Method for preparing ultra-fine high dispersing magnesium hydrate flame retardant from saline lake bittern or bischofite
A bischofite and salt lake brine technology, applied in the direction of magnesium hydroxide, etc., can solve the problems of environmental salt lake brine resource pollution, salt lake phase balance destruction, magnesium salt enrichment, etc., and achieve low production cost, convenient operation, and simple process Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
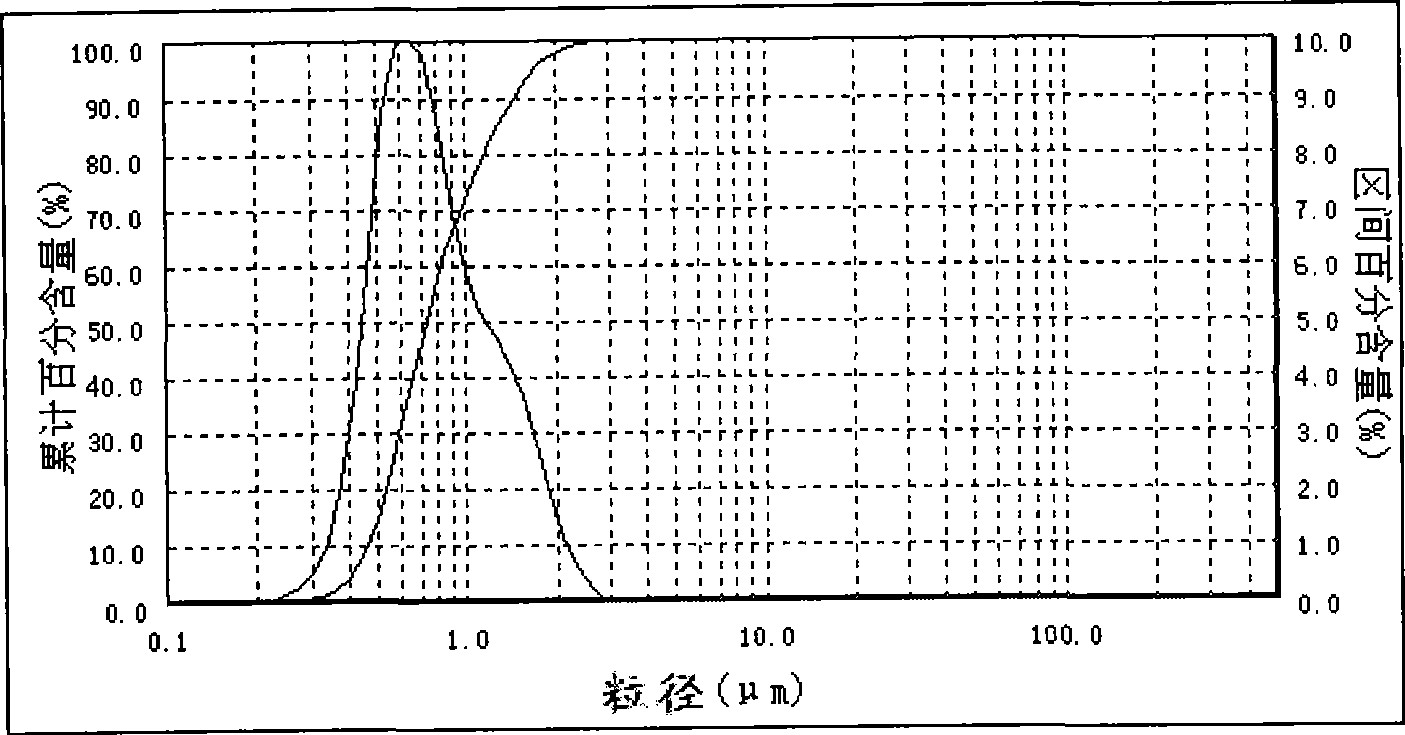
[0022] Example 1: Take a certain amount of water and put it in a beaker, and continuously add bischofite, which is rich in salt lakes, under low-speed stirring until the bischofite is no longer dissolved, and filter to remove insoluble impurities, and the concentration is 3.45mol / L of magnesium chloride solution. Get 500mL of magnesium chloride solution of 3.45mol / L and join in the constant temperature reaction kettle, heat up to 45 DEG C under the stirring rate of 2000rpm, add the ammoniacal liquor that concentration is 6.68mol / L with the flow rate of 70mL / min under the constant temperature condition of 45 DEG C, the ammoniacal liquor The feeding time is 11 minutes. After the ammonia water is completely fed, continue to react at a constant temperature of 45°C and a stirring rate of 2000rpm for 60 minutes. fuel products. The laser particle size analyzer test results are shown in Table 1, and the particle size distribution is as follows figure 1 shown.
[0023] Table 1 Part...
Embodiment 2
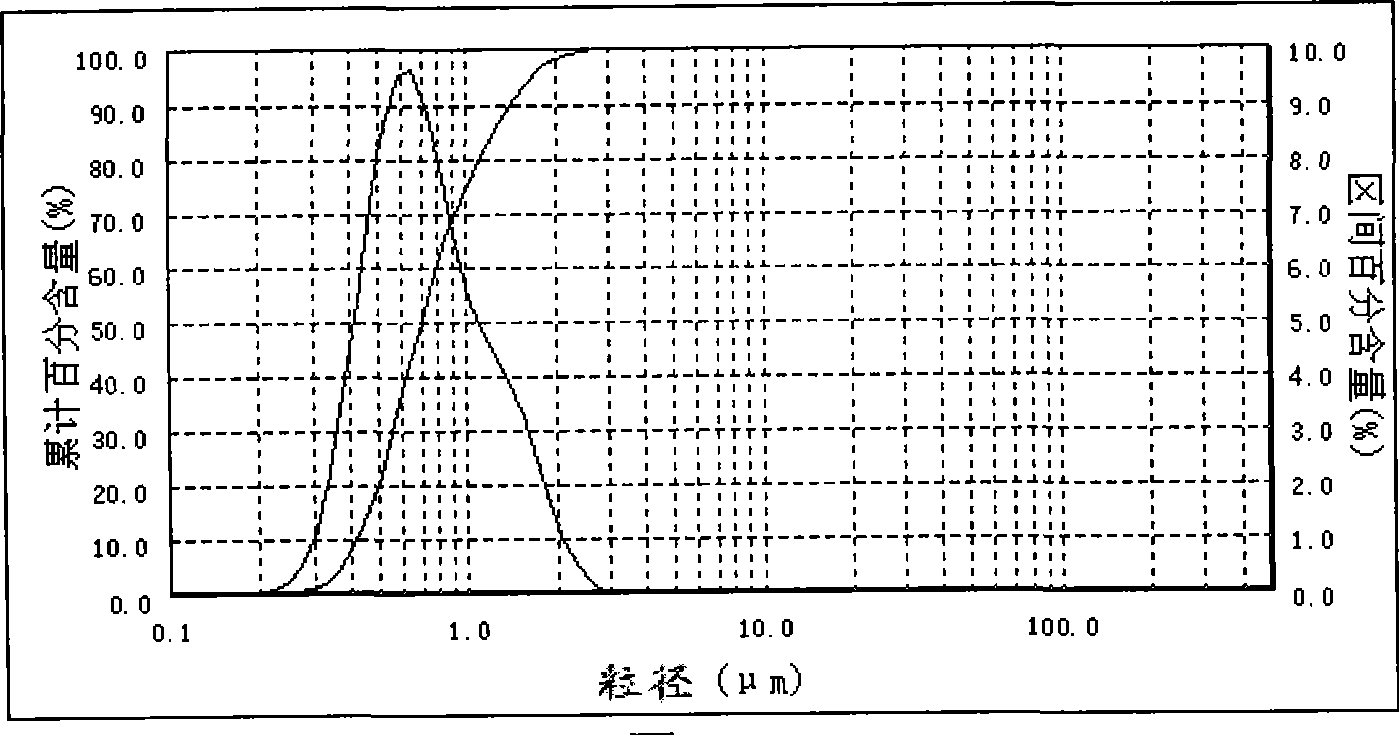
[0026] Example 2: Take a certain amount of water and put it in a beaker, and continuously add bischofite, which is rich in salt lakes, under low-speed stirring until the bischofite is no longer dissolved, filter to remove insoluble impurities, and make a concentration of 3.00mol / L of magnesium chloride solution. Take 500mL of 3.00mol / L magnesium chloride solution and add it to a constant temperature reaction kettle, raise the temperature to 75°C at a stirring rate of 2500rpm, and add 3mol / L ammonia water at a flow rate of 80mL / min at a constant temperature of 75°C, the feeding time of ammonia water The reaction time is 7.37min. After the ammonia water is completely fed, the reaction is continued at a constant temperature of 75°C and a stirring rate of 2500rpm for 20min, and then the slurry is washed, filtered, and vacuum-dried at 120°C to obtain a magnesium hydroxide product with a median diameter of 0.69μm. The laser particle size analyzer test results are shown in Table 2, ...
Embodiment 3
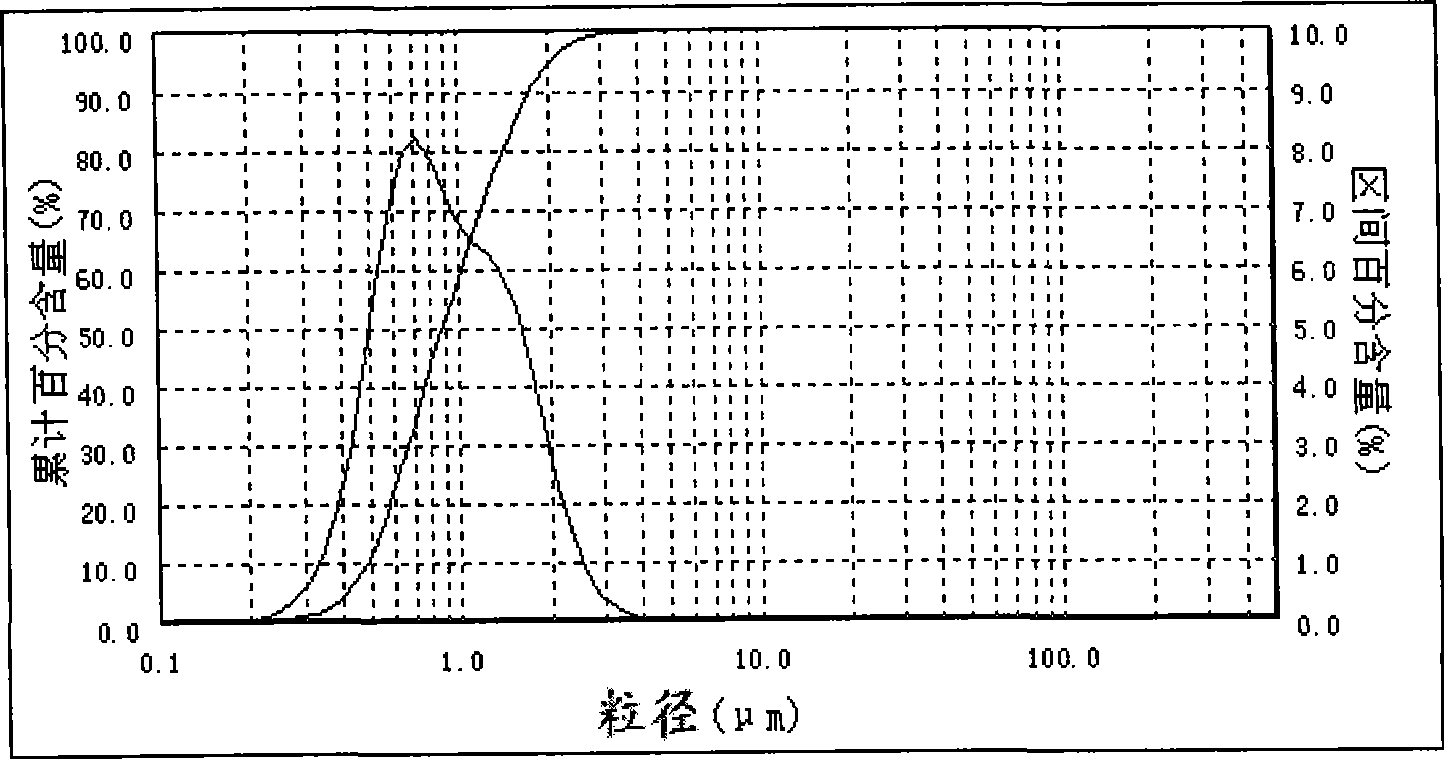
[0030] Example 3: Take a certain amount of water and put it in a beaker, continuously add bischofite, which is rich in salt lakes, under low-speed stirring, until the bischofite no longer dissolves, filter to remove insoluble impurities, and make a concentration of 3.21mol / L of magnesium chloride solution. Take 500 mL of 3.21 mol / L magnesium chloride solution and add it to a constant temperature reactor, heat up to 60°C at a stirring rate of 2000rpm, and add 6.28mol / L ammonia water at a flow rate of 70mL / min at a constant temperature of 60°C. The time is 11 minutes. After the ammonia water is completely fed, the reaction is continued for 60 minutes at a constant temperature of 60°C and a stirring rate of 2000rpm, and then the slurry is washed, filtered, and vacuum-dried at 120°C to obtain a magnesium hydroxide product with a median diameter of 0.87μm. The laser particle size analyzer test results are shown in Table 3, and the particle size distribution is as follows image 3...
PUM
| Property | Measurement | Unit |
|---|---|---|
| width | aaaaa | aaaaa |
| width | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com