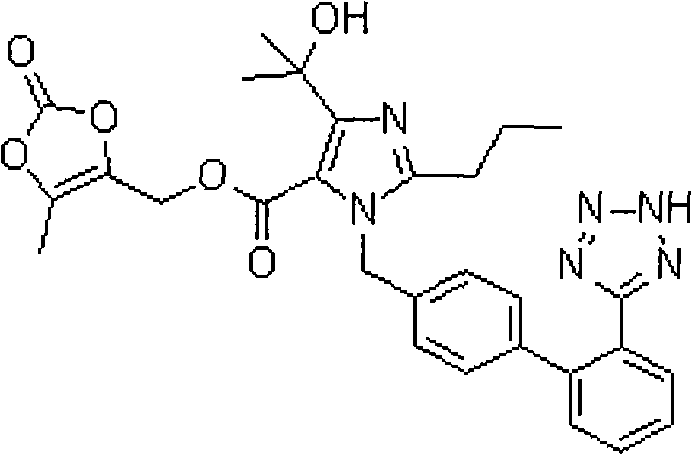
Stable olmesartan medoxomil solid preparation
A technology of olmesartan medoxomil and solid preparations, which is applied in the field of pharmaceutical preparations, can solve problems such as large influence, easy hydrolysis, and unstable properties, and achieve the effects of avoiding oxidation, preventing hydrolysis reactions, and ensuring stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
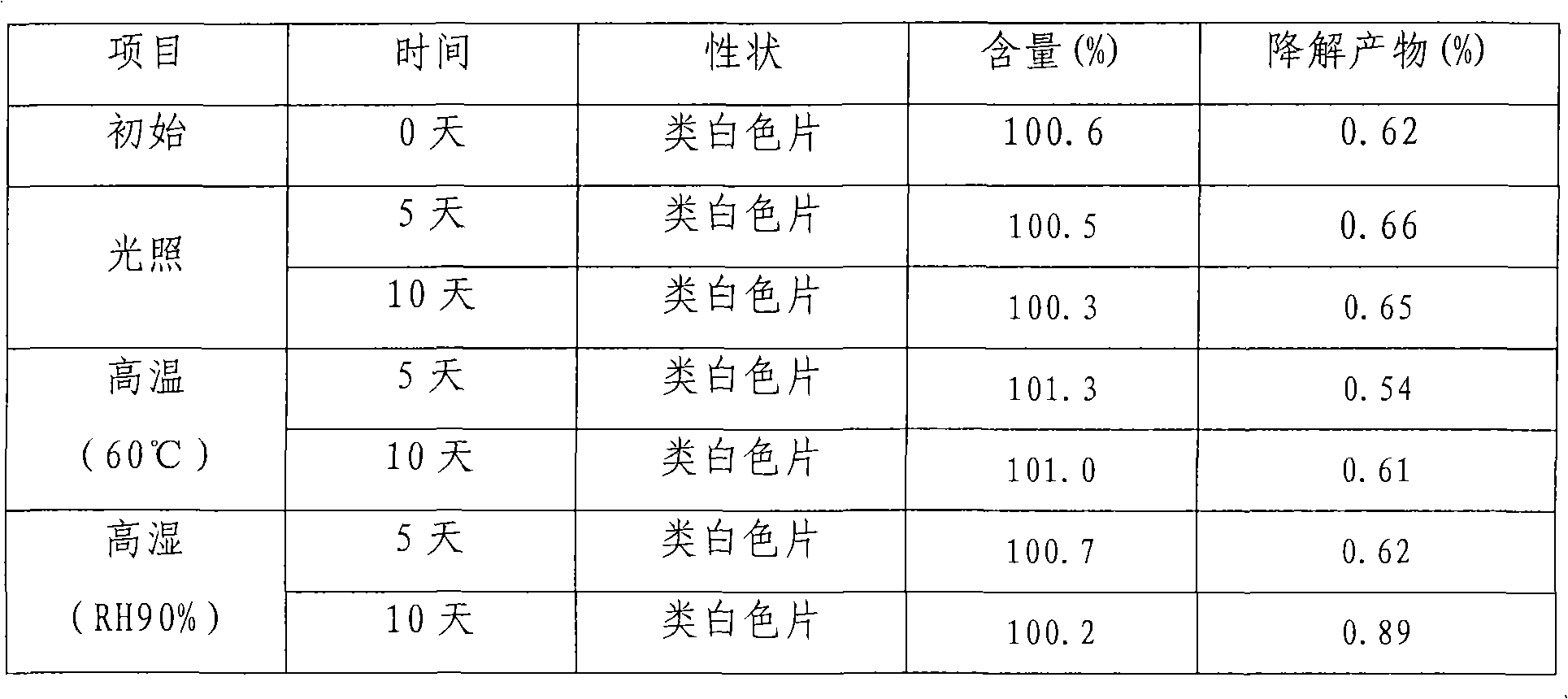
Embodiment 1
[0018] Olmesartan medoxomil 20g, BHA0.5g, BHT0.5g, citric acid 2g, lactose 60g, microcrystalline cellulose 100g, sodium carboxymethyl starch 10g, micropowder silica gel 5g, magnesium stearate 2g, absolute ethanol 60ml, poly Vitone 4.5g.
[0019] Preparation:
[0020] (1) Olmesartan medoxomil, lactose, microcrystalline cellulose, sodium carboxymethyl starch take by weighing prescription quantity, mix homogeneously;
[0021] (2) BHA, BHT, citric acid, and povidone are dissolved in absolute ethanol to make a binder solution;
[0022] (3) Add binder solution to the mixture obtained in (1), granulate, granulate, after drying, add silicon dioxide and magnesium stearate and mix uniformly;
[0023] (4) Determination of particle content, tableting.
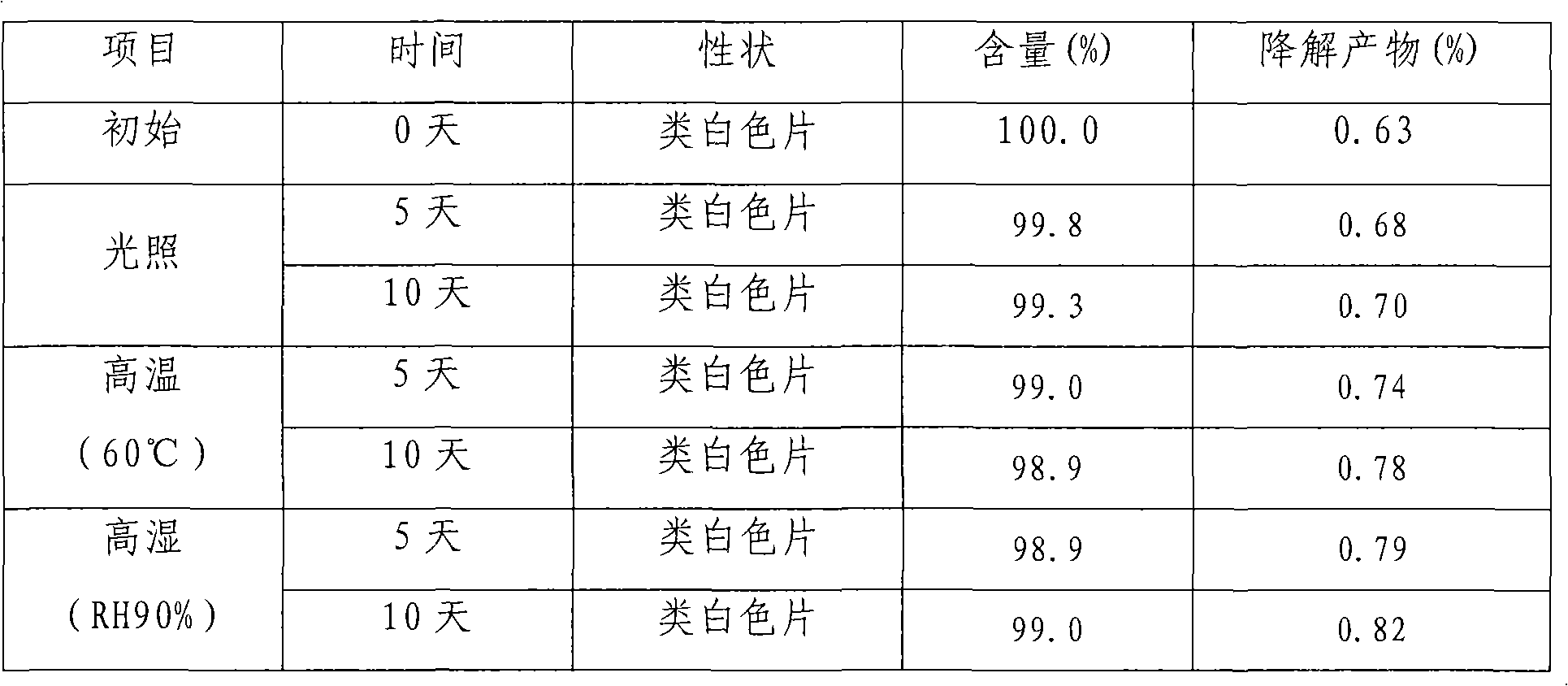
Embodiment 2
[0025] Olmesartan medoxomil 17g, BHA0.3g, BHT0.6g, citric acid 1.6g, lactose 54g, microcrystalline cellulose 98g, low-substituted hydroxypropyl cellulose 14g, micropowder silica gel 5g, absolute ethanol 60ml, povidone 4g.
[0026] Preparation method embodiment 1
Embodiment 3
[0028] Olmesartan medoxomil 20g, BHA 0.8g, malic acid 2.4g, lactose 30g, microcrystalline cellulose 115g, croscarmellose sodium 10g, crospovidone 5g, magnesium stearate 4g, absolute ethanol 70ml, povidone 5g.
[0029] Preparation method is as embodiment 1
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com