Freeze-drying protective agent for nucleic acid-lipid nanoparticles as well as preparation method and application of freeze-drying protective agent
A technology of lipid nanoparticles and drying protective agent, applied in the field of biomedicine, can solve the problems of energy consumption, increase the time and cost of product amplification and production, and achieve the effects of reducing energy consumption, inhibiting nucleic acid hydrolysis reaction, and high encapsulation rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Example 1 Preparation of Lyoprotectant
[0067] Prepare a lyoprotectant in a solvent according to the formula in Table 1, wherein the solvent is nuclease-free water, and filter and sterilize with a 0.22 μm sterile filter membrane. With the added amount of the lyoprotectant as 10% (w / w), the samples of Examples 1-1~1-12 and Comparative Examples 1-1~1-28 were used as the protective agent and the model sample lipid nanoparticles (ie mRNA- LNP) were mixed evenly, and then packed into vials; Comparative Example 1-29 was to mix the protective agent of Example 1-1 with the liposome sample (ie, nucleic acid-Lip), that is, to replace the model sample with the liposome sample Packed into vials.
[0068] Table 1 Prescription of lyoprotectant
[0069]
[0070]
[0071] Take 500 μl of samples from Examples 1-1~1-12 and Comparative Examples 1-1~1-29 into 3ml vials for freeze-drying. Freeze-drying procedure: pre-freezing stage: -60°C for 4 hours; sublimation drying stage: -45...
Embodiment 2
[0077] Example 2 Scanning Electron Microscope (SEM) Morphological Observation
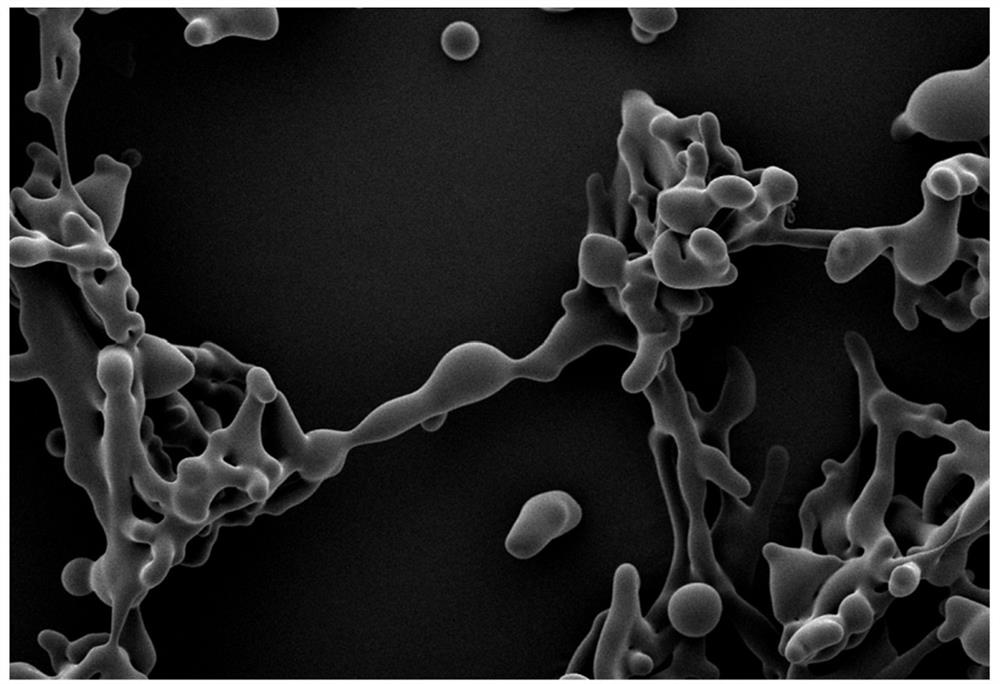
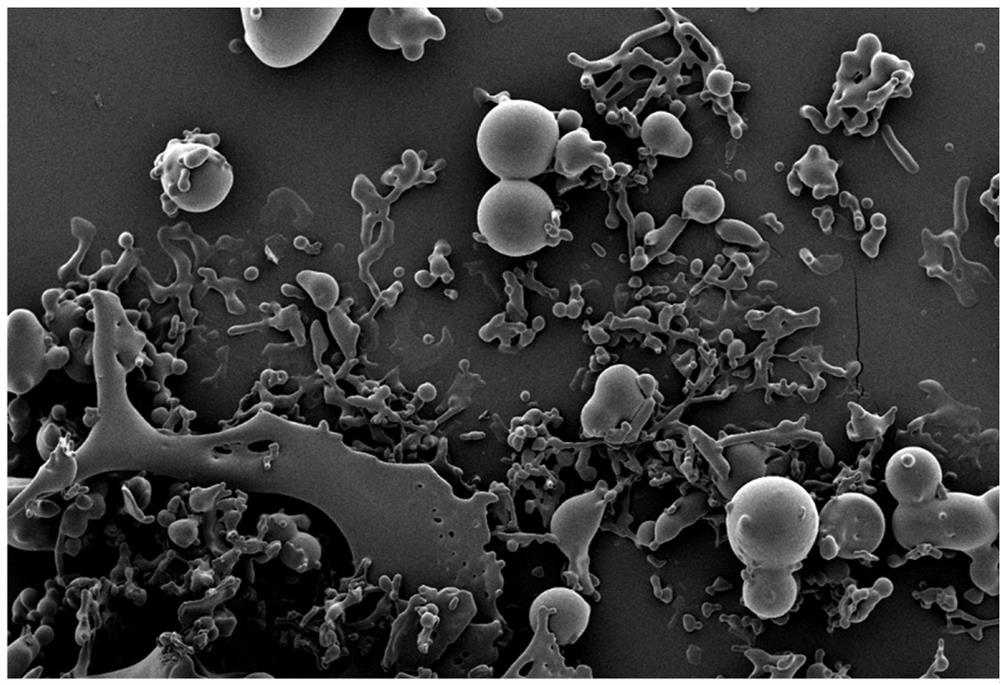
[0078] Scanning electron microscope (SEM) operation steps: use a needle to take a small amount of freeze-dried powder samples of Example 1-1 and Comparative Example 1-1 and place them on the conductive gel, and then use SEM to scan and image. For the results, see the attached Figure 2a , attached Figure 2b. It can be seen that Example 1-1 has a "ginger-shaped" wrapped scaffold microstructure, while in Comparative Example 1-1 no scaffold structure was formed between LNP and the protective agent, and the protective agent can only play the role of dispersive filling and reduce stress damage.
Embodiment 3
[0079] Example 3 Morphological observation by transmission electron microscope (TEM)
[0080] Dilute the model sample and Example 1-1, Example 1-4, Example 1-8, Example 1-12, and Comparative Example 1-10 by adding an appropriate amount of nuclease-free water, and then drip the diluted solution on the copper grid. For the sample, absorb the excess sample from the edge of the copper grid with filter paper, then add 2% phosphotungstic acid to stain for 5 seconds, absorb the excess phosphotungstic acid from the edge of the copper grid with filter paper, and use a transmission electron microscope to scan and image. The results are shown in the appendix Figure 3a , Figure 3b , Figure 3c , Figure 3d , Figure 3e , Figure 3f , visible embodiment 1-1 (see Figure 3b ), embodiment 1-4 (see Figure 3c ), Examples 1-8 (see Figure 3d ), Examples 1-12 (see Figure 3e ), Comparative Examples 1-10 (see Figure 3f ) have similar particle size distribution and microscopic morphol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com