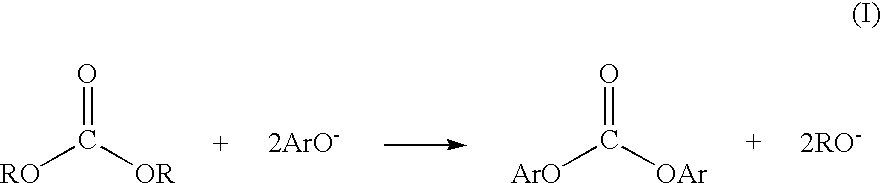
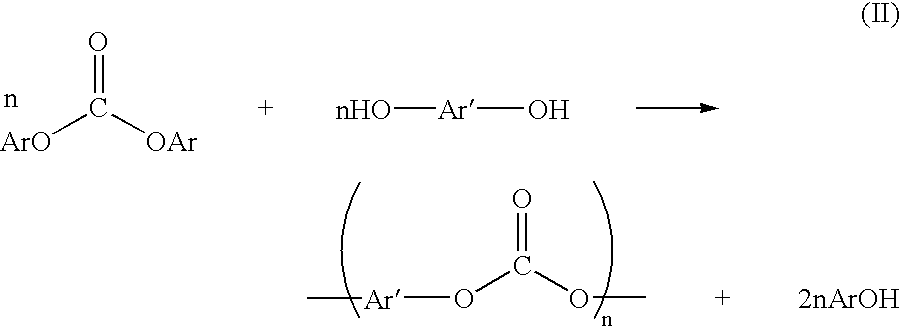
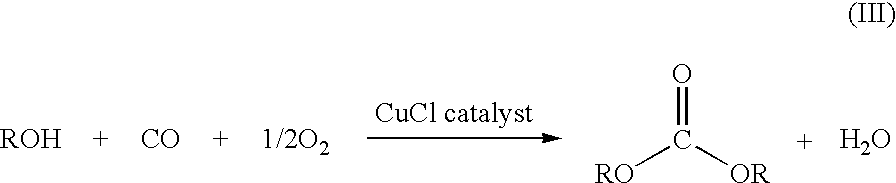
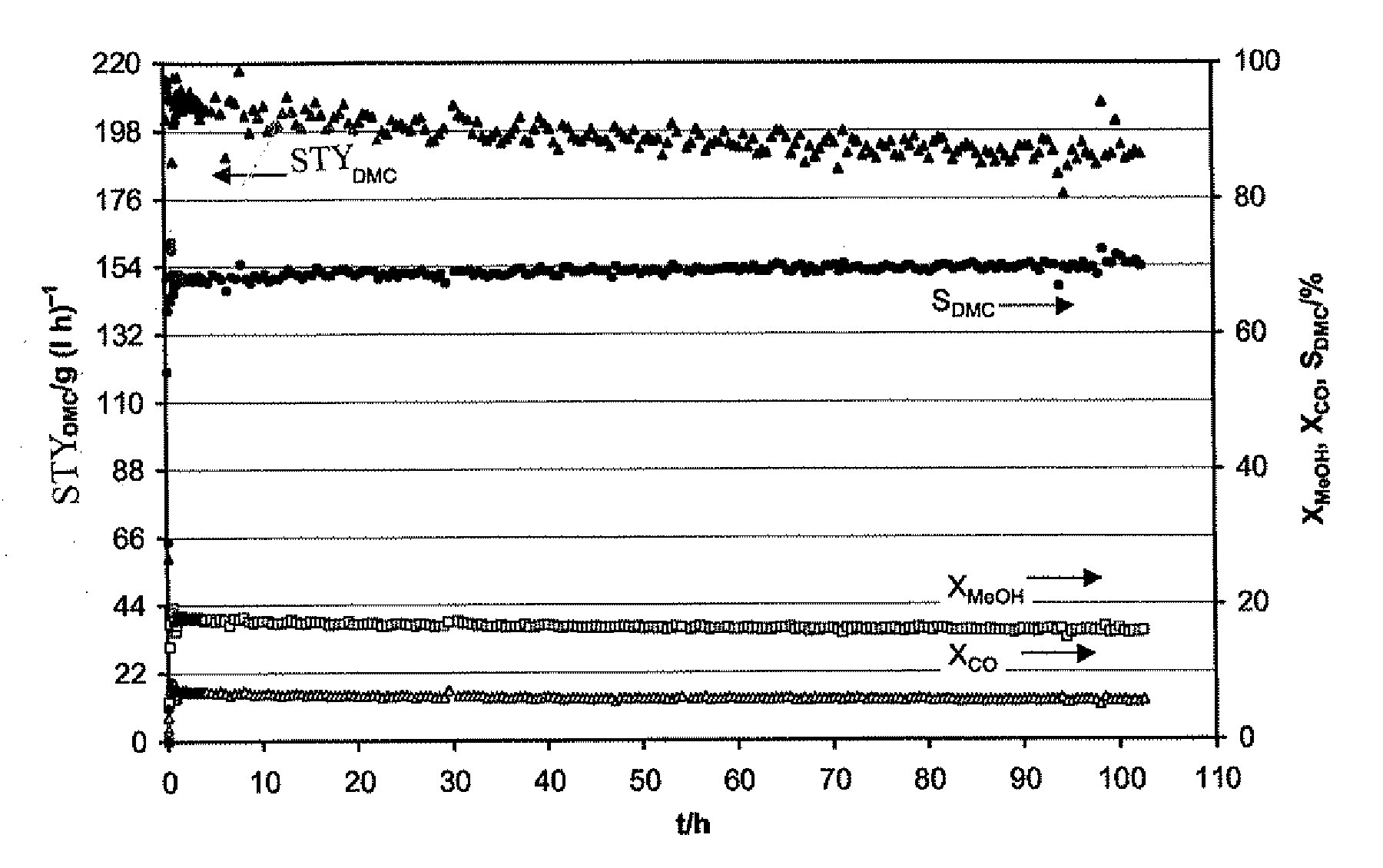
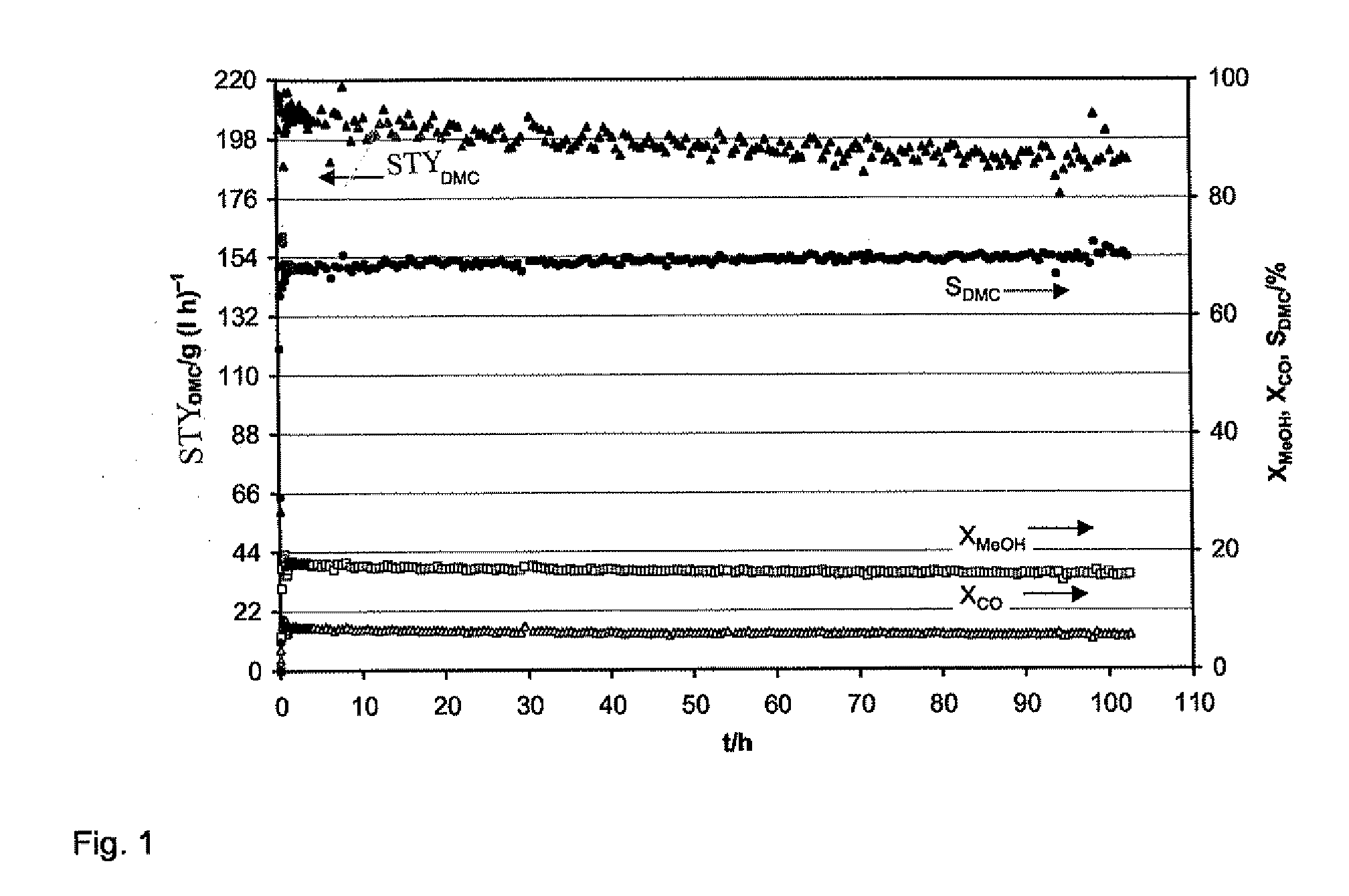
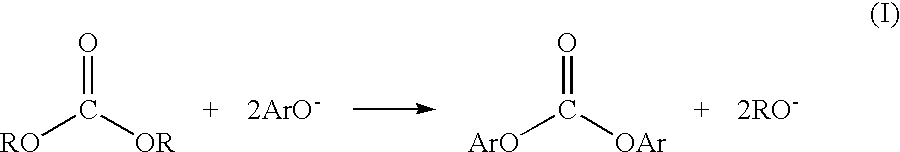
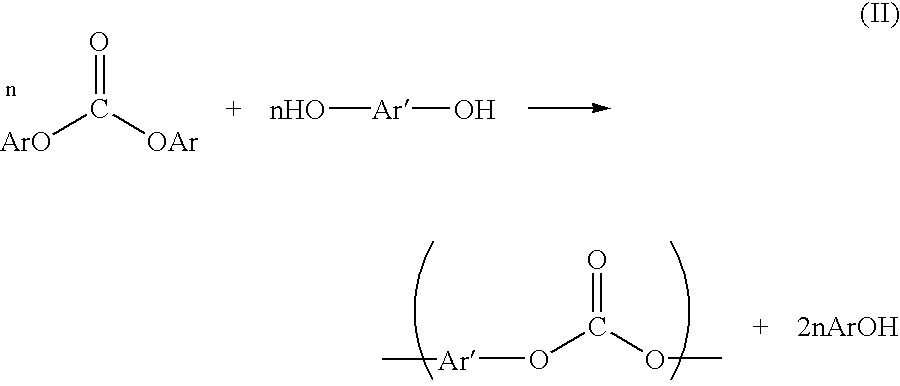
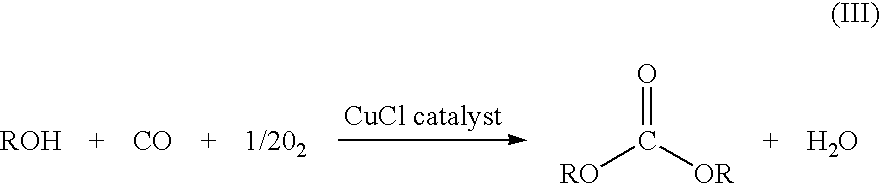
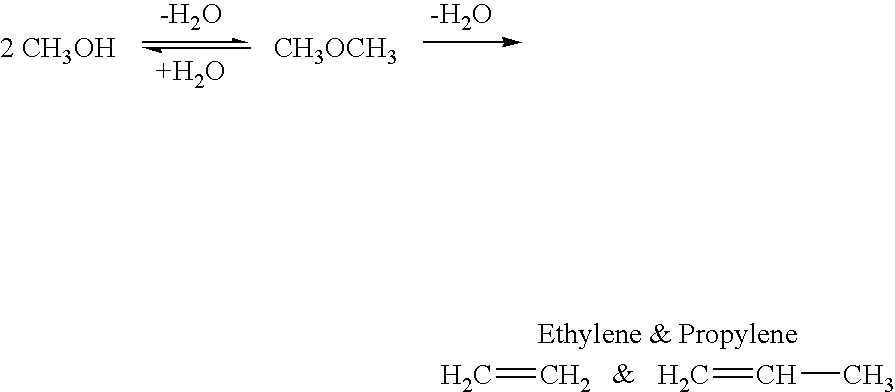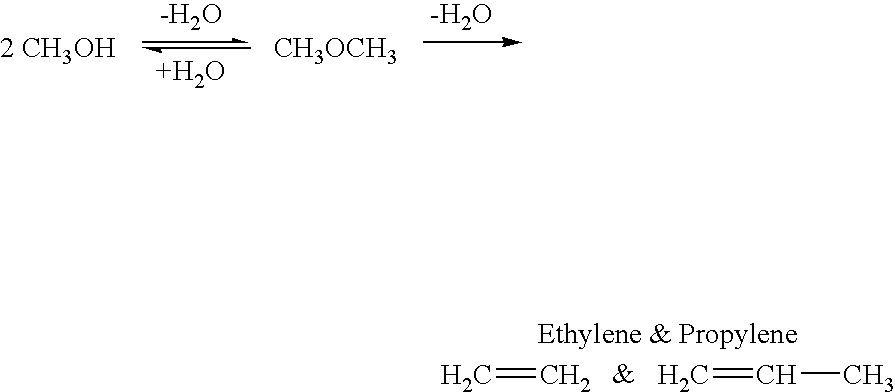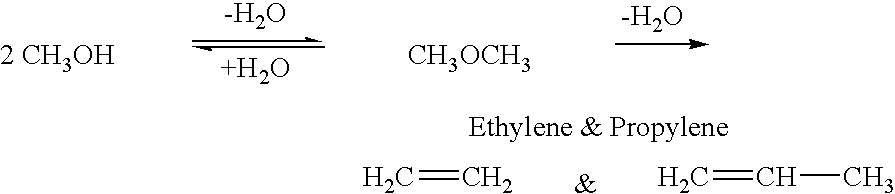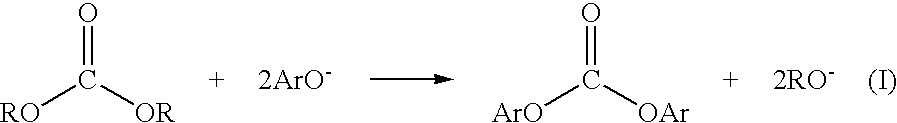Patents
Literature
114results about "Preparation from carbon monoxide and oxygen" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
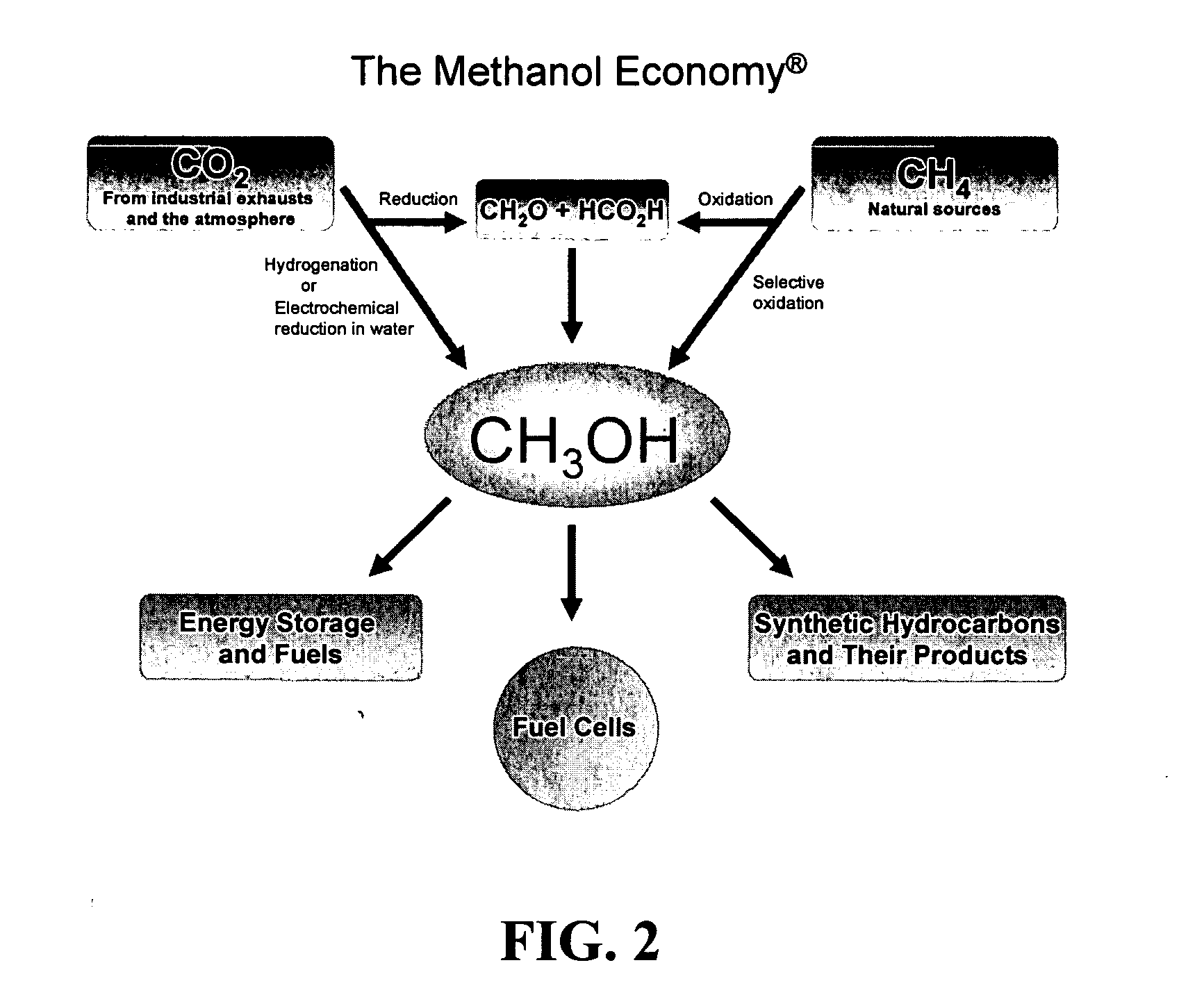
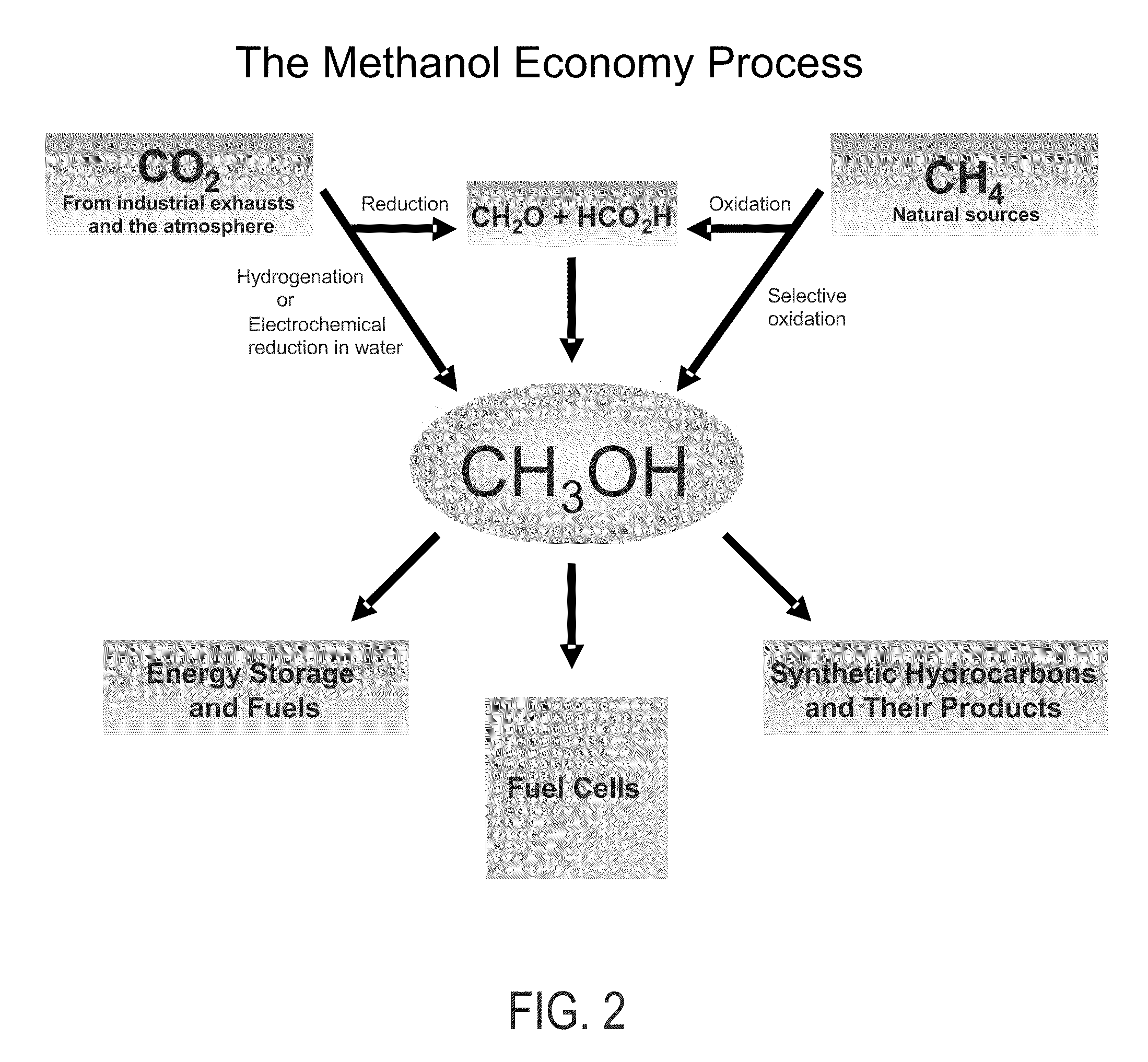
Efficient and selective chemical recycling of carbon dioxide to methanol, dimethyl ether and derived products
ActiveUS20070254969A1Avoid emissionsElectrolysis componentsOxygen compounds purification/separationElectrochemistryDimethyl ether
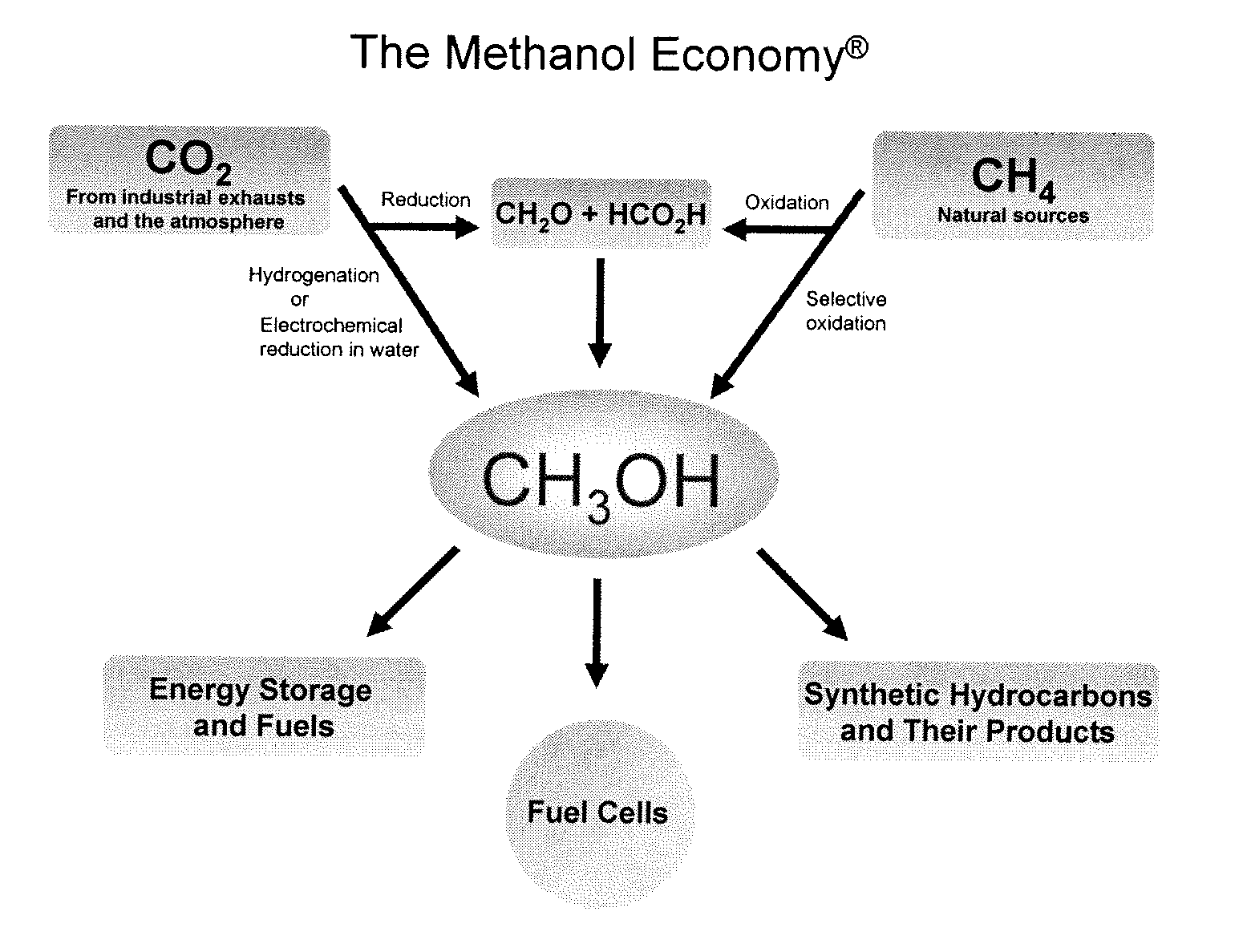
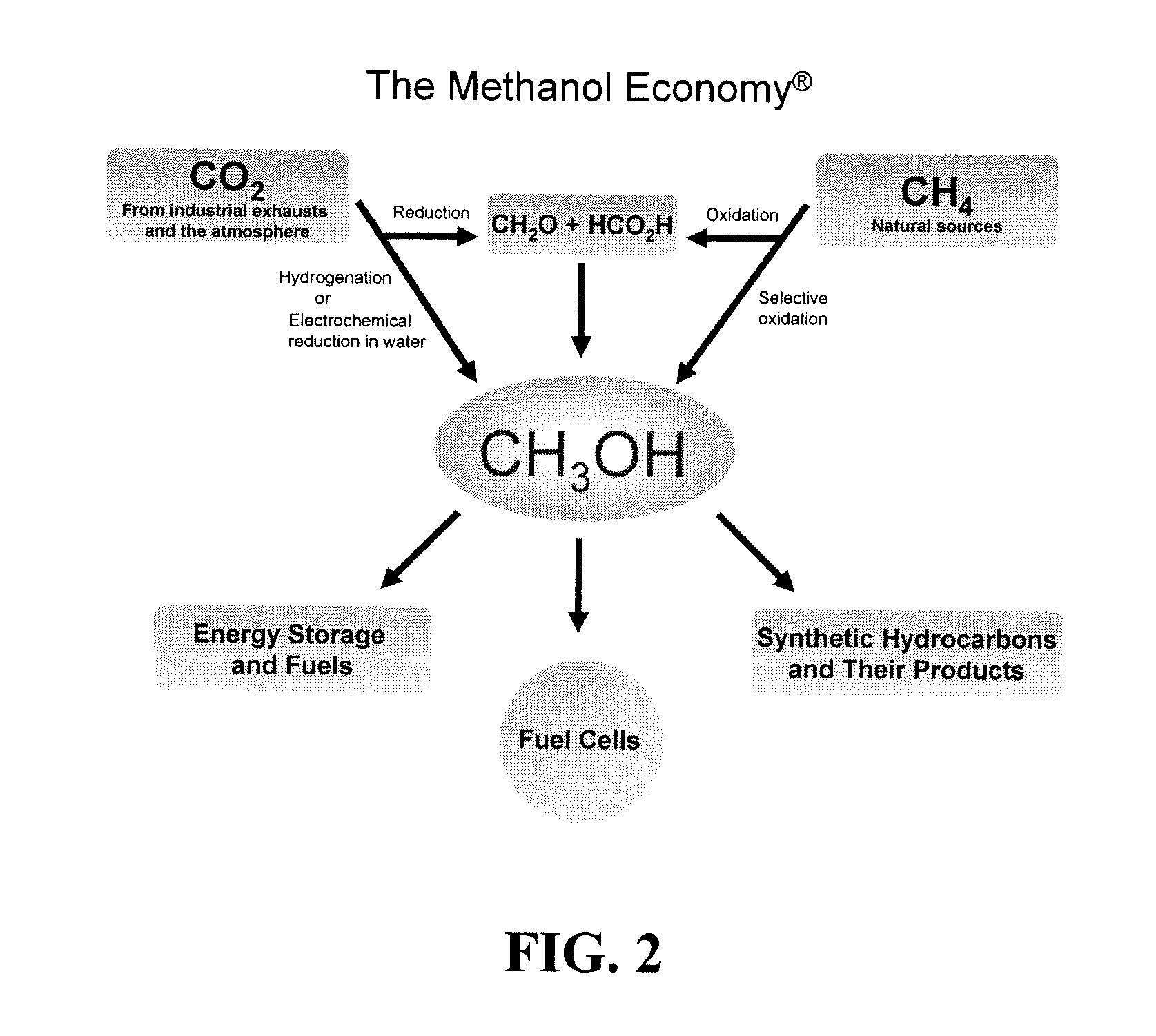
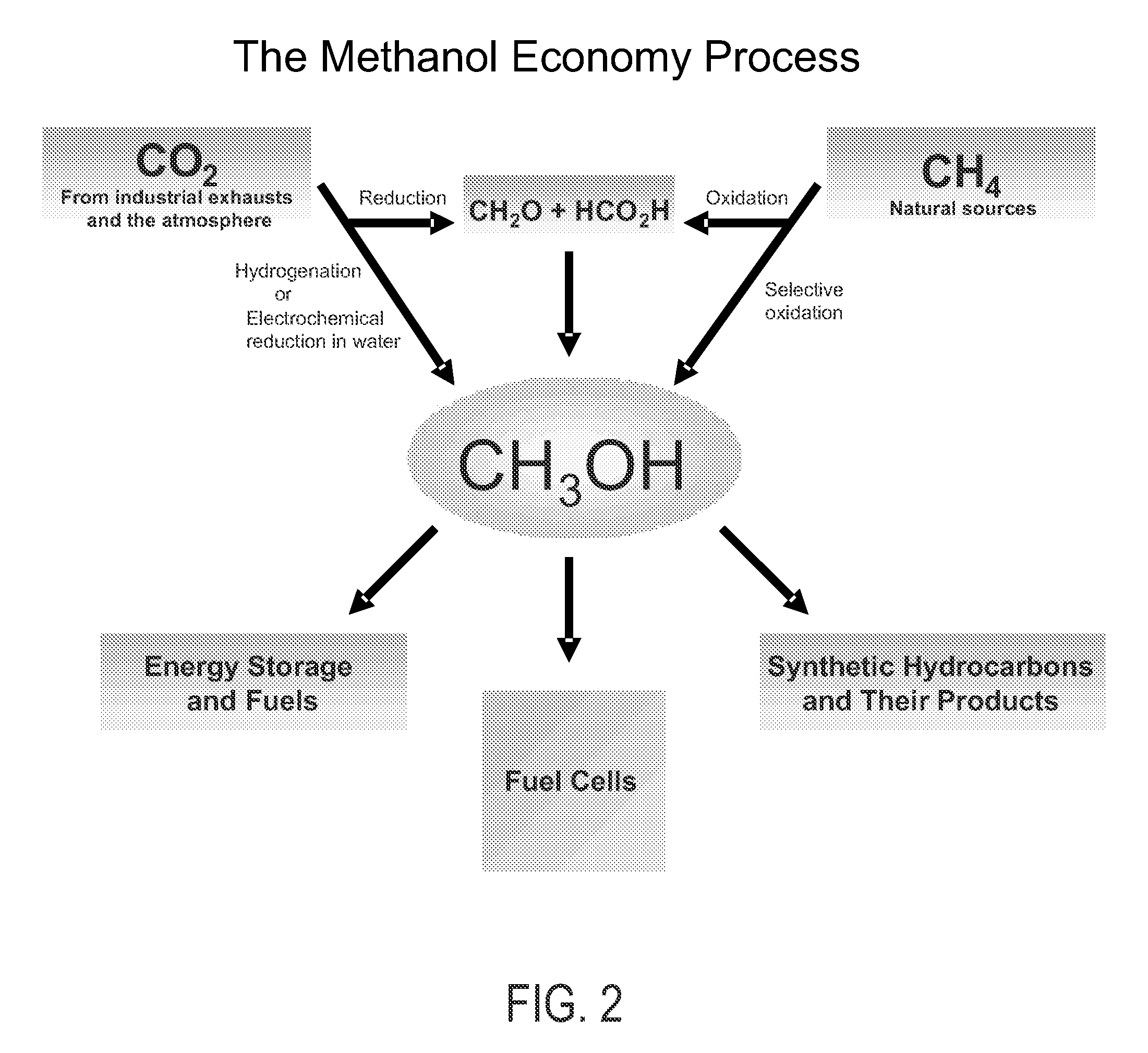
An efficient and environmentally beneficial method of recycling and producing methanol from varied sources of carbon dioxide including flue gases of fossil fuel burning powerplants, industrial exhaust gases or the atmosphere itself. Converting carbon dioxide by chemical or electrochemical reduction secondary treatment to produce essentially methanol, dimethyl ether and derived products.
Owner:UNIV OF SOUTHERN CALIFORNIA
Efficient and selective conversion of carbon dioxide to methanol, dimethyl ether and derived products
ActiveUS20060235091A1Minimize or eliminate the disadvantages or dangers inherentElectrolysis componentsCarbon compoundsHydrogenFlue gas
An environmentally beneficial method of producing methanol from varied sources of carbon dioxide including flue gases of fossil fuel burning powerplants, industrial exhaust gases or the atmosphere itself. Converting carbon dioxide by electrochemical reduction produces formic acid acid and some formaldehyde and methanol mixtures. The formic acid can be used as source of carbon as well as hydrogen to produce methanol, dimethyl ether and other products.
Owner:UNIV OF SOUTHERN CALIFORNIA
Efficient and selective conversion of carbon dioxide to methanol, dimethyl ether and derived products
ActiveUS7605293B2Minimize or eliminate the disadvantages or dangers inherentElectrolysis componentsCarbon compoundsHydrogenFlue gas
An environmentally beneficial method of producing methanol from varied sources of carbon dioxide including flue gases of fossil fuel burning powerplants, industrial exhaust gases or the atmosphere itself. Converting carbon dioxide by electrochemical reduction produces formic acid acid and some formaldehyde and methanol mixtures. The formic acid can be used as source of carbon as well as hydrogen to produce methanol, dimethyl ether and other products.
Owner:UNIV OF SOUTHERN CALIFORNIA
Efficient and selective chemical recycling of carbon dioxide to methanol, dimethyl ether and derived products
ActiveUS7608743B2Avoid emissionsElectrolysis componentsOxygen compounds purification/separationFlue gasExhaust fumes
An efficient and environmentally beneficial method of recycling and producing methanol from varied sources of carbon dioxide including flue gases of fossil fuel burning powerplants, industrial exhaust gases or the atmosphere itself. Converting carbon dioxide by chemical or electrochemical reduction secondary treatment to produce essentially methanol, dimethyl ether and derived products.
Owner:UNIV OF SOUTHERN CALIFORNIA
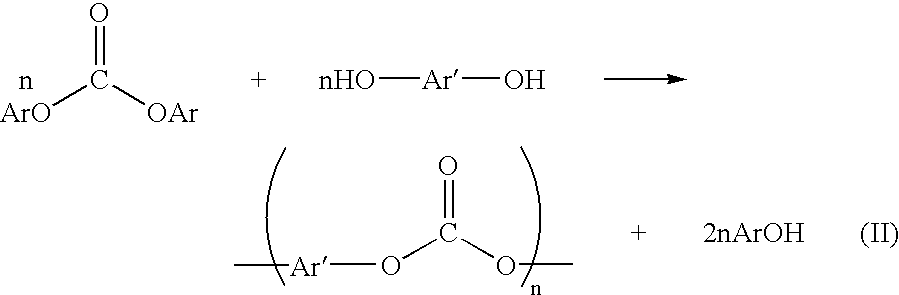
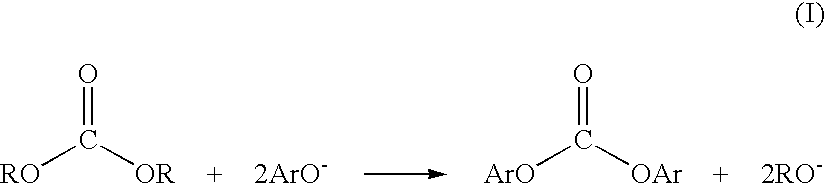
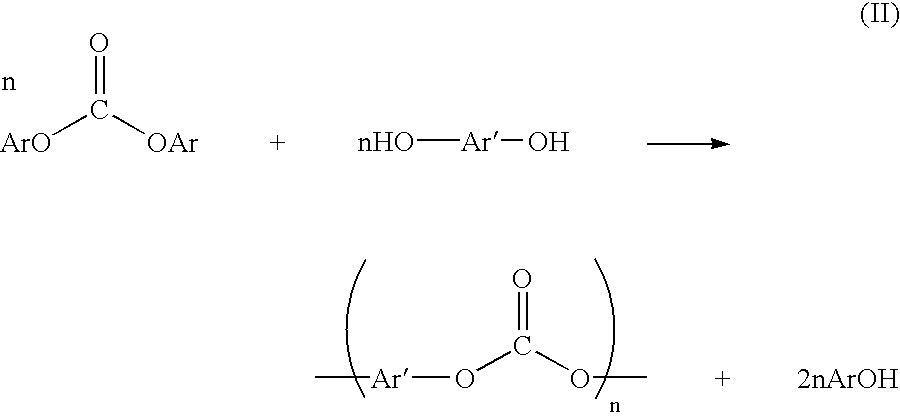
Method for preparing a dialkyl carbonate, and its use in the preparation of diaryl carbonates and polycarbonates
InactiveUS6784277B2Efficient use ofProcess control/regulationOrganic compound preparationPolycarbonateCarbonate
Owner:SABIC GLOBAL TECH BV
Method of separating metallic catalyst constituents from reaction mixtures
InactiveUS20050014965A1Effect reactivityEffect selectivityOrganic compound preparationPreparation from carbon monoxide and oxygenReaction temperatureSolvent
A process for the preparation of an aromatic carbonate is disclosed. The process entails reacting in the presence of a catalyst system an aromatic hydroxy compound with carbon monoxide and oxygen, and optionally in one or more solvents to produce a liquid phase. At least a portion of the liquid phase is subjected to a treatment to obtain a treated liquid phase. The treatment entails at least one of (a) heating to a temperature that is at most mean reaction temperature without passing oxygen thereto, and (b) adding one or more protic compounds thereto, and (c) passing through it one or more inert or reducing gases. Solid metallic catalyst constituents are then separated from the treated liquid phase.
Owner:BAYER MATERIALSCIENCE AG
Method for preparing a dialkyl carbonate, and its use in the preparation of diaryl carbonates and polycarbonates
InactiveUS20030055199A1Efficient use ofProcess control/regulationOrganic compound preparationPolycarbonateCarbonate
Unexpected corrosion of downstream sections of a dialkyl carbonate manufacturing apparatus has been traced to alkyl chloroformate impurities, which slowly decompose to yield hydrochloric acid. An improved process and apparatus for dialkyl carbonate synthesis reduce corrosion by physically removing or chemically decomposing the alkyl chloroformate impurities within the corrosion-resistant upstream sections of the apparatus.
Owner:SABIC GLOBAL TECH BV
Preparation method and application of high-efficiency nitrogen-doped carbon nanotube
ActiveCN107686105ASmall particle sizeEvenly dispersedOrganic compound preparationCarbon compoundsMultiwalled carbonFiltration
The invention discloses a preparation method of a high-efficiency nitrogen-doped carbon nanotube. The preparation method comprises the following steps: adding a multiwalled carbon nanotube into a nitric acid solution; closing the solution in an autoclave; pressurizing to 0.4 to 1.0 MPa, and stirring; then heating to 120 to 200 DEG C and keeping for 1 to 4 hours; finally, cooling to room temperature by the autoclave; washing to neutral and carrying out suction filtration and drying; mixing the carbon nanotube with a melamine solid according to the mass ratio being 1 to (1 to 4), and uniformly grinding by using mortar; and carrying out high-temperature sintering in nitrogen, washing to neutral and then drying to obtain the nitrogen-doped carbon nanotube. The nitrogen doping amount of the high-efficiency nitrogen-doped carbon nanotube disclosed by the invention is 4.6 to 10.3 weight percent.
Owner:TAIYUAN UNIV OF TECH
Method for preparing a dialkyl carbonate, and its use in the preparation of diaryl carbonates and polycarbonates
InactiveUS20030092872A1Efficient use ofProcess control/regulationOrganic compound preparationManufactured apparatusPolycarbonate
Unexpected corrosion of downstream sections of a dialkyl carbonate manufacturing apparatus has been traced to alkyl chloroformate impurities, which slowly decompose to yield hydrochloric acid. An improved process and apparatus for dialkyl carbonate synthesis reduce corrosion by physically removing or chemically decomposing the alkyl chloroformate impurities within the corrosion-resistant upstream sections of the apparatus.
Owner:SABIC GLOBAL TECH BV
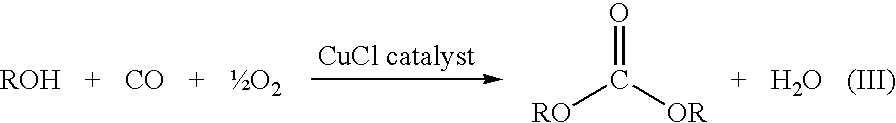
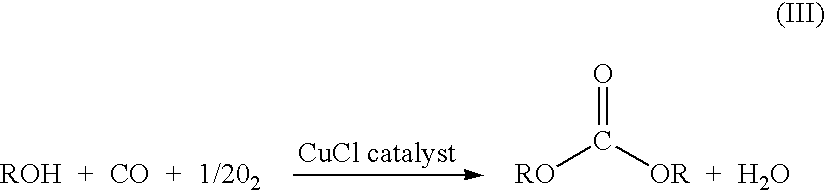
Catalyst for the Synthesis of Dimethyl Carbonate in the Gas Phase
InactiveUS20080249327A1Increased space-time yieldConstant operationAluminium compoundsMolecular sieve catalystsPtru catalystLiquid medium
The invention relates to an improved catalyst for the synthesis of dimethyl carbonate by reacting methanol, carbon monoxide and oxygen in the gas phase and to the use thereof. The catalyst consists of a copper-containing zeolite produced by admixing one or more halide-free copper(II) compounds to a zeolite in a liquid medium, drying the zeolite modified by the admixture, and tempering at 400-900° C. under inert conditions, essentially retaining the crystallinity of the zeolite, said admixing being effected by means of a method selected from the group consisting of impregnation of the zeolite, ion exchange, precipitation of copper(II) hydroxide in the presence of the zeolite, and a combination of these methods. The catalyst shows high space-time yields, is constant over the period of operation and has no corrosive action.
Owner:SUD CHEM AG
Method and apparatus for preparing a dialkyl carbonate, and its use in the preparation of diaryl carbonates and polycarbonates
InactiveUS20030060650A1Efficient use ofProcess control/regulationOrganic compound preparationManufactured apparatusPolycarbonate
Unexpected corrosion of downstream sections of a dialkyl carbonate manufacturing apparatus has been traced to alkyl chloroformate impurities, which slowly decompose to yield hydrochloric acid. An improved process and apparatus for dialkyl carbonate synthesis reduce corrosion by physically removing or chemically decomposing the alkyl chloroformate impurities within the corrosion-resistant upstream sections of the apparatus.
Owner:SABIC GLOBAL TECH BV
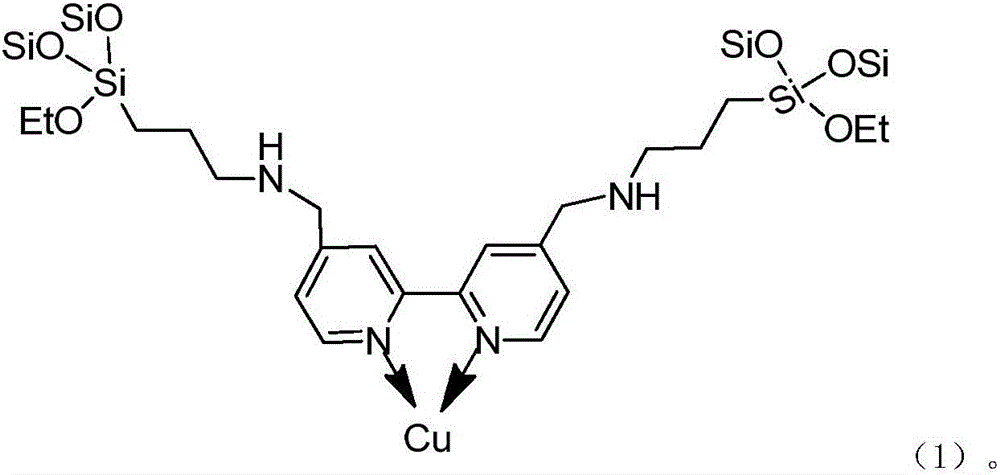
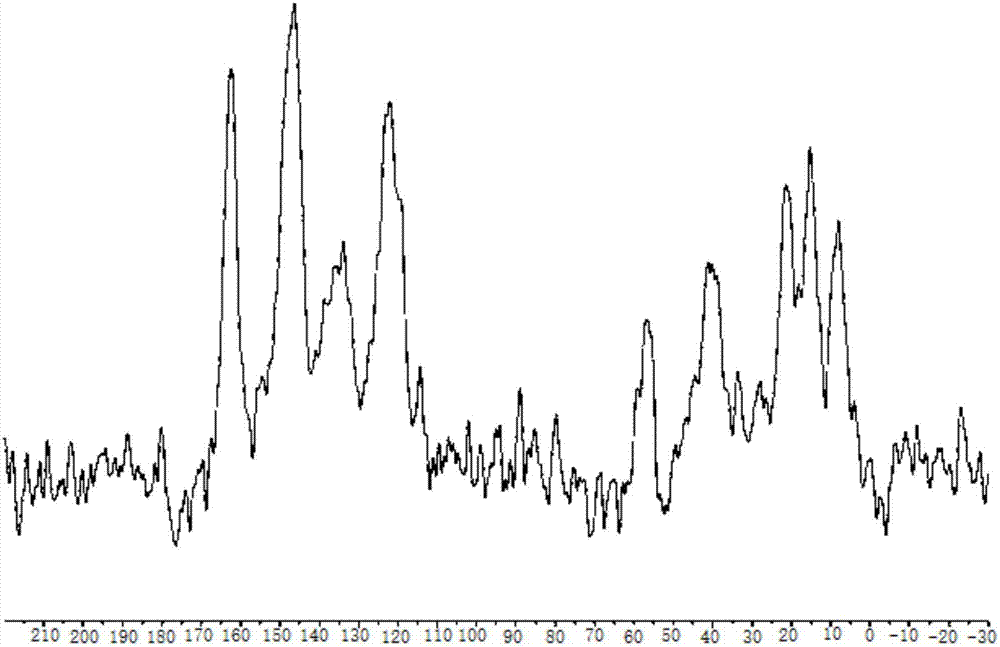
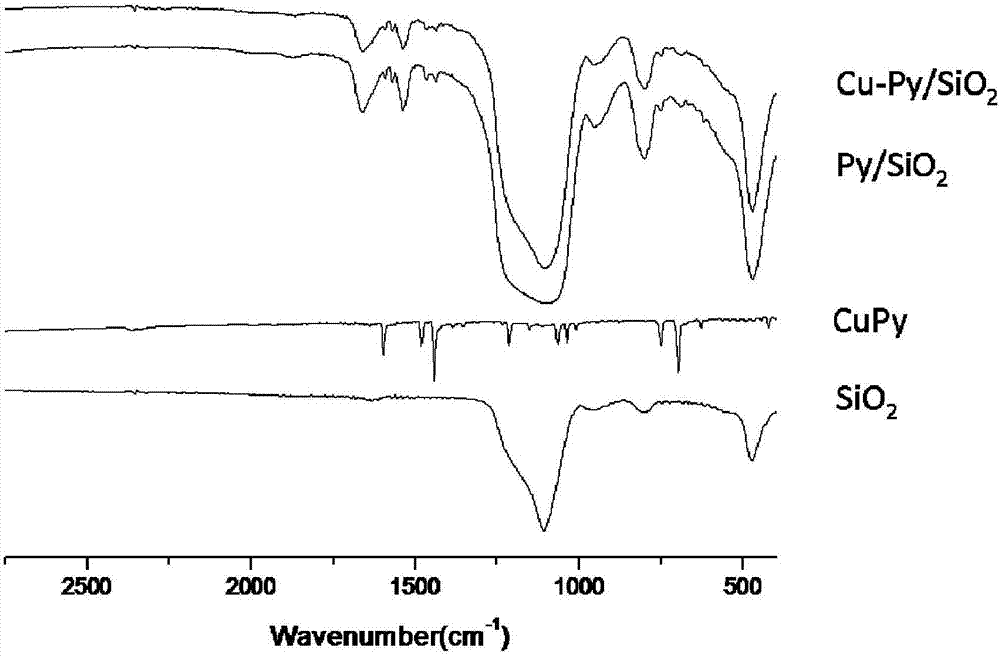
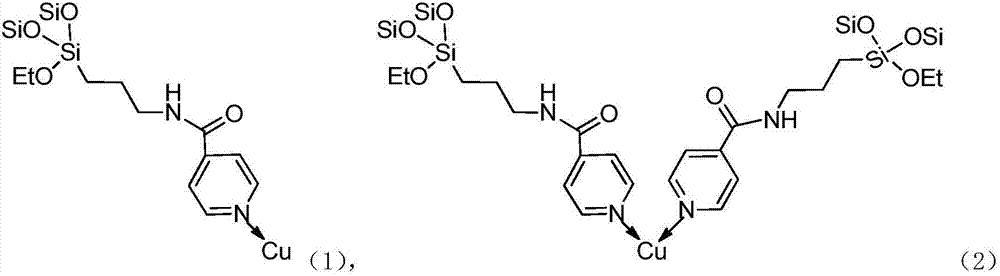
Silicon dioxide loaded copper-dipyridyl catalyst and its preparation method
ActiveCN107175133AOvercome the large amount of feedingOvercoming high valueOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsMethyl carbonateActive component
The invention relates to a silicon dioxide loaded copper-dipyridyl catalyst and its preparation method. The catalyst carrier is dipyridyl modified silicon dioxide, and the active component is copper; the mole ratio of the dipyridyl modified silicon dioxide to copper is 0.3-3.5: 1 and weight of Cu of catalyst is 4-6 wt% of the total weight of catalyst. In application of preparing dimethyl carbonate, the selectivity of dimethyl carbonate reaches 99.9%, and yield of dimethyl carbonate exceeds 53%.
Owner:FUJIAN INST OF RES ON THE STRUCTURE OF MATTER CHINESE ACAD OF SCI
Catalyst for synthesizing dimethyl carbonate by methanol liquid-phase oxidative carbonylation and methods
InactiveCN107694609AHigh selectivityReduce corrosionOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsHalogenMethyl carbonate
The invention discloses a catalyst for synthesizing dimethyl carbonate by methanol liquid-phase oxidative carbonylation and methods. The catalyst comprises a copper halide and an ionic liquid; the mass ratio of the copper halide to the ionic liquid is 1:(1-10); a cation of the ionic liquid is an imidazole cation, a quaternary ammonium salt cation or a pyridine cation; and an anion of the ionic liquid is a halogen ion. The catalyst disclosed by the invention has relatively high methanol conversion ratio and high dimethyl carbonate selectivity and has an industrial application prospect. In addition, due to the addition of the ionic liquid, corrosion to stainless steel reaction equipment can be relieved.
Owner:CHENGDU ORGANIC CHEM CO LTD CHINESE ACAD OF SCI
Method for improving concentration of methanol oxo-synthesized dimethyl carbonate crude product
InactiveCN104418749AIncrease concentrationReduce the difficulty of separation and purificationMolecular sieve catalystsOrganic compound preparationFluid phaseDimethyl carbonate
The invention relates to a method for improving concentration of a methanol oxo-synthesized dimethyl carbonate crude product, and the method comprises a first order synthetic reaction and a second order synthetic reaction; condensation and gas-liquid separation of a gas-liquid mixed material from an inlet of a first order dimethyl carbonate reactor of the first order synthetic reaction are performed; a separated liquid phase is pumped into a second order dimethyl carbonate reactor to participate into the second order synthetic reaction; and the separated CO-containing gas enters into the second order dimethyl carbonate reactor to participate into the second order synthetic reaction. The method adopts two order series of carbonyl oxidation process for co-production of dimethyl carbonate, through the adjustment of carbonyl oxidation synthesis process parameters, the concentration of the dimethyl carbonate crude product is increased to more than 30%, the difficulty of separation and purification of the dimethyl carbonate product is greatly reduced, and the production cost of the product is significantly reduced.
Owner:黄志忠
Intelligent reaction system and process for synthesizing dimethyl carbonate through liquid-phase oxidative carbonylation of methanol
InactiveCN112473566AImprove Oxylation EfficiencyIncreased phase boundary areaChemical/physical/physico-chemical microreactorsPreparation from carbon monoxide and oxygenEmulsionFluid phase
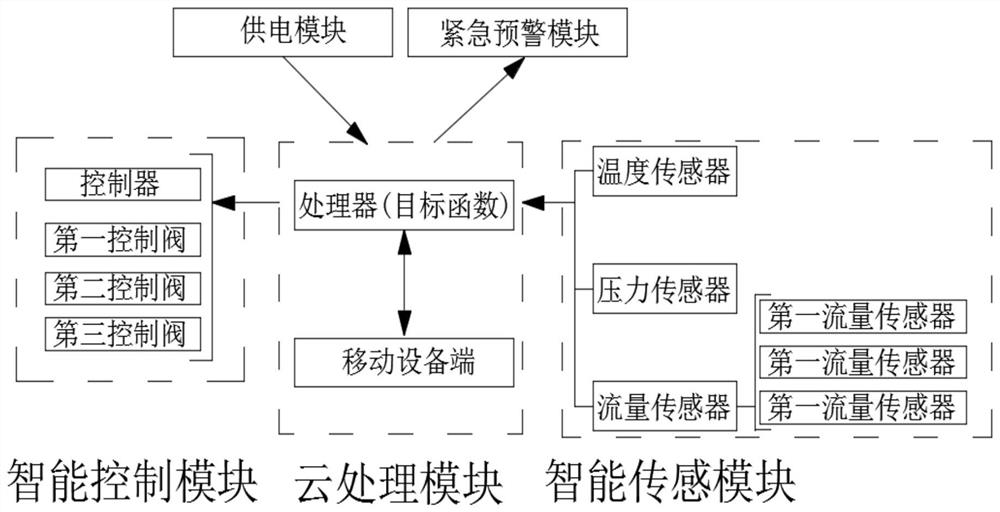
The invention relates to an intelligent reaction system and process for synthesizing dimethyl carbonate through liquid-phase oxidative carbonylation of methanol. The intelligent reaction system comprises a reaction tower, a buffer tank, micro-interface generators and an intelligent control unit. Oxygen and carbon monoxide are crushed to form micron-scale bubbles, so the reaction rate of oxygen andcarbon monoxide with liquid-phase methanol is increased; meanwhile, a gas-liquid emulsion is conveyed back to a reaction area through a second micro-interface generator for opposite impacting with agas-liquid emulsion output by a first micro-interface generator, and therefore, the retention time of the micron-scale bubbles in the reaction area is prolonged, gas and liquid phases are subjected toa secondary reaction, and the conversion rate of liquid-phase methanol is increased; and through the intelligent control unit, a worker can know the real-time situation of all data sent back by an intelligent sensing module at any time through a mobile device, and the inner temperature and the inner pressure of the whole reaction tower can be accurately controlled by changing preset values, so reaction efficiency is further improved.
Owner:NANJING YANCHANG REACTION TECH RES INST CO LTD
Preparation method of Cu nanoparticles-embedded ordered mesoporous carbon catalyst
InactiveCN105521782AUniform and orderly channelsLarge specific surface areaOrganic compound preparationPreparation from carbon monoxide and oxygenDispersityPtru catalyst
The invention discloses a preparation method of a Cu nanoparticles-embedded ordered mesoporous carbon catalyst and belongs to the field of chemical heterogeneous catalysis. The preparation method comprises the following steps: Step 1, template mesoporous silica material SBA-15 filling-rolling over; Step 2, carbonization; and Step 3, silica removal. The process of template mesoporous silica material SBA-15 filling-rolling over comprises impregnation, dispersion, drying, pre-carbonization and single repetitive operation. The problem that catalytic activity of a catalyst will decrease with the reaction process is solved. The prepared chloride-free copper-based catalyst of the invention is a nanoscale catalyst with 5-25nm copper particles embedded in pores of and on the surface of the ordered mesoporous carbon. Dispersity is good, and migration and agglomeration are not easy to occur in the reaction process. The catalyst has high reaction activity and good stability when used in an atmospheric continuous fixed bed for methanol gaseous oxidative carbonylation for synthesis of dimethyl carbonate.
Owner:CHINA UNIV OF MINING & TECH
Process for producing aromatic carbonate
InactiveUS6596896B2Efficiently obtainedMolecular sieve catalystsOrganic compound preparationPhosphonium saltHeteropoly acid
Owner:TEIJIN LTD
Catalysts system for producing aromatic carbonates
InactiveUS6380417B1Organic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsManganeseCerium
A method and catalyst system for economically producing aromatic carbonates from aromatic hydroxy compounds. In one embodiment, the present invention provides a method of carbonylating aromatic hydroxy compounds by contacting at least one aromatic hydroxy compound with oxygen and carbon monoxide in the presence of a carbonylation catalyst system that includes a catalytic amount of a combination of inorganic co-catalysts containing manganese and nickel; manganese and iron; manganese and chromium; manganese and cerium; manganese and europium; manganese, cerium, and europium; manganese, iron, and europium; or manganese and thorium. In various alternative embodiments, the carbonylation catalyst system can include an effective amount of a palladium source and an effective amount of a halide composition. Further alternative embodiments can include catalytic amounts of various other inorganic co-catalyst combinations.
Owner:GENERAL ELECTRIC CO
Method for reactivating a deactivated catalyst composition
The present invention is directed to a method for reactivating a deactivated carbonylation catalyst composition previously used in a carbonylation reaction involving an aromatic hydroxy compound, carbon monoxide and oxygen, such that the reactivated catalyst composition is effective at carbonylating an aromatic hydroxy compound in a subsequent oxidative carbonylation reaction.
Owner:GENERAL ELECTRIC CO
Efficient and safe method for synthesizing carbonic ester by using oxidative carbonyl
ActiveCN104529783AEnough contact areaSufficient reaction timeOrganic compound preparationPreparation from carbon monoxide and oxygenActive componentCarbonate ester
The invention discloses an efficient and safe method for synthesizing carbonic ester by using oxidative carbonyl. The method comprises the steps of oxidative glycosylation reaction, flash separation, gas-liquid separation and product separation. The method can be used for overcoming the defects of heterogeneous catalytic system, complex process, poor recyclability and potential safety hazard of reaction process in the prior art; a novel process device is adopted; the feeding mole ratio of carbon monoxide (CO) to oxygen (O2) can be controlled; CO and O2 are taken as the reaction raw material gases; the catalyst solution is recyclable; a catalyst system applied to the reaction is a catalyst solution prepared from a solvent and a complex which is formed by homogeneous active components and ligands. The method has the advantages that the process is efficient, safe and simple, and the catalyst solution is easily recyclable; and the method has a good prospect of industrial application.
Owner:NANJING UNIV OF TECH +2
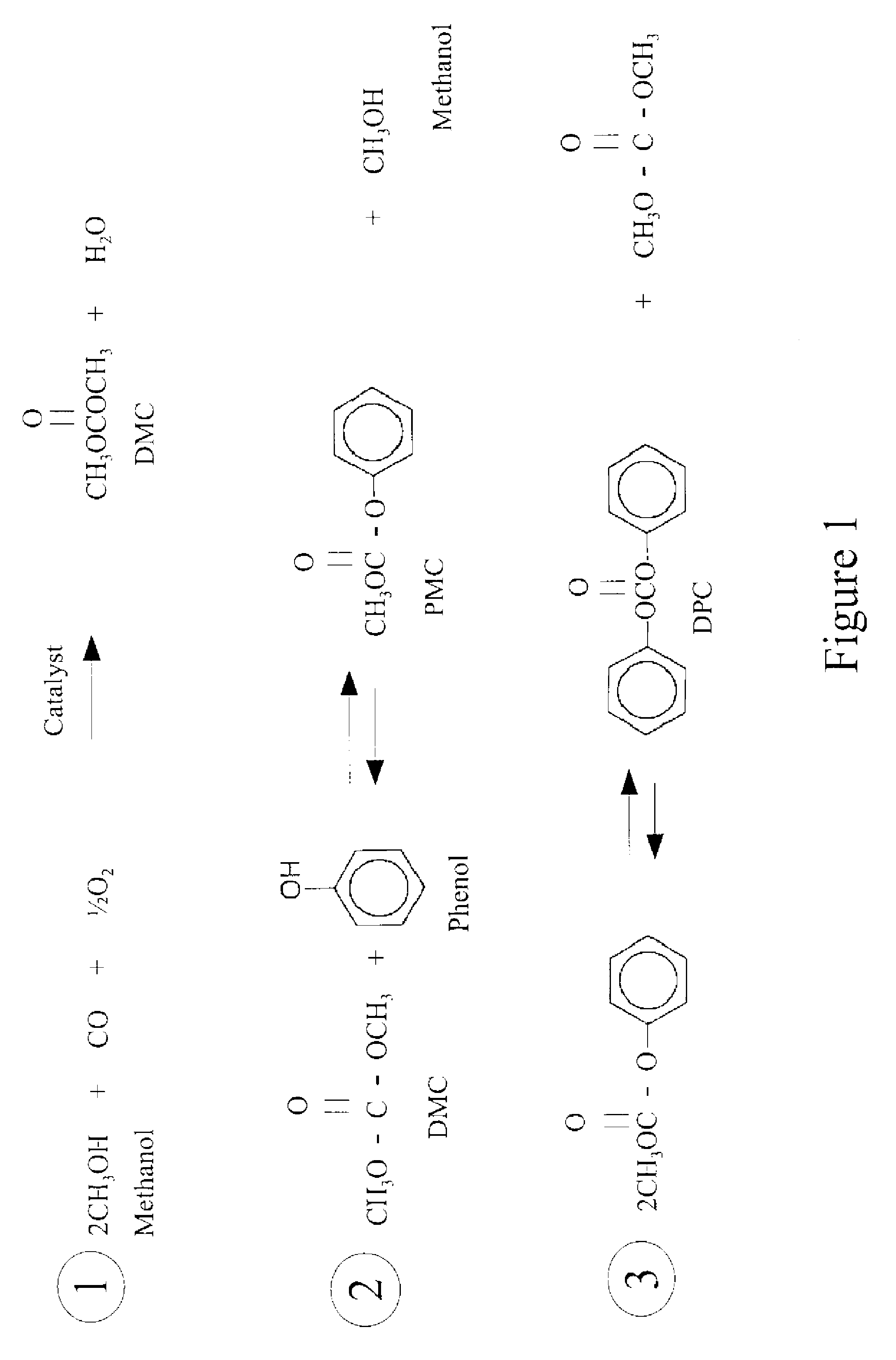
Process for producing diaryl carbonates
ActiveUS20110152558A1High reaction conversion rateLow reaction pressureOrganic compound preparationPreparation from carbon monoxide and oxygenNitrogenous heterocyclic compoundNitrogen
The present invention relates to a process for producing diaryl carbonates, which is to synthesize diaryl carbonates by oxidative carbonylation of phenols with carbon monoxide and oxygen, and in particular, to synthesize diphenyl carbonate from phenol. The present invention is characterized in that a catalytic system comprising a metal halide catalyst and one or more cocatalysts of nitrogenous heterocyclic compounds is used to increase the convertibility, selectivity and yield of this catalytic reaction.
Owner:CHINA PETROCHEM DEVMENT
Method for reactivating a catalyst composition
The present invention is directed to a method for reactivating a deactivated carbonylation catalyst composition previously used in a carbonylation reaction involving an aromatic hydroxy compound, carbon monoxide and oxygen, so that the re-activated catalyst composition is effective at carbonylating an aromatic hydroxy compound in a subsequent oxidative carbonylation reaction.
Owner:SABIC INNOVATIVE PLASTICS IP BV
Method for reactivating a deactivated catalyst composition
The present invention is directed to a method for reactivating a deactivated carbonylation catalyst composition previously used in a carbonylation reaction involving an aromatic hydroxy compound, carbon monoxide and oxygen, so that the re-activated catalyst composition is effective at carbonylating an aromatic hydroxy compound in a subsequent oxidative carbonylation reaction.
Owner:SABIC INNOVATIVE PLASTICS IP BV
Method and apparatus for preparing a dialkyl carbonate, and its use in the preparation of diaryl carbonates and polycarbonates
InactiveUS20030153782A1Efficient use ofProcess control/regulationOrganic compound preparationManufactured apparatusChloroformate
Unexpected corrosion of downstream sections of a dialkyl carbonate manufacturing apparatus has been traced to alkyl chloroformate impurities, which slowly decompose to yield hydrochloric acid. An improved process and apparatus for dialkyl carbonate synthesis reduce corrosion by physically removing or chemically decomposing the alkyl chloroformate impurities within the corrosion-resistant upstream sections of the apparatus. The alkyl chloroformate may be decomposed by passing it through a passageway at a temperature of about 30° C. to about 130° C. for a time of about 0.5 hour to about 10 hours. The passageway may include one or more holding vessels or a tubular section that promotes plug flow.
Owner:SABIC GLOBAL TECH BV
Process for manufacturing dimethyl carbonate
InactiveUS7803961B2Organic compound preparationPreparation from carbon monoxide and oxygenMethyl carbonateWater flow
A method of forming a dialkyl carbonate stream, includes obtaining a byproduct stream from a diaryl carbonate formation reaction that has alkanol, residual dialkyl carbonate, and residual aromatic compound. This byproduct stream is introduced to a distillation column to produce an alkanol tops stream and a first dialkyl carbonate bottoms stream. The alkanol tops stream is reacted with oxygen, carbon monoxide, and catalyst to form a second dialkyl carbonate stream that is introduced to the distillation column. The alkanol tops stream from the column contains alkanol, dialkyl carbonate, and less than 20 ppm aromatic compound. The first dialkyl carbonate bottoms stream from the column contains dialkyl carbonate, water, aromatic compound, and less than 2,000 ppm alkanol and is introduced to a water separation device to produce a product dialkyl carbonate stream and a water stream.
Owner:SABIC GLOBAL TECH BV
Method for preparing a dialkyl carbonate, and its use in the preparation of diaryl carbonates and polycarbonates
InactiveUS7514521B2Process control/regulationOrganic compound preparationManufactured apparatusPolycarbonate
Unexpected corrosion of downstream sections of a dialkyl carbonate manufacturing apparatus has been traced to alkyl chloroformate impurities, which slowly decompose to yield hydrochloric acid. An improved process and apparatus for dialkyl carbonate synthesis reduce corrosion by physically removing or chemically decomposing the alkyl chloroformate impurities within the corrosion-resistant upstream sections of the apparatus.
Owner:SABIC GLOBAL TECH BV
Pyridine coordinated copper heterogeneous catalyst and preparation method thereof
ActiveCN107199051AEasy to separateOvercome the large amount of feedingOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsMethyl carbonateActive component
The invention relates to a pyridine coordinated copper heterogeneous catalyst and a preparation method thereof. The carrier of the catalyst is pyridine modified silicon dioxide, and the active component is copper; the molar ratio of the pyridine modified silicon dioxide to the copper is (0.5-1.9):1; in the catalyst, the Cu accounts for 4 to 6 percent of the total weight of the catalyst. In application to preparation of dimethyl carbonate, the selectivity of the dimethyl carbonate reaches 99.9 percent and the yield of the dimethyl carbonate exceeds 54 percent.
Owner:FUJIAN INST OF RES ON THE STRUCTURE OF MATTER CHINESE ACAD OF SCI
Water resistant catalyst for the production of diaryl carbonates via the direct carbonylation of phenolic compounds
InactiveUS20050085656A1Increasing amount of producedIncrease the number of turnoversOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsAlkaline earth metalTurnover number
A method of increasing the amount of diphenylcarbonate produced per amount of catalyst consumed in a phenol carbonylation process is described. Phenolic carbonylation produces water as a reaction product which reduces the turnover number (TON) of the catalyst. A mixture of a phenolic precursor, a base containing catalyst and co-catalyst components and at least one chemical additive comprising a halide or hydroxide of alkali metal or alkaline earth metal when carbonylated together under specific conditions increases the turnover number (TON) and water resistivity of a palladium catalyst. The metal halide likely makes the catalyst less susceptible to degradation by water hence increasing the reaction yield per weight of catalyst consumed.
Owner:SABIC GLOBAL TECH BV
Dimethyl carbonate preparation method
InactiveCN103709040AFast transferImprove stabilityOrganic compound preparationPreparation from carbon monoxide and oxygenChemical industryIon
The invention belongs to the chemical industry catalysis field, and discloses a dimethyl carbonate preparation method. The method is characterized in that a reaction of methanol, CO and O2 is carried out in the presence of carbon-based supported nano-Cu as a catalyst to synthesize dimethyl carbonate, and the method comprises the following steps: adding the catalyst to a reactor, adding methanol, introducing CO and O2, and reacting at a temperature of 120-180DEG C under a pressure of 0.4-0.8Mpa for 2-5h to prepare dimethyl carbonate. Cl<-> existing in routine methods is not used in the reaction process of the method, so the method does not corrode apparatuses, the cost is reduced, and a problem that the catalyst inactivation caused by the Cl<-> loss is overcome.
Owner:唐雅蓉
Catalyst for oxidative carbonylation of ethanol to synthesize diethyl carbonate and preparation method thereof
ActiveCN110252406ATunable electronic structureThe relative distribution structure is adjustableOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsGas phaseFixed bed
Owner:BEIJING UNIV OF CHEM TECH
Popular searches
Preparation by hydroxy group addition Preparation by oxygen reduction Preparation from phosgene or haloformates Organic compounds purification/separation/stabilisation Oxygen compounds preparation by reduction Hydrocarbon from oxygen organic compounds Preparation by carbon monoxide or formate reaction Carboxylic compound preparation Electrolytic organic reduction Ether preparation by compound dehydration
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com