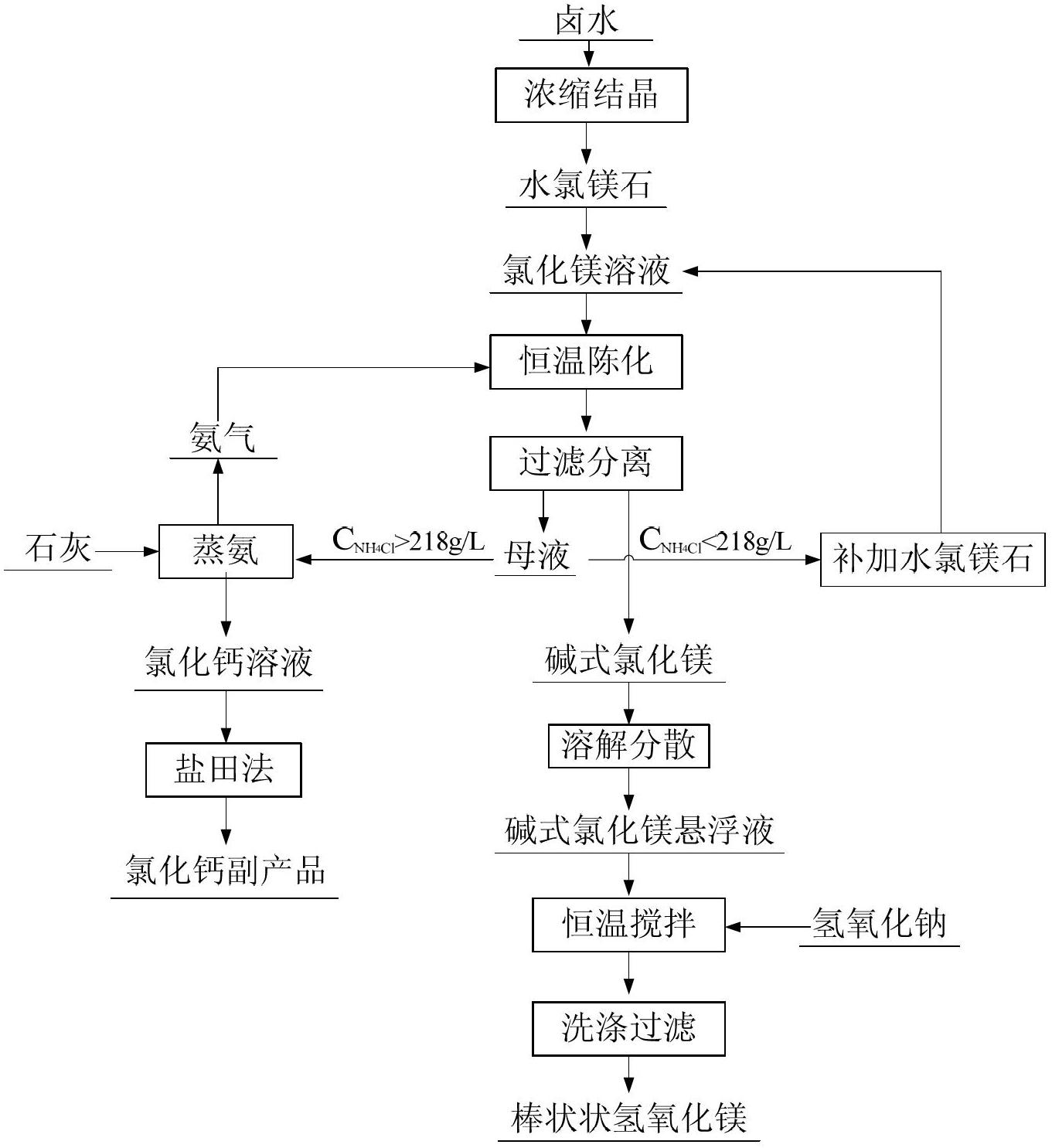
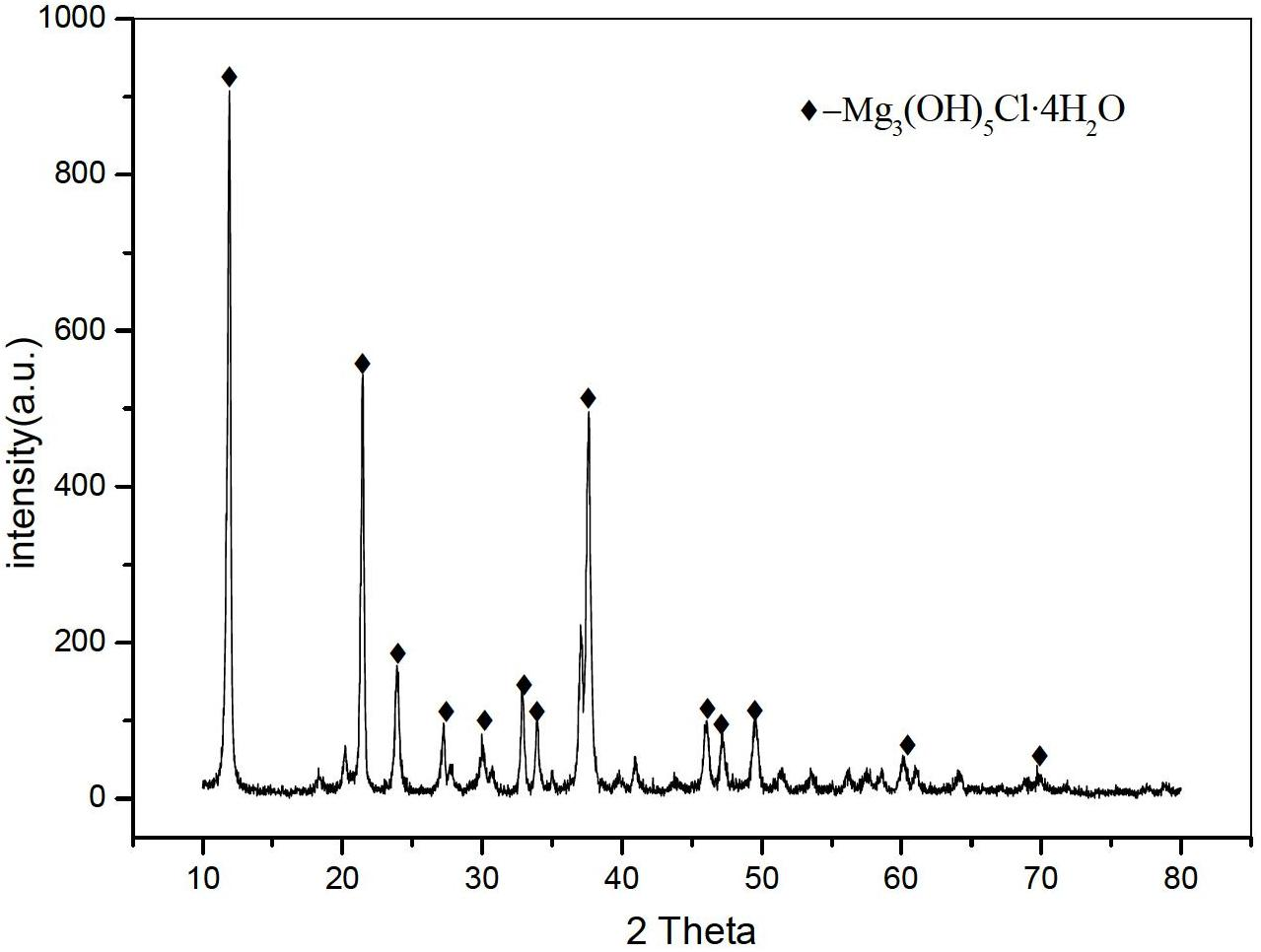
Method for preparing rod-like magnesium hydroxide from salt lake brine
A salt lake brine and magnesium hydroxide technology, applied in the field of comprehensive utilization of salt lake resources, can solve the problems of polluting products and difficult cleaning of surfactants, and achieve the effects of separation and removal, easy washing, and wide source of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0030] The salt lake brine is evaporated in the salt field, concentrated and crystallized to produce potassium sulfate, sodium chloride, and potassium chloride. After absorbing and extracting lithium, the old brine containing magnesium and boron is obtained. The composition is shown in Table 1.
[0031] 1) Concentrated crystalline bischofite
[0032] Add 10L of brine in the reaction tank, heat and concentrate at 118°C, when the volume of the brine is reduced to 6.5L, stop heating, seal and keep warm for 2-3 hours, then filter while it is hot, cool the filtrate to room temperature, and crystallize bischofite (composition See Table 2), the weight is 2.6kg.
[0033] 2) Preparation of basic magnesium chloride by ammonia precipitation
[0034] The bischofite obtained is dissolved in water, and the concentration of magnesium ions is made into a solution of 3.5mol / L, and the volume is 3.5L. In the ammonia distillation tank with a volume of 30L, 15L of ammonium chloride mother liquor...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com