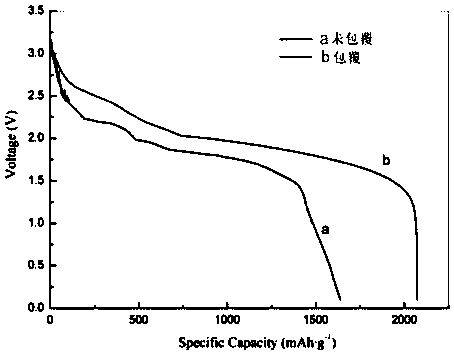
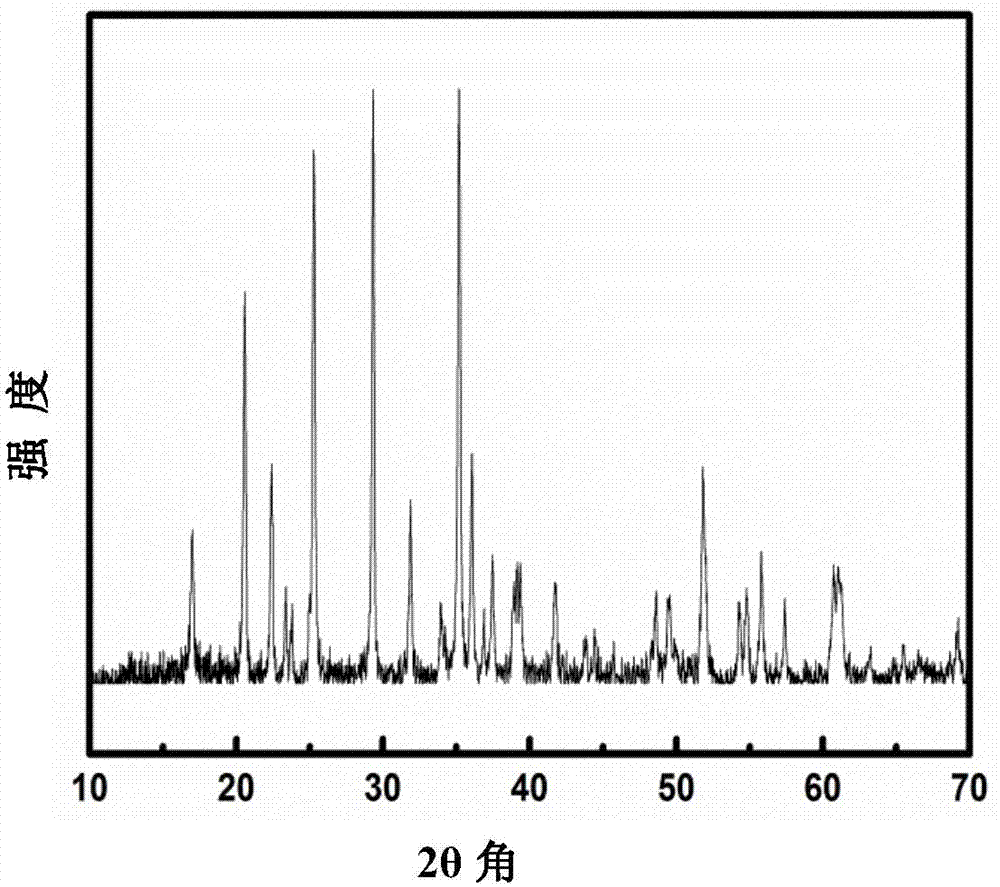
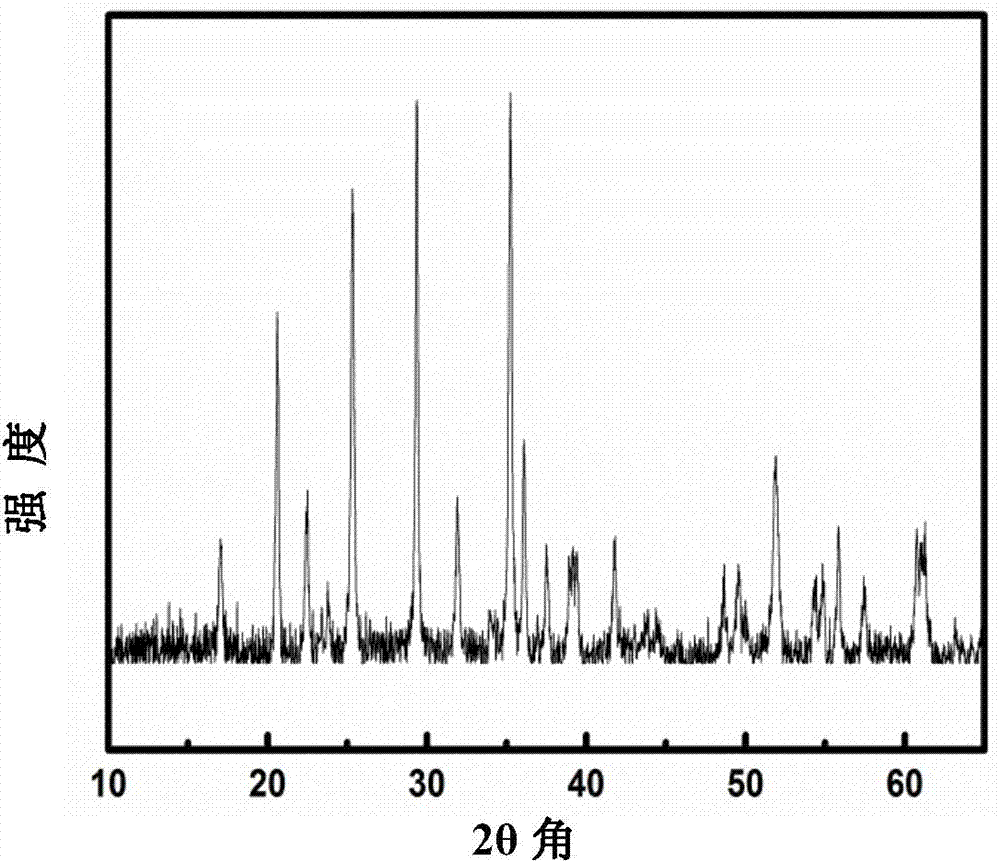
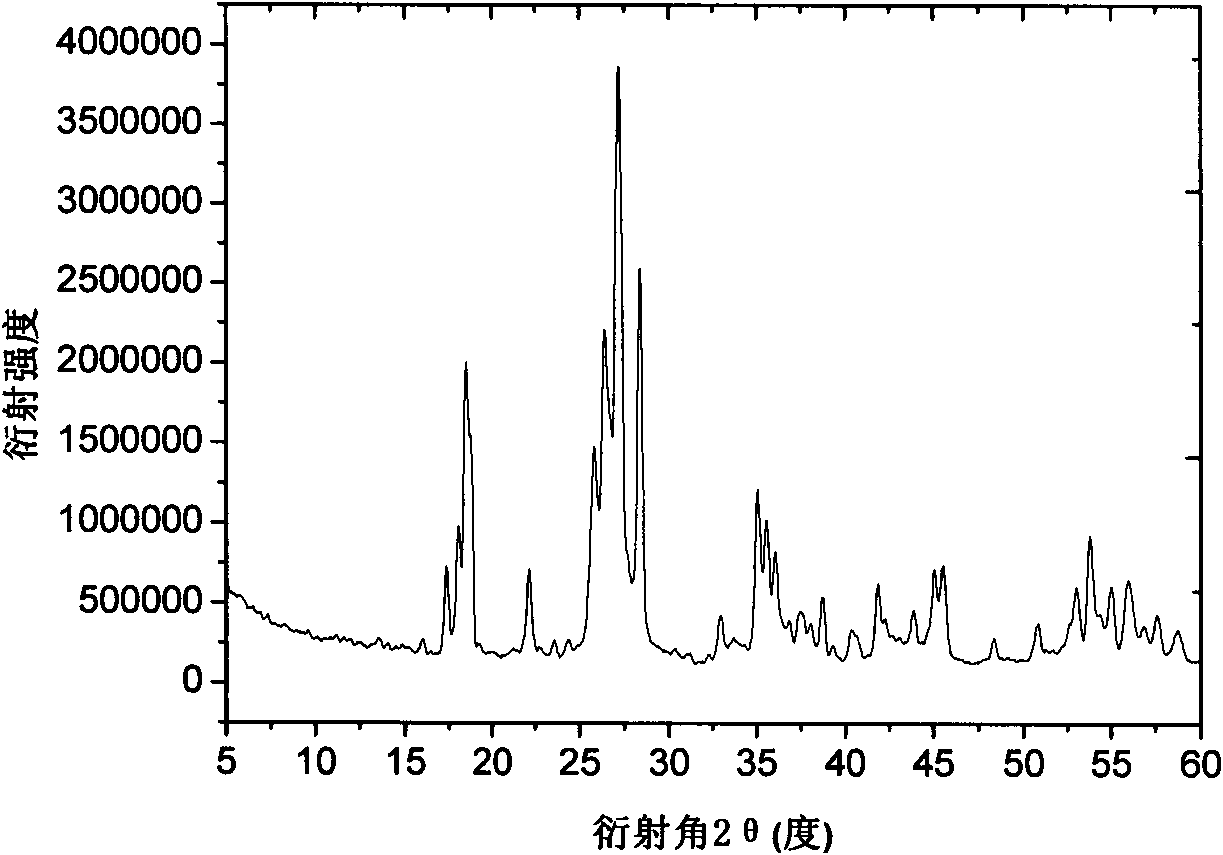
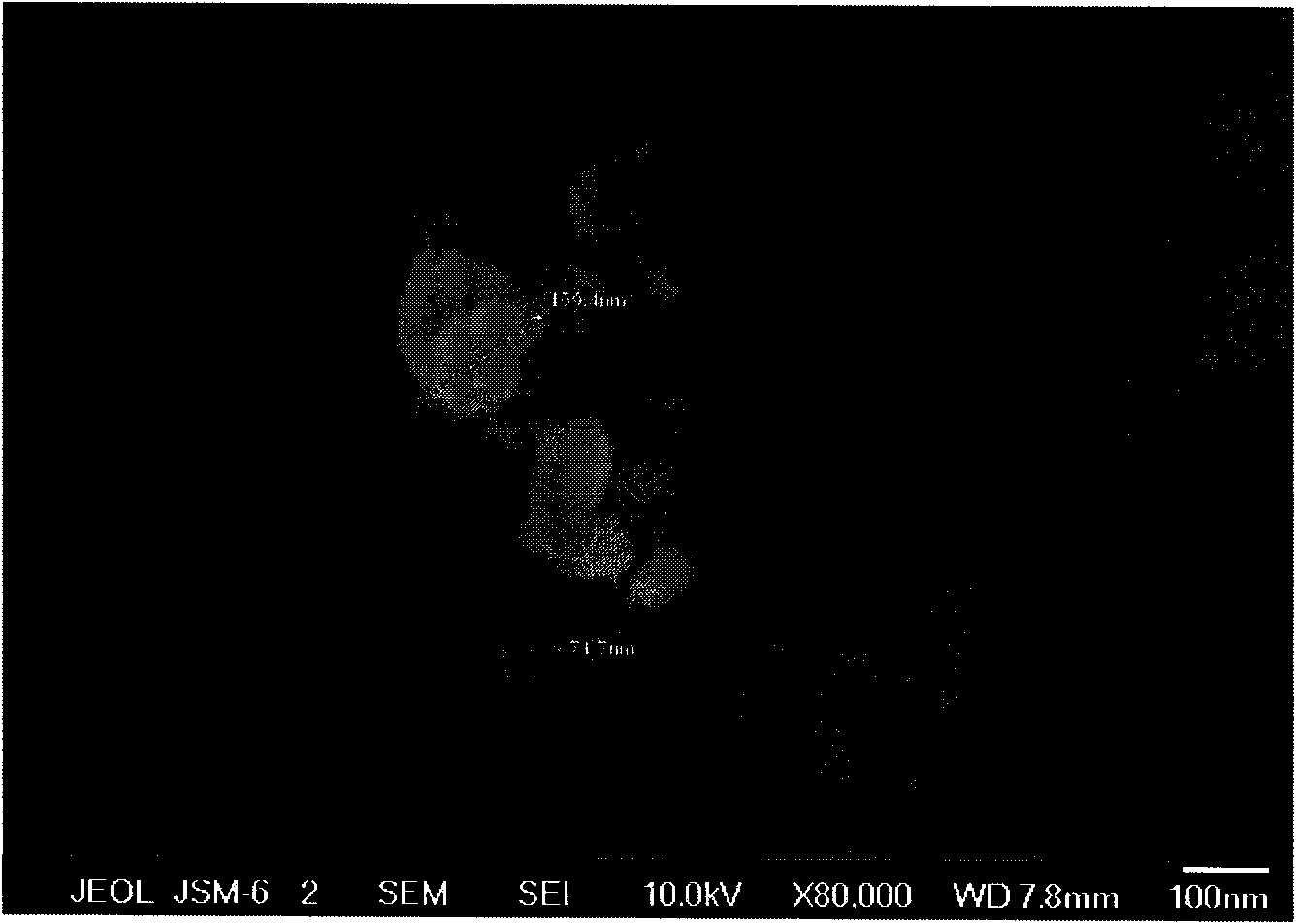
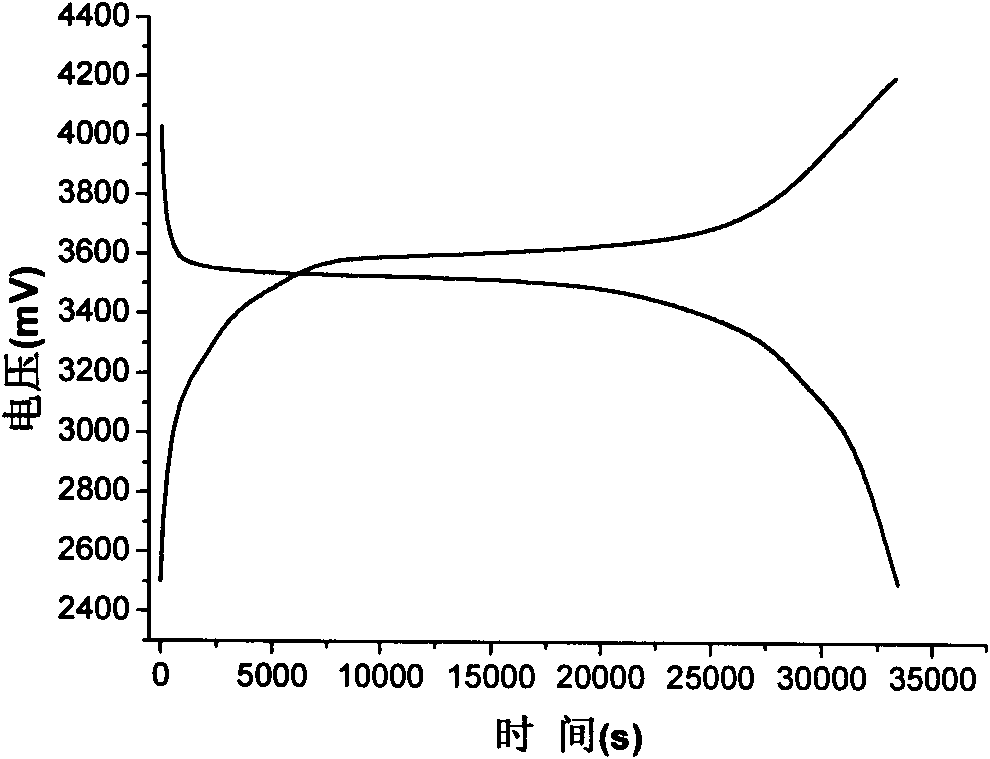
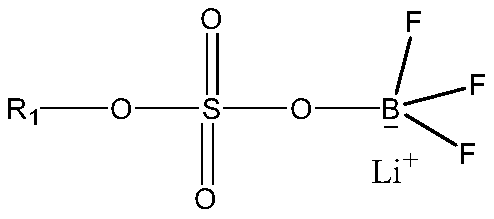
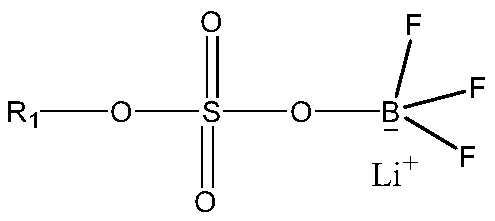
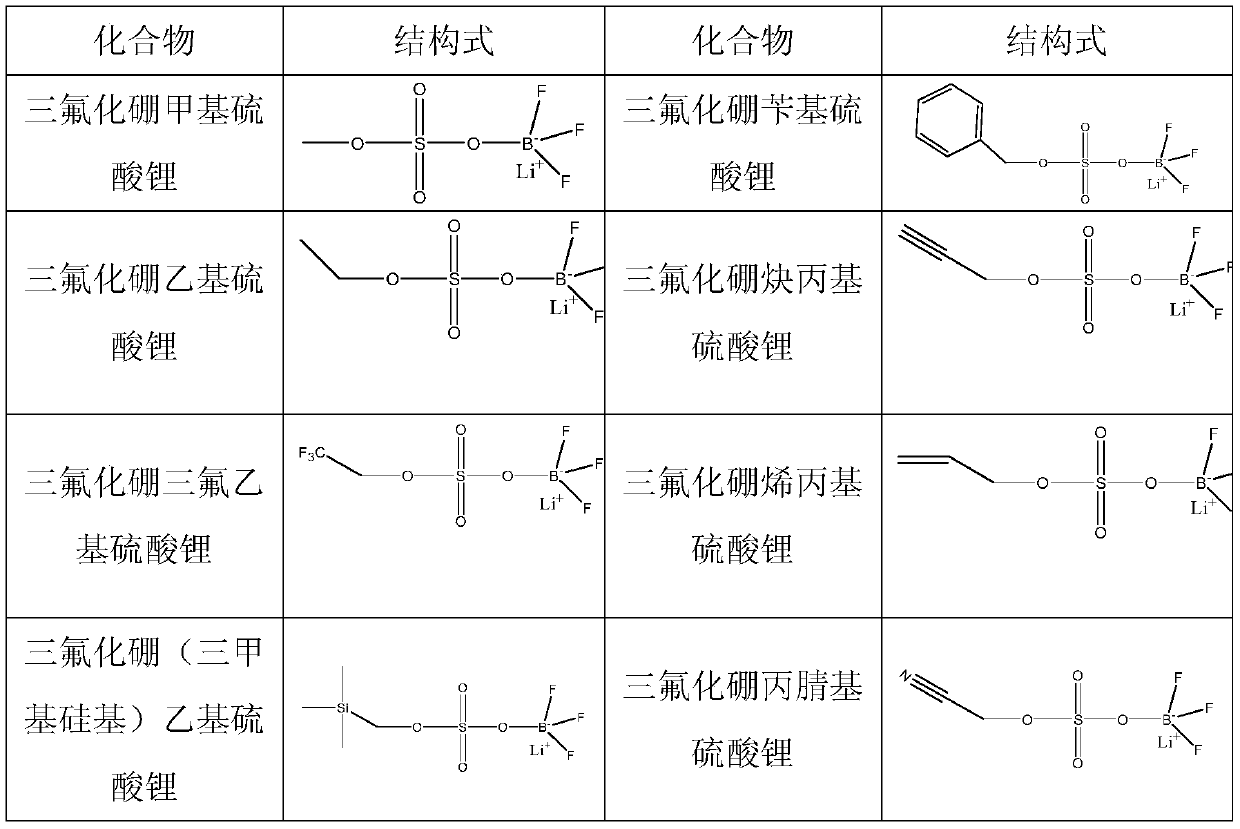
Patents
Literature
373 results about "Lithium sulphate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
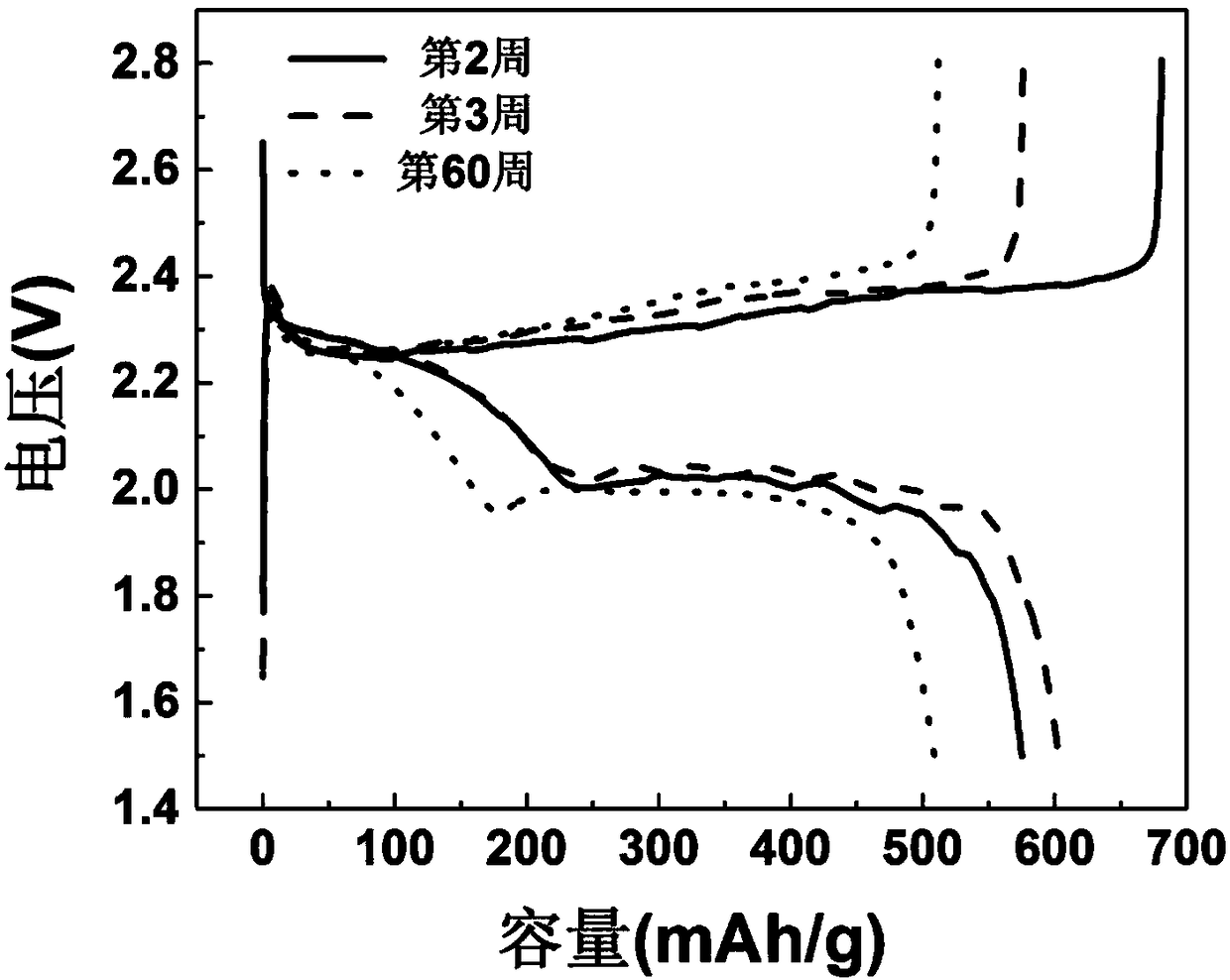
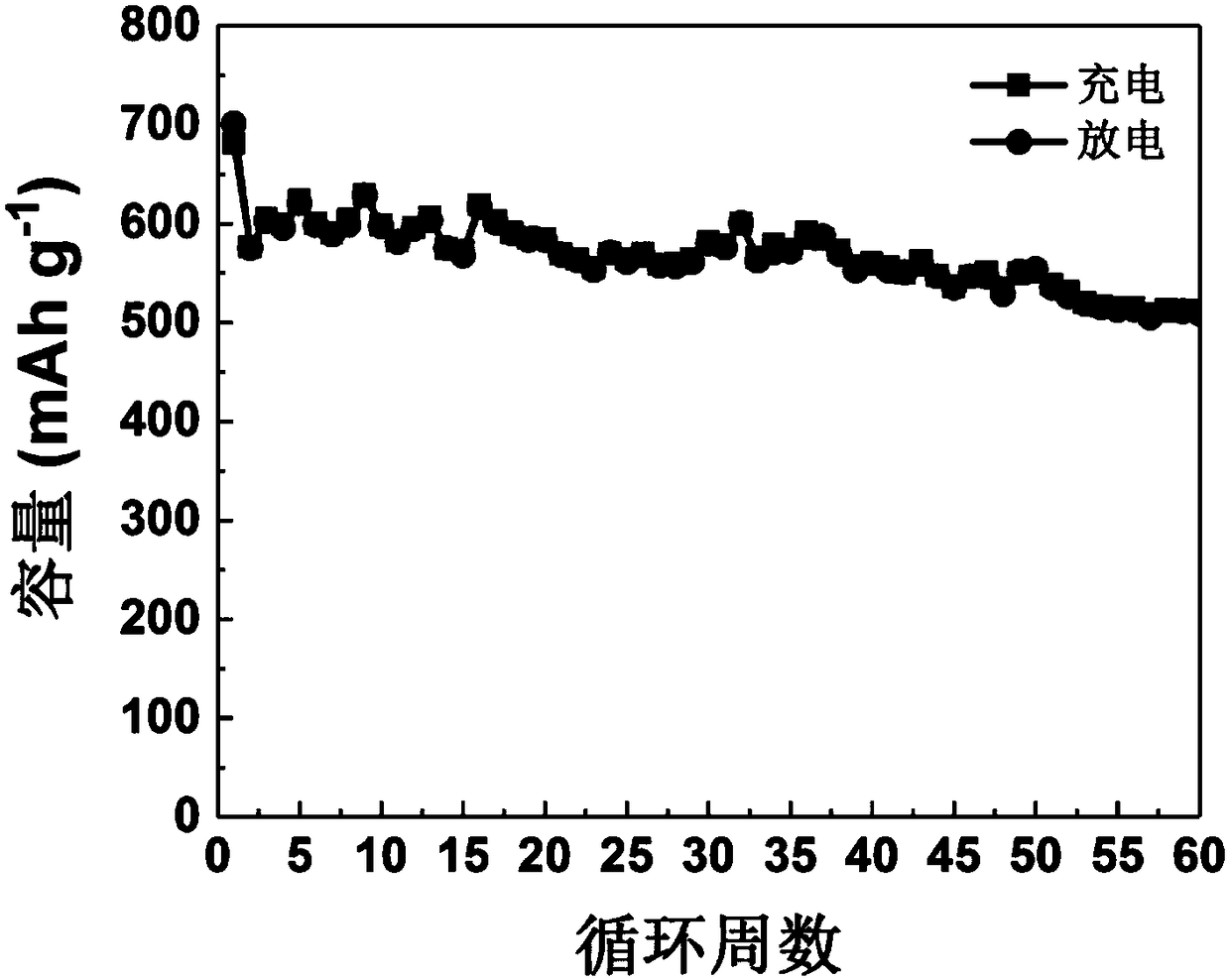
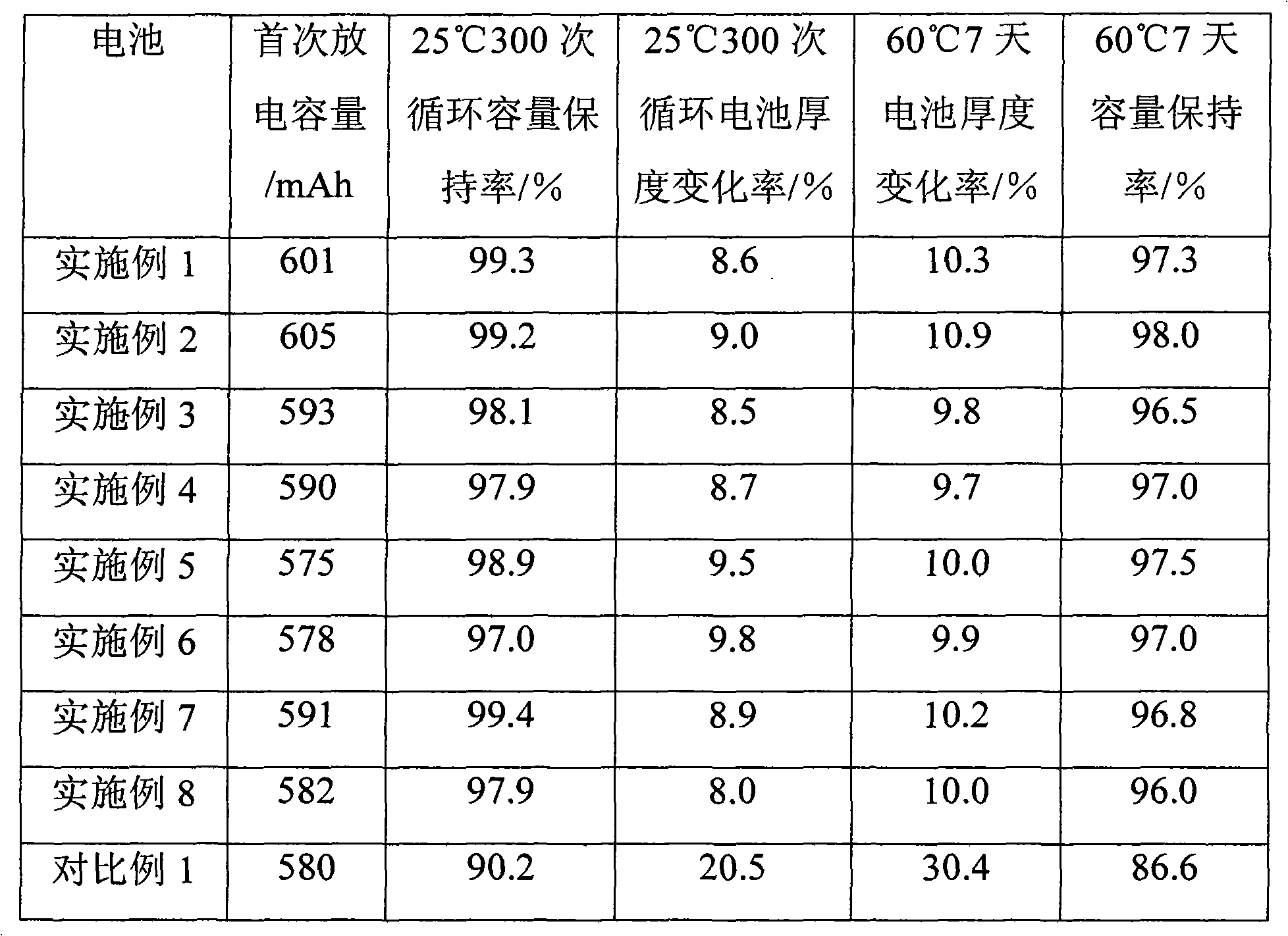
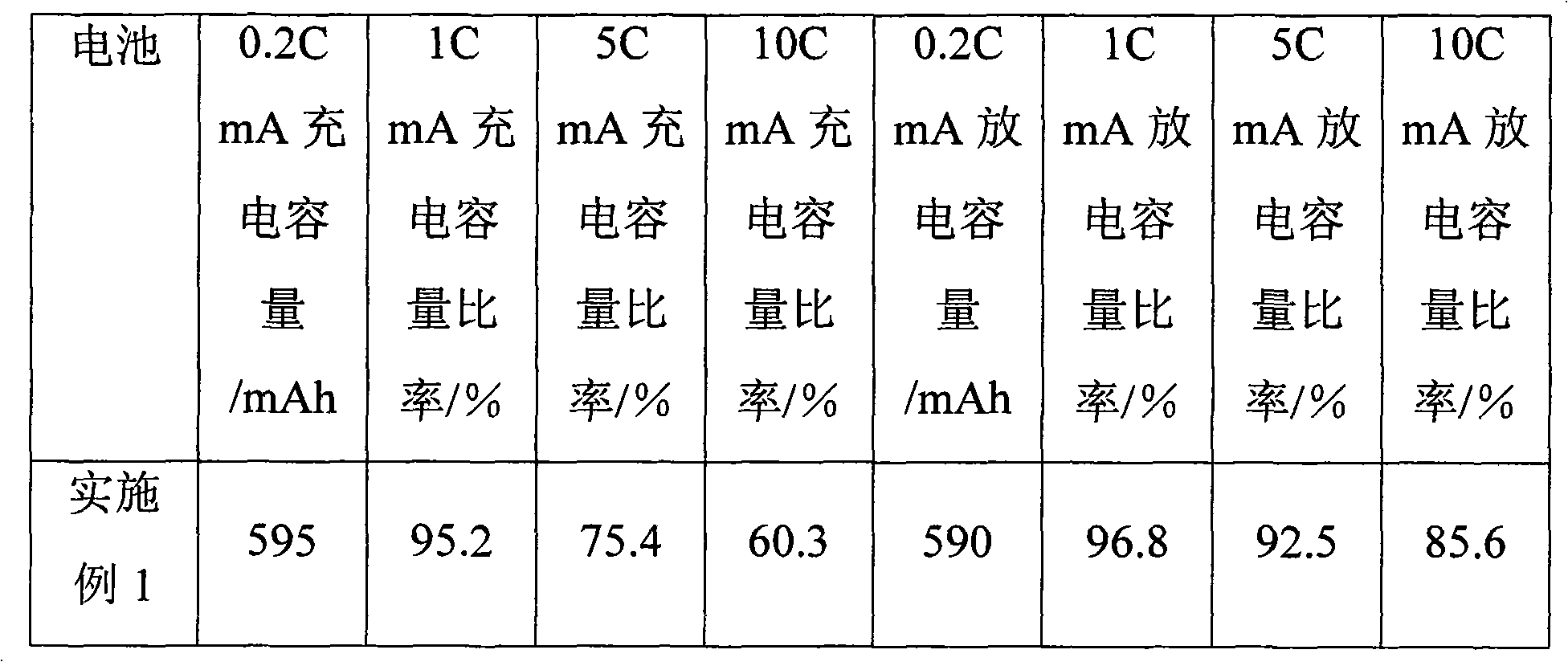
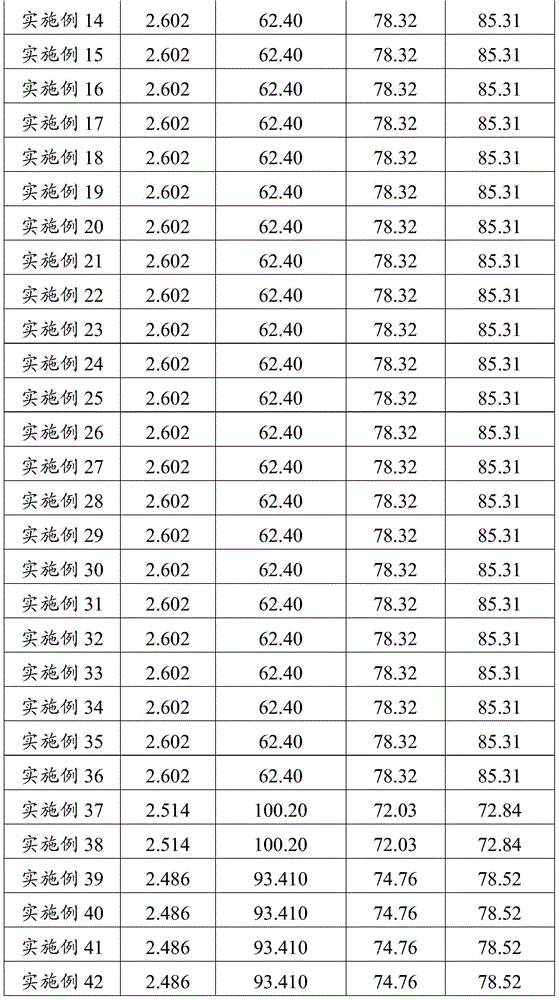
Since it has hygroscopic properties, the most common form of lithium sulfate is lithium sulfate monohydrate. Anhydrous Lithium sulfate has a density of 2.22 g/cm3 but, weighing lithium sulfate anhydrous can become cumbersome as it must be done in a water lacking atmosphere. Lithium Sulfate has pyroelectric properties.
Method for producing refined lithium sulfate solution used in lepidolite lithium-extracting technique by sulfuric acid process
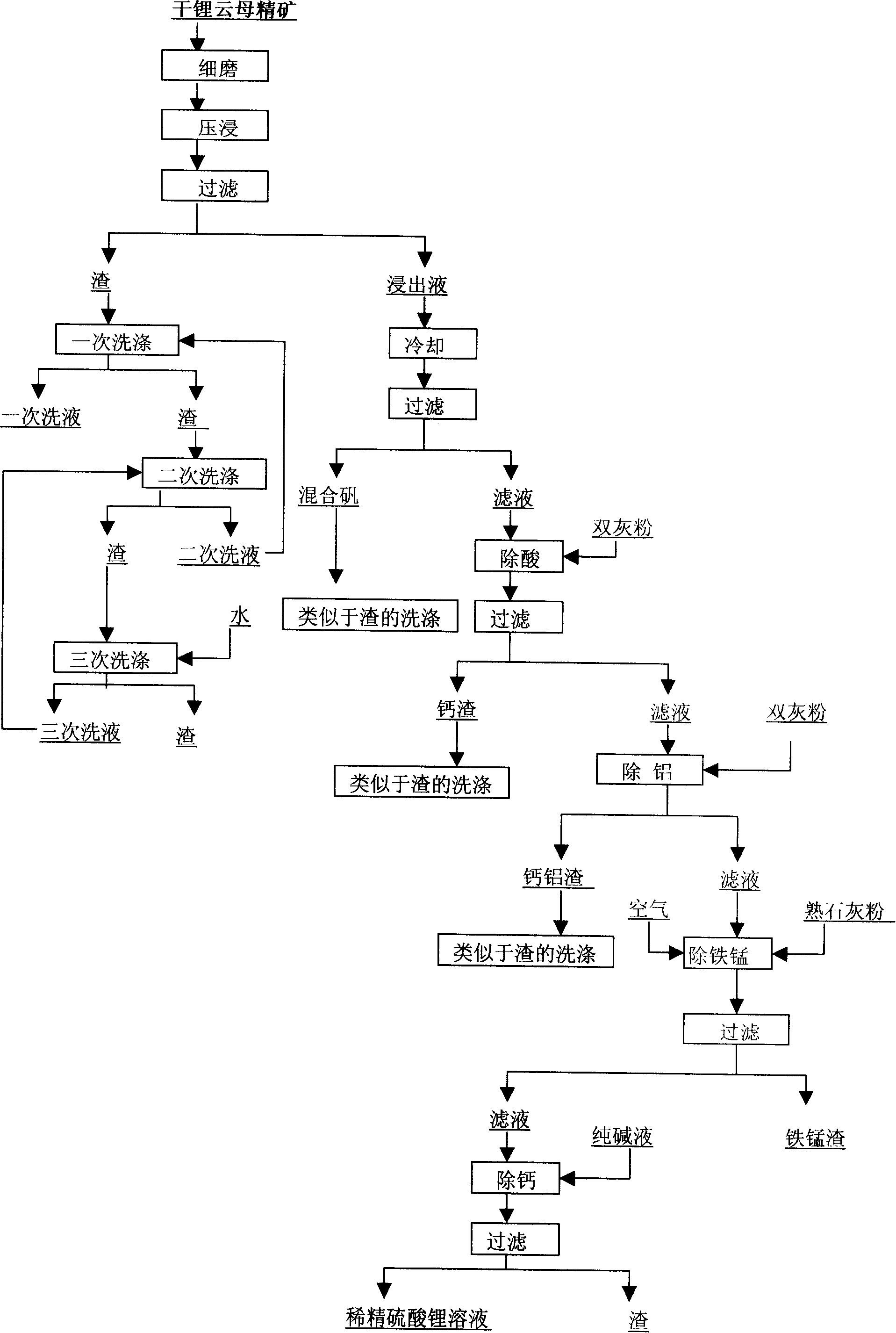
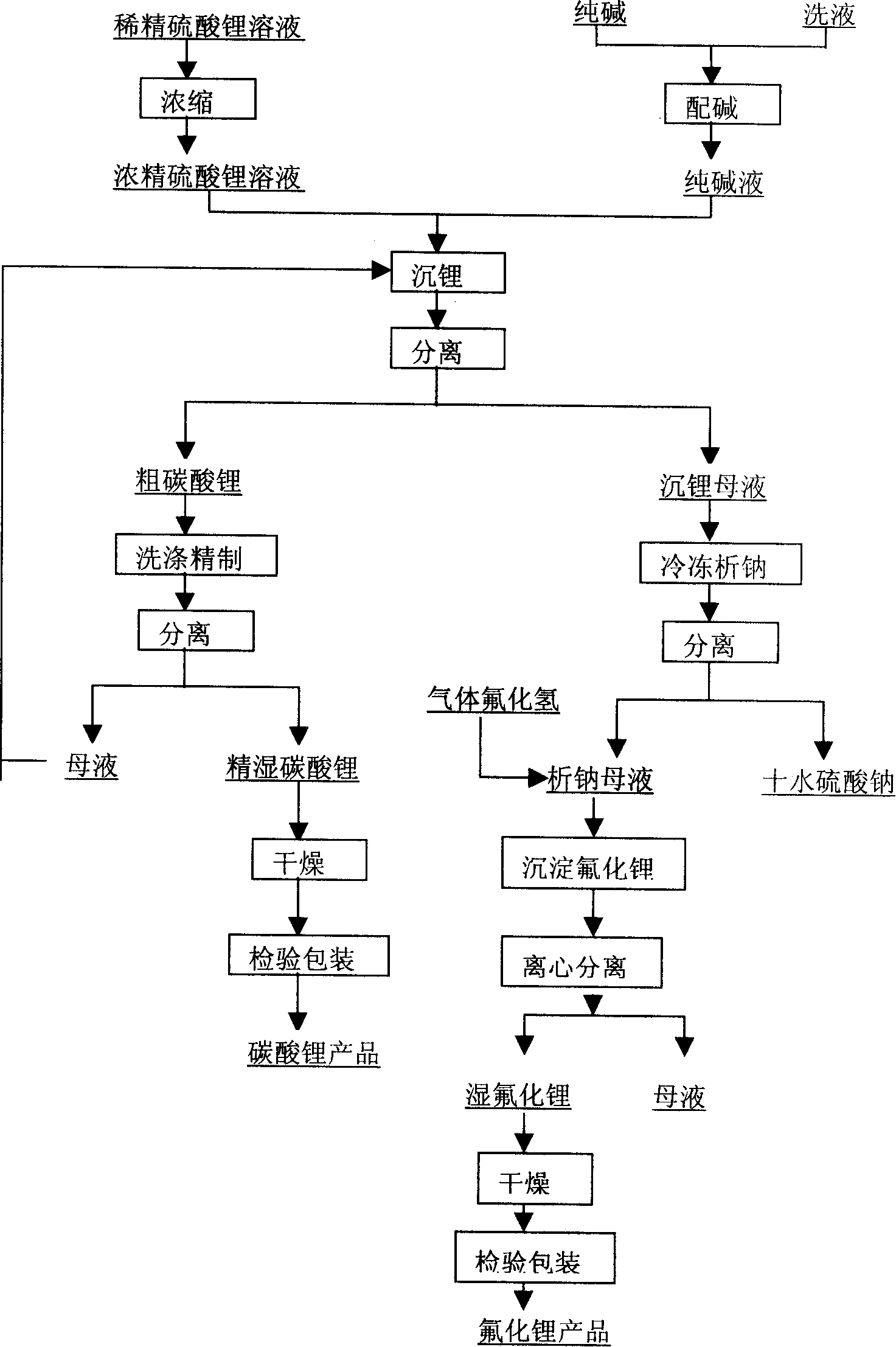
Provided is a process for producing refined lithium sulfate solution of lepidolite lithium extracting technology with sulfuric acid process, which takes lepidolite clean ore as raw material and sequentially includes the following steps, including leaching, alum cooling and decanting, acid removing, aluminum removing, decontaminating and deliming, thereby achieving refined lithium sulfate solution. The alum cooling and decanting process of the invention can precipitate kalium, rubidium and caesium in alum form, thereby the separation of lithium and kalium, rubidium and caesium is easily achieved, and the achieved alum dregs of kalium, rubidium and caesium are blend alum with high purity, which creates perfect condition for comprehensive utilization and simultaneously reduces the burdens of the separation of lithium and aluminum. The aluminum removing process can easily achieve the separation of lithium and aluminum. The process of the invention has the advantages that the energy consumption is relatively low, and the lithium yield is relatively high, most of the residues can be used and the process is favorable for comprehensive utilization. The invention further provides a process for producing lithium carbonate and lithium fluoride with the achieved refined lithium sulfate solution.
Owner:GANFENG LITHIUM CO LTD
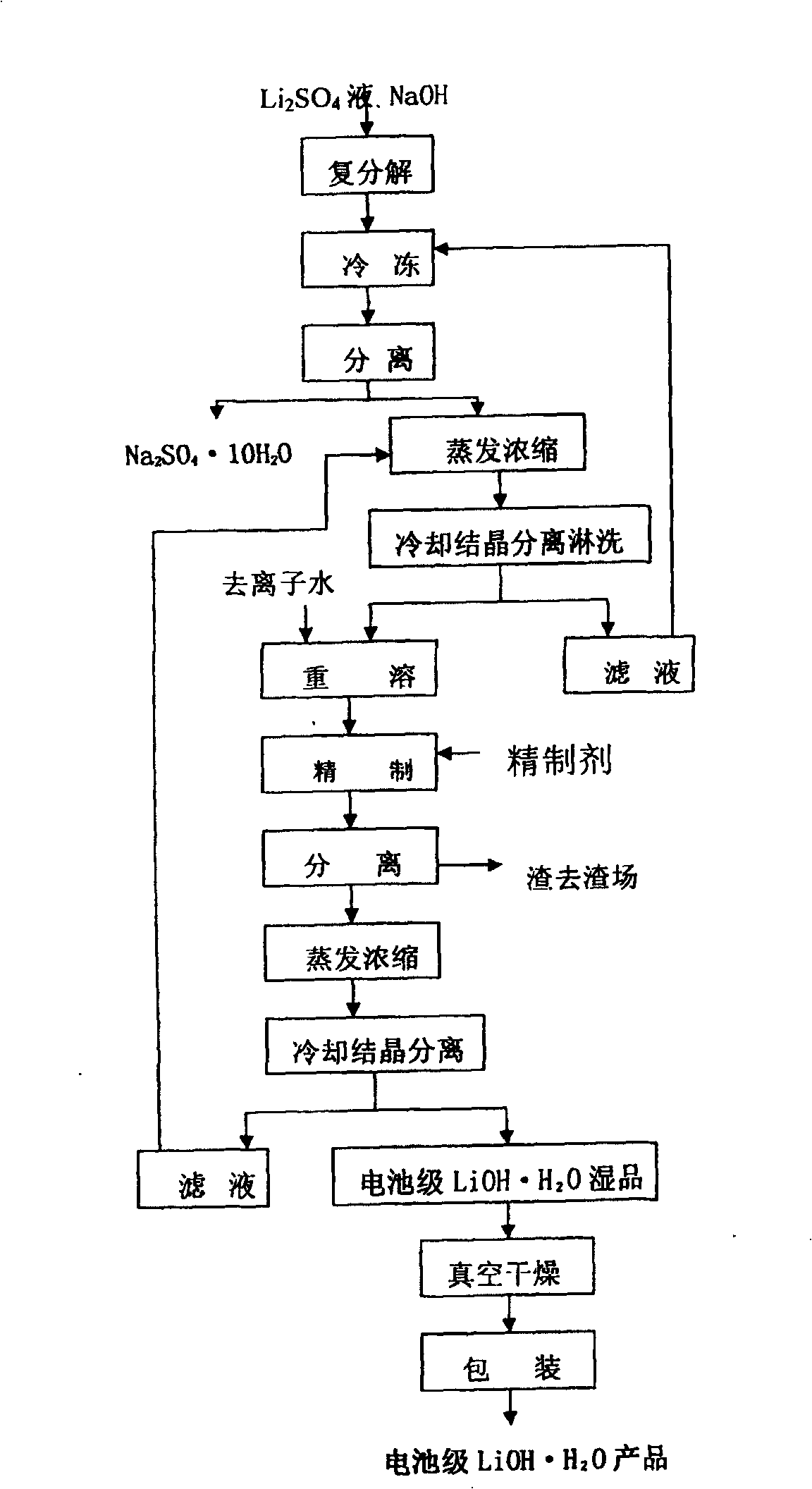
Production process of lithium hydroxide monohydrate
InactiveCN1486931AShort process routeHigh yieldSulfate/bisulfate preparationLithium oxides/hydroxidesSolubilityStrontium hydroxide octahydrate
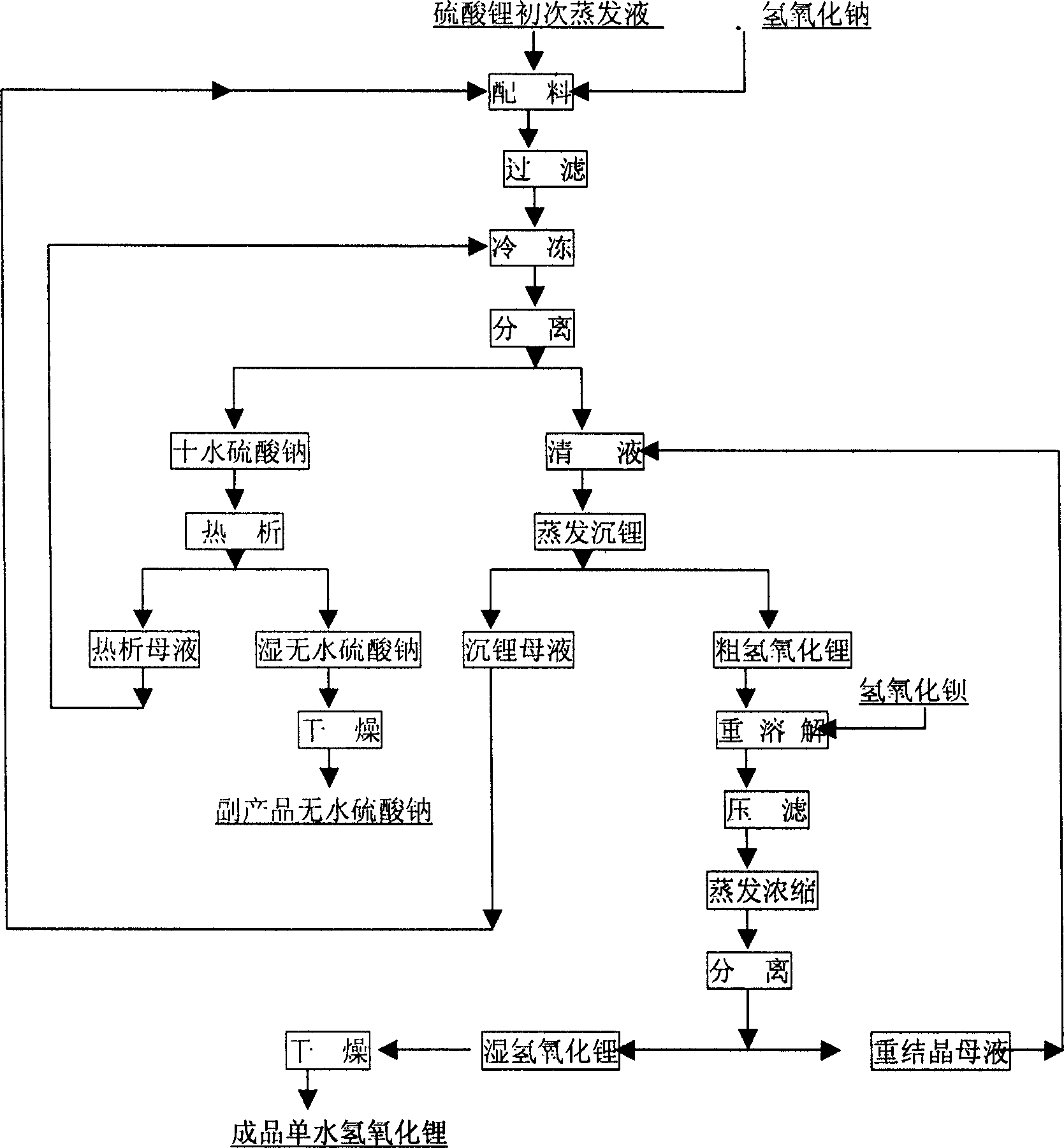
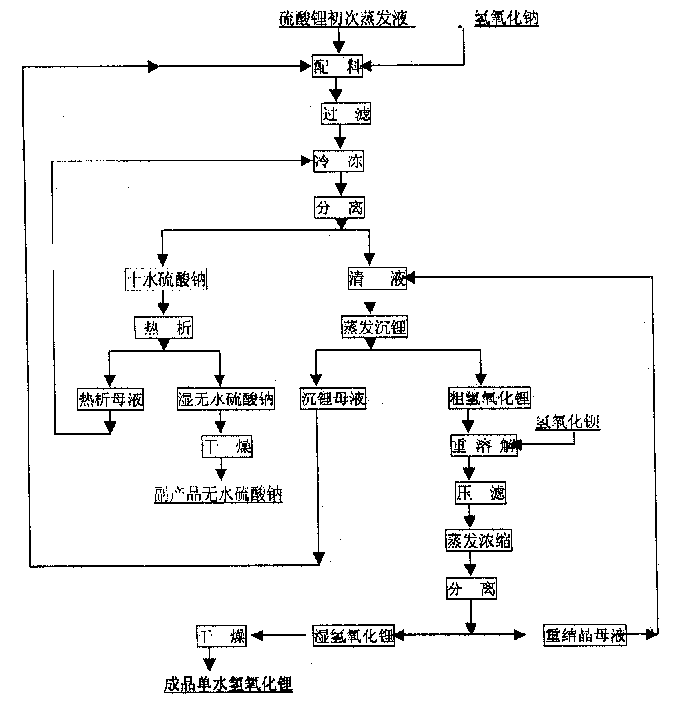
In the production process of lithium hydroxide monohydrate, lithium sulfate solution and caustic soda are made to produce metathetic reaction to form mixture solution of sodium sulfate and lithium hydroxide, and sodium sulfate and lithium hydroxide monohydrate are then separated by means of the obvious difference in low temperature solubility. The production process includes the following steps: adding sodium hydroxide into lithium sulfate solution obtained through serial production steps to obtain mixture solution of sodium sulfate and lithium hydroxide; cooling to minus 10 deg.c to 5 deg.c for the crystallization and separation of sodium sulfate; heating to concentrate the separated clear liquid; crystallization and separation to obtain coarse lithium hydroxide monohydrate product; water dissolving coarse lithium hydroxide monohydrate, adding barium hydroxide to form insoluble barium sulfate, filtering, concentrating filtrate, crystallizing to separate wet lithium hydroxide monohydrate; and drying.
Owner:JIANGSU RONGHUI GENERAL LITHIUM IND CO LTD
Method for producing high-purity lithium carbonate by using lithium concentrate
ActiveCN103318925AReduce consumptionGuaranteed RecoveryLithium compoundsLithium sulphatePhysical chemistry
The invention relates to a method for producing high-purity lithium carbonate by using lithium concentrate. The method is characterized by comprising the following steps of: preparing an acid clinker, preparing a mixing size, preparing a lithium sulfate leaching solution, preparing a lithium sulfate purification liquid, preparing a lithium sulfate finishing solution, preparing a sodium carbonate solution, performing primary lithium deposition reaction, preparing a sodium separating mother liquor, preparing excellent lithium carbonate in a sweating manner, and preparing 99.99% of high-purity lithium carbonate. By adopting the method, the principle of removing calcium and magnesium ions is ingeniously utilized in preparation of common lithium carbonate; a fussy procedure of removing calcium and magnesium by ion exchange resin is avoided when the common lithium carbonate is purified to prepare the high-purity lithium carbonate; a method of recycling after separating out sodium sulfate in a freezing manner is adopted to process the primary lithium settling mother liquor; the high-purity lithium carbonate mother liquor in precipitation of the high-purity lithium carbonate is used as an optimal lithium carbonate washing liquor for primary lithium sedimentation after being recycled for a plurality of times. Thus, the method has the characteristics of simple process, high production efficiency, high recovery rate and low production cost.
Owner:JIANGSU RONGHUI GENERAL LITHIUM IND CO LTD
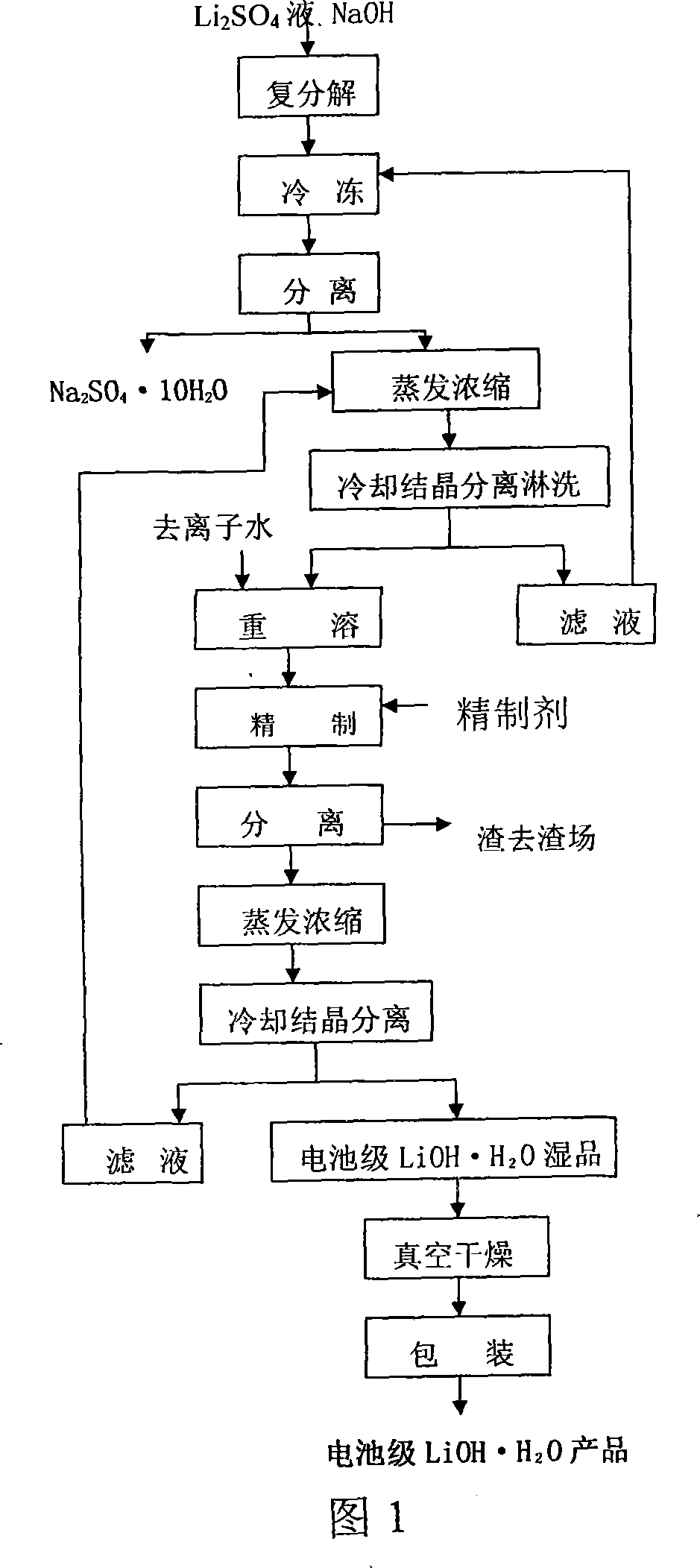
Method for preparing battery-stage monohydrate lithium hydroxide
ActiveCN101214978AHigh recovery rateSuit one's needsCell electrodesLithium oxides/hydroxidesLithium sulphateSodium hydroxide
The invention provides a process for preparing battery grade lithium hydroxide monohydrate, comprising: (1) adding sodium hydroxide in lithium sulfate purification fluid and obtaining solid of Na2SO4, 10H2O and liquid of LiOH after completely dissolving and cooling, (2) obtaining liquid of LiOH after filtering and separating, (3) evaporating and concentrating the liquid of LiOH and filtering, separating and leaching the liquid of LiOH after cooling and crystallizing to obtain one-time crude product of LiOH, H2O, (4) adding deionized water in the one-time crude product of LiOH, H2O and obtaining re-dissolving solution of the one-time crude product of LiOH, H2O, (5) adding refining agent in the re-dissolving solution of the one-time crude product of LiOH, H2O and obtaining filtrate of LiOH refined liquor after filtering and separating, (6) filtering and separating the LiOH refined liquor after evaporating, concentrating, cooling and crystallizing to obtain solid of battery grade wet product of LiOH, H2O and (7) taking out the battery grade wet product of LiOH, H2O after drying to obtain battery product of LiOH, H2O. The invention is simple in production process, easy operation and perfect product quality.
Owner:TIANQI LITHIUM CORP
Novel method for producing lithium carbonate and lithium hydroxide
InactiveCN102115101ASimple production processLow costLithium oxides/hydroxidesLithium carbonates/bicarbonatesLithium sulphateCalcium hydroxide
The invention relates to a novel method for producing lithium carbonate and lithium hydroxide, which belongs to the technical field of production of lithium salts. The method comprises the following steps of: baking spodumene concentrate; preparing a lithium sulfate solution; preparing lithium carbonate mother liquor; and preparing lithium hydroxide, wherein the lithium hydroxide can also be obtained by adding barium hydroxide into the lithium carbonate mother liquor. The method has the advantages that: lime is added into a lithium carbonate sinker mother liquor to produce the lithium hydroxide by causticizing and transforming, and the production of lithium carbonate is combined with the production of the lithium hydroxide, so that a process flow is simplified, investment and production costs are lowered, a production process is more flexible to regulate and control, and the quality of a lithium carbonate product is more stable. By adopting the method, the mother liquor of lithium carbonate is easier to treat, impurities in the lithium hydroxide mother liquor are easy to treat, and the product quality is not influenced.
Owner:屈俊鸿
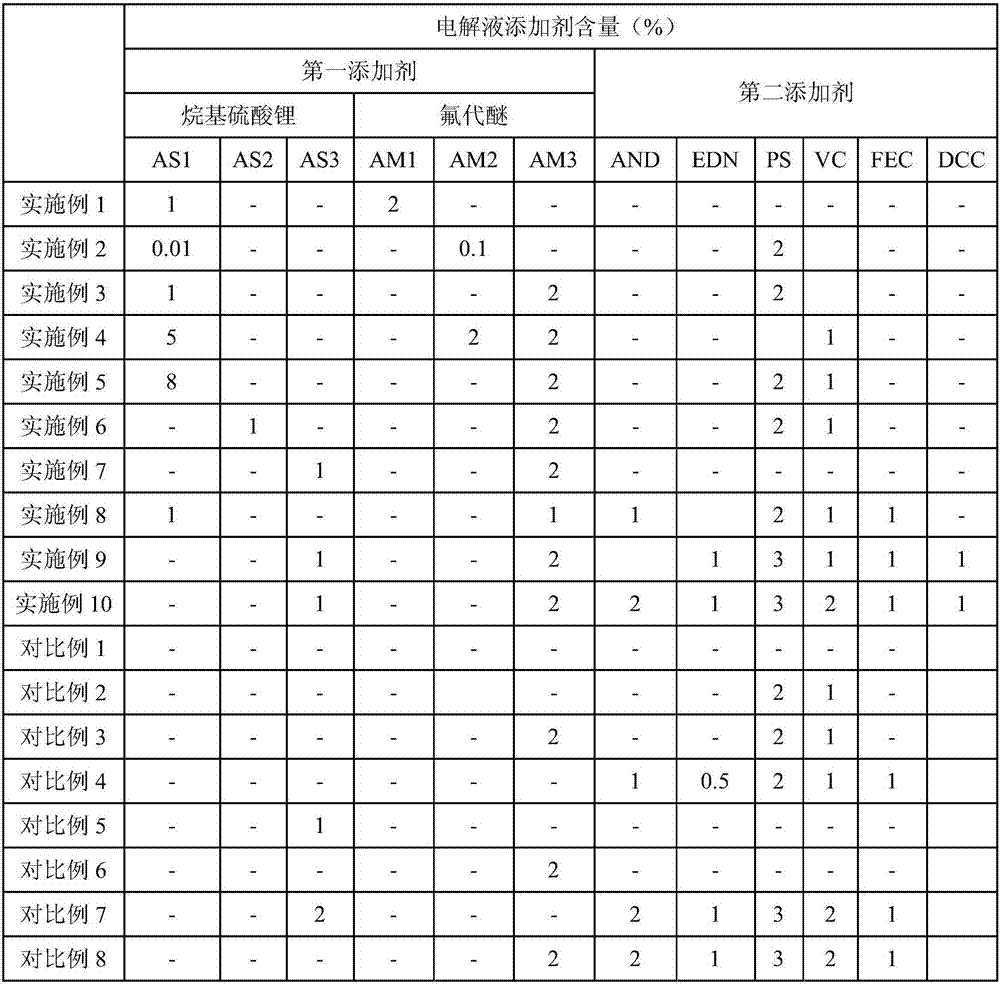
Electrolyte and lithium ion battery
ActiveCN107394269AEasy to storeImprove cycle performanceSecondary cellsLithium sulphateOrganic solvent
The invention provides an electrolyte and a lithium ion battery. The electrolyte comprises a lithium salt, an organic solvent and an additive, wherein the additive comprises a first additive, and the first additive comprises alkyl lithium sulfate and fluoro-ether. By the electrolyte, the storage performance, the cycle property, the rate performance and the low-temperature discharge performance of the lithium ion battery under high temperature and high voltage can be improved, meanwhile, the safety performance of the lithium ion battery is improved, and the service lifetime of the lithium ion battery is prolonged.
Owner:NINGDE AMPEREX TECH
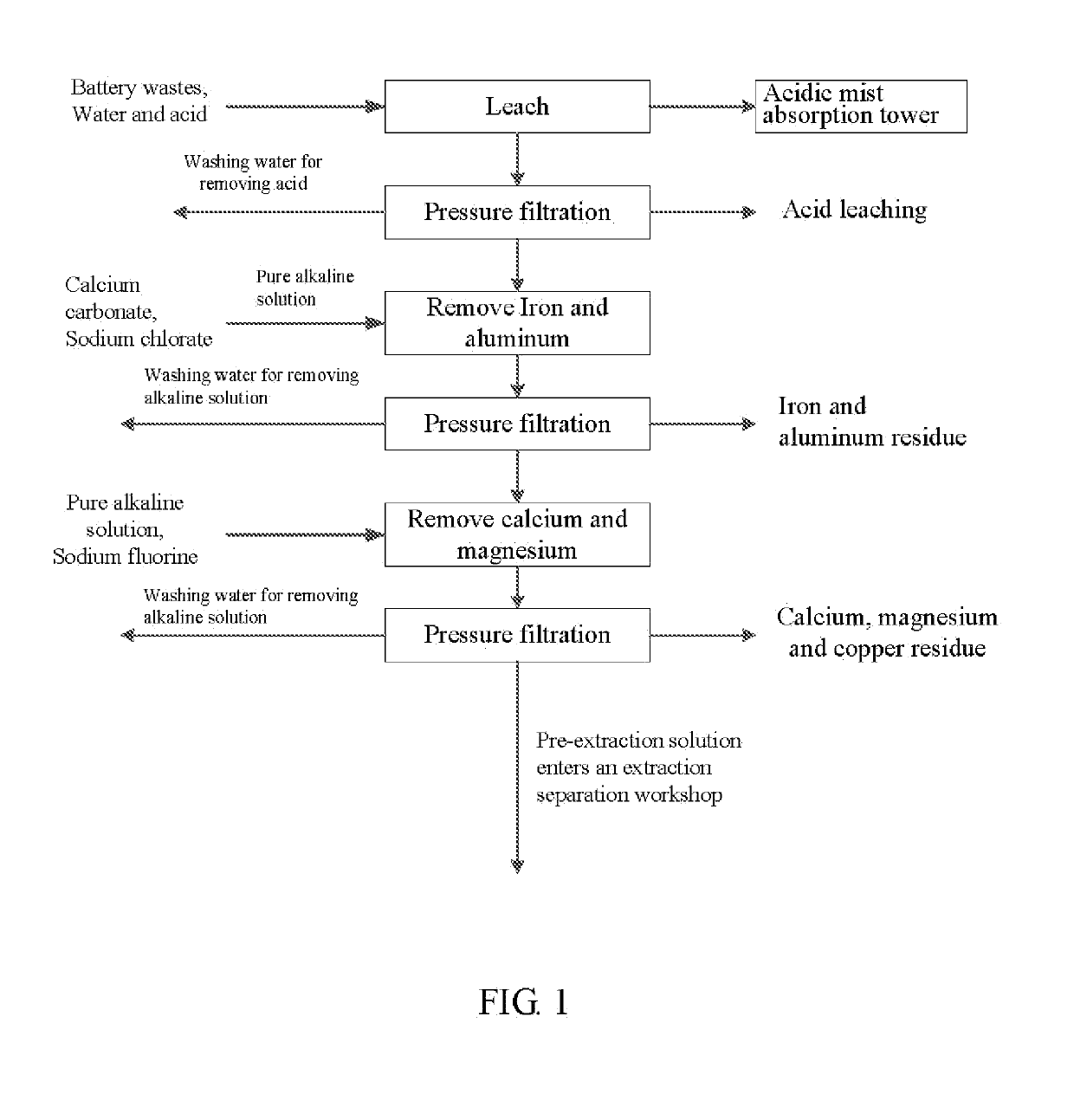
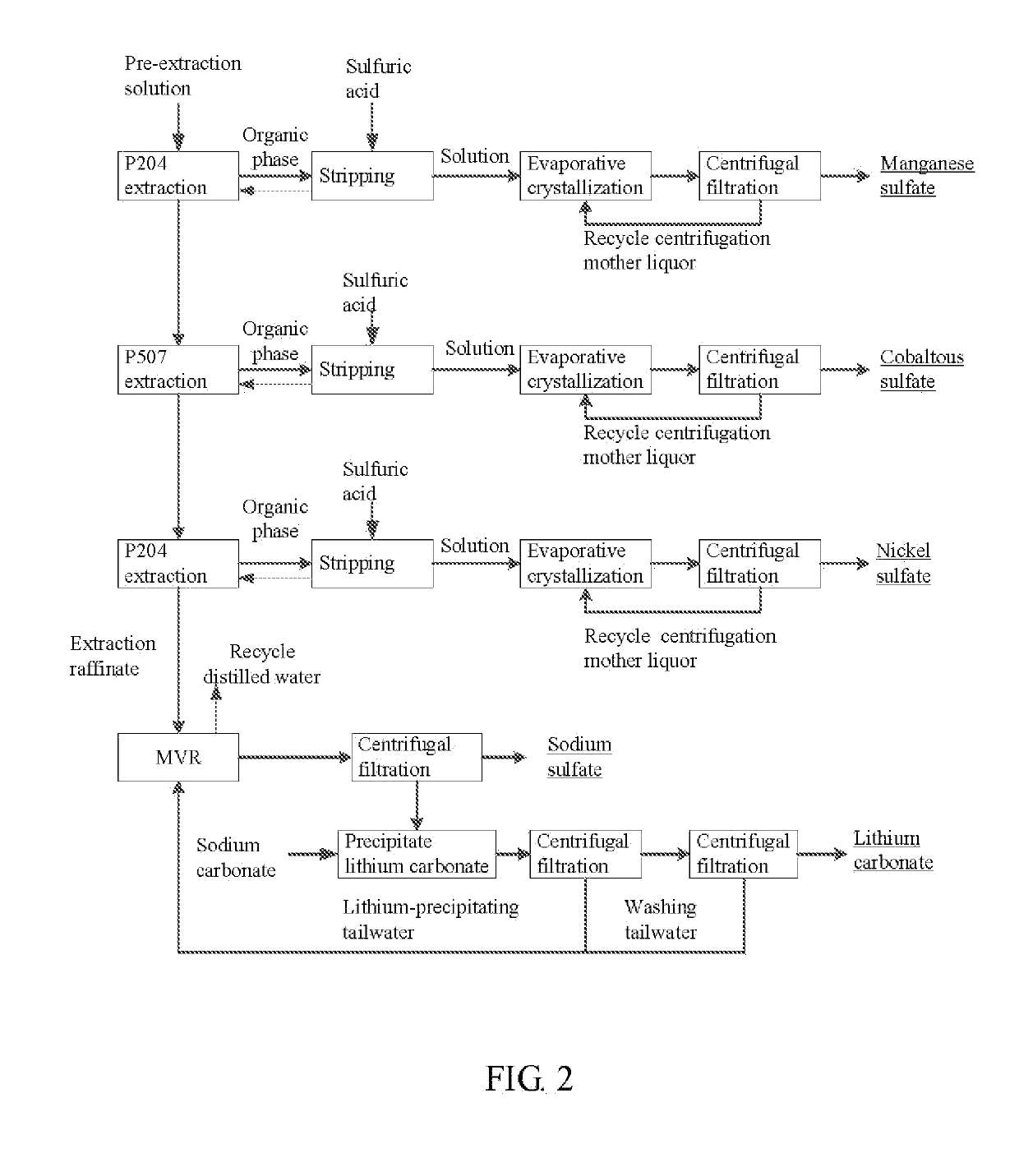
Method for preparing nickel/manganese/lithium/cobalt sulfate and tricobalt tetraoxide from battery wastes
ActiveUS20190152797A1Reduce productionHigh puritySolvent extractionCobalt sulfatesManganeseCobalt Sulfate
A method for preparing nickel / manganese / lithium / cobalt sulfate and tricobalt tetraoxide from battery wastes adopts the following process: dissolving battery wastes with acid, removing iron and aluminum, removing calcium, magnesium and copper, carrying extraction separation, and carrying out evaporative crystallization to prepare nickel sulfate, manganese sulfate, lithium sulfate, cobalt sulfate or / and tricobalt tetraoxide. By using the method, multiple metal elements, such as nickel, manganese, lithium and cobalt, can be simultaneously recovered from the battery wastes, the recovered products are high in purity and can reach battery grade, battery-grade tricobalt tetraoxide can also be directly produced. The method is simple in process, low in, energy consumption and free in exhaust gas pollution, and can realize zero release of wastewater.
Owner:HUNAN JINYUAN NEW MATERIALS CO LTD
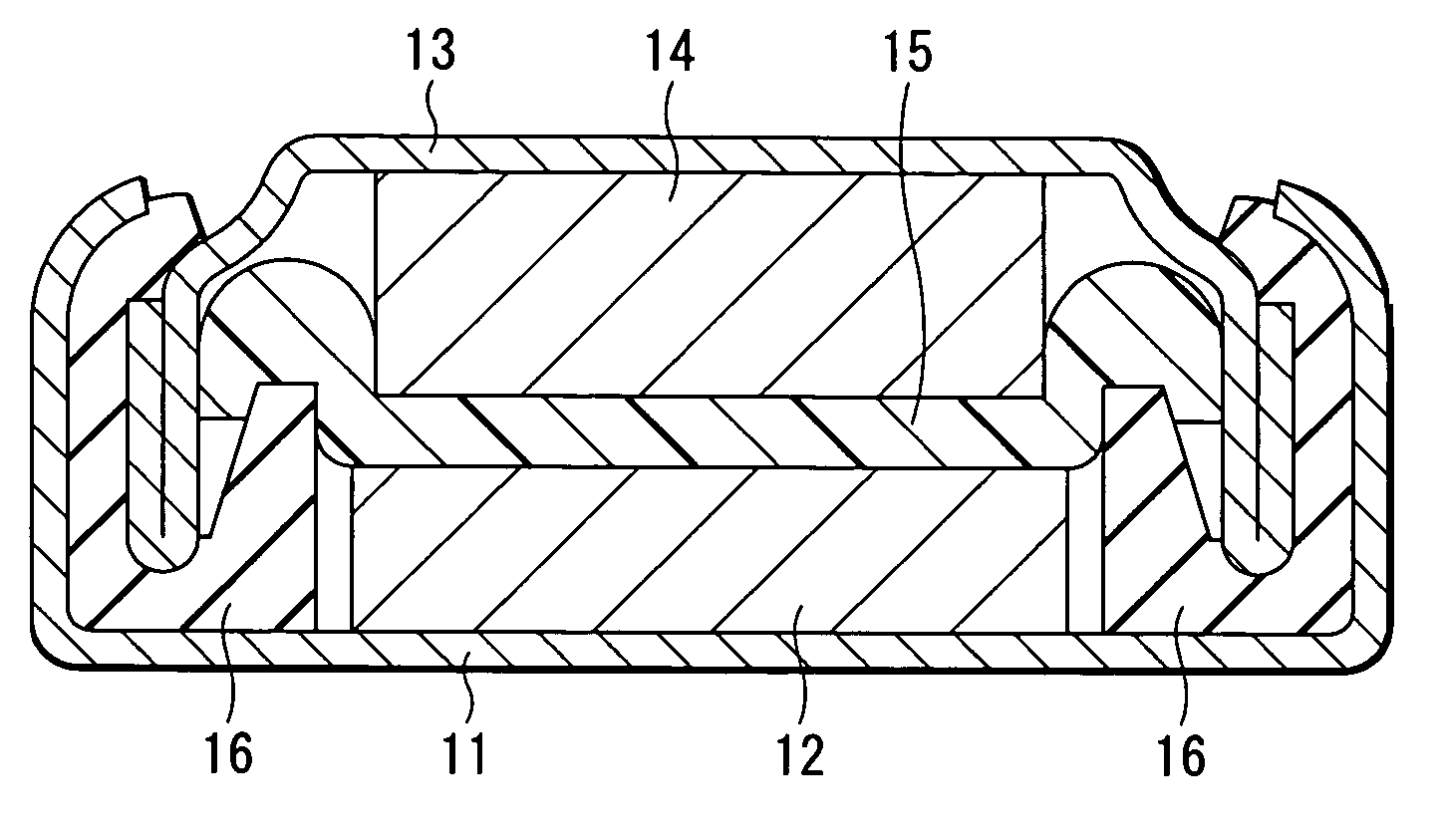
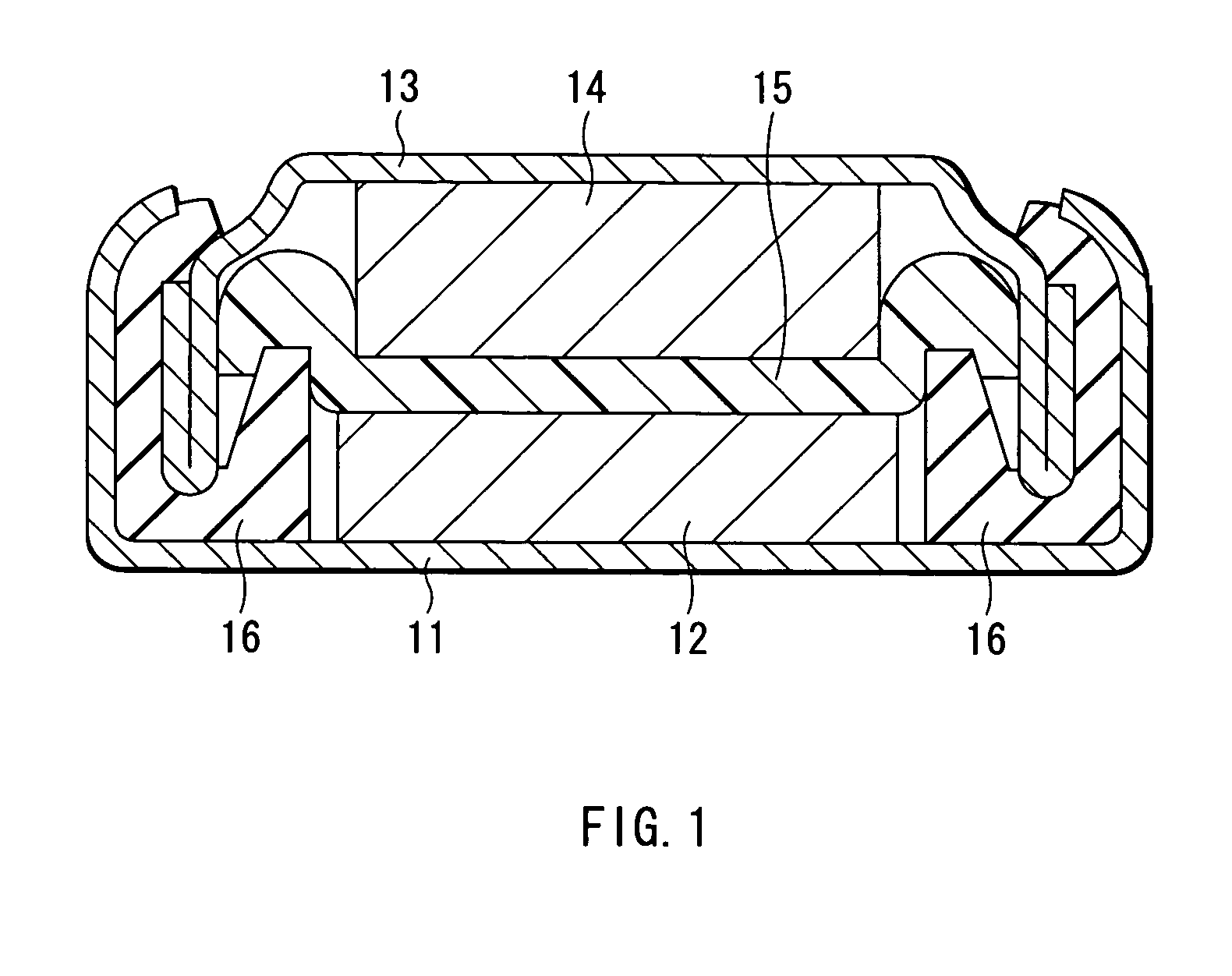
Battery
A battery with a high battery voltage at charging and improved energy density is provided. A cathode (12) and an anode (14) are laminated with a separator (15) sandwiched therebetween which is impregnated with an electrolyte. The cathode (12) has a cathode active material including a lithium composite oxide which contains lithium, at least either cobalt or nickel, and oxygen. The battery voltage at charging is 4.25 V or more. The total amount of lithium carbonate and lithium sulphate in the cathode (12) to the cathode active material is 1.0 wt % or less, a concentration of protic impurities in the electrolyte, which is converted to a mass ratio of protons to the electrolyte, is 20 ppm or less, or moisture content in the electrolyte is 20 ppm mass ratio or less to the electrolyte. This inhibits metal eluting from the lithium composite oxide even at high voltages.
Owner:SONY CORP
Method for preparing battery grade lithium hydroxide monohydrate
ActiveCN104944447AHigh yieldGuaranteed qualityLithium oxides/hydroxidesLithium sulphateCost effectiveness
The invention discloses a method for preparing battery grade lithium hydroxide monohydrate, which comprises steps: edulcoration and purification of lithium sulfate solution, freezing separation, preparation of lithium hydroxide monohydrate crude products, dissolution and purification of the lithium hydroxide monohydrate crude products and recrystallization. The method for preparing the battery grade lithium hydroxide monohydrate can obtain lithium hydroxide monohydrate finished products just through twice crystallization, and improves leaching efficiency of lithium. Sodium sulfafe decahydrate which is frozen and separated is washed through low temperature water, the lithium in the sodium sulfafe decahydrate is effectively recycled, the technological process is reduced, the yield of the lithium is further greatly improved, the investment of evaporator equipment is reduced, and the cost effectiveness is obvious.
Owner:JIANGSU RONGHUI GENERAL LITHIUM IND CO LTD
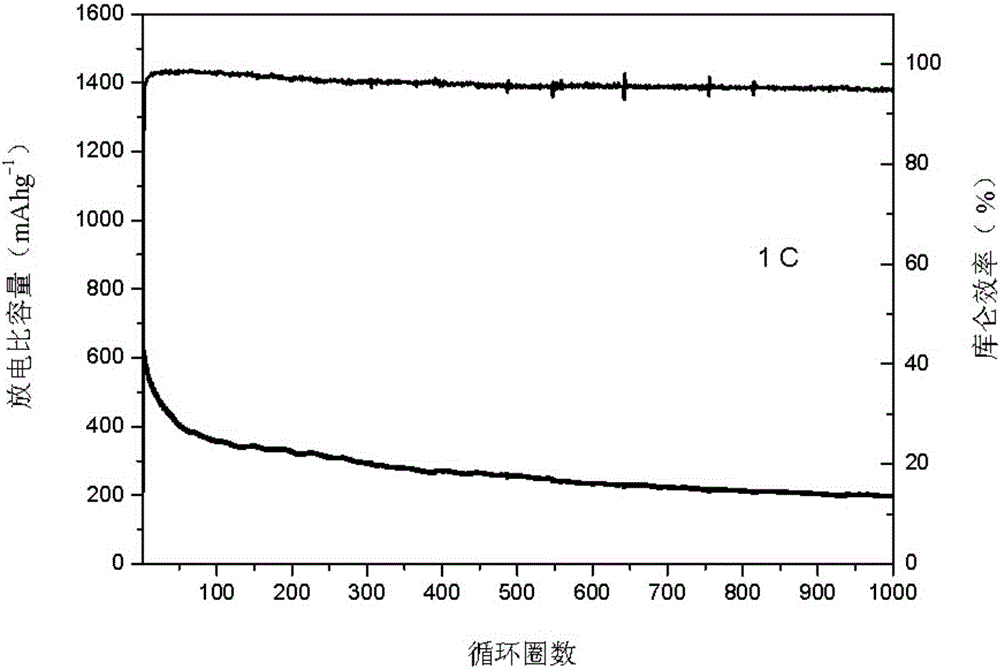
MXene/lithium sulfide/carbon composite cathode material and preparation method thereof
ActiveCN108258222AImprove conductivityHigh specific capacityCell electrodesSecondary cellsCarbon compositesLithium sulphate
The invention relates to an MXene / lithium sulfide / carbon composite cathode material and a preparation method of the MXene / lithium sulfide / carbon composite cathode material. The method comprises the following steps: (1) etching an MAX phase powder with an acid, filtering, washing and drying to obtain an MXene nanometer material with a porous structure; (2) preparing a solution from lithium sulfate,proportionally mixing with a carbon material or a carbon precursor, preparing a carbon / lithium sulfate or carbon precursor / lithium sulfate composite material; (3) heating the carbon / lithium sulfate or carbon precursor / lithium sulfate composite material in the step (2) under the protective atmosphere, reducing the above composite material into a lithium sulfide / carbon material by means of thermalreduction; (4) mixing the MXene nanometer material with the porous structure obtained in the step (1) with the lithium sulfide / carbon in the step (3), and ball-milling to obtain the MXene / lithium sulfide / carbon composite cathode material. Compared with the prior art, the prepared MXene / lithium sulfide / carbon composite cathode material has the advantages of high electrical conductivity, high specific capacity, good cycle performance, good rate capability, simple preparation process and the like.
Owner:SHANDONG UNIV
Titanium composite, preparation method thereof and application thereof
ActiveCN101901905AImprove performanceEasy to preparePigmenting treatmentAlkali titanatesLithium chlorideHigh rate
Owner:BYD CO LTD
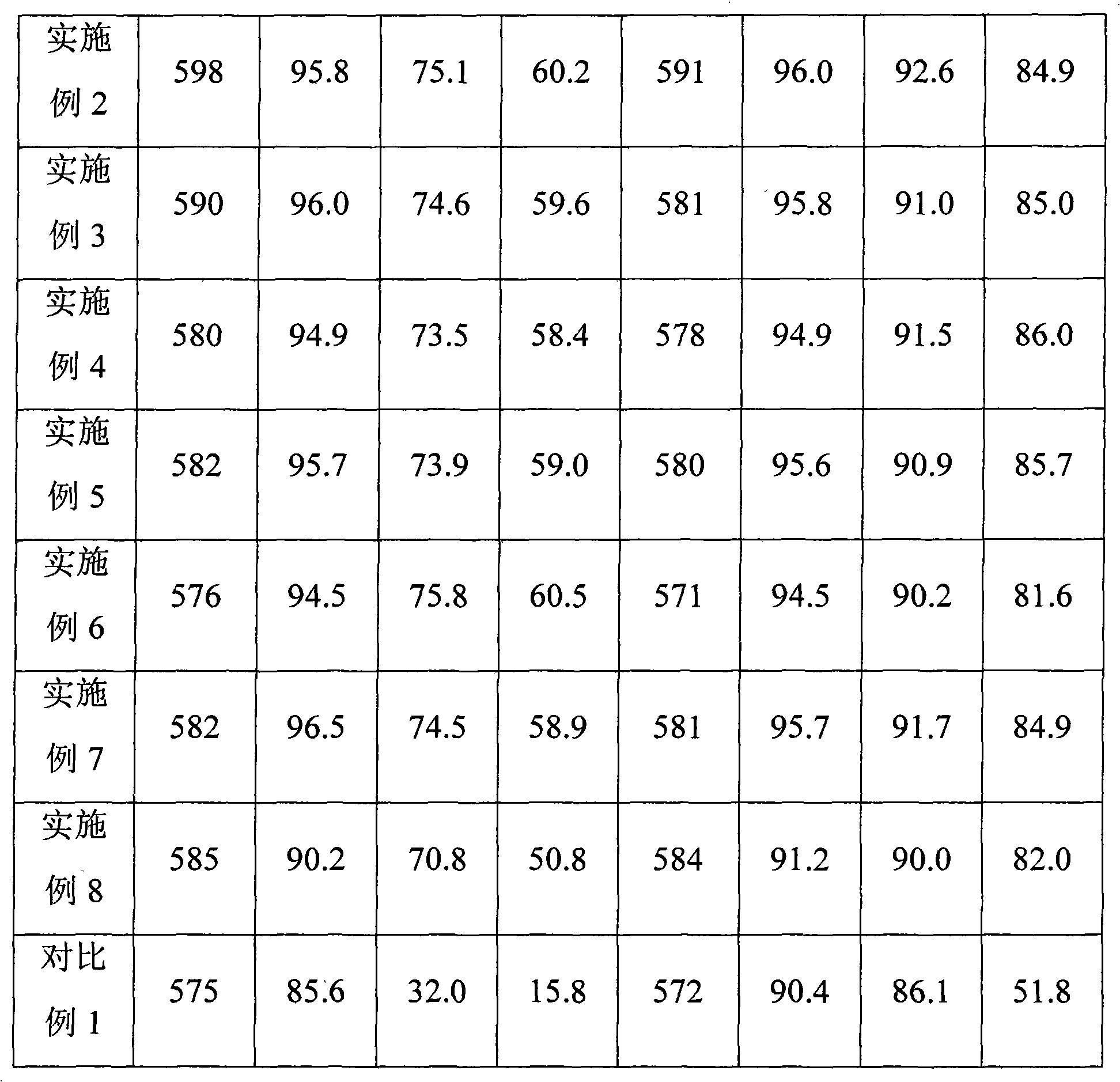
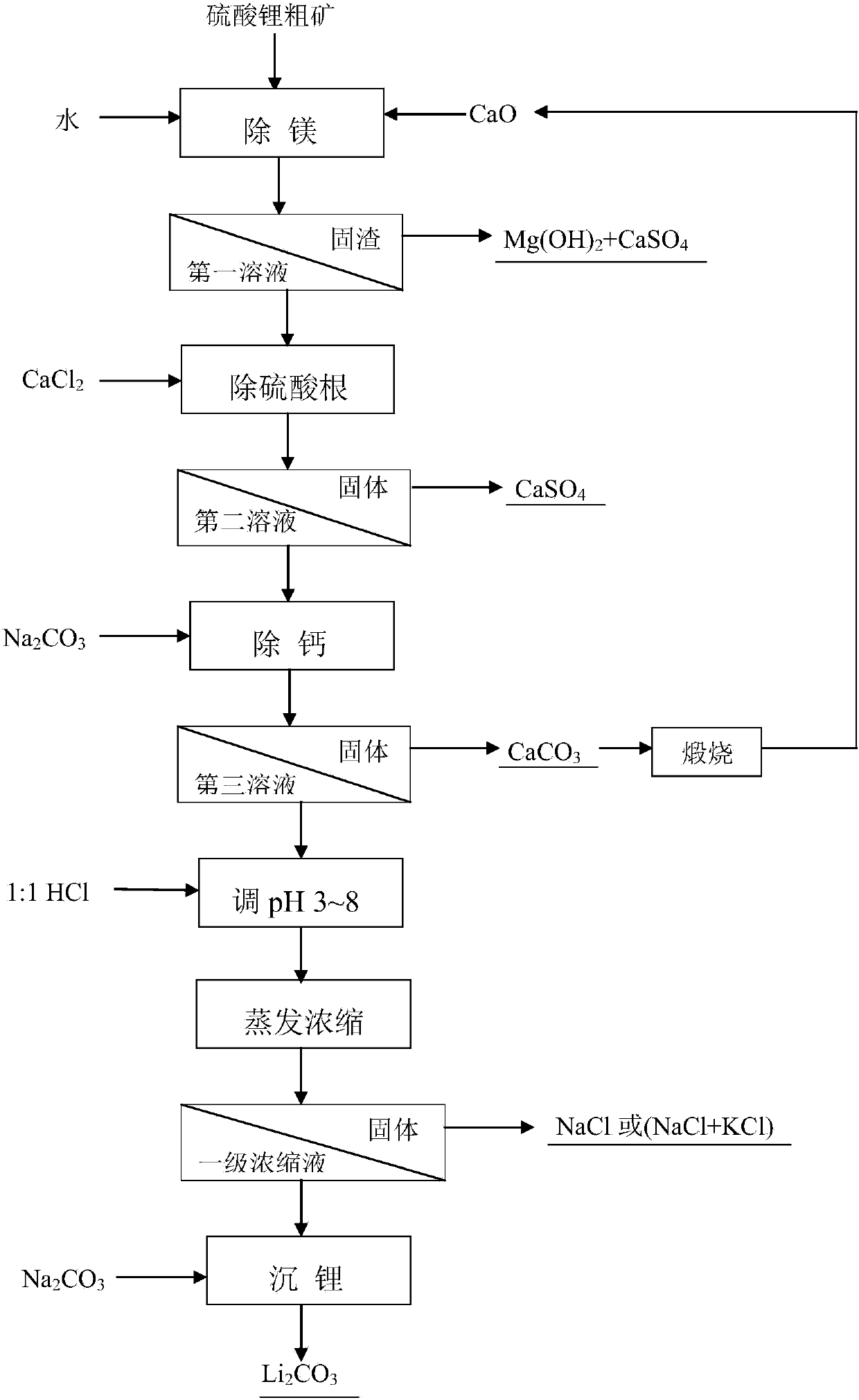
Method for separating and extracting lithium from lithium sulfate coarse ore
ActiveCN103898341ASimple processReduce energy consumptionLithium carbonates/bicarbonatesSulfate radicalsImpurity
The invention relates to a lithium extraction process and in particular discloses a method for separating and extracting lithium from lithium sulfate coarse ore. The method comprises the steps of removing magnesium, mixing lithium sulfate coarse ore powder with water, adding calcium oxide, reacting, ageing, and performing solid-liquid separation to obtain a first solution and solid residues; removing sulfate radicals, adding calcium chloride into the first solution, reacting, ageing, and performing solid-liquid separation to obtain a second solution and solid calcium sulfate; calcifying, adding sodium carbonate into the second solution, reacting, ageing, and performing solid-liquid separation to obtain a third solution and solid calcium carbonate; concentrating, adjusting the pH value of the third solution to be 3-8 by using hydrochloric acid, evaporating and concentrating, separating out solids, and performing solid-liquid separation to obtain a primary concentrated solution; extracting lithium carbonate, adding sodium carbonate into the primary concentrated solution, reacting, ageing, and performing solid-liquid separation to obtain solid lithium carbonate. According to the method, the energy consumption is low; a used impurity removal precipitant is low in price and easily available, and an impurity removal product, namely calcium carbonate can be recycled; the process is simple and easy to operate, the magnesium removal efficiency is high, and the lithium yield is high.
Owner:QINGHAI INST OF SALT LAKES OF CHINESE ACAD OF SCI
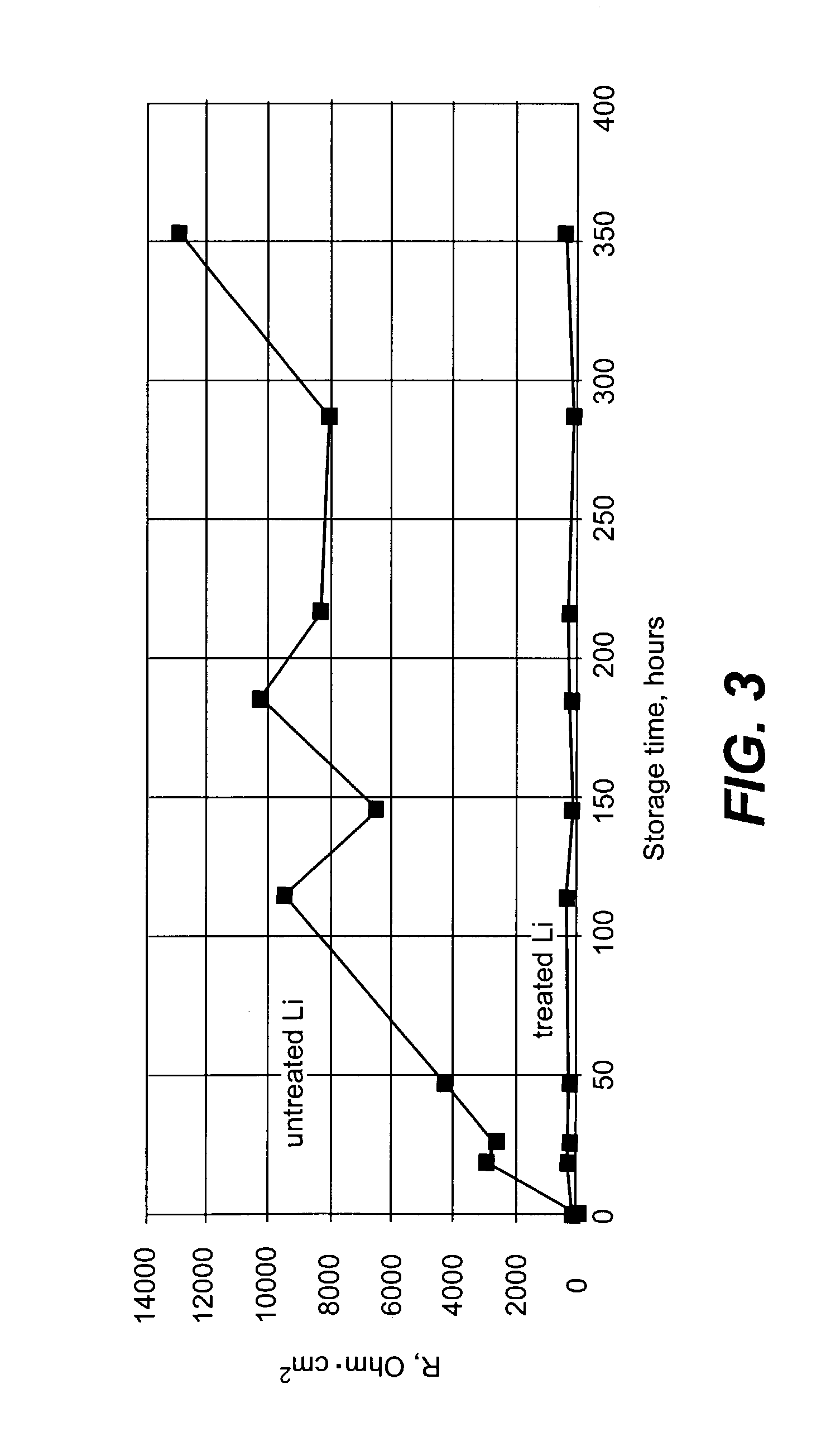
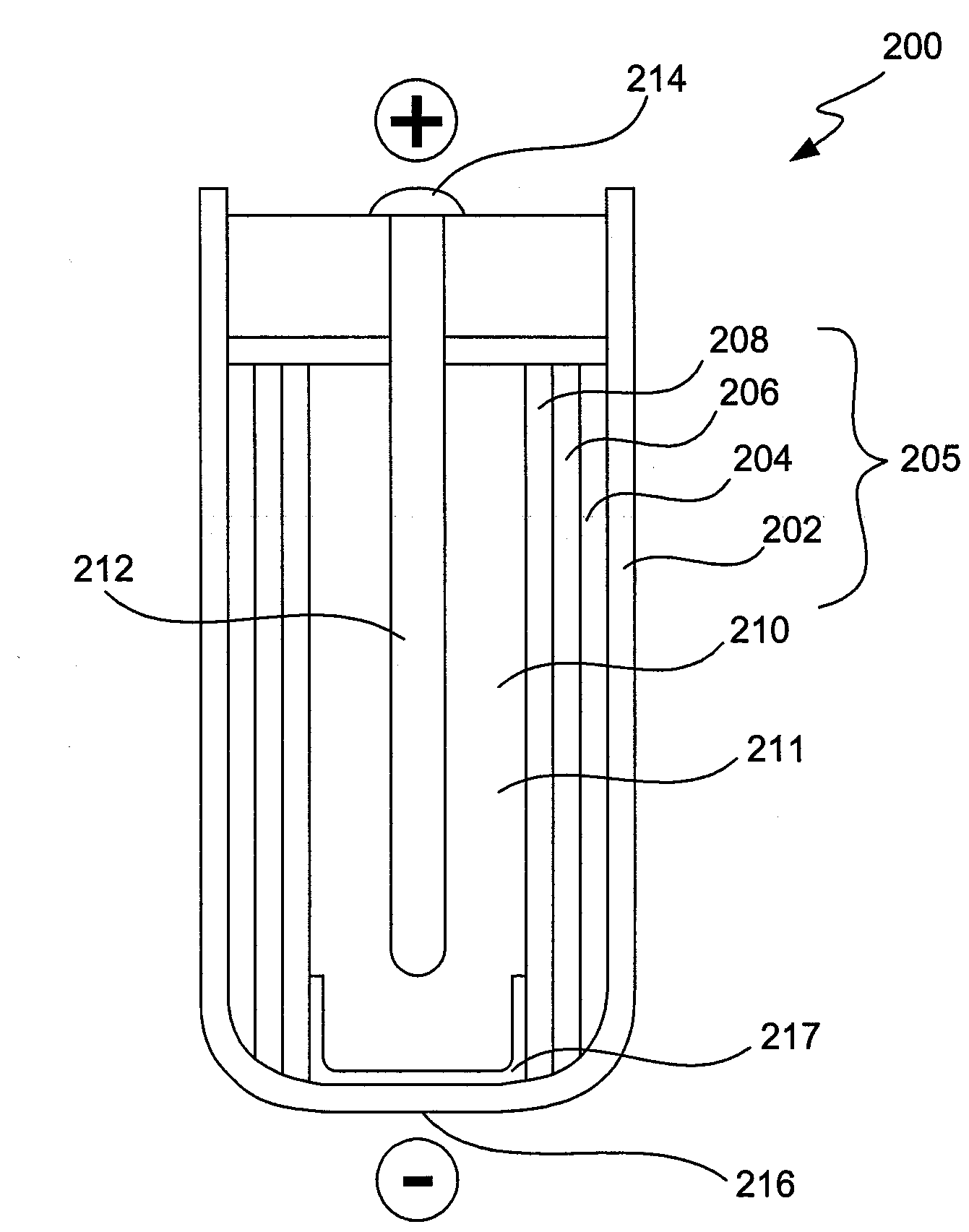
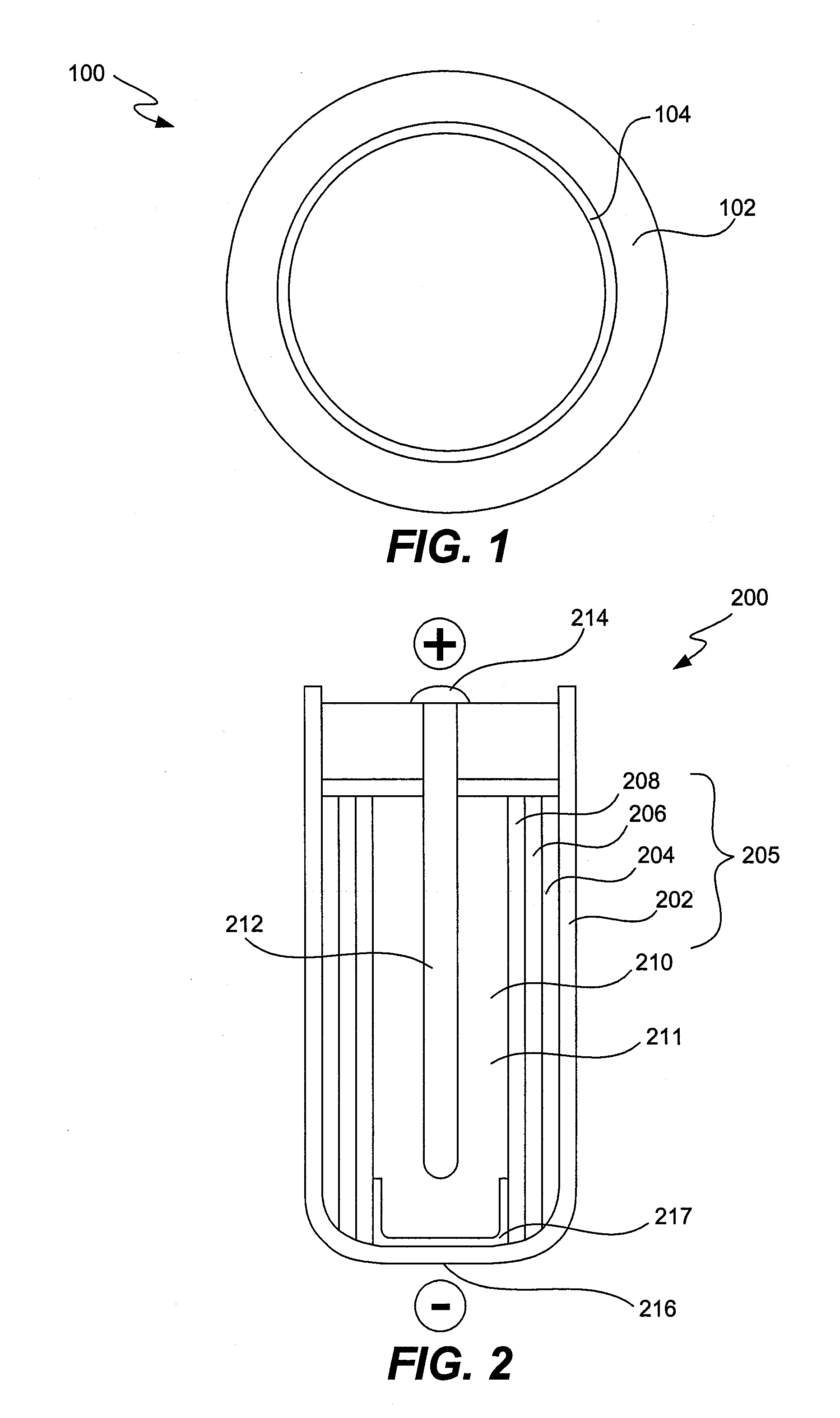
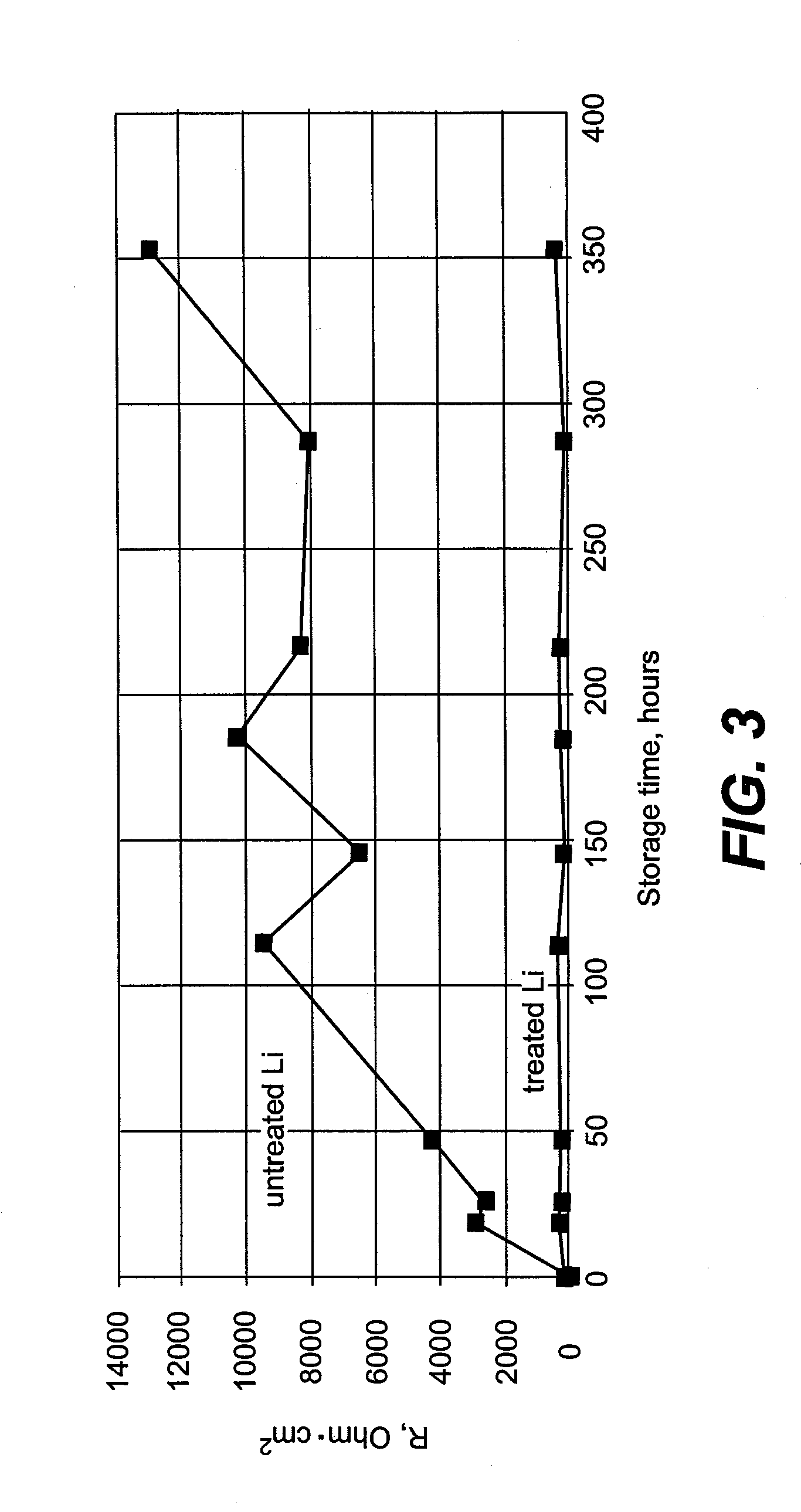
Alleviation of voltage delay in lithium-liquid depolarizer/electrolyte solvent battery cells
InactiveUS7482096B2Reduce degradationImprove stabilityElectrode manufacturing processesSolid electrolyte cellsChemical treatmentInorganic compound
Voltage delay in an active metal anode / liquid cathode battery cell can be significantly reduced or completely alleviated by coating the active metal anode (e.g., Li) surface with a thin layer of an inorganic compound with Li-ion conductivity using chemical treatment of Li surface. Particularly, preferred examples of such compounds include lithium phosphate, lithium metaphosphate, and / or their mixtures or solid solutions with lithium sulphate. These compounds can be formed on the Li surface by treatment with diluted solutions of the following individual acids: H3PO4, HPO3 and H2SO4, their acidic salts, or their binary or ternary mixtures in a dry organic solvent compatible with Li, for instance in 1,2-DME; by various deposition techniques. Such chemical protection of the Li or other active metal electrode significantly reduces the voltage delay due to protected anode's improved stability toward the electrolyte.
Owner:POLYPLUS BATTERY CO INC
Lithium sulfide/carbon composite nanometer material and preparation method and application thereof
ActiveCN106299261AImprove conductivityEvenly dispersedMaterial nanotechnologyCell electrodesCarbon compositesFiber
The invention discloses a lithium sulfide / carbon composite nanometer material and a preparation method and application thereof. In a relatively typical embodiment, the method comprises the steps of fully mixing lithium sulfate and a carbon material precursor or a carbon material, and performing thermal treatment, wherein the thermal treatment condition comprises a temperature rising rate of 1-20 DEG C per minute, a constant temperature of 600 to 1000 DEG C for 2-12 hours in an inert atmosphere; and natural cooling of the material to a room temperature to acquire the lithium sulfide / carbon composite material. The invention provides a process for synthesizing the lithium sulfide / carbon nanocomposite material by reducing lithium sulfate with carbon, the process is simple and easy to operate, is high in controllability and low in cost, the raw materials are low in price and easy to get, moreover, the obtained product is the lithium sulfide / carbon nanocomposite material which is uniformly dispersed, has good performance and is controllable in morphology, the lithium sulfide / carbon nanocomposite material comprises a one-dimensional nanometer fiber, a two-dimensional nanosheet and the like, and the lithium sulfide / carbon nanocomposite material is high in conductivity and can be widely applied to an electrochemical energy storage device such as a lithium-sulfur battery.
Owner:SUZHOU INST OF NANO TECH & NANO BIONICS CHINESE ACEDEMY OF SCI
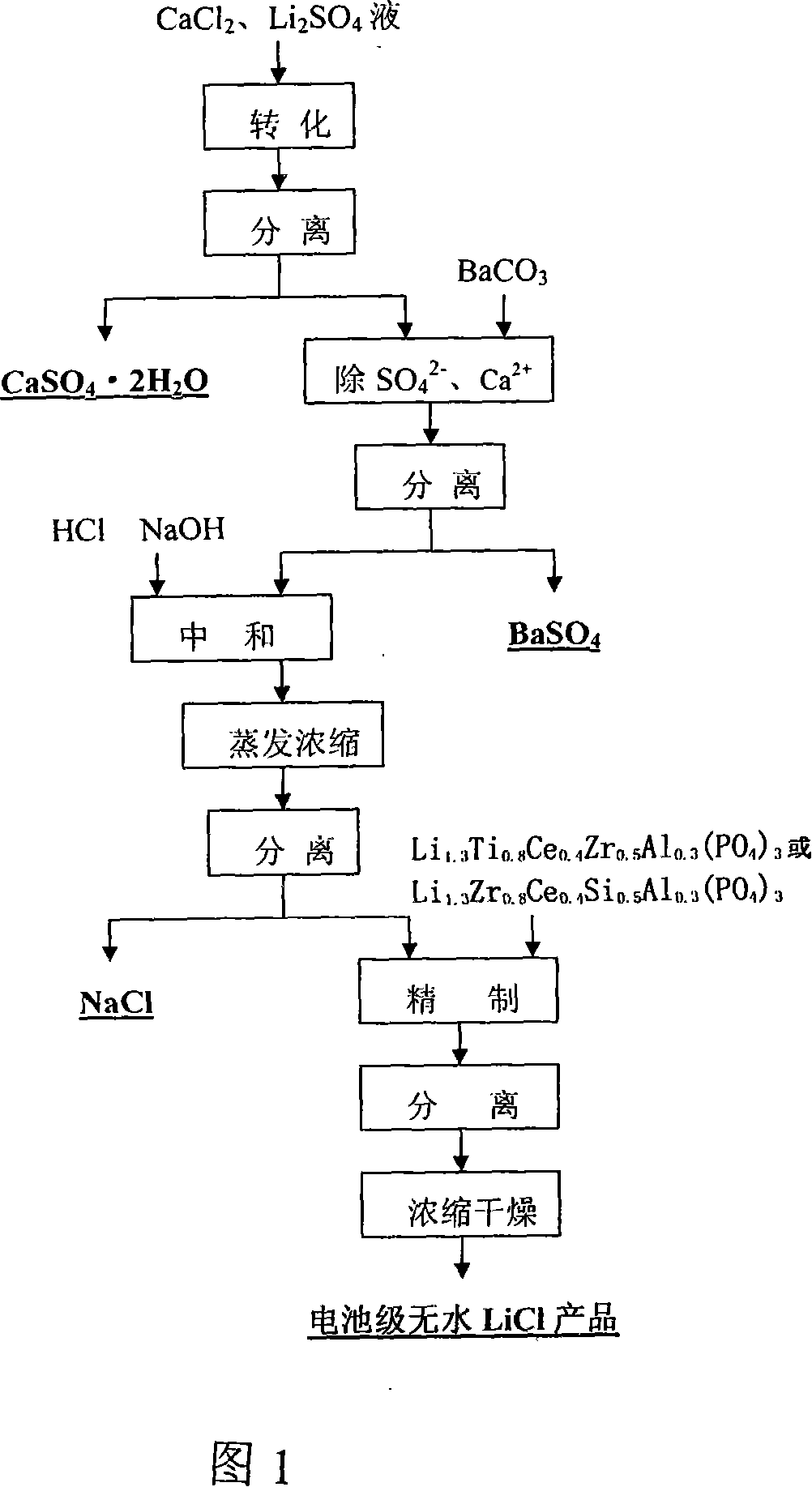
Method for preparing battery-stage anhydrous lithium chloride
ActiveCN101125667AReduce lossesHigh recovery rateAlkali metal chloridesLithium sulphateLithium chloride
The invention provides a preparation method of a battery grade lithium chloride, comprising: (1) calcium chloride is added into the acid clinker extracting solution of lithium concentrate - lithium sulfate solution, the PH value of the solution is regulated by NaOH to eliminate Fe and Mg, and the precipitation of CaSO4.2H2O, Fe(OH)3 and Mg(OH)2 and lithium chloride solution are obtained after the reaction; (2) the products of step (1) are filtered and cleaned to eliminate the precipitation of CaSO4.2H2O, Fe(OH)3 and Mg(OH)2, and the LiCl solution, i.e. a conversion solution is prepared; (3) the conversion solution is added with BaCO3, and filtered and cleaned after the reaction, to eliminate SO42- and Ca2+, and the LiCl concentrate liquid 1 is prepared; (4) the LiCl concentrate liquid is added with HCL and boiled to eliminate CO32-, and added with NaOH solution to back blend PH, then be evaporated, condensed, cooled, crystallized and separated, and the LiCl concentrate liquid 2 is obtained; (5) the LiCl concentrate liquid is added with refining agent, and filtered and cleaned after the reaction to eliminate Na, and the LiCl finish liquid is prepared, the LiCl finish liquid is condensed and dried, and finally the battery grade LiCl product is prepared. The invention has simple production process and can be easily operated.
Owner:TIANQI LITHIUM CORP
Method for preparing lithium carbonate by extracting lithium salt from aluminium electrolytic high-lithium electrolyte waste
InactiveCN108569711AReduce manufacturing costReduce dependenceLithium carbonates/bicarbonatesLithium sulphateLithium carbonate
The invention discloses a method for preparing lithium carbonate by extracting lithium salt from aluminum electrolytic high-lithium electrolyte waste, which specifically comprises the following stepsof S1, taking the aluminum electrolytic high-lithium electrolyte waste as a raw material to prepare a lithium sulfate solution; S2, filtering the lithium sulfate solution prepared in S1 to obtain filter residue and filter liquor, returning the obtained filter residue to an aluminum electrolytic cell to be used as aluminum electrolyte, and enabling the obtained filter liquor to be standby for lateruse; S3, performing impurity removal, lithium precipitation and secondary filtering to obtain crude lithium carbonate for later use; S4, washing and drying the crude lithium carbonate prepared in theS3 to obtain a lithium carbonate finished product. By taking the aluminum electrolytic high-lithium electrolyte waste as the main raw material to produce the lithium carbonate product, the more expensive and scarce lithionite is replaced, so that the dependence of the lithium-electric energy materials in China on the lithionite is reduced, and the production cost of lithium carbonate is greatly reduced; the obtained filter residue are returned to the electrolytic cell for use, the lithium concentration of the original electrolyte is reduced, the property of the electrolyte is optimized, and the energy is saved.
Owner:HENAN UNIV OF SCI & TECH
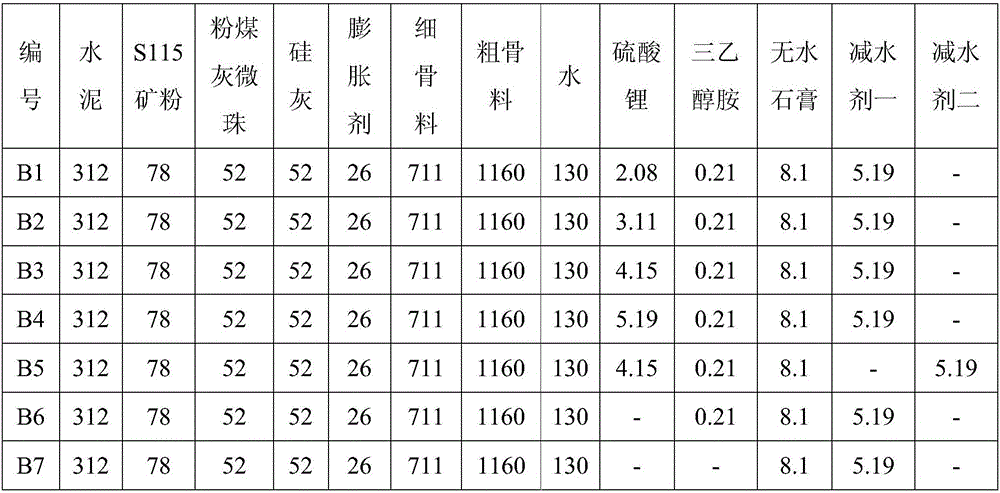
Curing-free PHC pipe pile concrete
The invention discloses a curing-free PHC pipe pile concrete. Production raw materials of every cubic meter of the concrete comprise 300-450kg of portland cement, 25-110kg of granulated blast-furnace slag powder, 10-100kg of fly ash microspheres, 10-100kg of silica fume, 20-60kg of an expanding agent, 640-725kg of fine aggregates with the fineness modulus of 2.4-3.0, 1120-1200kg of coarse aggregates with the granular composition of 5-25mm, 0.1-0.5kg of triethanolamine, 2-6kg of lithium sulfate, 7-13kg of anhydrous gypsum, 4-7kg of a water reducer, and 125-140kg of water. The concrete is used for producing PHC pipe piles, and can reach the demolding strength requirement of the curing-free PHC pipe piles and the strength requirement of C80 concrete at normal temperature in a short time.
Owner:CCCC SHANGHAI THIRD HARBOR SCI RES INST CO LTD +3
Process for producing lithium carbonate from spodumene concentrate by sulfuric acid method
The invention discloses a process for producing lithium carbonate from spodumene concentrate by a sulfuric acid method, and is used for solving the problems caused by the fact that lithium sulfate is leached in a strong acid environment in the existing lithium carbonate process. The process provided by the invention comprises the following steps: in a lithium sulfate preparation process, adding slag washing water and ground calcium carbonate in to a leaching tank and uniformly stirring, then, adding an acid clinker to uniformly stir to obtain a mixture, and filtering when the pH value of the mixture is 5.5-6.0 to obtain a lithium sulfate leachate, wherein the molar weight of ground calcium carbonate is greater than that of residual acid in the acid clinker. The process has the characteristics that neutralization reaction between the residual acid (sulfuric acid) in the acid clinker and ground calcium carbonate is firstly carried out, so that the lithium sulfate leaching environment is in a weak acid environment; the residual acid is prevented from reacting with oxides of Fe and Al in the material to reduce generation of gelatinous precipitates of Al(OH)3 and Fe(OH)3, so that the filtering performance is improved, the separating and filtering effect is improved and the water content in the filter cake (the filter residue) is effectively controlled, therefore, the output of lithium is reduced and the utilization of lithium is improved.
Owner:甘孜州泸兴锂业有限公司
Gel polymer electrolyte, polymer battery and preparing method
ActiveCN101747642AImprove conductivityPrevent crystallizationSecondary cellsPolymer electrolytesLithium sulphate
The present invention provides a gel polymer electrolyte. The gel polymer electrolyte comprises a polymer, an electrolyte, an organic solvent and inorganic fillers. The electrolyte, the organic solvent and the inorganic fillers are dispersed in the polymer. The inorganic fillers are lithium sulfate and / or lithium bisulfate. The present invention also provides a polymer battery containing the gel polymer electrolyte and a preparing method thereof. The preparing method of the polymer battery comprises the steps: mixed solution containing the electrolyte, the inorganic fillers, a polymerizable monomer and the organic solvent is added between the positive pole and the negative pole of the battery, the polymerizable monomer in the mixed solution is then polymerized under polymerization conditions, and a substance in a gel state is obtained. As lithium sulfate and / or lithium bisulfate are used as the inorganic fillers in the gel polymer electrolyte, the conductivity of the gel polymer electrolyte and the rate discharge performance of the battery containing the gel polymer electrolyte are obviously improved.
Owner:BYD CO LTD
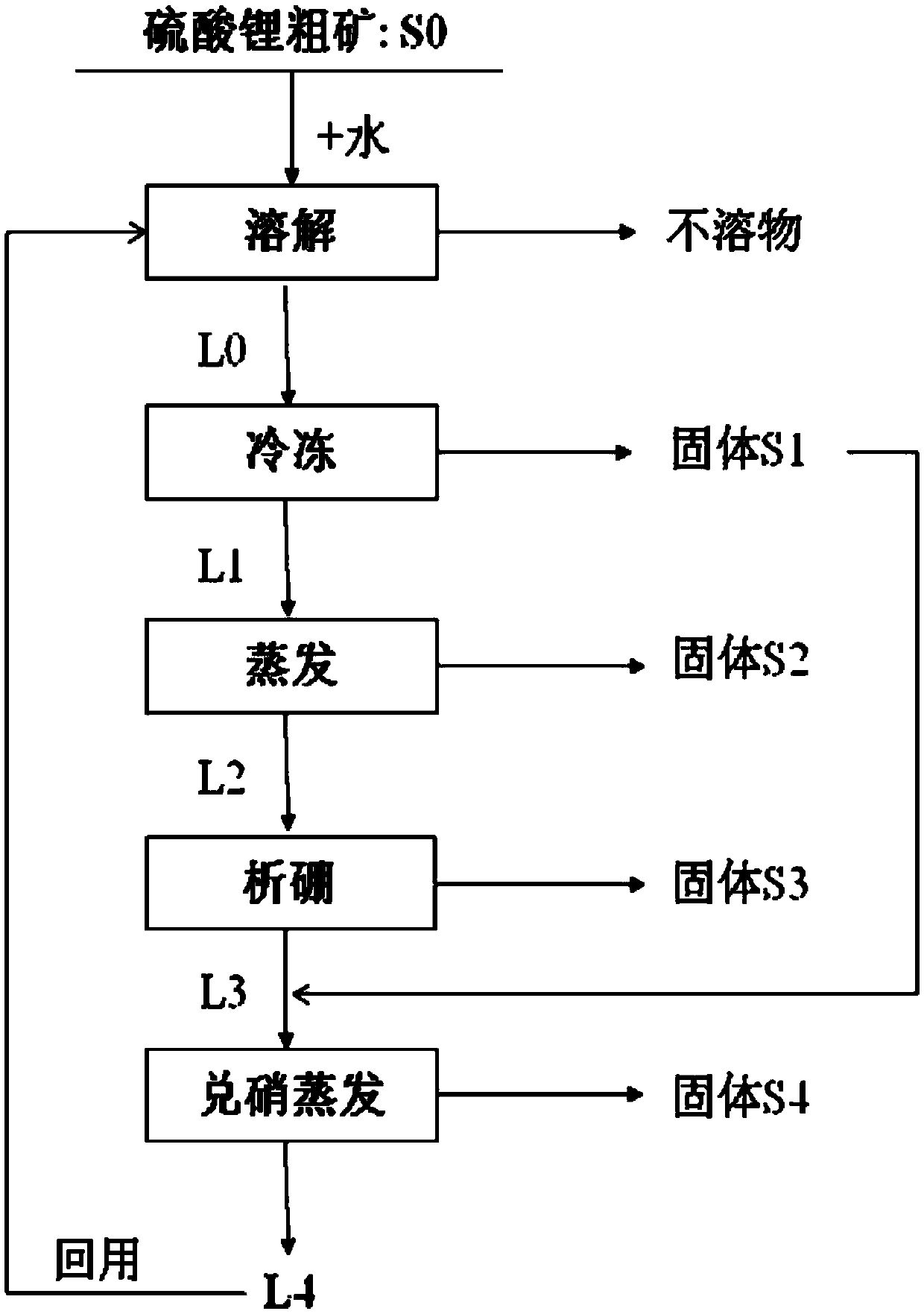
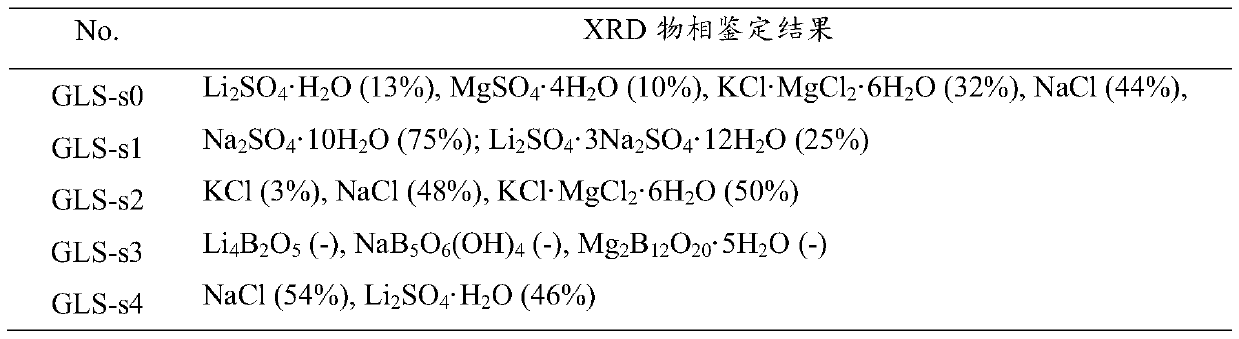
Method for purifying lithium sulfate crude ore
ActiveCN105502440AHigh yieldSimple process routeMagnesium chloridesAlkali metal chloridesLithium sulphateMirabilite
The invention provides a method for purifying lithium sulfate crude ore. The method comprises the following steps: step I, mixing the lithium sulfate crude ore S0 with excessive water to ensure that soluble ingredients in the lithium sulfate crude ore are just dissolved completely, and carrying out solid-liquid separation to obtain a solution L0; step II, freezing the solution L0 to separate out mirabilite, and carrying out solid-liquid separation to obtain a solution L1 and a solid S1; step III, evaporating the solution L1 to separate out a solid phase, and carrying out solid-liquid separation to obtain a solution L2 and a solid S2; step IV, allowing the solution L2 to stand still in a sealed condition for 7 to 50 days at 0 to 40 DEG C to separate out borate, and carrying out solid-liquid separation to obtain a solution L3; step V, mixing the solid S1 obtained in the freezing process in the step II with the solution L3, and evaporating the mixture at 0 to 40 DEG C to separate lithium sulfate concentrate.
Owner:QINGHAI INST OF SALT LAKES OF CHINESE ACAD OF SCI
Preparation method of lithium manganese phosphate nano-microsphere and product
InactiveCN104393289ASimple processEasy to controlMaterial nanotechnologyCell electrodesAfter treatmentMicrosphere
The invention discloses a preparation method of a lithium manganese phosphate nano-microsphere. The method comprises the steps of mixing ethylene glycol with water according to a volume ratio of 1:(1-2) to obtain a mixed solvent, and mixing part of ethylene glycol / water mixed solvent with manganese sulfate to obtain a mixed solution I of which the concentration is 0.1-0.2 M; mixing the part of ethylene glycol / water mixed solvent with lithium sulfate and ammonium dihydrogen phosphate, performing uniform stirring, adding potassium hydroxide, and continuously performing stirring to obtain a mixed solution II, wherein the concentration of the lithium sulfate in the mixed solution II is 0.125-0.25 M, the concentration of the ammonium dihydrogen phosphate is 0.112-0.1665 M, and the concentration of the potassium hydroxide is 0.25-0.3125 M; adding the mixed solution I into the mixed solution II, performing uniform stirring to obtain a precursor solution, performing hydrothermal reaction at the temperature of 160-240 DEG C, and then performing after-treatment to obtain the lithium manganese phosphate nano-microsphere. By accurately controlling a charging sequence and reaction conditions, the method for preparing the lithium manganese phosphate nano-microsphere is obtained.
Owner:ZHEJIANG UNIV
Reinforcement method of aluminosilicate glass
ActiveCN104556648AImprove wear resistanceImprove the strengthening effectGlass tempering apparatusLithium chlorideAlkali metal oxide
The invention relates to a reinforcement method of aluminosilicate glass, which comprises the following steps: preheating aluminosilicate glass to 500-600 DEG C, wherein the aluminosilicate glass contains silicon dioxide, aluminum oxide and alkali oxide, and the alkali oxide is selected from at least one of sodium oxide and potassium oxide; coating a reinforcement treating agent on the surface of the aluminosilicate glass, wherein the reinforcement treating agent contains molten salts, the molten salts comprise 70-100% of lithium salt and 0-30% of sodium salts, the lithium salt is a mixture of at least one of lithium nitrate and lithium sulfate with lithium chloride or lithium chloride, and the sodium salt is selected from at least one of sodium chloride, sodium nitrate and sodium sulfate; and carrying out ion exchange on the reinforcement treating agent and aluminosilicate glass at 650-825 DEG C for 3-15 minutes to form a crystallized layer. The reinforcement method of aluminosilicate glass can enhance the strength of the reinforced glass, and has the advantage of short time consumption.
Owner:CSG HOLDING
Alleviation of voltage delay in lithium-liquid depolarizer/electrolyte solvent battery cells
InactiveUS20090071835A1Reduce degradationImprove stabilityElectrode manufacturing processesVacuum evaporation coatingChemical treatmentInorganic compound
Voltage delay in an active metal anode / liquid cathode battery cell can be significantly reduced or completely alleviated by coating the active metal anode (e.g., Li) surface with a thin layer of an inorganic compound with Li-ion conductivity using chemical treatment of Li surface. Particularly, preferred examples of such compounds include lithium phosphate, lithium metaphosphate, and / or their mixtures or solid solutions with lithium sulphate. These compounds can be formed on the Li surface by treatment with diluted solutions of the following individual acids: H3PO4, HPO3 and H2SO4, their acidic salts, or their binary or ternary mixtures in a dry organic solvent compatible with Li, for instance in 1,2-DME; by various deposition techniques. Such chemical protection of the Li or other active metal electrode significantly reduces the voltage delay due to protected anode's improved stability toward the electrolyte.
Owner:POLYPLUS BATTERY CO INC
Method for extracting lithium from lithium-containing solution by solvent extraction
PendingCN111057848AHigh yieldEasy to separateProcess efficiency improvementLithium sulphateLithium chloride
The invention provides a method for extracting lithium from a lithium-containing solution by solvent extraction. The method comprises the following steps: (1) adjusting the pH value of the lithium-containing solution to 11-14; (2) mixing the adjusted solution with a synergistic extraction system to extract lithium; (3) washing an extracted organic phase; and (4) carrying out organic phase reverseextraction on loaded lithium by adopting a reverse extraction solution to obtain a high-purity lithium reverse extraction solution. The method can be used for preparing battery-grade lithium carbonatein one step at low cost, and can also be used for preparing high-purity lithium chloride, lithium sulfate and lithium hydroxide products. The method is low in energy consumption, short in process, high in yield and less in pollutant emission. The method can also be used for efficiently recovering lithium from a lithium-containing residual liquid after lithium carbonate precipitation.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
Negative electrode material of high-voltage thermal battery, high-voltage thermal battery and preparation method thereof
InactiveCN108172757AIncreased diffusion rateImprove ionic conductivityDeferred-action cellsPrimary cell electrodesElectrical conductorSilicon alloy
The invention discloses a negative electrode material of a high-voltage thermal battery, and the high-voltage thermal battery. The thermal battery consists of a positive electrode material, a negativeelectrode material and an electrolyte diaphragm material; the positive electrode material is a manganese dioxide material; the negative electrode material is a lithium quick ion conductor lithium borate-lithium sulfate-coated lithium silicon alloy material; and the electrolyte diaphragm material is a lithium nitrate-potassium nitrate-magnesium oxide material. By coating the lithium silicon alloyby lithium quick ion conductor lithium borate-lithium sulfate, the interface composition of the negative electrode lithium silicon alloy and the electrolyte lithium nitrate-potassium nitrate and the transmission process of metal positive ions in the interface in the battery working process are changed, so that the diffusion rate and ion electrical conductivity of lithium ions in the interface passivation film layer are greatly improved, and the working voltage of the thermal battery is further improved.
Owner:INST OF ELECTRONICS ENG CHINA ACAD OF ENG PHYSICS
Preparation method and product of flowerlike lithium manganese phosphate nano-particles
InactiveCN104326467ASimple processEasy to controlMaterial nanotechnologyPhosphorus compoundsLithium sulphateNanoparticle
The invention discloses a preparation method of flowerlike lithium manganese phosphate nano-particles. The preparation method comprises the following steps: mixing ethylene glycol with water in a volume ratio of 1 to (1-2) to obtain an ethylene glycol / water mixed solvent; taking one part of the ethylene glycol / water mixed solvent to mix with manganese sulfate to obtain a mixed solution I with concentration of 0.1-0.2mol / L; then, taking one part of the ethylene glycol / water mixed solvent to mix with lithium sulfate, ammonium dihydrogen phosphate and sodium hydroxide to obtain a mixed solution II, wherein the concentration of the lithium sulfate in the mixed solution II is 0.15-0.3mol / L; adding the mixed solution I into the mixed solution II, uniformly stirring to obtain a precursor solution, performing hydrothermal reaction for 8-24 hours at 160-240 DEG C, and then, performing post-treatment to obtain the flowerlike lithium manganese phosphate nano-particles. According to the preparation method disclosed by the invention, by precisely controlling the charging sequences and the reaction conditions, a method of preparing the flowerlike lithium manganese phosphate nano-particles is obtained, wherein the method is simple in process and easy to control.
Owner:ZHEJIANG UNIV
Ferrous lithium sulphate fluoride as well as preparation method and application thereof
ActiveCN101935072ASimple methodRaw materials are easy to getCell electrodesSecondary cellsPolyethylene glycolLyonium ion
The invention discloses ferrous lithium sulphate fluoride as cathode materials of lithium batteries as well as a preparation method and application thereof. The ferrous sulphate lithium fluoride is prepared according to the following steps of: adding FeSO4.xH2O, LiF and a solvent into a reaction container and mixing the added substances to obtain a reaction solution, and heating the reaction solution to 200-350 DEG C for reaction to obtain the ferrous sulphate fluoride, wherein x in the FeSO4.xH2O is an integer greater than or equal to 0, and the solvent is alcohol or polyethylene glycol containing at least one hydroxide radical. In the invention, the preparation method is simple and the raw materials are rich and easy to obtain; thus, the preparation method is suitable for mass production and has high practicality degree. In addition the obtained ferrous lithium sulphate fluoride is in nanoparticles with high actual capacity and can be directly used as cathode materials of lithium-ion batteries.
Owner:INST OF CHEM CHINESE ACAD OF SCI
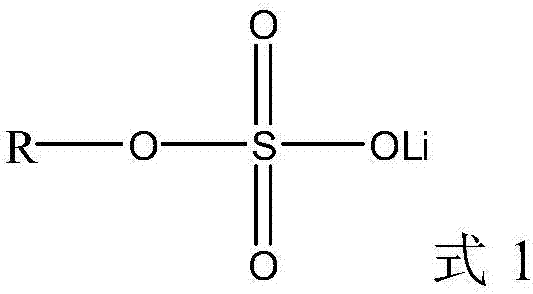
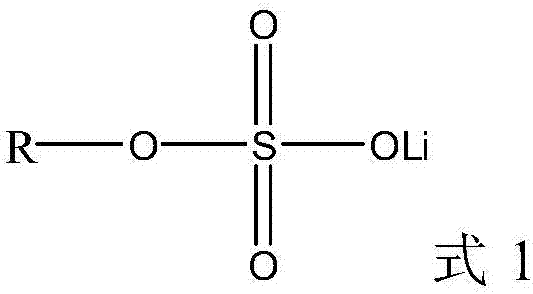
Lithium-ion battery electrolyte and lithium-ion battery containing same
InactiveCN111029653AStable structureImprove thermal stabilitySecondary cellsOrganic electrolytesBoron trifluorideLithium sulphate
The invention discloses a lithium-ion battery electrolyte which comprises a lithium salt, an additive and a non-aqueous organic solvent, and the additive comprises the following components in percentage by mass in the lithium-ion battery electrolyte: 1.5-3.0% of a film-forming additive, 0.5-2.0% of a phosphate additive and 0.5-2.0% of a compound with a boron trifluoride alkyl lithium sulfate structure. The invention also provides a lithium-ion battery containing the lithium-ion battery electrolyte. According to the invention, the three additives are organically combined to realize better normal-temperature and high-temperature cycling stability in a high-voltage or high-nickel system, and meanwhile, the impedance of the battery is lower, so that the high-temperature and low-temperature performances can be considered at the same time.
Owner:DONGGUAN SHANSHAN BATTERY MATERIALS +1
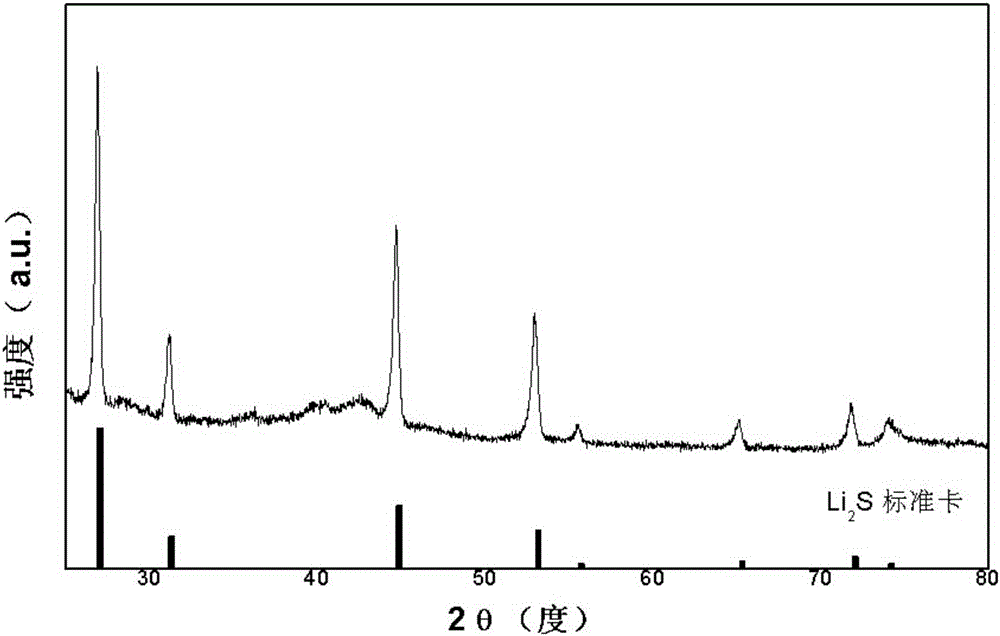
Production method of high-purity lithium sulfide
InactiveCN103552990AReduce usageImprove efficiencyAlkali metal sulfides/polysulfidesLithium sulphateLithium chloride
A production method of high-purity lithium sulfide is disclosed. The production method comprises the following steps: using hydrogen sulfide and lithium chloride used as raw materials to carry out a hydrosulfide reaction in N-methyl 2 pyrrolidone and under inert gas protection so as to obtain lithium hydrogen sulfide; filtering a precipitate, heating a N-methyl 2 pyrrolidone solution of lithium hydrogen sulfide under inert gas protection for decomposition and conversion so as to obtain a lithium sulfide precipitate, and separating, wherein mol ratio of the raw material hydrogen sulfide to the raw material lithium chloride is 0.95-1:1. According to the method, a high-risk hydrogen sulfide gas is not used such that the production process is safer and more convenient; lithium sulfide obtained is a white crystal; and the yield is greater than 98%, and the content of lithium sulfide is greater than 99%, wherein the content of lithium sulfite is less than 0.1% and the content of lithium sulfate is less than 0.5%.
Owner:四川宝利丰科技有限公司
Method for preparing battery-stage monohydrate lithium hydroxide
ActiveCN100455512CHigh recovery rateMeet actual needsCell electrodesLithium oxides/hydroxidesLithium sulphateSodium hydroxide
The invention provides a process for preparing battery grade lithium hydroxide monohydrate, comprising: (1) adding sodium hydroxide in lithium sulfate purification fluid and obtaining solid of Na2SO4, 10H2O and liquid of LiOH after completely dissolving and cooling, (2) obtaining liquid of LiOH after filtering and separating, (3) evaporating and concentrating the liquid of LiOH and filtering, separating and leaching the liquid of LiOH after cooling and crystallizing to obtain one-time crude product of LiOH, H2O, (4) adding deionized water in the one-time crude product of LiOH, H2O and obtaining re-dissolving solution of the one-time crude product of LiOH, H2O, (5) adding refining agent in the re-dissolving solution of the one-time crude product of LiOH, H2O and obtaining filtrate of LiOH refined liquor after filtering and separating, (6) filtering and separating the LiOH refined liquor after evaporating, concentrating, cooling and crystallizing to obtain solid of battery grade wet product of LiOH, H2O and (7) taking out the battery grade wet product of LiOH, H2O after drying to obtain battery product of LiOH, H2O. The invention is simple in production process, easy operation and perfect product quality.
Owner:TIANQI LITHIUM CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com