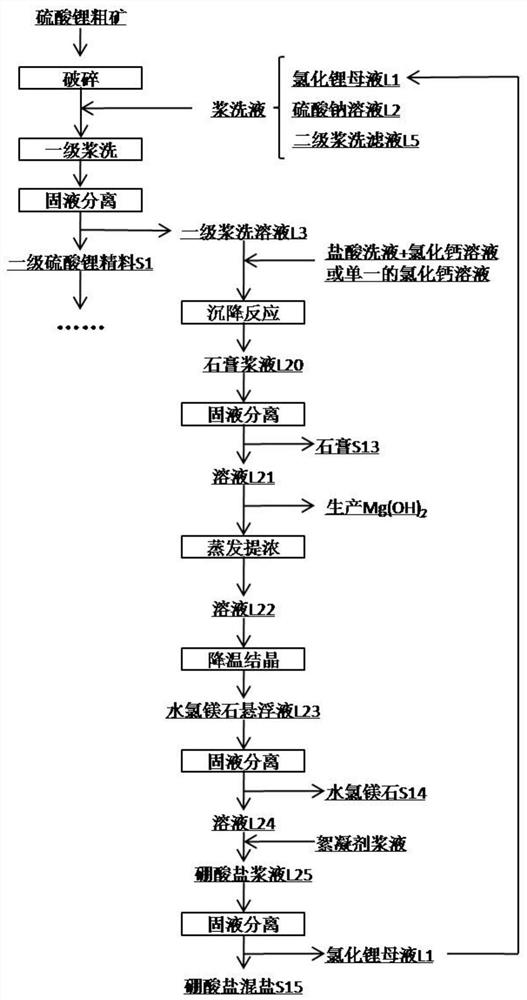
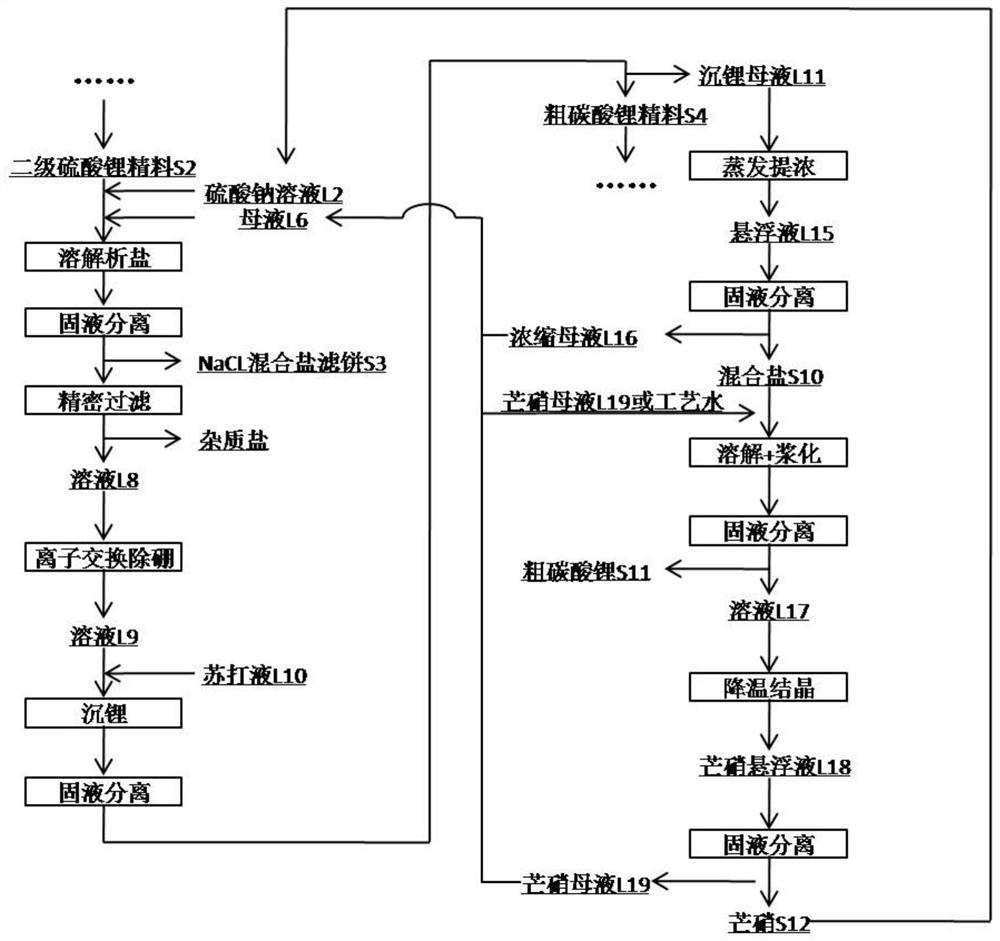
Method for preparing battery-grade lithium carbonate from lithium sulfate crude ores and recovering byproducts
ActiveCN111960445AReduce lossesHigh recovery rateMagnesium chloridesCalcium/strontium/barium sulfatesLithium sulphateLithium chloride
The invention discloses a method for preparing battery-grade lithium carbonate from lithium sulfate crude ores and recovering byproducts. The method comprises the following steps: preparing battery-grade lithium carbonate; preparing mixed salt of gypsum, bischofite and borate; and preparing salt cake. According to the method, lithium chloride mother liquor generated by lithium precipitation and asodium sulfate solution are adopted for primary pulp washing, so that the loss of lithium sulfate can be reduced, and the recovery rate is increased; a recycled solution L4 containing lithium carbonate is taken as a pulp washing solution to carry out secondary pulp washing, soluble calcium and magnesium ions are removed, and lithium in the recycled solution is recovered; the filtrate L5 obtained after solid-liquid separation of the secondary pulp washing contains lithium, and is returned to the primary pulp washing as supplement, the loss of the primary pulp washing is reduced while soluble impurity ions are dissolved; the secondary lithium sulfate concentrate is dissolved by mirabilite preparation mother liquor L6, NaCl mixed salt is separated out in the dissolving process, and lithium isprecipitated. According to the method, the crude lithium carbonate is subjected to pulp washing by adopting the recycling solution containing lithium carbonate, so that the lithium yield can be increased while the system discharge is reduced.
Owner:CINF ENG CO LTD
Method for preparing anhydrous magnesium chloride
ActiveCN103922371ASimple processLow equipment requirementsMagnesium chloridesPhysical chemistryChloride
Owner:QINGHAI INST OF SALT LAKES OF CHINESE ACAD OF SCI
Preparation method of anhydrous magnesium chloride
InactiveCN107500319AInhibition of hydrolysis reactionAvoid the problem of severe corrosion equipmentMagnesium chloridesHydrolysisBischofite
The invention discloses a preparation method of anhydrous magnesium chloride. The preparation method comprises the following steps: S1, mixing bischofite with ammonium chloride to obtain a dehydration raw material; S2, enabling the dehydration raw material to be subjected to primary dehydration at 180-240 DEG C and secondary dehydration at 250-300 DEG C to obtain a crude product of the anhydrous magnesium chloride; S3, heating the crude product of the anhydrous magnesium chloride to a temperature, which is not lower than 250 DEG C, under an protective atmosphere after white smoke around the crude product of the anhydrous magnesium chloride disappears, and performing heat preservation for at least 2h to obtain the anhydrous magnesium chloride, wherein in the steps S2 and the S3, no gas overflows from the reaction system. According to the preparation method disclosed by the invention, the ammonium chloride serves as a protective agent, ammonia gas and hydrogen chloride gas, which are generated after the ammonium chloride is heated and decomposed, are utilized, the hydrogen chloride gas can inhibit hydrolysis reaction, and the ammonia gas can be used for replacing water molecules in hydrated magnesium chloride, so as to further facilitate the obtaining of the anhydrous magnesium chloride. The preparation method has the advantages of simple and easy performing and low cost, and the problem of seriously corroded equipment caused by a pure hydrogen chloride gas method in a high-temperature airtight condition is avoided.
Owner:QINGHAI INST OF SALT LAKES OF CHINESE ACAD OF SCI
Method for preparing magnesium hydroxide and chlorine by coupling magnesium chloride reactive crystallization and electrolysis
InactiveCN103626210ADemand adjustmentEfficient use ofElectrolysis componentsMagnesium hydroxideElectrolysisChloride
The invention relates to a method for preparing magnesium hydroxide and chlorine by coupling magnesium chloride reactive crystallization and electrolysis. A magnesium hydroxide product is obtained by firstly adopting bischofite and sodium hydroxide as raw materials, adding a chloride as an additive and controlling crystallization. The purity of the magnesium hydroxide product is more than 99.5%, and the magnesium hydroxide product is uniform in grain size distribution and has better dispersibility. Sodium hydroxide and chlorine are prepared by electrolyzing the byproduct sodium chloride in the process. Sodium hydroxide is used as a raw material for reactive crystallization. Magnesium hydroxide and chlorine can be sold as products. Compared with the prior art, the method has the positive effects that the adding of the chloride as the additive is beneficial to improving the crystallization property and dispersing property of magnesium hydroxide, thus solving the problems that magnesium hydroxide filter cakes produced by original sodium hydroxide methods are difficult to filter and wash and have low purity; meanwhile, the process achieves comprehensive utilization of resources and promotes circular economic development; the method is simple in process flow and convenient to operate and easily achieves large-scale industrial production.
Owner:EAST CHINA UNIV OF SCI & TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com