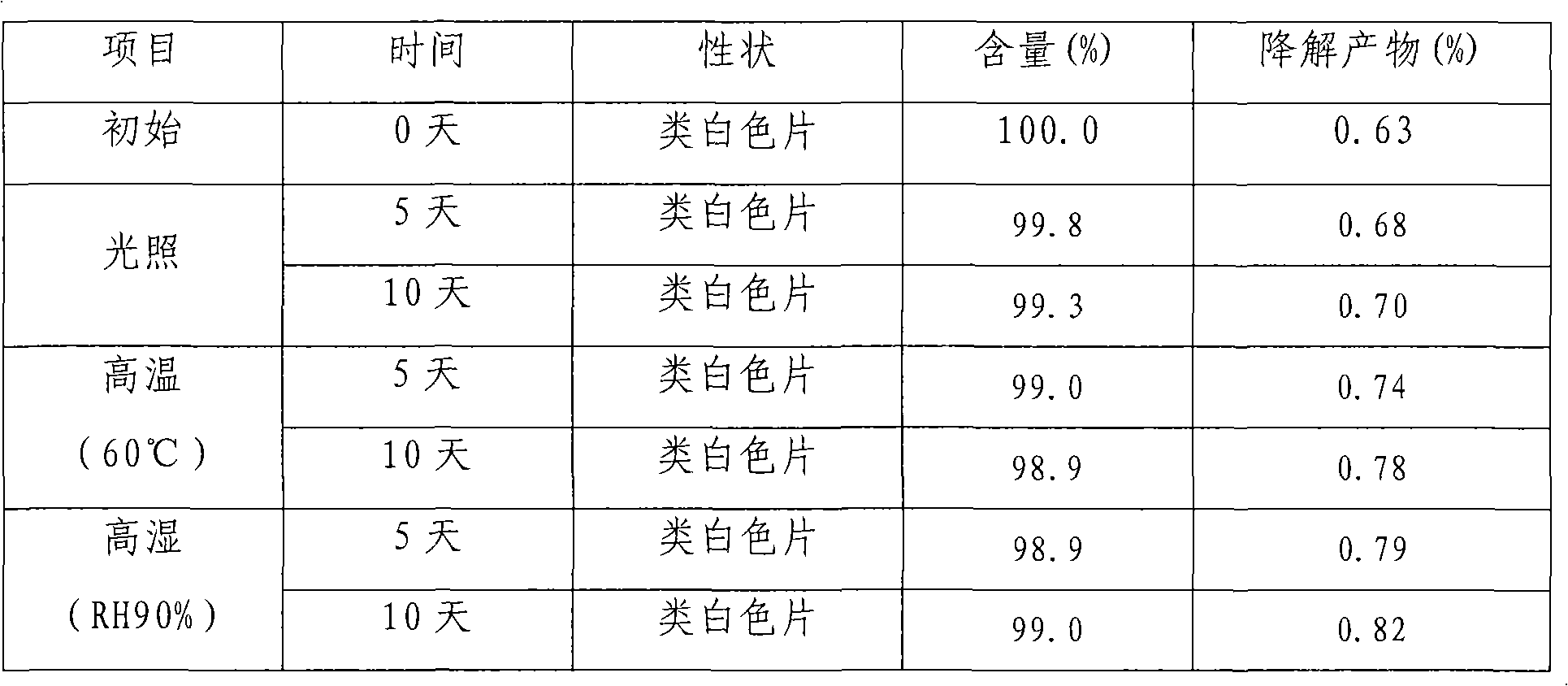
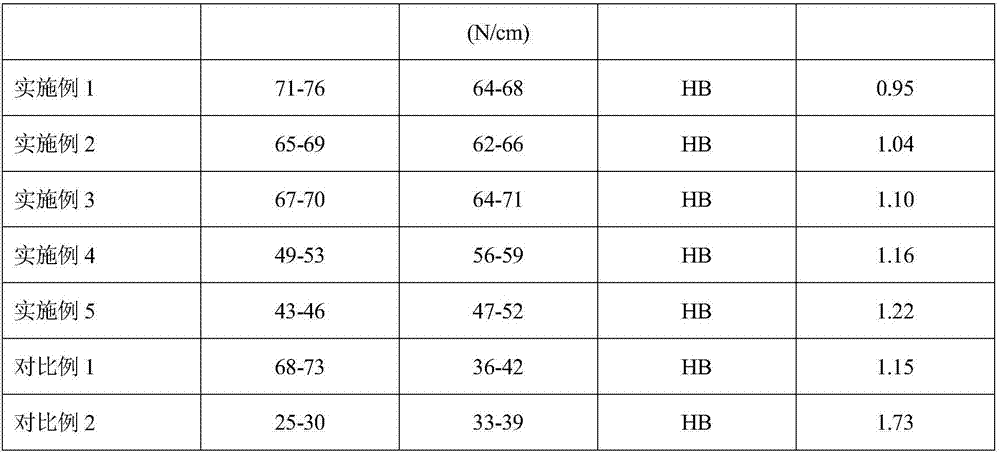
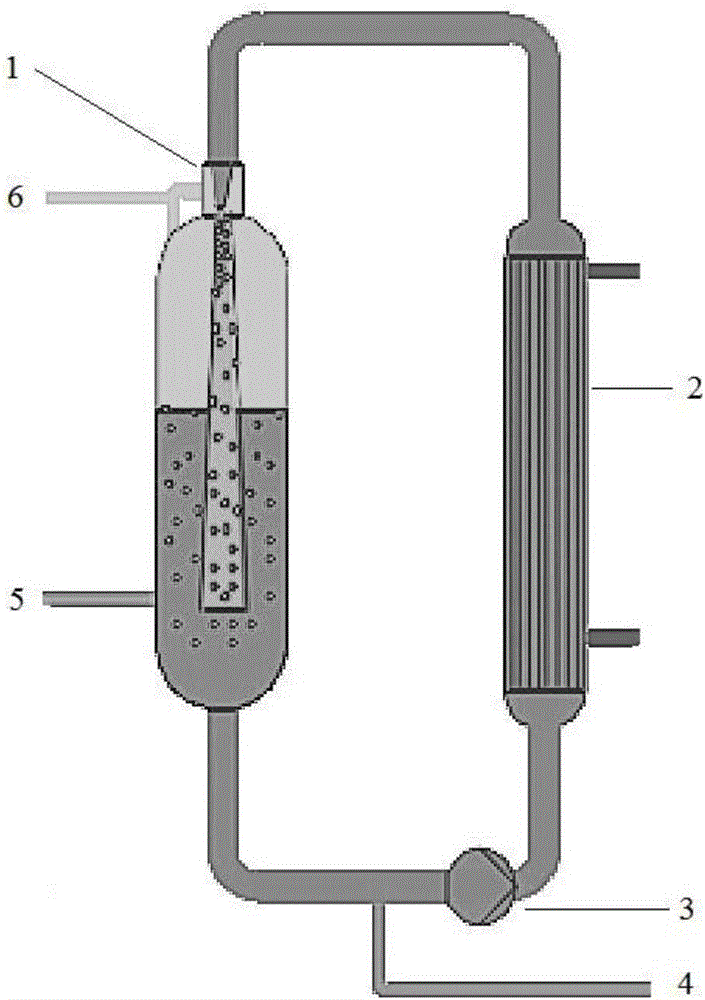
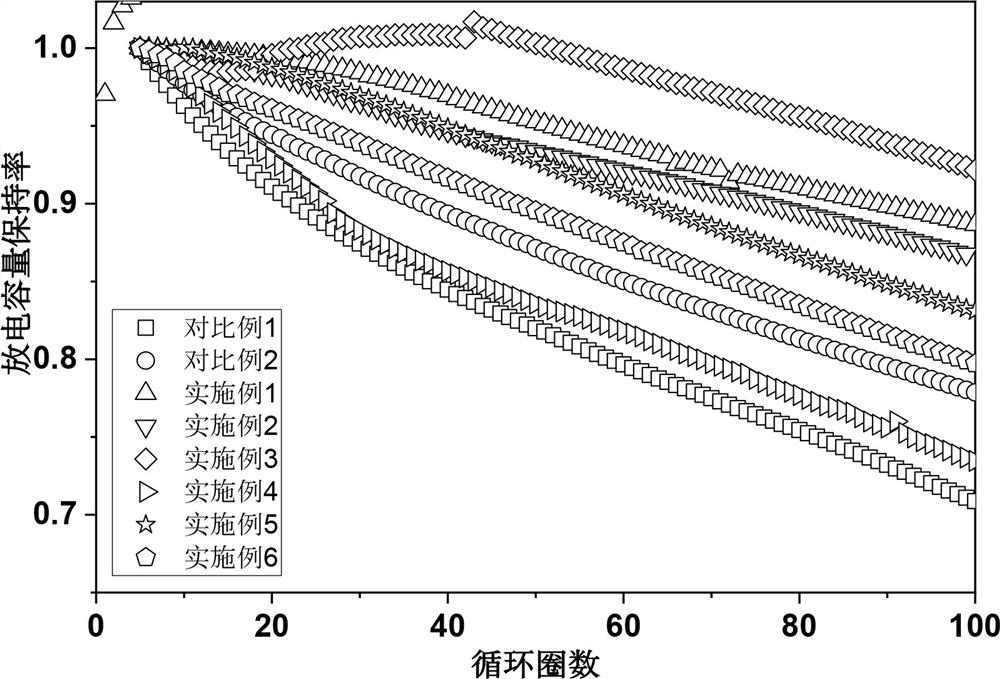
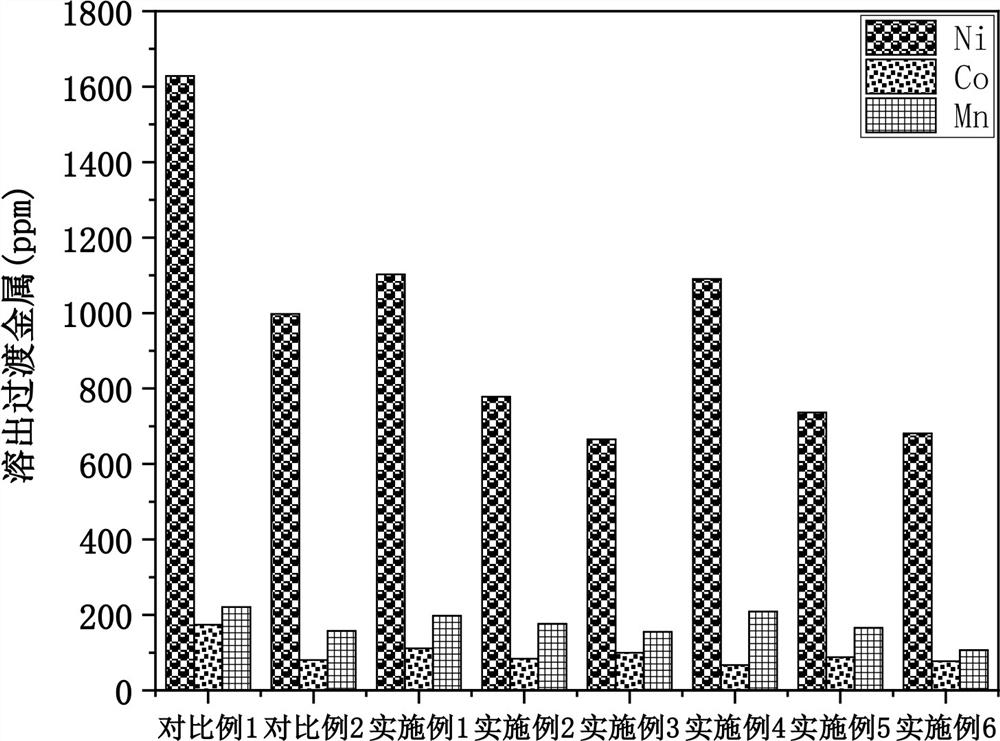
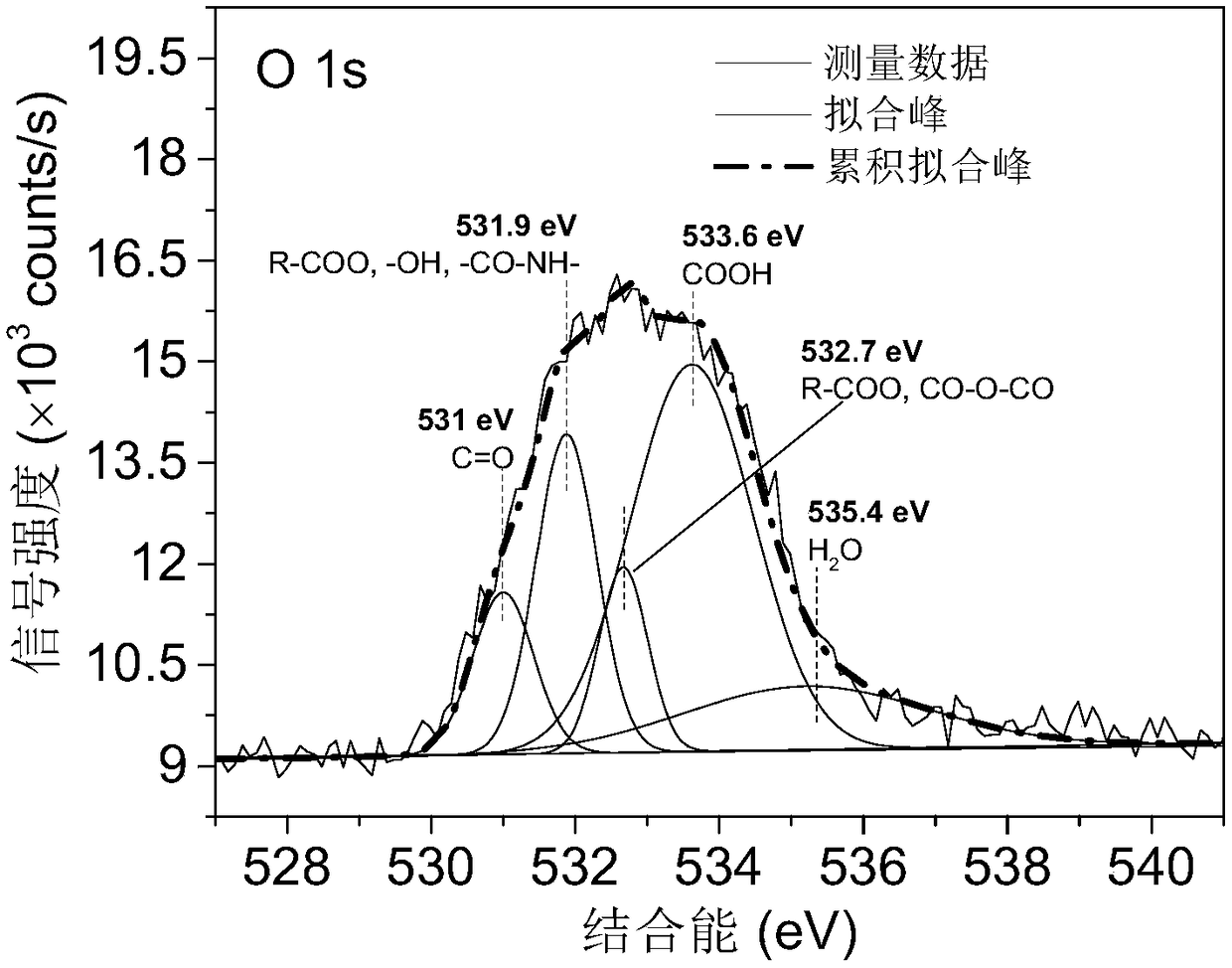
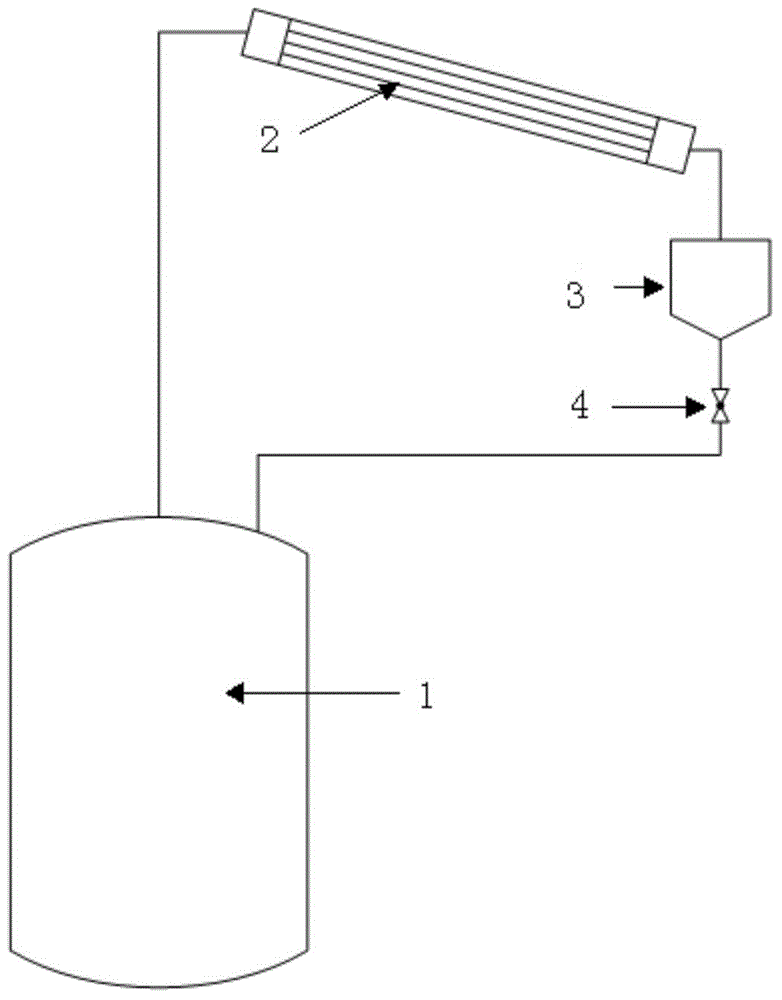
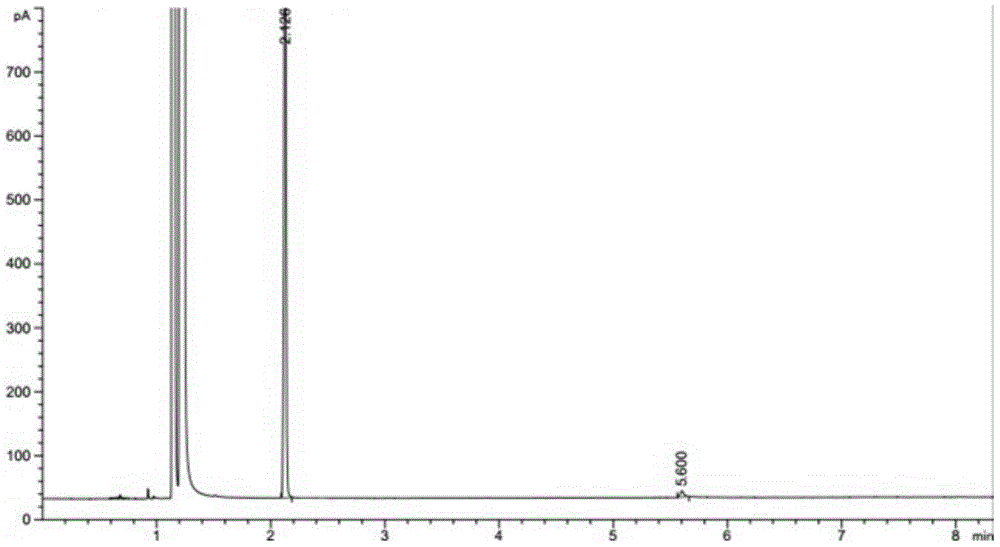
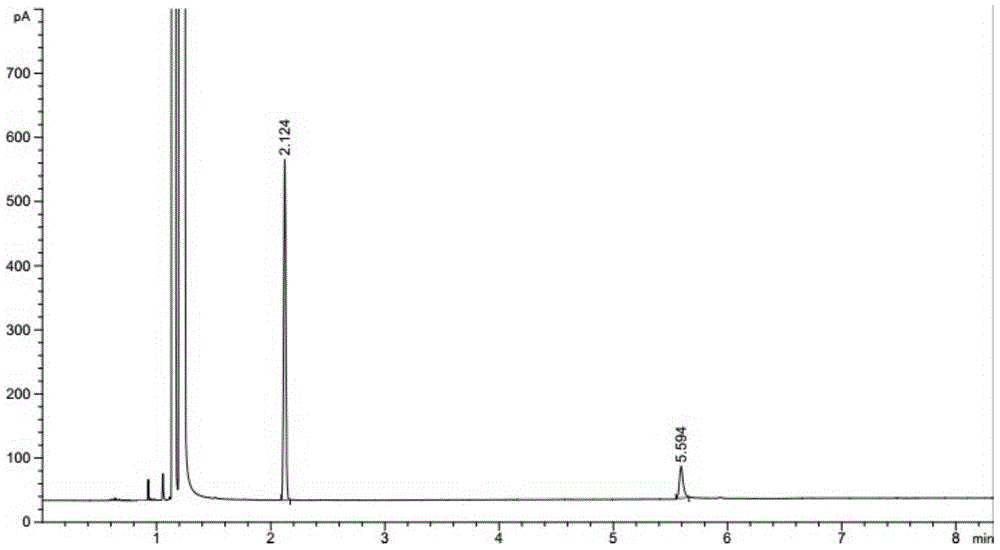
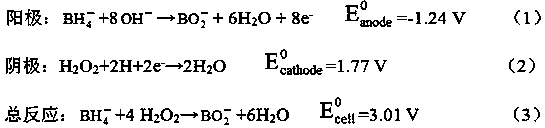Patents
Literature
77results about How to "Inhibition of hydrolysis reaction" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Sulfate-resistant organosilicone modified polycarboxylic superplasticizer and preparation method thereof
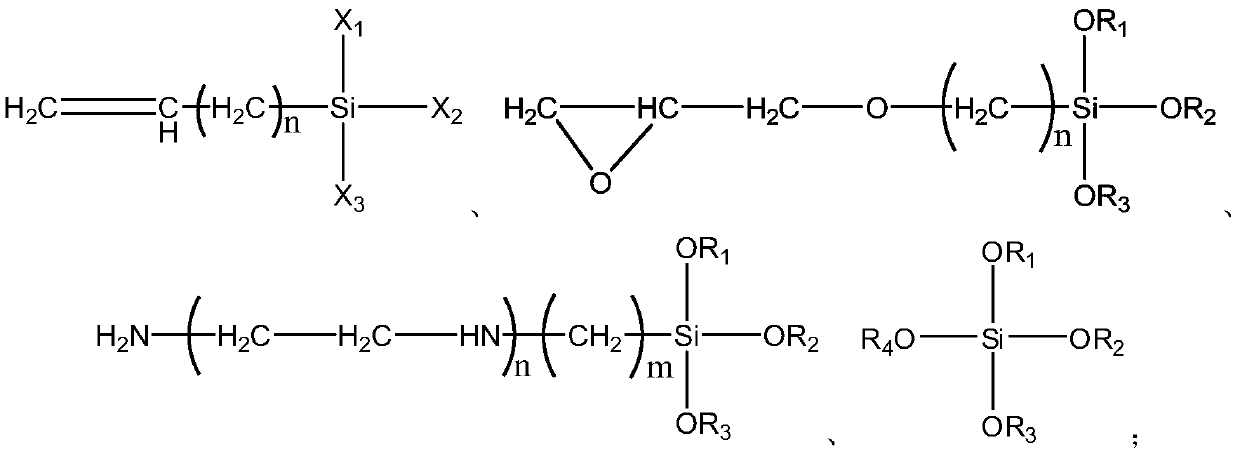
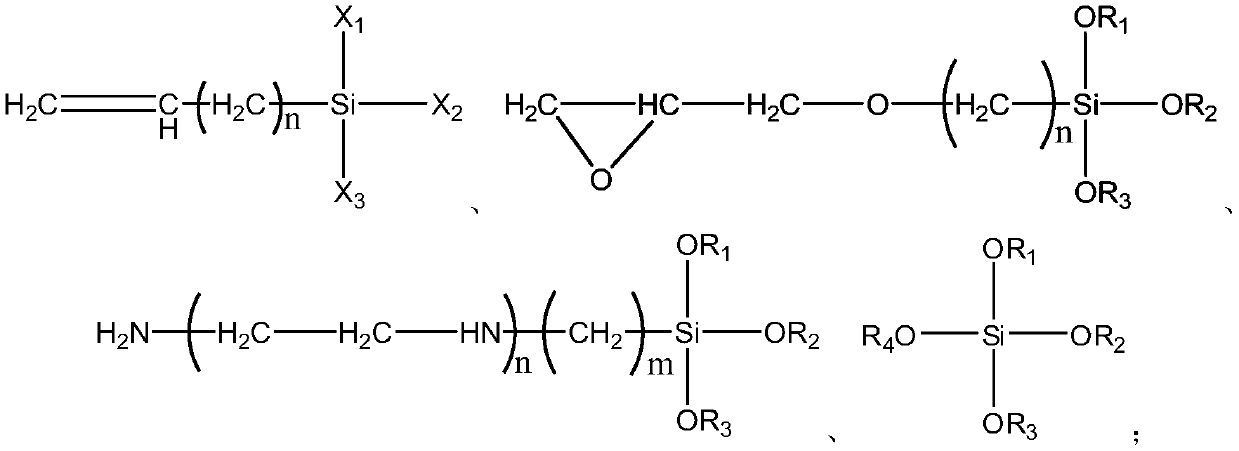
The invention relates to a sulfate-resistant organosilicone modified polycarboxylic superplasticizer and a preparation method thereof. A novel polycarboxylic superplasticizer is prepared from methoxy-polyoxyethylene (methyl) acrylic esters, organosilicone monomers, carboxylic acid monomers, carboxylic ester monomers and sulfonic acid monomers and the like through free radical polymerization. According to the invention, organosilicone monomers and sulfonic acid monomers are adopted for copolymerization; and by introduced silane bonds and silicate, strong chemical bonds are formed so as to resist the competitive adsorption action of sulfate-resistant ions, thereby reaching the purpose of improving a situation that the polycarboxylic superplasticizer is incompatible with a concrete material with high sulphate content.
Owner:CHINA UNIV OF MINING & TECH (BEIJING)
Low-smoke halogen-free flame-retardant silane crosslinked cable material capable of being crosslinked at room temperature and preparation method of cable material
InactiveCN104262883AReduce performanceLow smoke productionPlastic/resin/waxes insulatorsElastomerLinear low-density polyethylene
The invention relates to a low-smoke halogen-free flame-retardant silane crosslinked cable material capable of being crosslinked at the room temperature and a preparation method of the cable material. The method is characterized by comprising the steps as follows: firstly, a twin-screw mixing extruder is used for mixing polyolefin elastomers and linear low-density polyethylene with part of unsaturated silane and part of a grafting initiator respectively and extruding the mixtures into particles; then a high-speed mixer is used for mixing the two kinds of prepared particles with ethylene-vinyl acetate copolymer resin, functional polyolefin resin, a flame retardant, an antioxidant and a processing aid; and finally, the twin-screw mixing extruder is used for extruding the mixture into particles, so that the low-smoke halogen-free flame-retardant silane crosslinked cable material capable of being crosslinked at the room temperature is obtained. The cable material is high in silane grafting rate and high in crosslinking activity, crosslinking can be directly performed at the room temperature when the cable material is prepared into wires and cables, time-consuming and energy-consuming procedures such as water boiling, sauna and the like can be avoided, a crosslinking method is simple and easy to implement, and more than half of the crosslinking time can be saved even when a water boiling or steam sauna crosslinking method is adopted. The cable material is low in cost, high in production efficiency, easy to operate and free from structural limits when being used for producing the wires and the cables, and the product performance is stable and reliable after crosslinking.
Owner:WUXI JAKE PLASTIC
Low-conductivity cooling liquid and preparation method thereof
ActiveCN109762642AGuaranteed storage stabilityAvoid hydrolysisLubricant compositionCorrosionOrganosilicon
The invention relates to a low-conductivity cooling liquid and a preparation method thereof, and belongs to the field of chemical engineering. The total mass of each component in a cooling liquid formula is 100%, and the low-conductivity cooling liquid is prepared from the components in percentage by mass: 30-80% of dihydric alcohol, 0.05-5.0% of organosilicon compound, 0.01-2.0% of nitrogen-containing compound, 0-1.0% of azole-based compound and the balance of deionized water. The organosilicon compound, the nitrogen-containing compound and the azole-based compound are uniformly mixed and then added into the dihydric alcohol for normal temperature stirring for more than 20min, and finally the deionized water is added for uniform mixing to obtain the low-conductivity cooling liquid. The low-conductivity cooling liquid has good effect of corrosion inhibition on a multi-metal system, has better storage and using stability, and can be used for cooling and heat exchanging of fuel cells, electric automobile motors and wind power generators.
Owner:中国船舶集团有限公司第七一八研究所
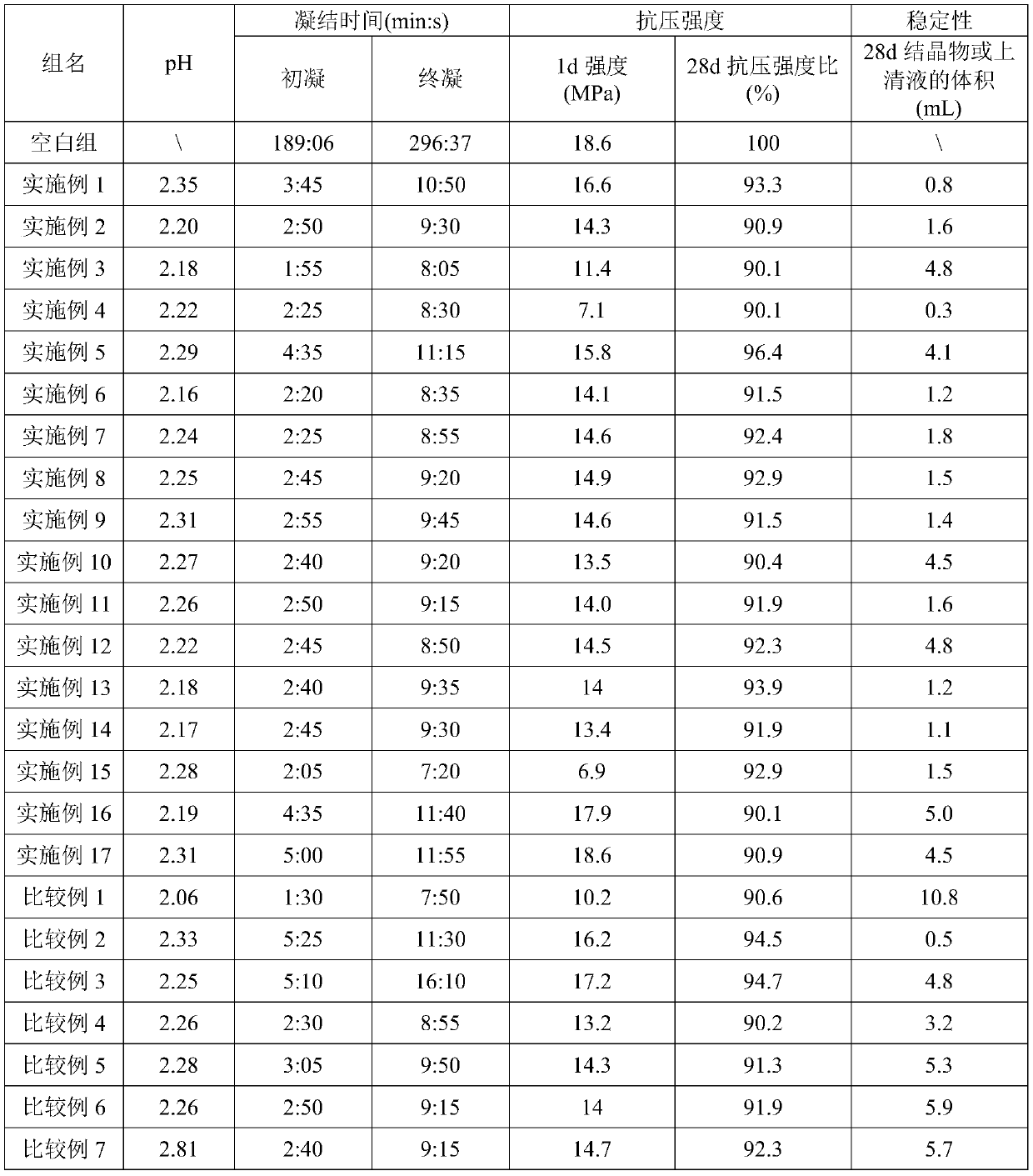
Aluminum sulfate supersaturated suspended alkali-free liquid accelerator and preparation method thereof
ActiveCN110922085AInhibition of hydrolysis reactionIncrease crystallization resistanceAluminum IonSulfate radicals
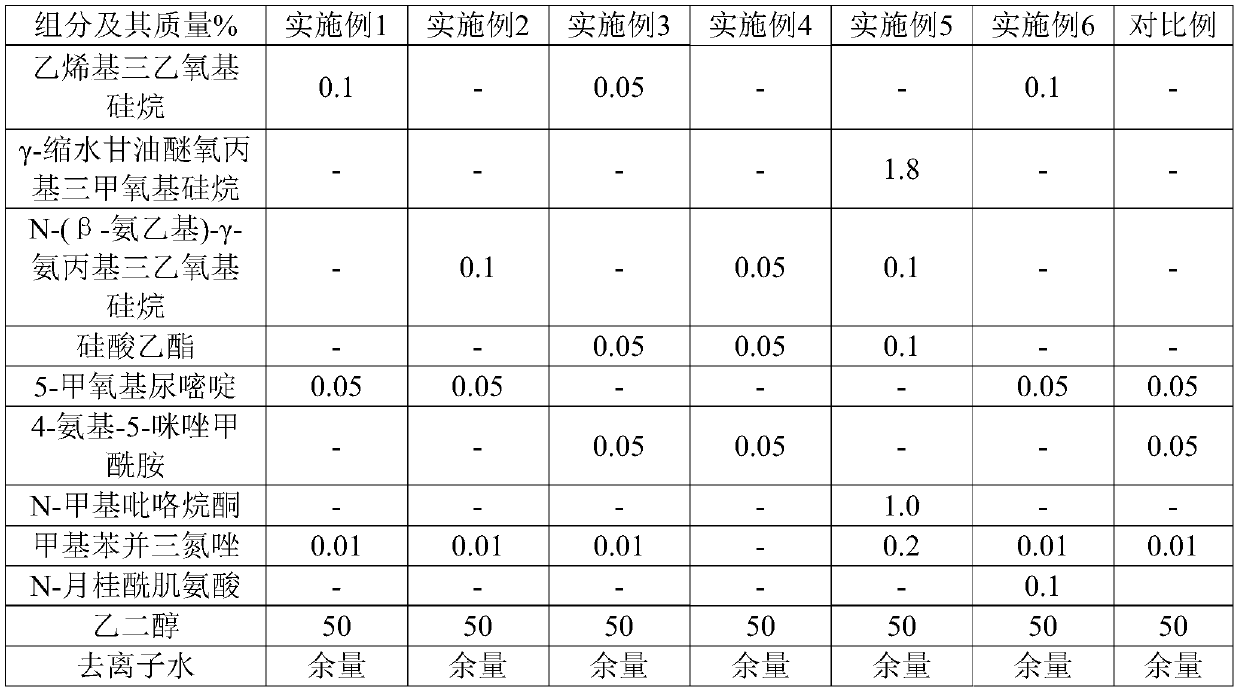
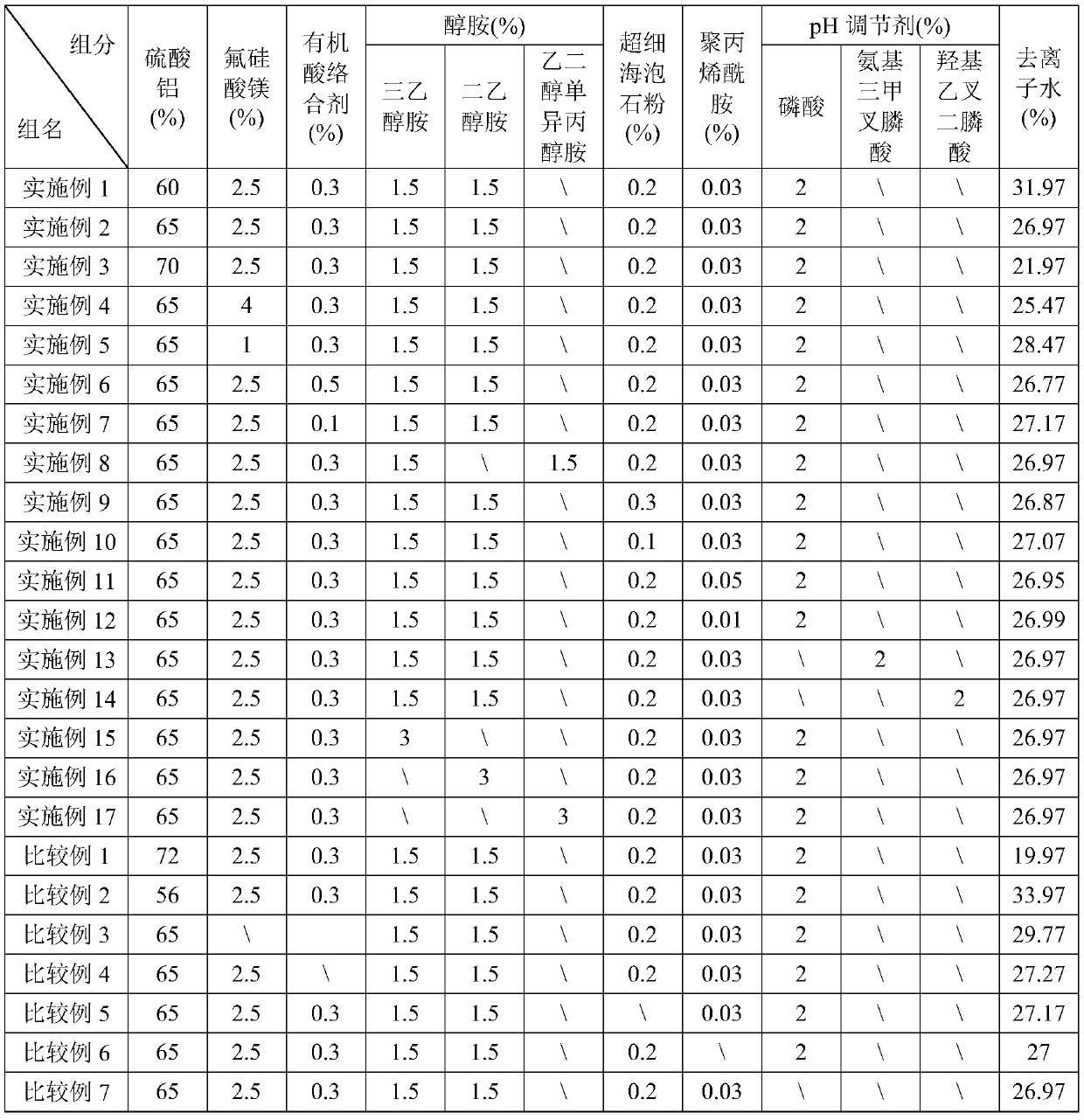
The invention belongs to the field of accelerator preparation, and relates to an aluminum sulfate supersaturated suspended alkali-free liquid accelerator and a preparation method thereof. The alkali-free liquid accelerator comprises 60-70 wt% of aluminum sulfate, 1-4 wt% of magnesium fluosilicate, 0.1-0.5 wt% of an organic acid complexing agent, 1-3 wt% of alkylol amine, 0.1-0.3 wt% of superfine sepiolite powder, 0.01-0.05 wt% of polyacrylamide, 0.5-2 wt% of a pH regulator, and the balance of deionized water. The synergistic effects of multiple components can inhibit the hydrolysis reaction ofAl<3+> and enhance the crystallization resistance of sulfate radicals and aluminum ions, and obviously increases the effective aluminum sulfate content of the alkali-free liquid accelerator, the aluminum sulfate proportion reaches up to 60-70%, and the final product is an aluminum sulfate supersaturated solution, so that the addition amount of the alkali liquid accelerator is reduced; the alkali-free liquid accelerator has the advantages of good stability and long storage period, and can be stored at normal temperature stably for 8 months or above.
Owner:武汉优城科技有限公司
Method for synthesizing polyepoxysuecinic acid salt
InactiveCN101538362AReduce hydrolysis rateIncrease polymerization rateScale removal and water softeningCalcium hydroxideFiltration
The invention discloses a method for synthesizing a polyepoxysuecinic acid salt, which is characterized by comprising the following steps of: dissolving maleic anhydride in deionized water; adding a solution of sodium hydroxide at 30 to 60 DEG C with stirring; adding sodium tungstate and hydrogen peroxide when the temperature is controlled to be between 60 to 100 DEG C; adjusting the pH value to 5 to 7 for reaction for 2 to 100 hours; cooling the reaction solution to below 20 DEG C; adding a precipitator, mixing and stirring the precipitator and the solution; performing suction filtration, adding water into filtrate obtained after suction filtration; keeping the pH value between 6 and 7; heating the filtrate added with water to 70 to 100 DEG C to obtain an epoxysuecinic acid salt; preparing a 0.2 to 4.0 mol / L aqueous solution of the epoxysuecinic acid salt; adding calcium hydroxide and a polymerization promoter into the aqueous solution for performing polymerization reaction till liquid turns yellowish and pasty; and cooling the yellowish and pasty liquid to room temperature to obtain the polyepoxysuccinic acid salt. The method has the advantages of good product quality, low raw material cost and product scale inhibition performance advantageous over that of the prior art.
Owner:北京合创同盛科技有限公司
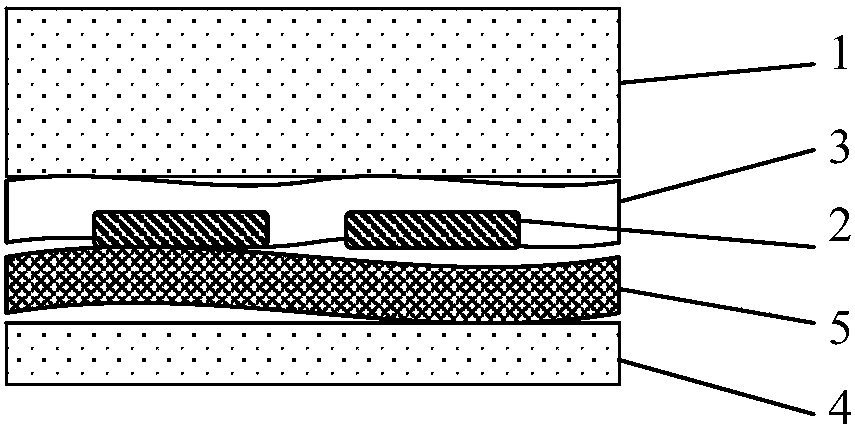
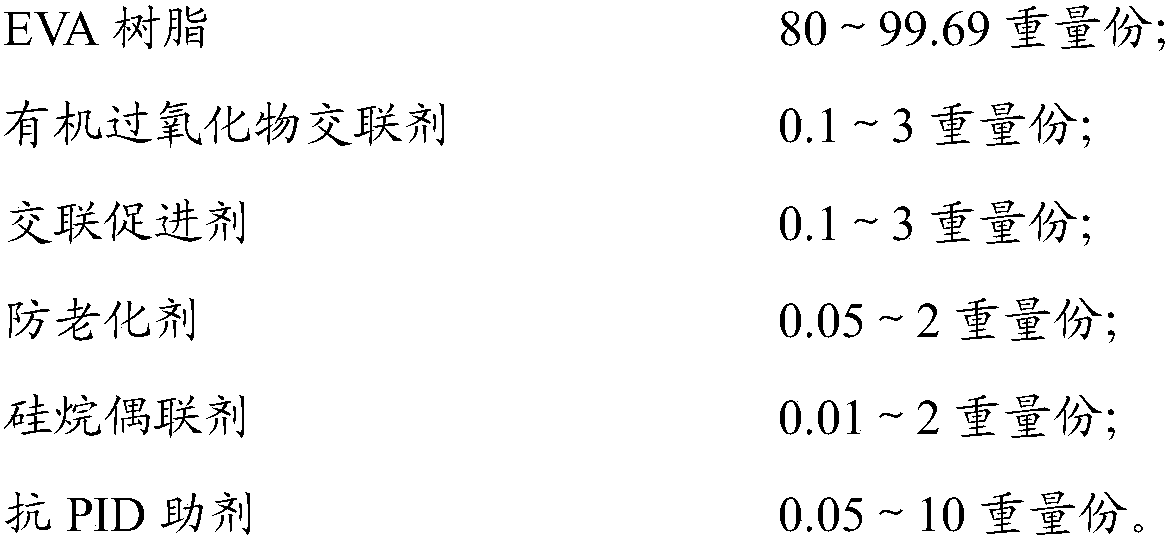
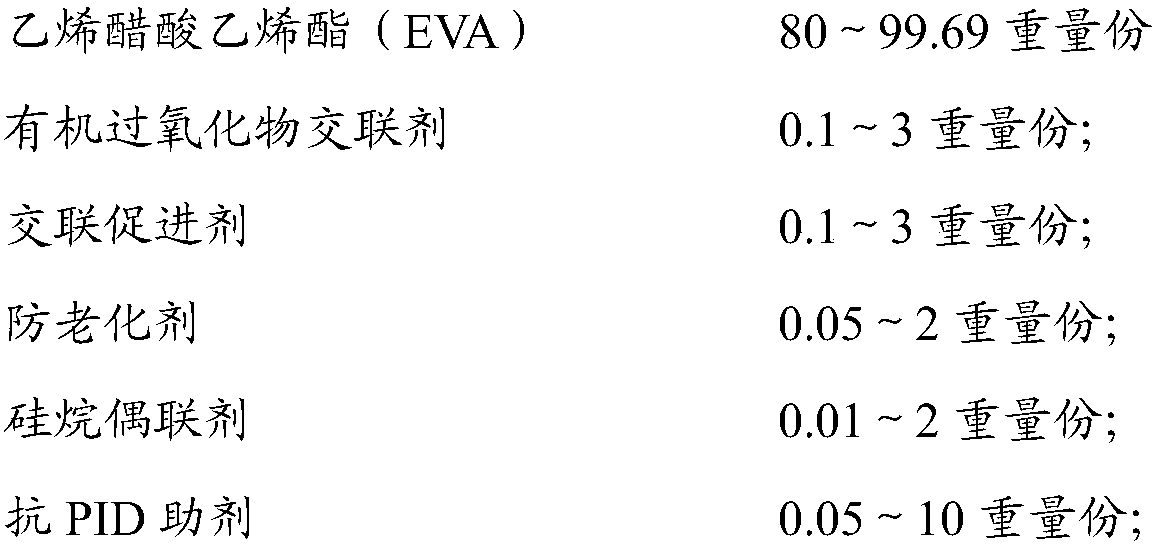
Anti-PID ethylene vinyl acetate film, package assembly and packaging method
ActiveCN109554141AReduce hydrolysisInhibition of hydrolysis reactionNon-macromolecular adhesive additivesMacromolecular adhesive additivesCarbon chainHydrolysis
The invention relates to an anti-PID ethylene vinyl acetate film comprising the following components: 80-99.69 parts by weight of EVA resin; 0.1-3 parts by weight of an organic peroxide crosslinking agent; 0.1 to 3 parts by weight of a cross-linking accelerator; 0.05 to 2 parts by weight of an anti-aging agent; 0.01 to 2 parts by weight of a silane coupling agent; and 0.05 to 10 parts by weight ofan anti-PID auxiliary agent. The invention also provides a package assembly and a packaging method thereof. The anti-PID auxiliary agent of the anti-PID film is resin having a carbon chain and a branched chain containing a plurality of hydroxyl groups, the structure of the resin is similar to the structure of a hydrolyzed product of the EVA resin, progress of EVA hydrolysis reaction can be suppressed, the EVA hydrolysis reaction can be effectively reduced, the crosslink density of the EVA film can be ensured, ions on the surface of the EVA and glass can be effectively blocked from accumulating on the surface of a battery, and the PID phenomenon of the assembly can be suppressed.
Owner:SHANGHAI HIUV NEW MATERIALS
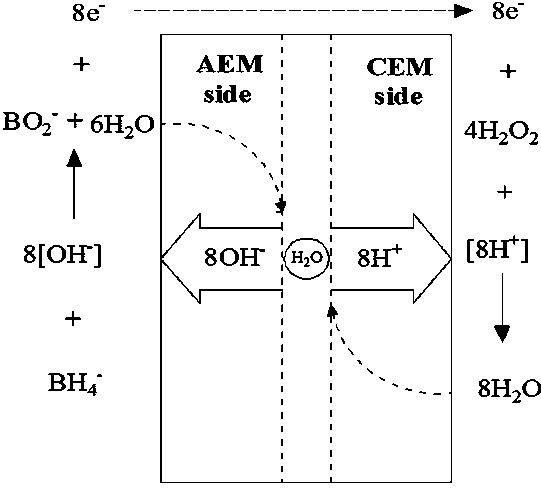
Bipolar membrane type direct borohydride fuel cell
ActiveCN107706435APrevent penetrationPrevent arrivalElectrolyte holding meansSolid state electrolyteInterface layer
Disclosed is a bipolar membrane type direct borohydride fuel cell. The bipolar membrane type direct borohydride fuel cell comprises at least one cell unit; each cell unit consists of two layers of solid-state electrolyte membranes with ion selectivity transmission property; the solid-state electrolyte membranes are divided into two parts by the bipolar membrane; and the bipolar membrane adopts a three-layer structure which includes a cation exchange membrane layer, an anion exchange membrane layer and an intermediate interface layer positioned between the two exchange membrane layers. By adoption of the bipolar membrane type direct borohydride fuel cell, the negative electrode and the positive electrode can adopt non-uniform electrolytes, and a mixed potential caused by permeation of an intermediate product generated by the positive electrode fuel or the positive electrode to the negative electrode can be prevented; and meanwhile, permeation of a negative electrode product to the positive electrode also can be prevented, polarization loss of the electrode is lowered, the mixed potential is eliminated, and the fuel utilization rate and the output power are improved.
Owner:TAIYUAN UNIV OF TECH
Metal-free boron-doped-charcoal-material hydrogen peroxide (H2O2) electroreduction catalyst and preparation method thereof
ActiveCN104084226AWide variety of sourcesLow pricePhysical/chemical process catalystsCell electrodesElectrochemistryBoron atom
The invention provides a metal-free boron-doped-charcoal-material hydrogen peroxide (H2O2) electroreduction catalyst and a preparation method thereof. The method comprises the steps of uniformly mixing triphenyl borane and a charcoal material according to the mass ratio of (3-5):(97-95), milling the mixture for 3-5 hours in a ball mill at the speed of 3,000-5,000r / min, putting the milled mixture in a heating furnace, firstly introducing argon gas for 10 minutes, reacting for 5-6 hours at the temperature of 750-850 DEG C under the protection of the argon gas, and cooling to room temperature under the protection of the argon gas, thereby obtaining the metal-free boron-doped-charcoal-material H2O2 electroreduction catalyst. According to the metal-free boron-doped-charcoal-material H2O2 electroreduction catalyst and the preparation method thereof, a B-C bond is formed through substituting carbon with electron-deficient boron, and superfluous positive charges are generated nearby a boron atom, so that H2O2 adsorption is facilitated; the raw materials are wide in source and are cheap; the boron-doped activated charcoal catalyst is high in electrochemical activity, good in stability and strong in toxicity resisting capability; due to the absence of precious metals or transition metals, the occurrence of H2O2 hydrolysis reaction can be inhibited, and the generation of oxygen gas is reduced; the electro-oxidation property and utilization ratio of H2O2 are greatly improved.
Owner:HARBIN ENG UNIV
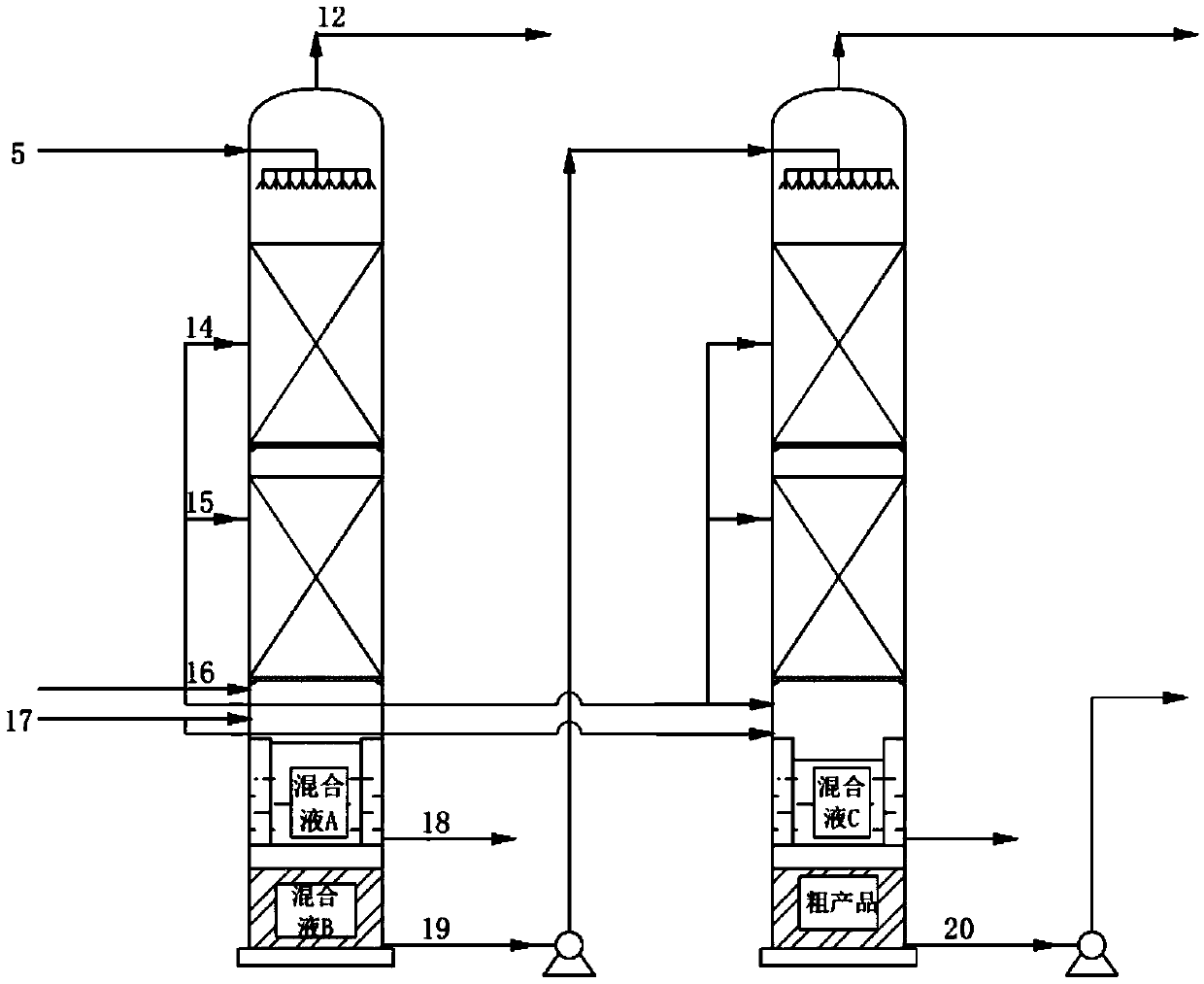
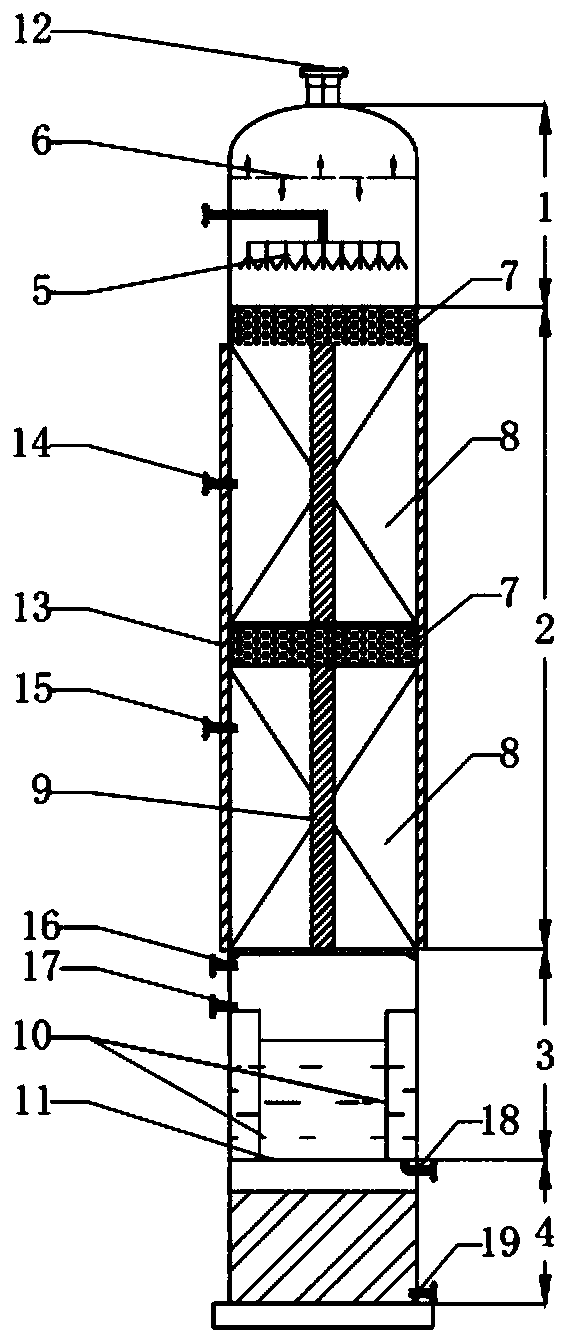
Technology and device for synthesizing dimethyl carbonate
ActiveCN109534999AIncrease contentReduce energy consumptionOrganic compound preparationPreparation from carbon monoxide and oxygenMethyl carbonateTower
A technology for synthesizing dimethyl carbonate adopts a two-tower continuous reaction, and comprises the following steps: a raw material liquid methanol is fed from the upper part of a gas and liquid distributor of a first reactor, flows out of the gas and liquid distributor and enters the reaction section of a reactor, a raw material gas CO is introduced to the first reactor from the bottom ofthe reaction section of the reactor, O2 is divided into 2-5 passages, O2 in the 2-5 passages except one is distributed in the reaction section of the reactor, a reaction is continuously carried out under the action of a catalyst in the reaction section to generate dimethyl carbonate, a formed mixed liquid A flows into the water separation section of the first reactor, methanol and dimethyl carbonate in the mixed A go through a bottom hydrophobic membrane and continuously downwards flow into a tower bottom to form a mixed liquid B, water permeates a surrounding hydrophilic membrane, and then iscollected and discharged, the mixed liquid B in the bottom of the first reactor enters a second reactor, a crude product is extracted from the bottom of the second reactor, and non-condensable gasesCO, O2 and CO2 obtained after the reactions in the first reactor and the second reactor are discharged from the top of the reactors. The technology has the advantages of simple process, high methanolconversion rate and low energy consumption.
Owner:潞安化工集团有限公司 +1
Preparation method of anhydrous magnesium chloride
InactiveCN107500319AInhibition of hydrolysis reactionAvoid the problem of severe corrosion equipmentMagnesium chloridesHydrolysisBischofite
The invention discloses a preparation method of anhydrous magnesium chloride. The preparation method comprises the following steps: S1, mixing bischofite with ammonium chloride to obtain a dehydration raw material; S2, enabling the dehydration raw material to be subjected to primary dehydration at 180-240 DEG C and secondary dehydration at 250-300 DEG C to obtain a crude product of the anhydrous magnesium chloride; S3, heating the crude product of the anhydrous magnesium chloride to a temperature, which is not lower than 250 DEG C, under an protective atmosphere after white smoke around the crude product of the anhydrous magnesium chloride disappears, and performing heat preservation for at least 2h to obtain the anhydrous magnesium chloride, wherein in the steps S2 and the S3, no gas overflows from the reaction system. According to the preparation method disclosed by the invention, the ammonium chloride serves as a protective agent, ammonia gas and hydrogen chloride gas, which are generated after the ammonium chloride is heated and decomposed, are utilized, the hydrogen chloride gas can inhibit hydrolysis reaction, and the ammonia gas can be used for replacing water molecules in hydrated magnesium chloride, so as to further facilitate the obtaining of the anhydrous magnesium chloride. The preparation method has the advantages of simple and easy performing and low cost, and the problem of seriously corroded equipment caused by a pure hydrogen chloride gas method in a high-temperature airtight condition is avoided.
Owner:QINGHAI INST OF SALT LAKES OF CHINESE ACAD OF SCI
Preparation method of dioctyl sodium sulfosuccinate
ActiveCN103709078AReduce water productionInhibition of hydrolysis reactionSulfonic acids salts preparationSulfonic acid preparationMaleic anhydrideFine chemical
The invention belongs to the technical field of fine chemical production, and discloses a preparation method of dioctyl sodium sulfosuccinate. The preparation method mainly comprises the steps of an esterification stage: implementing alcoholysis reaction to isooctanol and maleic anhydride, adding a catalyst and a color fixative, implementing primary dual-esterification reaction at 115 DEG C, increasing temperature to 185-205 DEG C and carrying out reaction to generate diisooctyl maleate; and a sulfonation stage: implementing reflux reaction to diisooctyl maleate, sodium hydrogen sulfite and water, and removing residual isooctanol in a refluxing process to obtain dioctyl sodium sulfosuccinate. The preparation method disclosed by the invention is low in isooctanol addition, thus reducing raw material cost, and is free from vacuum reaction and isooctanol removal under high vacuum, so as to greatly reduce requirement on equipment; the isooctanol is removed in the sulfonation reaction, thus effectively reducing residue. The prepared dioctyl sodium sulfosuccinate is light in color, small in smell, good in wettability and low in isooctanol content.
Owner:广州星业科技股份有限公司
Method for making low-temperature refined pickled lard oil
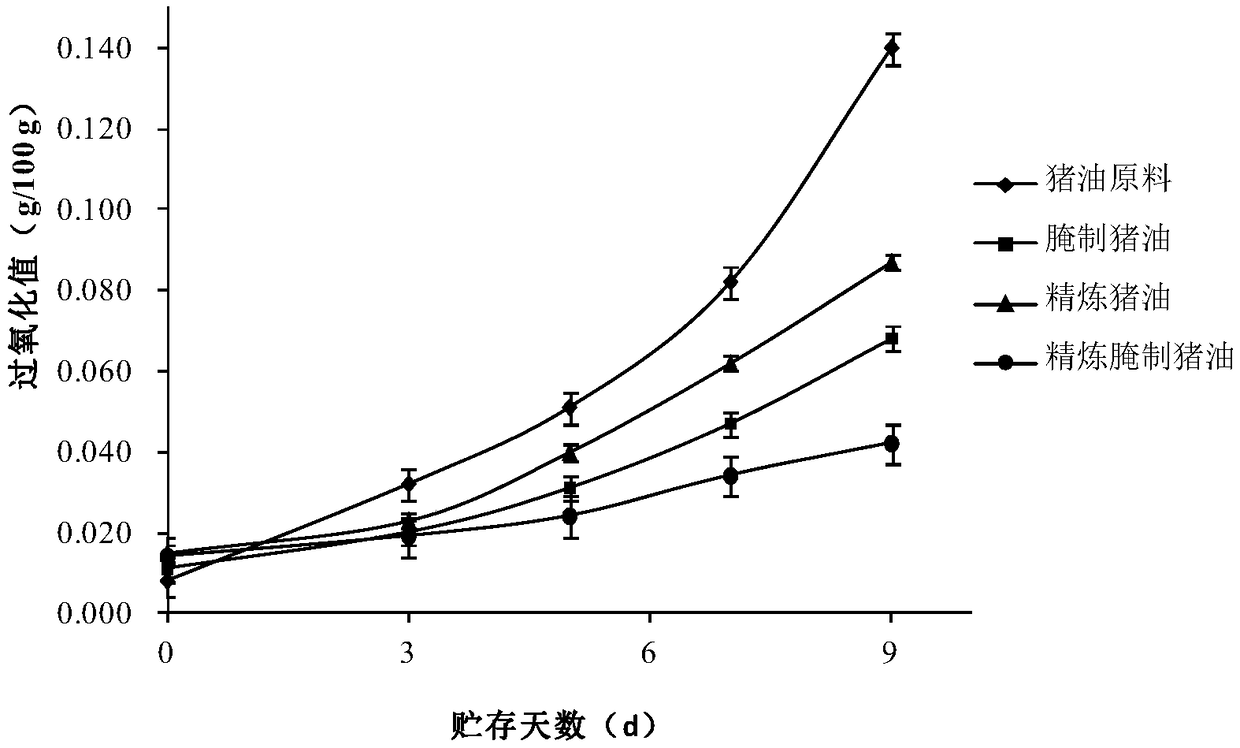
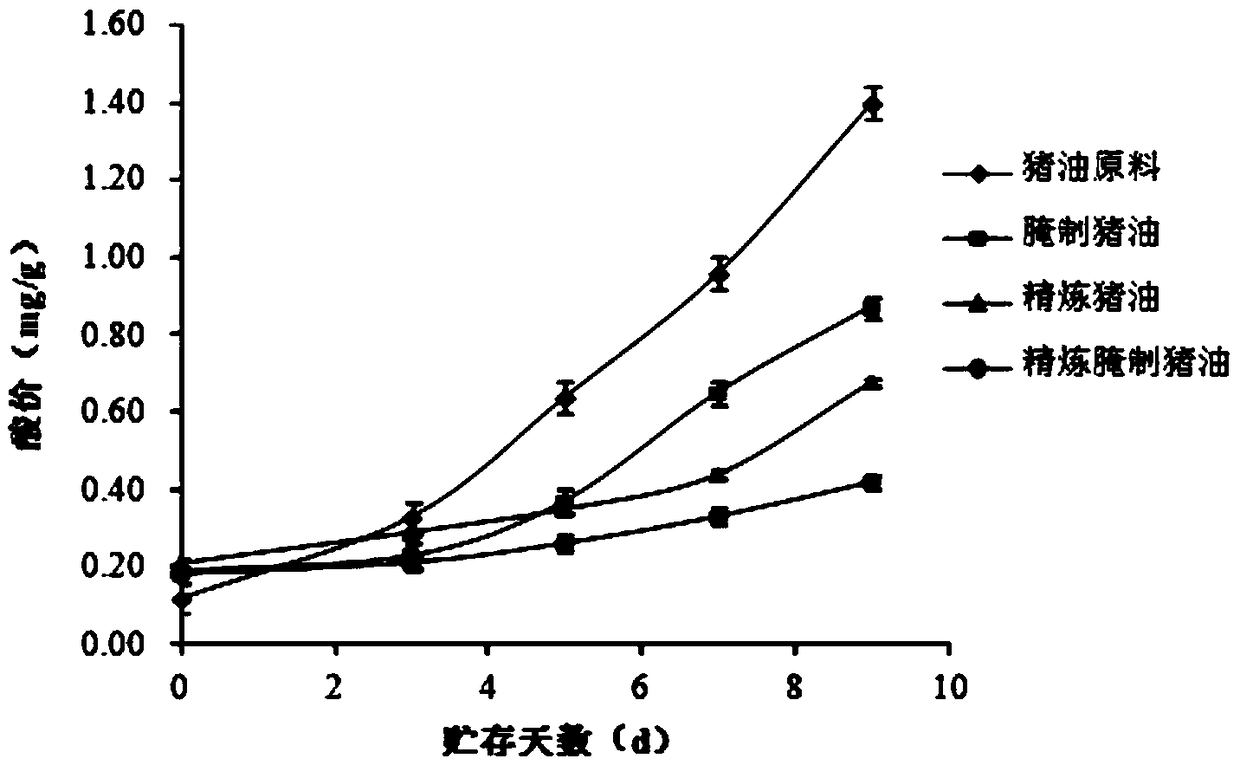
PendingCN109055002AImprove qualitySure seepFatty oils/acids recovery from wasteFatty substance recoveryAcid valuePeroxide value
The invention belongs to the technical field of lard oil processes and in particular relates to a method for making low-temperature refined pickled lard oil. The method comprises the following steps:selecting raw materials, trimming and cleaning, cutting into pieces, pickling, carrying out vacuum refining, separating oil from residues, filtering, filling, preserving, and the like, thereby obtaining a product. By adopting the method, the problems that in the prior art, lard oil is liable to deterioration in the production process and a product cannot be quantitatively added can be solved; withthe combination of pickling and vacuum refining, the acid value and the peroxide value of a pickled lard oil product can be remarkably reduced, the preservation time of the lard oil can be effectively prolonged, and meanwhile, quantitative packaging and addition of the pickled lard oil product can be achieved.
Owner:INST AGRO PROD PROCESSING ANHUI ACADEMY AGRI SCI
Method for synthesizing polyepoxysuecinic acid salt
InactiveCN101538362BHigh degree of polymerizationGood for anti-scaling performanceScale removal and water softeningCalcium hydroxideFiltration
The invention discloses a method for synthesizing a polyepoxysuecinic acid salt, which is characterized by comprising the following steps of: dissolving maleic anhydride in deionized water; adding a solution of sodium hydroxide at 30 to 60 DEG C with stirring; adding sodium tungstate and hydrogen peroxide when the temperature is controlled to be between 60 to 100 DEG C; adjusting the pH value to 5 to 7 for reaction for 2 to 100 hours; cooling the reaction solution to below 20 DEG C; adding a precipitator, mixing and stirring the precipitator and the solution; performing suction filtration, adding water into filtrate obtained after suction filtration; keeping the pH value between 6 and 7; heating the filtrate added with water to 70 to 100 DEG C to obtain an epoxysuecinic acid salt; preparing a 0.2 to 4.0 mol / L aqueous solution of the epoxysuecinic acid salt; adding calcium hydroxide and a polymerization promoter into the aqueous solution for performing polymerization reaction till liquid turns yellowish and pasty; and cooling the yellowish and pasty liquid to room temperature to obtain the polyepoxysuccinic acid salt. The method has the advantages of good product quality, low raw material cost and product scale inhibition performance advantageous over that of the prior art.
Owner:北京合创同盛科技有限公司
Metal-free sulfur-doped carbon material hydrogen peroxide reduction catalyst and preparation method
ActiveCN104051748AWide variety of sourcesLow priceMaterial nanotechnologyPhysical/chemical process catalystsElectrochemistryNitrogen gas
The invention provides a metal-free sulfur-doped carbon material hydrogen peroxide reduction catalyst and a preparation method. The preparation method is characterized by comprising the following steps: putting benzyl disulfide and a carbon material into sufficient ethanol according to a mass ratio of (5-6): (95-94), ultrasonically treating the mixture for 0.5 to 1 hour, centrifuging the mixture to remove the upper substances, vacuum drying the mixture for 5 to 6 hours at the temperature of 50 to 60 DEG C to form solid powder, putting the solid powder into a high-temperature furnace to be reacted for 7 to 8 hours at the temperature of 800 to 900 DEG C under the protection of nitrogen, cooling the solid powder to room temperature under the protection of nitrogen to obtain the sulfur-doped carbon material hydrogen peroxide reduction catalyst. The sulfur is used for substituting carbon to form S-C bonds to form an activity center of the electroreduction of H2O2. The source of the raw material is wide, and the price is low. Not only is the electrochemical activity high, but also the stability is high, and the toxicity resistance is high. The hydrolysis reaction of the H2O2 can be prevented, and the oxygen can be reduced. The electric oxidation performance and utilization rate of the H2O2 can be greatly improved.
Owner:HARBIN ENG UNIV
Production method of sulfoacid firming agent for casting sand mold ink-jet printing resin
The invention relates to a production method of a firming agent, and in particular relates to a production method of a sulfoacid firming agent for casting sand mold ink-jet printing resin. The production method is characterized by comprising the following steps: turning off a discharging valve at the bottom of a reaction kettle, starting a power supply, turning on a methylbenzene feeding valve, sequentially adding methylbenzene and anhydrous sodium sulfate, starting a stirrer, and meanwhile slowly adding concentrated sulfuric acid; after feeding materials, raising temperature and refluxing; preserving the reaction temperature and removing 10-25 parts of water, subsequently turning on a methylbenzene recovery valve, turning off a reflux valve, adding de-ionized water, and decreasing temperature when methylbenzene is recovered to 40-96 parts; when the temperature of material liquid is reduced to 70 DEG C, refilling the de-ionized water which is evaporated when evaporating methylbenzene; when the temperature of the material liquid is reduced to 40 DEG C, sampling and inspecting, and discharging materials after the materials are qualified. The method disclosed by the invention is unique, the corrosion of the firming agent can be remarkably weakened, the technological requirements of the firming agent for 3D resin are achieved, the rate of a sulfonation reaction is improved and a sulfurizing defect on casting surface is remarkably relieved.
Owner:NINGXIA KOCEL MOLD
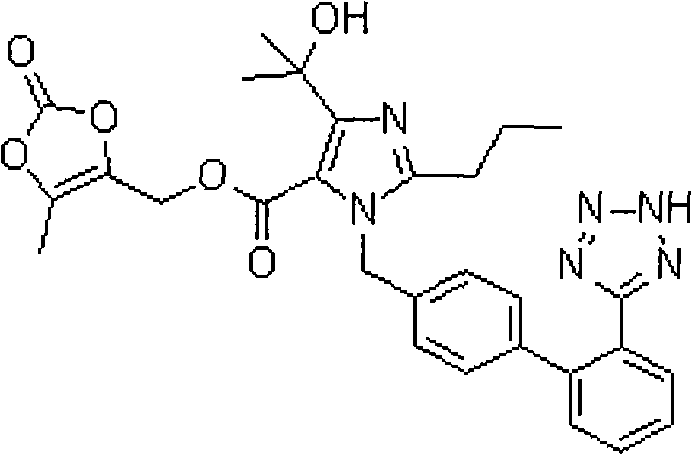
Stable olmesartan medoxomil solid preparation
ActiveCN102028663APrevent oxidationAvoid hydrolysisOrganic active ingredientsPill deliverySafety effectivenessChemistry
The invention provides a stable olmesartan medoxomil solid preparation and a preparation method thereof. Olmesartan medoxomil is unstable within a certain pH range and is easy to undergo hydration reaction. The hydrolysis product as an impurity is retained in the medicine, thereby influencing the safety effectiveness of the medicine. A pH adjusting agent and a stabilizing agent are added in a prescription, active components of the preparation are ensured to be free from undergoing the hydration reaction in the production process, the impurities are avoided, and the quality of the products and the medicine application safety of patients can be ensured.
Owner:BEIJING WINSUNNY PHARMA CO LTD
Freeze-drying protective agent for nucleic acid-lipid nanoparticles as well as preparation method and application of freeze-drying protective agent
ActiveCN114557971AImprove integrityInhibition of hydrolysis reactionPowder deliveryViral antigen ingredientsFreeze-dryingNanoparticle
Based on physical and chemical principles of a freeze drying process, freeze drying conditions, namely freeze drying procedures and freeze drying protective agents, of the nucleic acid-lipid nanoparticles are systematically studied, and an efficient freeze drying method suitable for the nucleic acid-lipid nanoparticles is preferably designed. According to the method, the total time of a nucleic acid-lipid nanoparticle freeze-drying process can be shortened to 8-18 hours, the energy consumption and the time cost of product amplified production are remarkably reduced, the freeze-dried nucleic acid-lipid nanoparticles are rapid in rehydration (within 10 seconds) and high in nucleic acid total amount, entrapment efficiency and nucleic acid integrity, and in addition, the freeze-dried nucleic acid-lipid nanoparticles have good application prospects. The cell transfection efficiency of the freeze-dried and rehydrated preparation is not significantly different from that of an unfreeze-dried nucleic acid-lipid nanoparticle stock solution, and the in-vivo immune response is high and even exceeds that of the unfreeze-dried nucleic acid-lipid nanoparticle stock solution.
Owner:CANSINO BIOLOGICS INC +1
Modified hydrotalcite and solar battery back panel
ActiveCN107163880AImprove hydrolysis resistanceInhibition of hydrolysis reactionNon-macromolecular adhesive additivesFilm/foil adhesivesWater vaporEngineering
The invention provides preparation of an organic modified hydrotalcite and application of the organic modified hydrotalcite to a solar battery back panel. The solar battery back panel is excellent in hydrothermal aging resistance, fire resistance and barrier performance. The solar battery back panel is composed of a weather-resisting layer, an insulating layer and a fluorine-containing bonding coating, wherein the fluorine-containing bonding coating is formed by coating a fluorine-containing paint, which is prepared from fluorocarbon resin, the organic modified hydrotalcite, polyurethane acrylate (PUA) and isocyanate. By means of the modified hydrotalcite applied in the fluorine-containing bonding coating, the solar battery back panel is good in EVA (ethylene-vinyl acetate copolymer) initial bonding performance and hydrothermal-aged bonding performance and obtains excellent fire resistance, weather resistance and water vapor barrier property.
Owner:乐凯胶片股份有限公司
Catkin hollow carbonization tube composite precious metal fuel-cell catalyst and preparation method of catkin hollow carbonization tube
ActiveCN104795576AIncrease profitSimple preparation processCell electrodesElectrochemical responseCatkin
The invention provides a catkin hollow carbonization tube composite precious metal fuel-cell catalyst and a preparation method of a catkin hollow carbonization tube. The preparation method of the catkin hollow carbonization tube comprises the steps of: adding catkins to acetone, and carrying out ultrasonic treatment; violently stirring and washing by using distilled water; carrying out vacuum drying; adding the treated catkins to a potassium hydroxide solution, grinding into powder after drying by distillation, feeding the powder into a tubular furnace, heating, then naturally cooling after heat preservation to obtain the catkin hollow carbonization tube. The catkin hollow carbonization tube is put in precious metal solution-containing electrodeposition liquid and subjected to electrolytic deposition so as to obtain the catkin hollow carbonization tube composite precious metal fuel-cell catalyst. The catkin hollow carbonization tube composite precious metal fuel-cell catalyst can inhibit the hydrolysis reaction of an oxidizing agent and fuels and reduce the generation of gases. Lots of voids exist on the tube wall of the catkin hollow carbonization tube and can provide channels for electrolyte, and electrochemical reactions happen through catalysis of precious metals. Oxygen generated through hydrolysis can be sealed in the catkin hollow carbonization tube, reacts continuously and does not release O2, and the electrochemical property and utilization rate of an oxidizing agent and fuels are greatly increased.
Owner:HARBIN ENG UNIV
Novel method for terminating allyl alcohol irregular polyether methyl
InactiveCN106565949AIncrease mass transfer rateIncreased mass transfer rates over conventional stirred tank reactorsEther preparation by ester reactionsIsomerizationKinetic control
The invention discloses a novel method for terminating allyl alcohol irregular polyether methyl. The novel method comprises the following steps of: adding allyl alcohol irregular polyether raw materials through a material inlet in a jetting loop reactor based on Venturi effect, deoxidizing and introducing inert gases for replacement and protection, and starting an external circulating pump to carry out high-speed jetting mixing; and at a control temperature ranging from 20 DEG C to 70 DEG C, slowly adding a methane chloride raw material and an alkali raw material into the jetting loop reactor through a methane chloride raw material feeding hole and a material feeding hole separately, and the like. According to the novel method disclosed by the invention, this system belongs to a dynamic control system, side reactions are restrained sufficiently, a low-temperature method is further adopted to carry out dynamic reaction control according to the characteristic that activation energy of side reactions is high, hydrolysis reaction of methane chloride is restrained, and isomerization reaction of allyl is further restrained, and therefore, selectivity of a reaction system is higher. Finally obtained allyl alcohol irregular polyether methyl terminated product has color and luster smaller than 30, and has a terminating rate greater than 98%.
Owner:ZHEJIANG UNIVERSITY OF SCIENCE AND TECHNOLOGY +1
Preparation method of carbon surface modified hydrogen storage alloy and method for improving anode catalytic performance of direct borohydride fuel cell
The invention provides a preparation method of a carbon surface modified hydrogen storage alloy and a method for improving the anode catalytic performance of a direct borohydride fuel cell. The preparation method includes: mixing hydrogen storage alloy powder and carbon evenly, then placing the mixture into a ball milling tank, conducting ball milling for 1-2h at a speed of 3000-5000rpm under the protection of nitrogen, then taking the mixture out, gradually adding a 60wt.% polytetrafluoroethylene solution and a 1.5wt.% sodium carboxymethylcellulose colloid that are in a mass ratio of 1:1, keeping the mass ratio of the hydrogen storage alloy powder and the carbon quality to the polytetrafluoroethylene solution and the sodium carboxymethyl cellulose at 9-10:1, and stirring them uniformly to prepare a slurry containing the hydrogen storage alloy and carbon, coating foamed nickel with the slurry, and carrying out drying for 2.5h at 70DEG C, thus obtaining the carbon surface modified hydrogen storage alloy, which can be used for improving the anode catalytic performance of a direct borohydride fuel cell.
Owner:HARBIN ENG UNIV
Non-aqueous electrolyte containing cyano cyclic amine compound, lithium ion battery and application thereof
ActiveCN114678592AInhibition of hydrolysis reactionSpeed up water removalSecondary cellsOrganic electrolytesElectrolytic agentOrganic solvent
The invention discloses a non-aqueous electrolyte containing a cyano cyclic amine compound, a lithium ion battery and application of the non-aqueous electrolyte and the lithium ion battery, the non-aqueous electrolyte comprises electrolyte lithium salt, an organic solvent and a functional additive, the functional additive is the cyano cyclic amine compound shown in the formula I, and the structural formula of the formula I is shown in the specification; wherein n1 is greater than or equal to 0 and is a natural number; n2 > = 1 and is a natural number. According to the invention, the cyano-containing cyclic amine compound is adopted as a functional additive, due to the existence of cyano, the cyano-containing cyclic amine compound can be easily combined with water molecules and can be combined with lithium salt molecules before water and an organic solvent in the electrolyte, so that the hydrolysis reaction of lithium hexafluorophosphate in the electrolyte is inhibited, and the water removal capacity and the acid generation inhibition capacity of the non-aqueous electrolyte are improved. Besides, the cyano-containing cyclic amine compound is an amine compound, and due to the characteristics of Lewis base, the cyano-containing cyclic amine compound can be combined with hydrofluoric acid existing in the electrolyte to generate fluorine ammonium salt, so that the purpose of removing free hydrofluoric acid is achieved.
Owner:SHENZHEN ORI TECH CO LTD
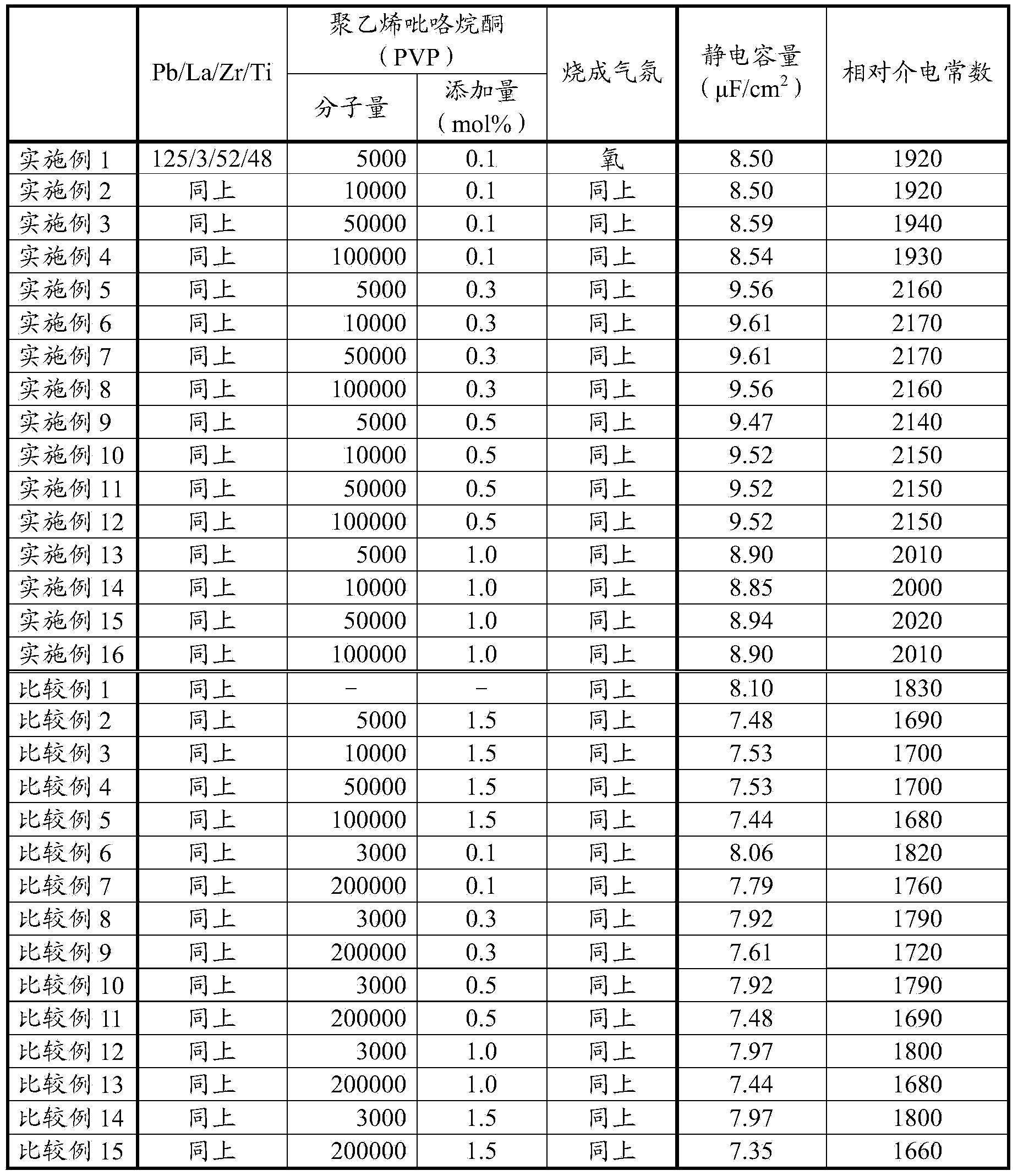
Composition for forming ferroelectric thin film, ferroelectric thin film and forming method thereof, and composite electronic assembly
ActiveCN103664169AIncrease capacityHigh densitySemiconductor/solid-state device manufacturingLiquid/solution decomposition chemical coatingLead zirconate titanateFerroelectric thin films
Cracking does not occur in a ferroelectric thin film even when Ce is not doped in a composition for forming ferroelectric thin films and a composition for forming relatively thick ferroelectric thin films contains lead acetate instead of lead nitrate. A ferroelectric thin film made of a titanate lead-based perovskite film or a titanate zirconate lead-based complex perovskite film is formed using the composition for forming ferroelectric thin films. The composition includes lead acetate, a stabilizing agent made of lactic acid and polyvinyl pyrrolidone. In addition, a monomer-equivalent molar ratio of polyvinyl pyrrolidone to a perovskite A site atom included in the composition is more than 0 to less than 0.015. Furthermore, a weight average molecular weight of the polyvinyl pyrrolidone is 5000 to 100000.
Owner:MITSUBISHI MATERIALS CORP
Modified carbon nano tube material and preparation method and application thereof
ActiveCN108502870AImprove adsorption capacityStable structureSludge treatmentCarbon compoundsEthylenediamineActivated carbon
The invention discloses a modified carbon nano tube material and a preparation method and application thereof. The modified carbon nano tube material comprises a carbon nano tube, the surface of the carbon nano tube is modified with ethylenediamine tetraacetic acid, and the mass content of carboxyl in the modified carbon nano tube is 3%-5%. The preparation method comprises the steps that oxidationtreatment is conducted on the carbon nano tube; an activated carbon nano tube intermediate is prepared; an activated ethylenediamine tetraacetic acid intermediate is prepared; the activated carbon nano tube intermediate is mixed with the activated ethylenediamine tetraacetic acid intermediate, and diethylenetriamine is added for preparing the modified carbon nano tube material. The modified carbon nano tube material has the advantages that the technology is simple, operation is easy, the reaction condition is mild and easy to control, the cost is low, less energy is consumed, and less time isconsumed. The modified material can stabilize refractory organic matter and heavy metal in bottom mud, the stabilization effect is significant, and the modified carbon nano tube material has a wide application prospect.
Owner:HUNAN UNIV
Sulfate-resistant organosilicone modified polycarboxylic superplasticizer and preparation method thereof
Owner:CHINA UNIV OF MINING & TECH (BEIJING)
Preparation method of Ni-loaded hollow carbon microsphere NaBH4 electro-oxidation catalyst
ActiveCN104084207AImprove electrocatalytic performanceReduce escapeCell electrodesMetal/metal-oxides/metal-hydroxide catalystsResin microsphereIon-exchange resin
The invention provides a preparation method of a Ni-loaded hollow carbon microsphere NaBH4 electro-oxidation catalyst. The method comprises the steps of (1) mixing styrene and divinylbenzene with deionized water, introducing N2 into the mixed solution, feeding CC14, and maintaining the temperature of 60 DEG C; after that, feeding K2S2O8 and NaCl, stirring, gradually heating up to 70 DEG C, and carrying out reaction for 10-12 hours to obtain an emulsion; cleaning by alcohol and centrifuging; carrying out vacuum drying for 8-10 hours at the temperature of 50 DEG C to obtain polystyrene microspheres; (2) putting the obtained polystyrene microspheres into dichloromethane, swelling for 30 minutes, feeding concentrated sulfuric acid, and carrying out reaction for 2-4 hours at the temperature of 80-90 DEG C; adjusting the pH value to be 10-12, and drying for 5-6 hours at the temperature of 50 DEG C to obtain sodium polystyrenesulfonate cation exchange resin microspheres; (3) soaking the obtained sodium polystyrenesulfonate cation exchange resin microspheres into NiCl solution for 8-10 hours, then taking out the product, and drying for 4-5 hours at the temperature of 50 DEG C to obtain Ni-loaded cation exchange resin microspheres; and (4) calcining the Ni-loaded cation exchange resin microspheres for 5-6 hours under the nitrogen atmosphere at the temperature of 650-750 DEG C to obtain the Ni-loaded hollow carbon microsphere catalyst. After the method is adopted, the electro-oxidation performance and the utilization rate of the NaBH4 can be greatly improved.
Owner:HARBIN ENG UNIV
Recycling method for recycling brominated aromatic hydrocarbon-containing hazardous waste
InactiveCN113321227AInhibition of hydrolysis reactionAvoid it happening againWater contaminantsWater/sewage treatment by neutralisationAqueous sodium hydroxideWaste treatment
The invention relates to the technical field of bromine-containing hazardous waste treatment, and particularly discloses a recycling method for recycling brominated aromatic hydrocarbon-containing hazardous waste. The method comprises the following steps: (1) pretreatment: separating inorganic bromine from organic bromine in the waste to obtain an upper-layer alkali washing liquid and a lower-layer waste liquid; (2) hydrolysis: controlling the temperature at 300-400 DEG C and the pressure at 20-30 MPa, adding a sodium hydroxide aqueous solution with the concentration of 20-30% into the lower-layer waste liquid in the step (1), and carrying out hydrolysis reaction; (3) separating; and (4) purifying to obtain a sodium bromide product. According to the method, the recycling rate of sodium bromide can reach 95% or above, the purity meets the first-grade product standard, the hydrolysis reaction time is greatly shortened, and the treatment efficiency is improved; and the waste liquid is prevented from being directly incinerated to generate a large amount of acid gas such as hydrogen bromide, corrosion to treatment equipment is reduced, and harmless and resourceful bromine treatment and recovery of the brominated aromatic hydrocarbon-containing hazardous waste are achieved.
Owner:GO HIGHER ENVIRONMENT GRP CO LTD +1
A kind of cyclodextrin-metal complex and its preparation method and application
ActiveCN112844485BEasy to makeThe material is cheap and non-toxicOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsMeth-Carbonate ester
The invention discloses a cyclodextrin-metal complex and its preparation method and application. The cyclodextrin-metal complex is obtained by adding hexamethylenetetramine and metal halides into an aqueous solution of cyclodextrin, stirring and reacting . The invention adopts a one-pot method to use water as a solvent, and utilizes renewable cyclodextrin, cheap hexamethylenetetramine and metal halides as raw materials to synthesize a cyclodextrin-metal complex with a hydrophobic active center for catalysis CO 2 Cyclic carbonate is synthesized by cycloaddition reaction with epoxide, and the activity of the cyclodextrin-metal complex hardly changes after repeated recycling.
Owner:HUNAN INSTITUTE OF ENGINEERING
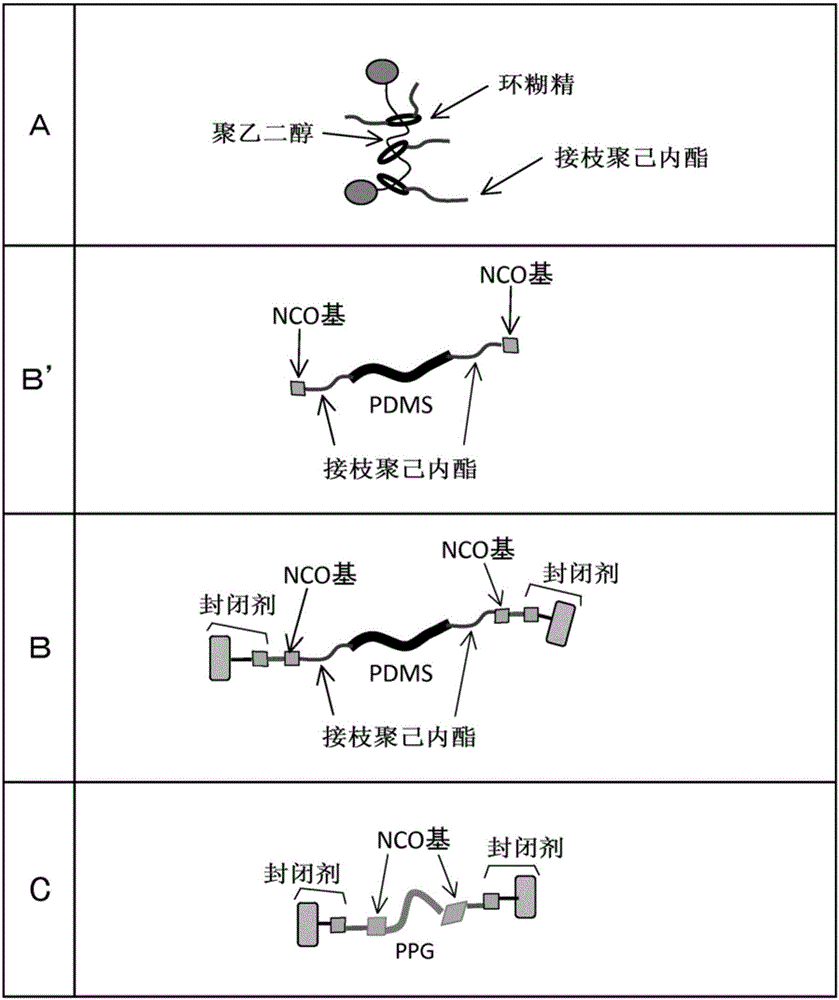
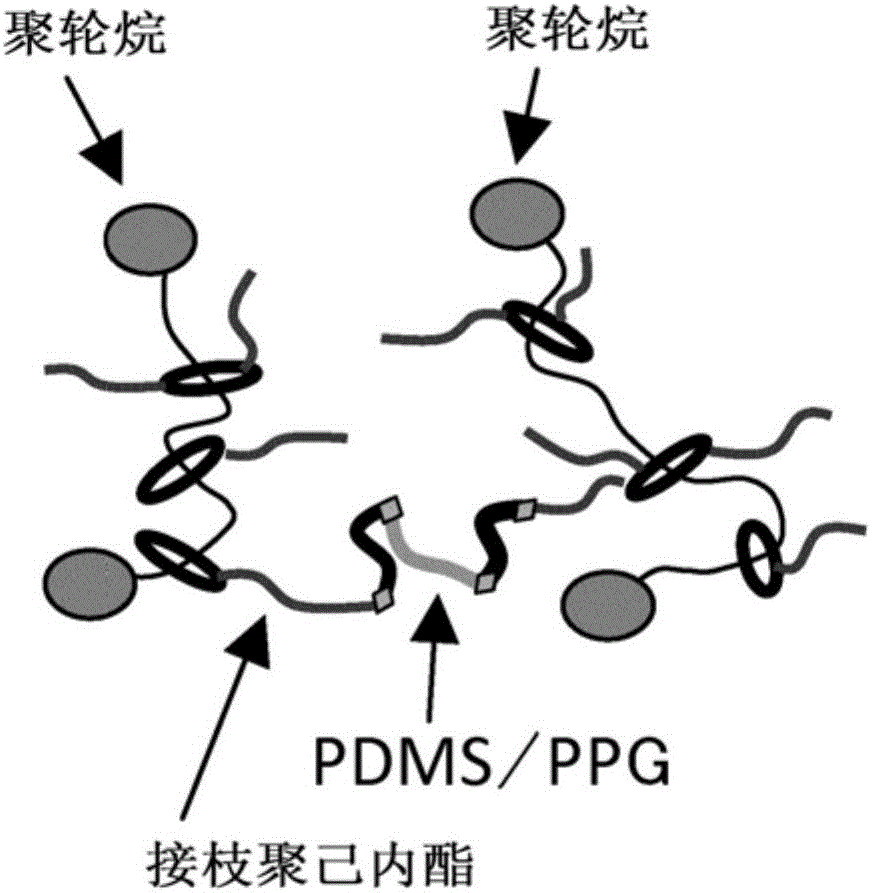
Composition Including Polyrotaxane And Utilization Object Thereof
ActiveCN106560485AInhibition of hydrolysis reactionImprove moisture resistanceConductive layers on insulating-supportsPiezoelectric/electrostrictive/magnetostrictive devicesPolyethylene glycolPolyrotaxane
In a composition including polyrotaxane and a utilization object thereof, the water molecules are difficult to mix, thereby suppressing the generation of a hydrolysis reaction, and improving the wet resistance. The composition including polyrotaxane comprises a polyrotaxane (A) which includes cyclodextrin as a ring molecule and polyethylene glycol as a linear molecule, and in which a blocking group is arranged at both ends of the linear molecule; a block copolymer (B) including polysiloxane; and a polymer (C) including no polysiloxane. In a curing body formed by the composition, a plurality of annular molecules of the polyrotaxane are combined by a crosslinking part, at least a part of the crosslinking part is the block copolymer (B) having the polysiloxane. A dielectric medium attached with an electrode layer uses a curing film formed by the curing body as the dielectric medium, and electrode layers are attached to the two surfaces of the curing film respectively. An actuator uses the dielectric medium attached with the electrode layers.
Owner:TOYODA GOSEI CO LTD +1
A kind of preparation method of dioctyl sodium sulfosuccinate
ActiveCN103709078BReduce generationReduce the total feed amountSulfonic acids salts preparationSulfonic acid preparationIsooctyl alcoholHydrogen
The invention belongs to the technical field of fine chemical production, and discloses a preparation method of dioctyl sodium sulfosuccinate. The preparation method mainly comprises the steps of an esterification stage: implementing alcoholysis reaction to isooctanol and maleic anhydride, adding a catalyst and a color fixative, implementing primary dual-esterification reaction at 115 DEG C, increasing temperature to 185-205 DEG C and carrying out reaction to generate diisooctyl maleate; and a sulfonation stage: implementing reflux reaction to diisooctyl maleate, sodium hydrogen sulfite and water, and removing residual isooctanol in a refluxing process to obtain dioctyl sodium sulfosuccinate. The preparation method disclosed by the invention is low in isooctanol addition, thus reducing raw material cost, and is free from vacuum reaction and isooctanol removal under high vacuum, so as to greatly reduce requirement on equipment; the isooctanol is removed in the sulfonation reaction, thus effectively reducing residue. The prepared dioctyl sodium sulfosuccinate is light in color, small in smell, good in wettability and low in isooctanol content.
Owner:广州星业科技股份有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com