Ferrous lithium sulphate fluoride as well as preparation method and application thereof
A lithium ferrous fluorosulfate, reaction technology, applied in the direction of ferric sulfate, electrical components, electrochemical generators, etc., can solve the problems of restricting competitive advantages and wide application, unfavorable large-scale production, high equipment requirements, etc., to achieve suitable The effect of large-scale production, simple method and high degree of practicality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Embodiment 1, preparation lithium ferrous fluorosulfate
[0022] by FeSO 4 ·H 2 The molar ratio of O:LiF=1:1.1 was weighed, and tetraethylene glycol was added as a solvent, and mixed evenly. React at 300°C for 5h. After the reaction was completed, it was separated and dried to obtain a powdery solid.
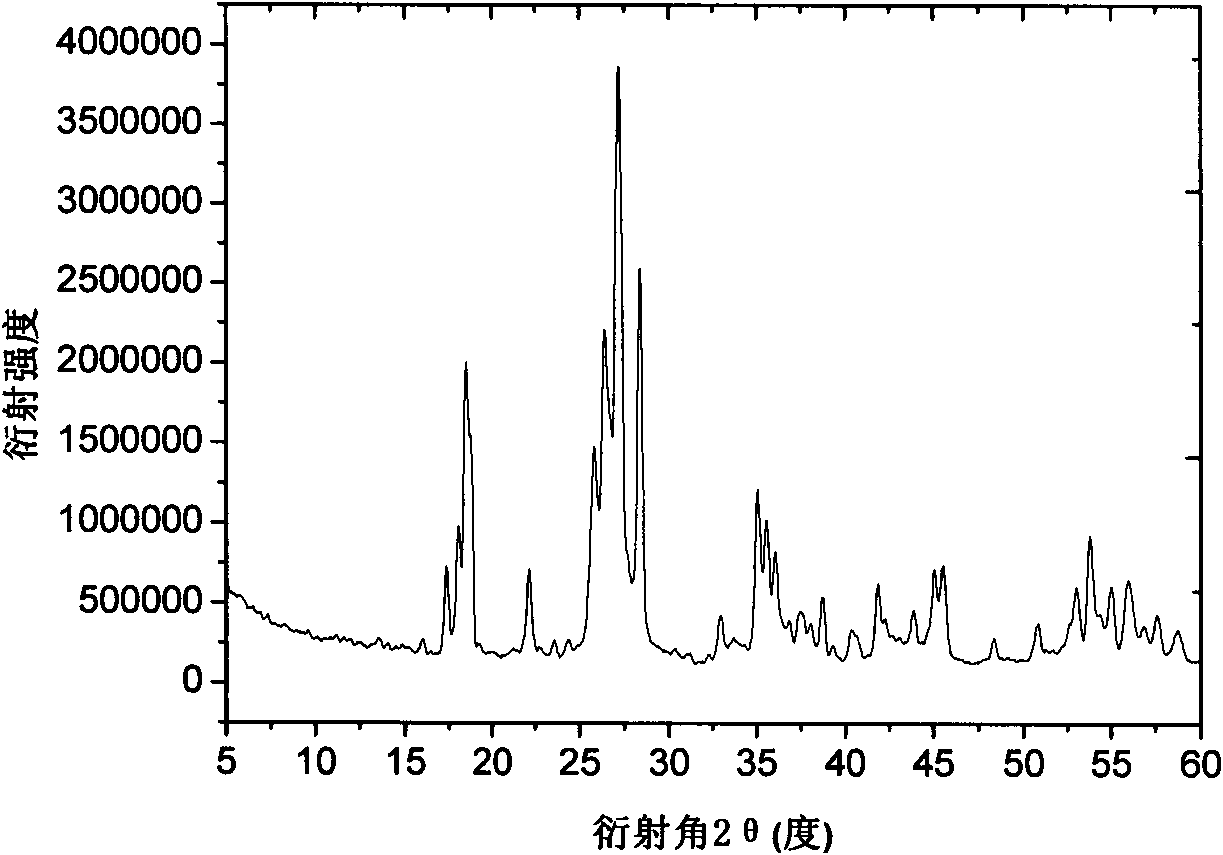
[0023] Powder X-ray diffractometer (Rigaku DmaxrB, CuK α X-ray) analysis confirmed the structure. The result is as figure 1 shown. As can be seen from the figure, there is no impurity peak in the spectrogram, indicating that the product is of high purity.
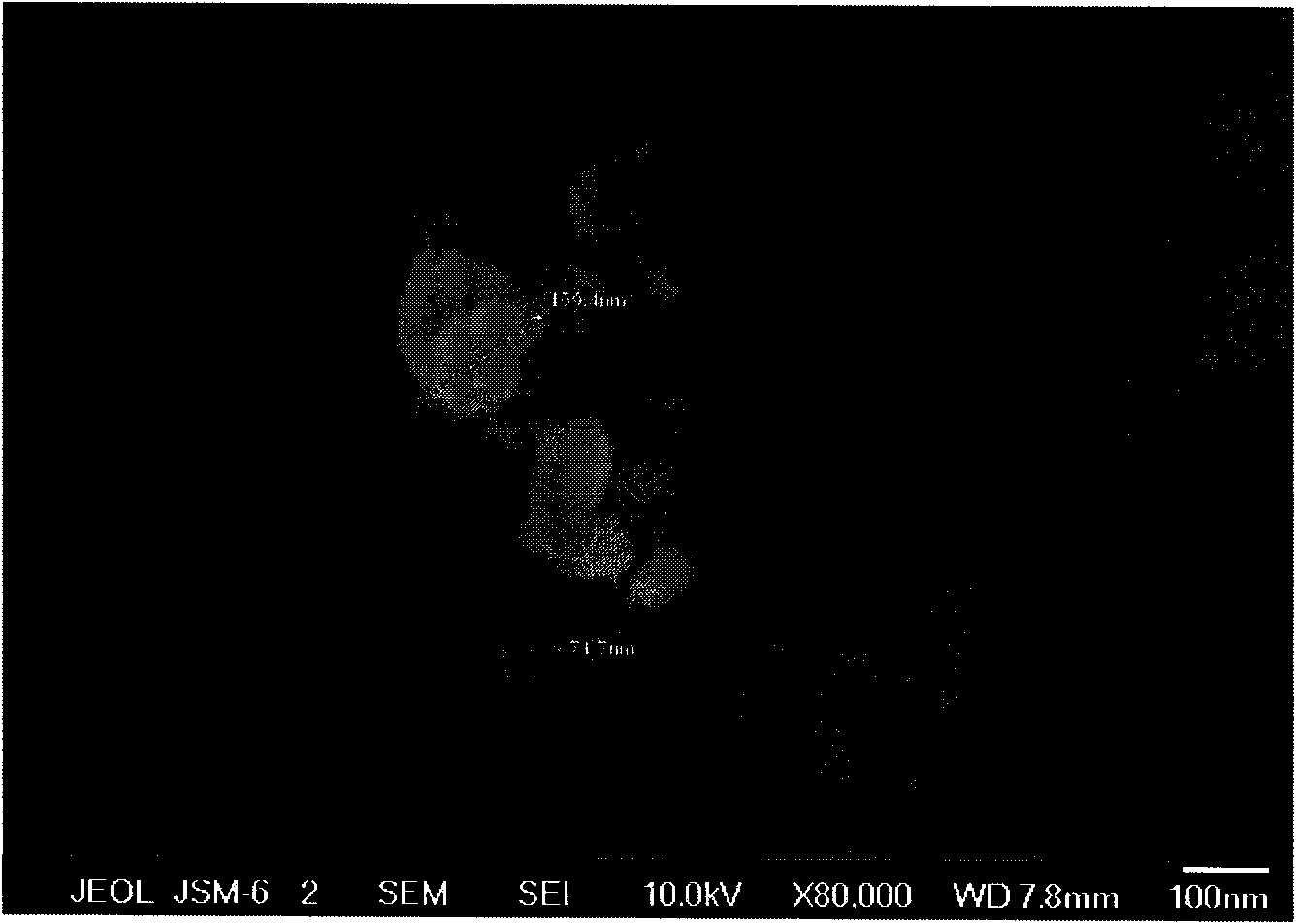
[0024] The morphology of lithium ferrous fluorosulfate was characterized by a scanning electron microscope (JEOL-6700F), as shown in figure 2 shown. It can be seen from the figure that the particle size range of this material is 20-200nm, and the particles with a particle size of 159.4nm and particles with a particle size of 71.7nm are marked in the figure.
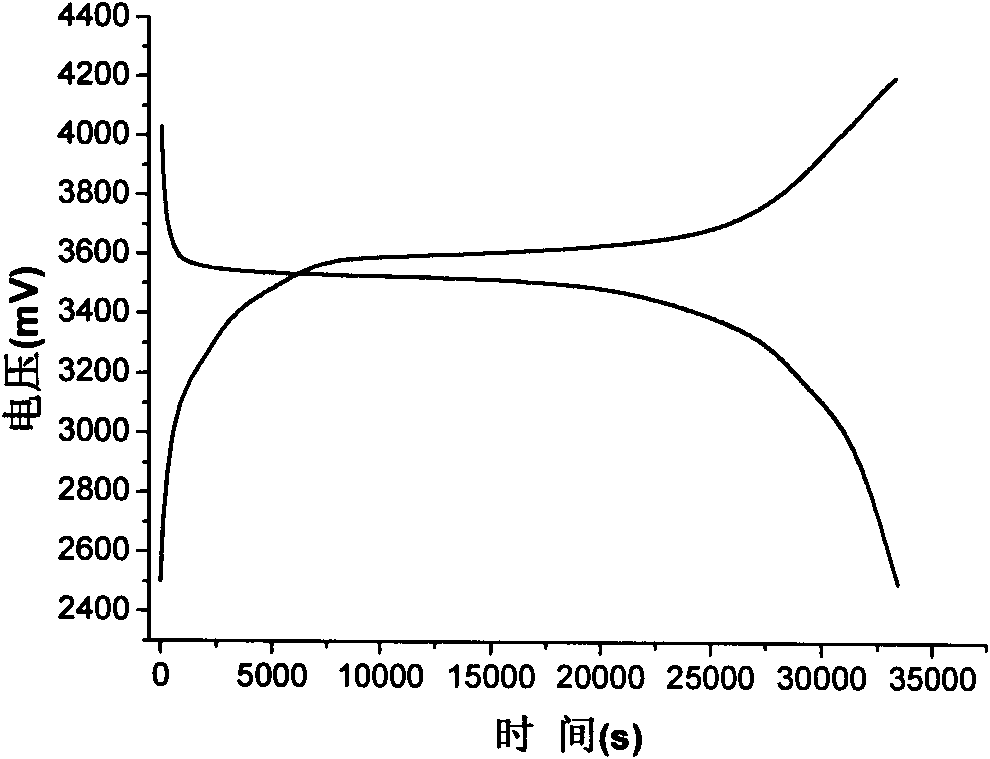
[0025] Electrochemical performance characterizati...
Embodiment 2
[0029] Embodiment 2, preparation lithium ferrous fluorosulfate
[0030] by FeSO 4 ·H 2 Weigh the molar ratio of O:LiF=1:1.15, add glycerol as a solvent, and mix well. React at 285°C for 36h. Complete, separated and dried to obtain a powdery solid. The lithium ferrous fluorosulfate prepared in this example and the test results of the simulated battery are listed in Table 1.
Embodiment 3
[0031] Embodiment 3, preparation lithium ferrous fluorosulfate
[0032] by FeSO 4 ·H 2 The molar ratio of O:LiF=1:1.2 was weighed, and polyethylene glycol (weight-average molecular weight: 200) was added as a solvent, and mixed evenly. React at 250°C for 60h. Complete, separated and dried to obtain a powdery solid. The lithium ferrous fluorosulfate prepared in this example and the test results of the simulated battery are listed in Table 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com