Method for separating and extracting lithium from lithium sulfate coarse ore
A technology for lithium sulfate and lithium extraction, applied in the direction of lithium carbonate; High, simple process effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
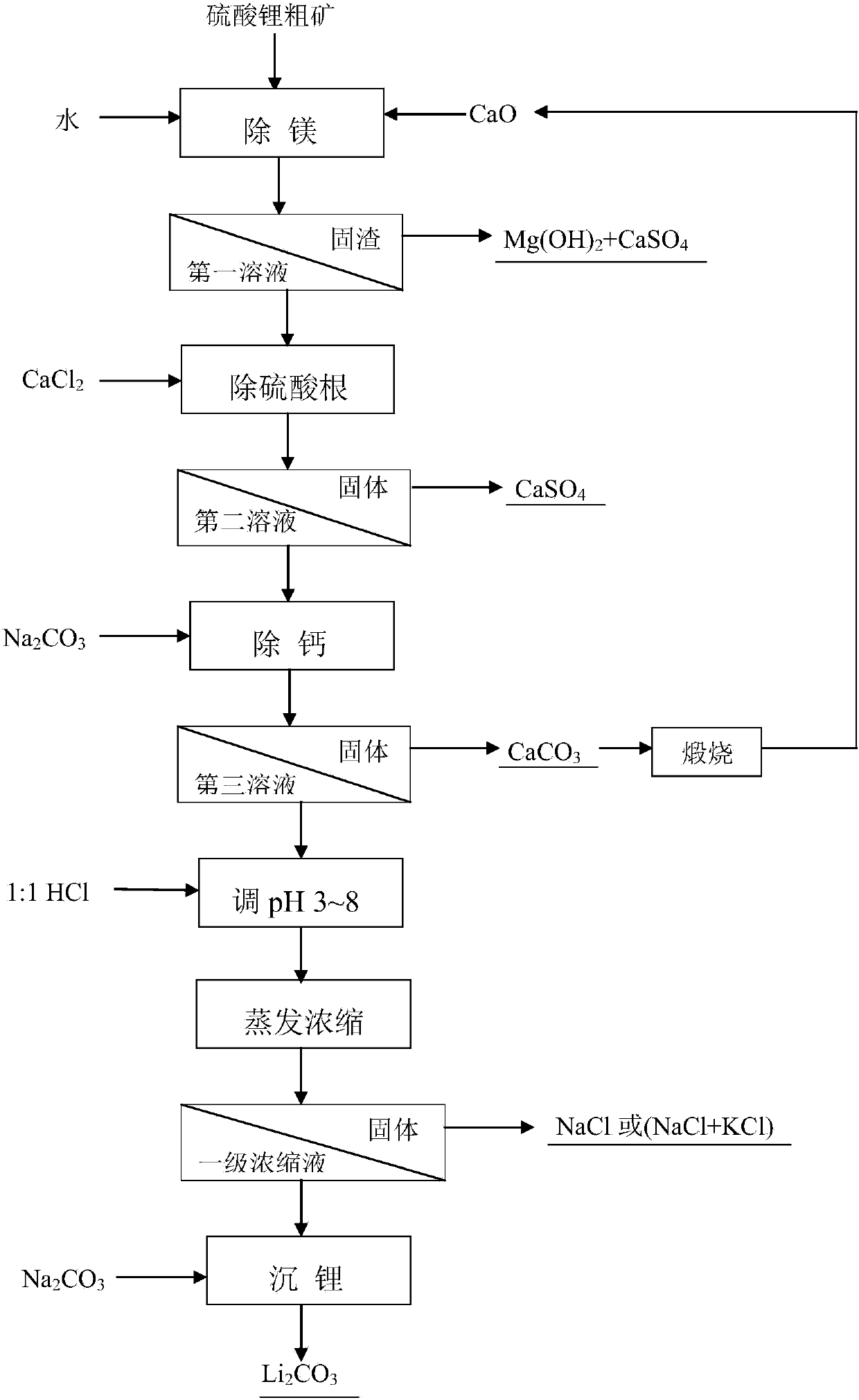
[0033] In addition to magnesium, mix 200.00g of lithium sulfate coarse ore powder with 1500mL of water, add 100% calcium oxide, stir and react for 2 hours, separate after aging for 3 hours, and obtain 1485mL of the first solution. where Mg 2+ The content is 0.17g / L, and the measured magnesium removal rate is 96.88%; Li + Concentration is 3.25g / L, and lithium yield is 92.41%; SO 4 2- The concentration was 12.14g / L, and the sulfate radical removal rate was 63.58%.
[0034] In addition to sulfate, take 1460mL of the first solution, add 100% calcium chloride to it under magnetic stirring, react for 60 minutes, and separate after aging for 60 minutes to obtain 1450mL of the second solution, whose sulfate concentration is 2.65g / L , Remove 78.31% sulfate.
[0035] For calcium precipitation, put 1430mL of the second solution in a water bath at 60°C, add 100% sodium carbonate, stir and react for 60 minutes, and separate after aging for 30 minutes to obtain 1373mL of the third solut...
Embodiment 2
[0041] In addition to magnesium, mix 200.00g of lithium sulfate coarse ore powder with 3000mL of water, add 100% calcium oxide, stir and react for 3 hours, separate after aging for 2 hours, and obtain 2990mL of the first solution. where Mg 2+ The content is 0.13g / L, and the measured magnesium removal rate is 95.37%; Li + Concentration is 1.68g / L, and lithium yield is 96.46%; SO 4 2- The concentration is 8.02g / L, and the sulfate removal rate is 51.53%.
[0042] In addition to sulfate radicals, take 2970mL of the first solution, add 105% calcium chloride to it under magnetic stirring, react for 40 minutes, separate after aging for 30 minutes, and obtain 2962mL of the second solution, whose sulfate radical concentration is 3.28g / L , Remove 59.21% sulfate.
[0043] For calcium precipitation, put 2940mL of the second solution in a water bath at 80°C, add 105% sodium carbonate, stir and react for 50 minutes, and separate after aging for 60 minutes to obtain 2880mL of the third s...
Embodiment 3
[0049] In addition to magnesium, mix 200.00g of lithium sulfate coarse ore powder with 2000mL of water, add 105% calcium oxide, stir and react for 3 hours, separate after aging for 2 hours, and obtain 1990mL of the first solution. where Mg 2+ The content is 0.073g / L, and the measured magnesium removal rate is 98.24%; Li + Concentration is 2.51g / L, and lithium yield is 95.73%; SO 4 2- The concentration is 9.77g / L, and the sulfate removal rate is 60.71%.
[0050] In addition to sulfate, take 1970mL of the first solution, add 110% calcium chloride to it under magnetic stirring, react for 60 minutes, separate after aging for 30 minutes, and obtain 1963mL of the second solution, whose sulfate concentration is 2.74g / L , Remove 72.06% sulfate.
[0051] For calcium precipitation, take 1940mL of the second solution and place it in a water bath at 90°C, add 110% sodium carbonate, stir and react for 50 minutes, and separate after aging for 30 minutes to obtain 1880mL of the third sol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com