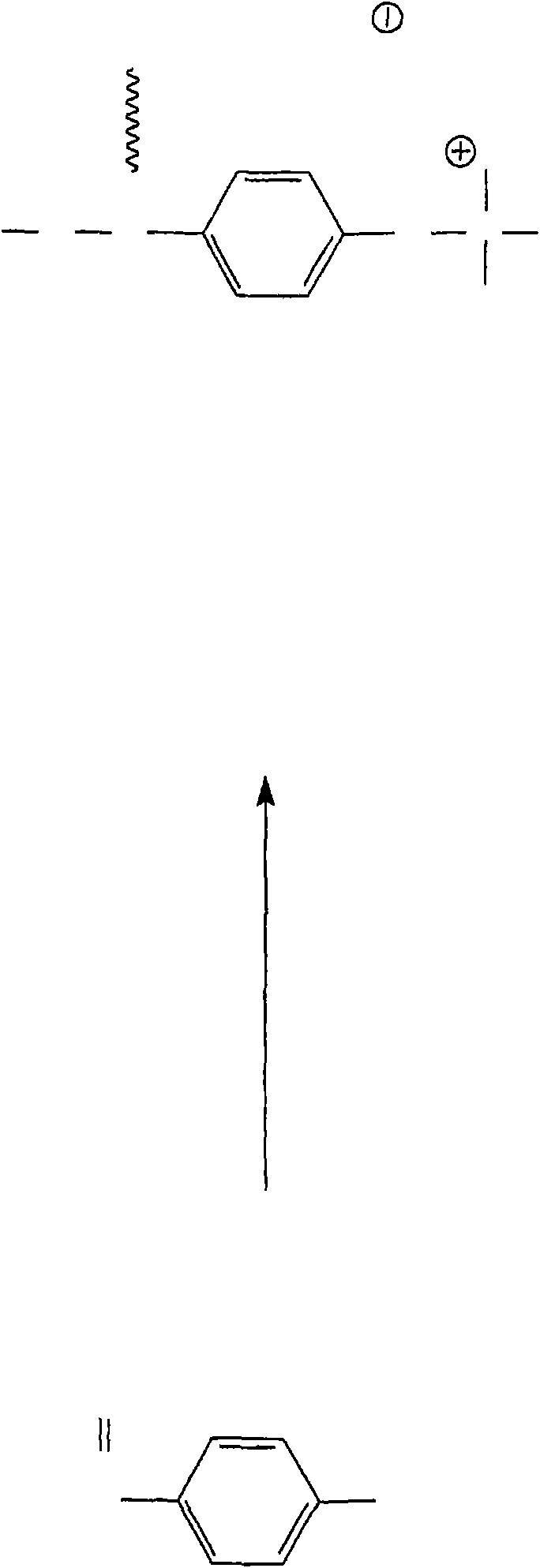
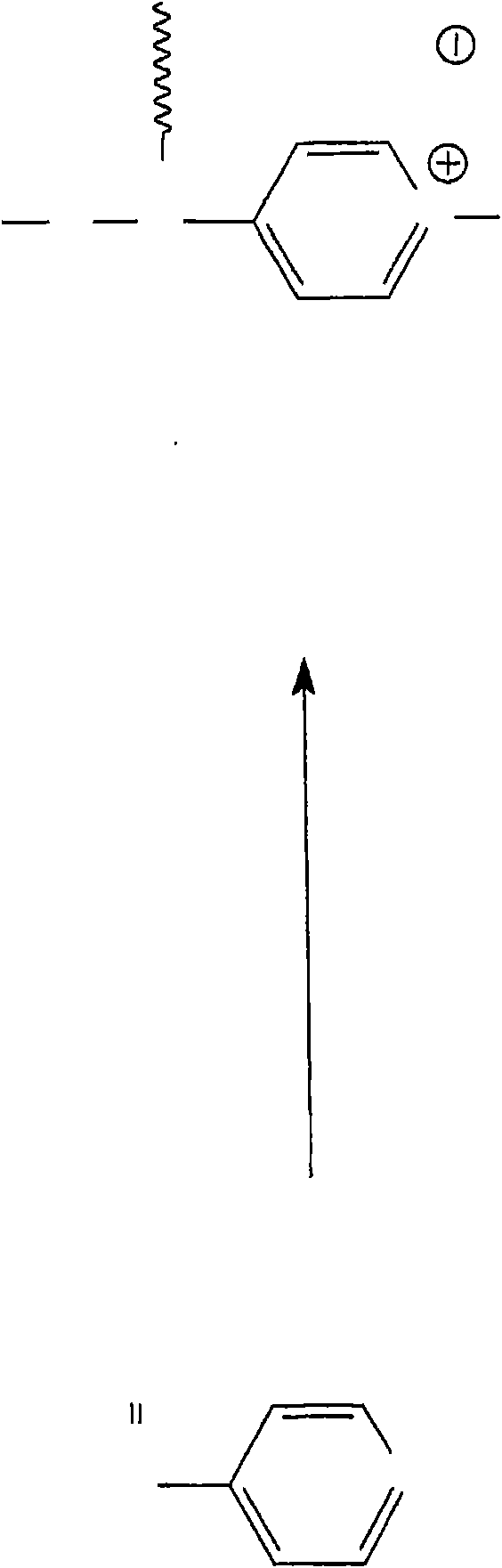
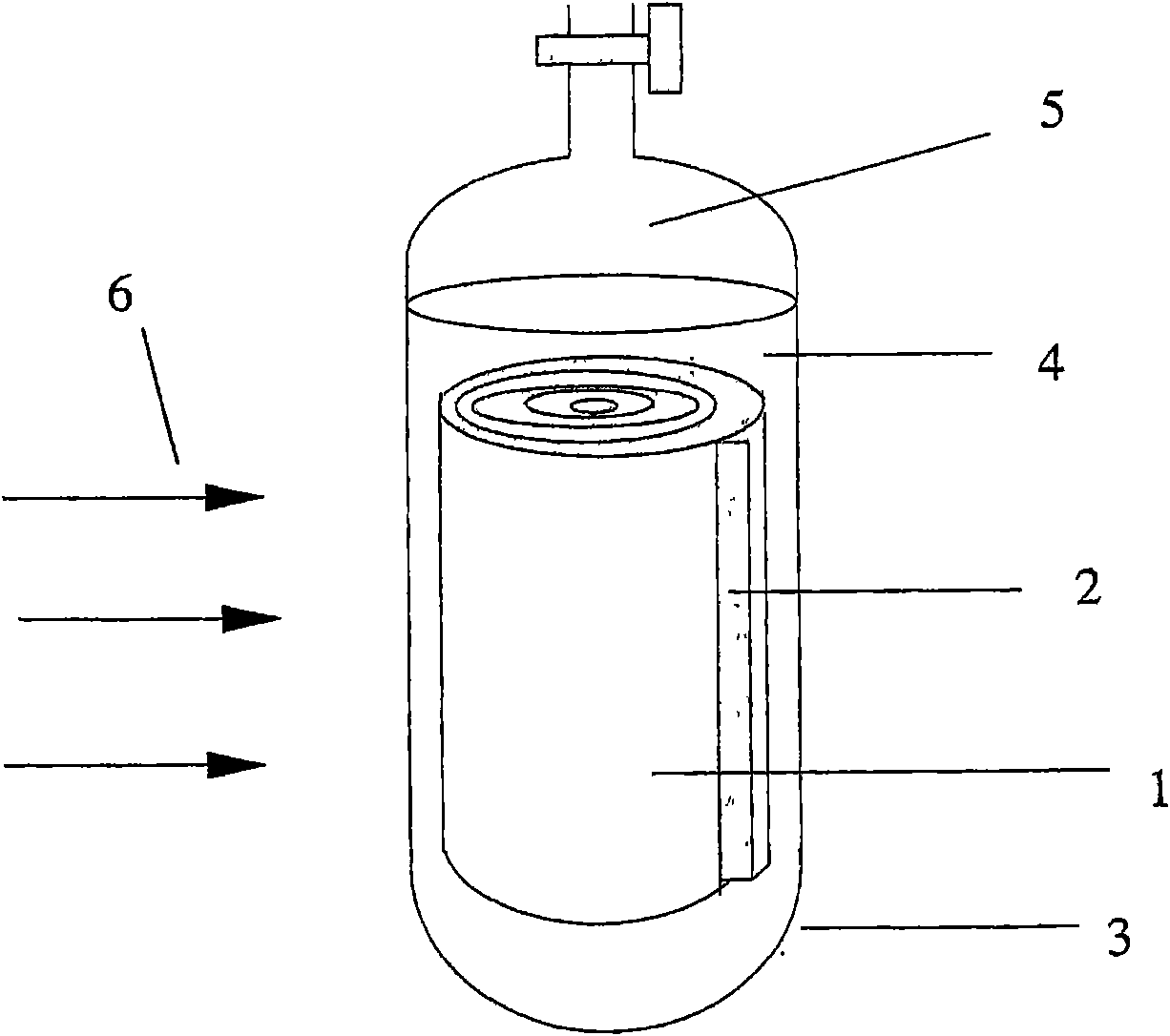
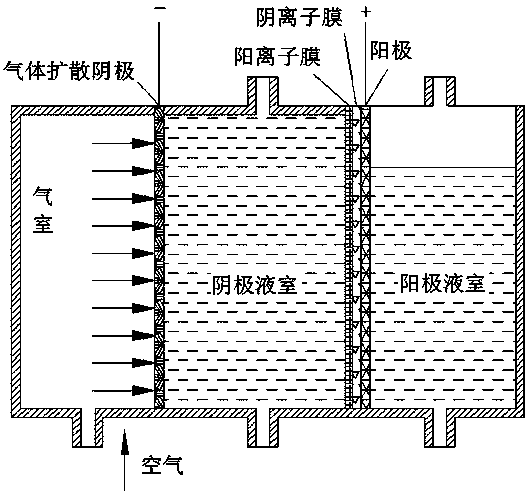
Patents
Literature
39 results about "Direct borohydride fuel cell" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Direct borohydride fuel cells (DBFCs) are a subcategory of alkaline fuel cells which are directly fed by sodium borohydride or potassium borohydride as a fuel and either air/oxygen or hydrogen peroxide as the oxidant. DBFCs are relatively new types of fuel cells which are currently in the developmental stage and are attractive due to their high operating potential in relation to other type of fuel cells. Recently, DBFCs that rival proton-exchange membrane fuel cells (PEMFCs) in peak power but operating at double the voltage have been reported.
Anodic electrocatalyst for direct borohydride fuel cell and preparation method thereof
InactiveCN101485982ASimple preparation processNo need for high temperature calcinationCell electrodesMetal/metal-oxides/metal-hydroxide catalystsFiberGold particles
The invention relates to an anode electrocatalyst for a direct borohydride fuel cell and a method for preparing the same, in particular to a method for preparing a porous carbon loading nanometer gold catalyst. The preparation method comprises that: by adopting the metal sol loading method, the surface of the porous carbon carrier after purification and surface oxidizing treatment is loaded with a nanometer gold particle, and the size of the gold particle can be controlled by controlling the adding amount of a reducing agent and the gold concentration in the metal sol. The loading capacity of the gold is 5 to 30 percent, and the particle size of the gold is 2 to 6nm. The porous carbon is one of or a mixture of more than one of carbon nanometer pipe, carbon nanometer fiber, active carbon fiber, graphitized carbon black, active carbon and intermediate phase carbon microsphere, and the specific surface area of the carrier is between 100 and 2,000m / g. The method has the advantages of simple preparation process, unnecessary high-temperature calcination, and easy mass production. The prepared gold particle has the advantages of high-degree dispersion on the surface of the carbon carrier and even size distribution. When used as the anode electrocatalyst of the direct borohydride fuel cell, the anode electrocatalyst has good BH4 electric oxidation catalysis activity.
Owner:NO 63971 TROOPS PLA
Non-film type direct borohydride fuel cell pack
InactiveCN101388468ALow costSimple structureFuel cells groupingFinal product manufactureElectrochemical responsePeristaltic pump
The invention relates to a leptomonasform direct borohydride fuel cell stack, which comprises an electrode, a bipolar plate, fuel cavities, end plates and a fastening device, wherein the fuel cavities are arranged between the cathodes and the anodes of monocells, any proton exchange membranes are not adopted. The cell stack is formed through connecting the monocells in series by the bipolar plate. Fuel and oxygen (air) respectively enter through a fuel hole and a gas hole on one of the end plates, fuel enters the fuel cavity of each monocell, oxygen (air) enters each monocell in turn along a gas flow field on the bipolar plate after entering, and fuel solution and gas after reacting respectively flow out from the fuel hole and the gas hole on the other end plate. The continuous supplies of fuel and oxygen (air) are achieved through other accessory equipment (such as a peristaltic pump, a peristaltic pump and the like). Since the leptomonasform direct borohydride fuel cell stack directly adopts cathode catalyst with high selectivity to achieve a leptomonasform fuel cell structure, the production cost is lowered in a greater degree, and meanwhile, the speed of electrochemical reaction is further improved, thereby improving the property of a cell.
Owner:XI AN JIAOTONG UNIV
Application of Ni-based catalyst in anode of direct borohydride fuel cell
InactiveCN101667645AImprove stabilityImprove antioxidant capacityCell electrodesMetal/metal-oxides/metal-hydroxide catalystsActive componentDirect borohydride fuel cell
The invention provides application of a Ni-based catalyst in an anode of a direct borohydride fuel cell. Active components of the catalyst consist of Ni and one or more metal elements in I B and VIIIB groups; the atomic ratio of the Ni to other metal active components in the catalyst is 99:1-8:1; and the active components in the catalyst account for 5 to 80 percent and the balance is a C carrier.A small amount of one or more metal elements in the I B and VIII B groups are added into the Ni-based catalyst to obviously improve the stability and activity of the catalyst; and when the catalyst is used as a catalyst for the anode of the direct borohydride fuel cell, the direct borohydride fuel cell shows excellent performance.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Bipolar membrane type direct borohydride fuel cell
ActiveCN107706435APrevent penetrationPrevent arrivalElectrolyte holding meansSolid state electrolyteInterface layer
Disclosed is a bipolar membrane type direct borohydride fuel cell. The bipolar membrane type direct borohydride fuel cell comprises at least one cell unit; each cell unit consists of two layers of solid-state electrolyte membranes with ion selectivity transmission property; the solid-state electrolyte membranes are divided into two parts by the bipolar membrane; and the bipolar membrane adopts a three-layer structure which includes a cation exchange membrane layer, an anion exchange membrane layer and an intermediate interface layer positioned between the two exchange membrane layers. By adoption of the bipolar membrane type direct borohydride fuel cell, the negative electrode and the positive electrode can adopt non-uniform electrolytes, and a mixed potential caused by permeation of an intermediate product generated by the positive electrode fuel or the positive electrode to the negative electrode can be prevented; and meanwhile, permeation of a negative electrode product to the positive electrode also can be prevented, polarization loss of the electrode is lowered, the mixed potential is eliminated, and the fuel utilization rate and the output power are improved.
Owner:TAIYUAN UNIV OF TECH
Anion exchange membranes
InactiveCN101622305AImprove ionic conductivityReduce penetrationSolid electrolytesCell seperators/membranes/diaphragms/spacersHydrocarbon solventsAlcohol
The invention relates to an anion exchange membrane. The method for preparing an anion exchange membrane suitable for use in an alkaline fuel cell and particularly in a direct borohydride fuel cell, involves radiation grafting a hydrocarbon polymer film with a monomer and adding a quaternising agent. The degree of grafting is improved by mixing the monomer with a diluent comprising alcohol and a hydrocarbon solvent.
Owner:THE SEC OF STATE FOR DEFENCE IN HER BRITANNIC MAJESTYS GOVERNMENT OF THE UK OF GREAT BRITAIN & NORTHERN IRELAND
Direct borohydride fuel cell capable of simultaneously treating organic wastewater
InactiveCN107919484AEfficient degradationWith production capacityElectrolyte holding meansElectricityFuel cells
The invention discloses a direct borohydride fuel cell capable of simultaneously treating organic wastewater. The fuel cell is formed through assembling a bipolar membrane compounded by an anion-cation exchange membrane as a membrane; hydrogen peroxide is generated through oxygen reduction for a cathode of the direct borohydride fuel cell; a fenton reagent is formed through adding ferrous ions; and pollutants in organic wastewater are degraded during electricity generation, so that the direct borohydride fuel cell has two effects of power generation and organic wastewater treatment. A lot of H2O2 does not need to be added on site while organic matters are degraded, so that the whole process is natural and environment-friendly.
Owner:TAIYUAN UNIV OF TECH
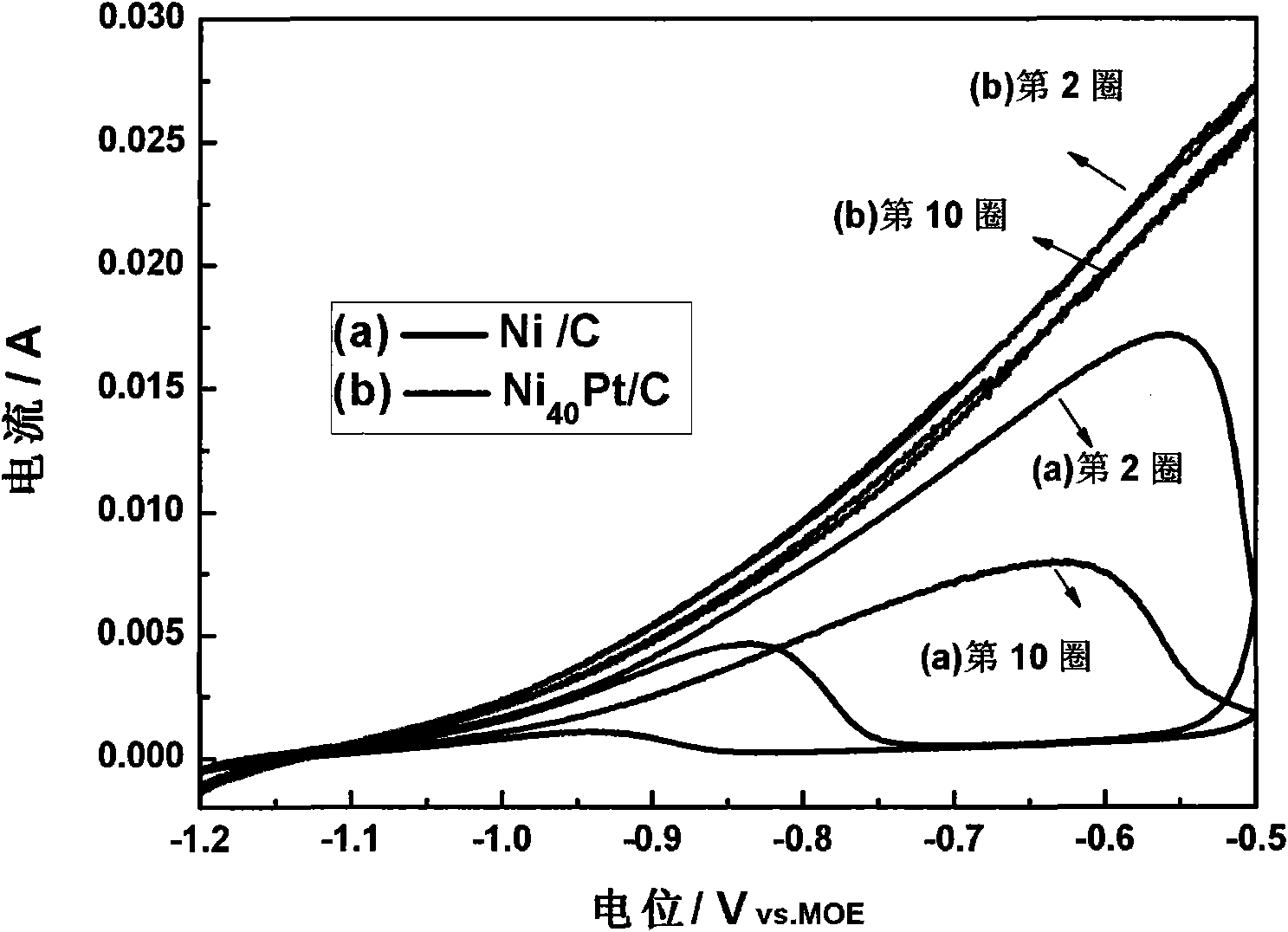
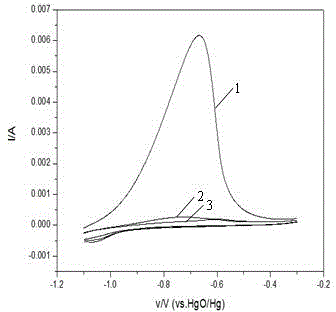
Nickel-based catalyst for improving performance of direct borohydride fuel cell
InactiveCN105070926AImprove discharge efficiencyEnhanced direct oxidation performanceCell electrodesDischarge efficiencyFuel cells
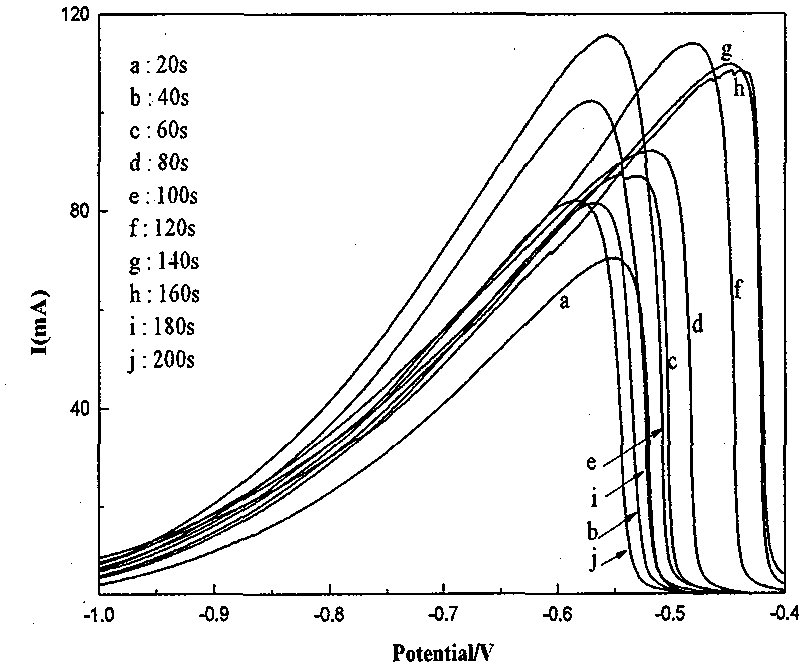
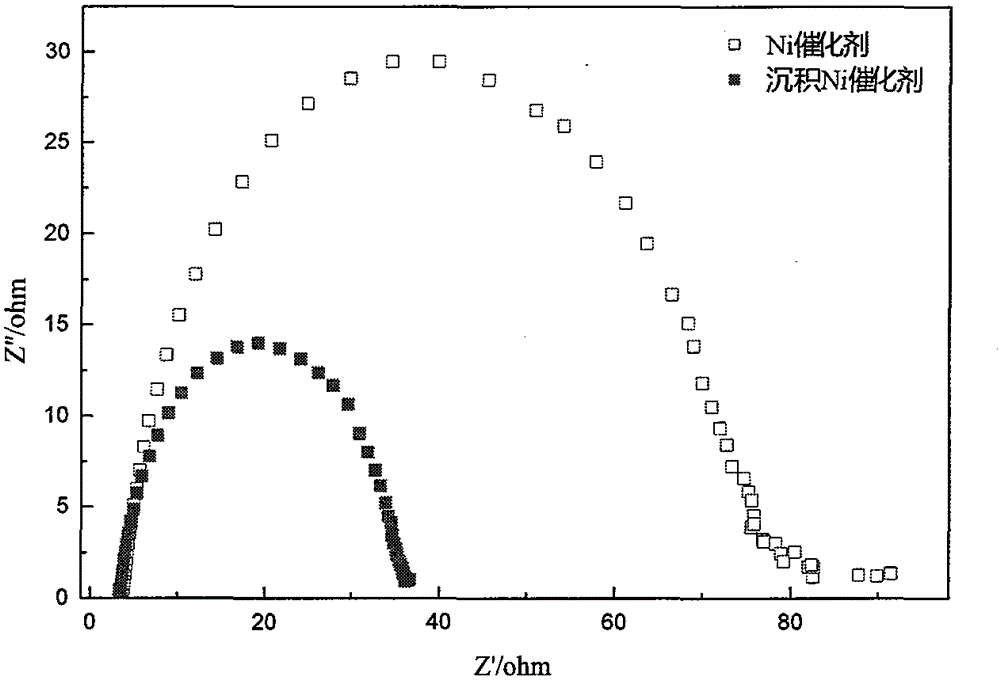
A nickel-based catalyst for improving the performance of a direct borohydride fuel cell is characterized in that the catalyst is prepared according to the following simple method of: (1) preparing a NiSO4 solution of 0.2 mol / dm<3> at a normal pressure and a temperature ranging from 293.15K to 313.15K; (2) assembling a three-electrode system, namely placing a smooth Ni sheet (serving as a working electrode) of 2 square meters in the above solution, wherein the Ni sheet is taken as a counter electrode, and a silver / silver chloride electrode is taken as a reference electrode; and (3) depositing Ni on a metal nickel sheet to prepare the nickel-based catalyst by using a constant potential (-0.8V) method. The surface morphology of the catalyst is changed due to deposition of the Ni, the specific surface area is obviously increased, catalytic active sites are greatly increased, and thus, the direct oxidizability of BH4<-> is enhanced; and meanwhile, the charge transfer resistance of electrode reaction is further reduced due to the deposition of the Ni, and fuel discharging efficiency is obviously improved.
Owner:CHONGQING UNIV
Preparation method of film electrode material using graphite coated paper to load NiAu
ActiveCN103943869AImprove anode catalytic performanceSolve the problem of poor anode activityCell electrodesFuel cellsGraphite
The invention provides a preparing method of a film electrode material using graphite coated paper to load NiAu. Clay is used as a curing agent, the clay and graphite are mixed evenly, then the surface of ordinary paper is coated with the evenly-mixed clay and graphite; 5-5.5g of NH4Cl and 1-1.5g of NiCl2 are dissolved in 50mL of water for preparing an electro-deposition solution; the paper coated with the graphite is maintained in the electro-deposition solution under 1.0V voltage for 20-30min for activation of the coated graphite, then a paper-graphite-Ni film electrode is obtained by 160-180min of electro-deposition of Ni under-1.0 V voltage; the paper-graphite-Ni film electrode is stood in a 1mmol L<-1> HAuClO4 solution for 2 to 4 minutes to obtain a paper-graphite-NiAu film electrode. According to the method, the graphite coated paper the is used, Ni is electrically deposited on the surface of the conductive graphite coated paper, then part of Ni is replaced with Au to prepare a NiAu-loading pencil coated paper catalyst, and the catalytic performance of the positive pole of a direct hydronoron fuel cell can be improved. The problem of poor activity the positive pole of a sodium borohydride fuel cell can be solved.
Owner:HEILONGJIANG HACHUAN CARBON MATERIAL TECH CO LTD
Preparation method of core-shell structured catalyst for direct hydroboron fuel cell anode
ActiveCN104218249AReduce sizeReduce the amount of AuMaterial nanotechnologyCell electrodesCatalytic oxidationControllability
A preparation method of a core-shell structured catalyst for a direct hydroboron fuel cell anode comprises the following steps: 1, preparing alloy nanoparticles of a core: preparing a core reactant micro-emulsion, preparing a reducing agent inverse micro-emulsion, and preparing alloy nanoparticles; and 2, preparing the core-shell structured catalyst: preparing a shell reactant micro-emulsion, and preparing a shell structured catalyst. The core-shell structured catalyst prepared through the method adopts a micro-emulsion method to effectively control the size of the particles in order to obtain particles with uniform dimensions, and a non-noble metal is used as a core element, so the noble metal is substantially reduced, the catalytic oxidation activity is improved, the surface morphology controllability of the metal nanoparticles is realized, and the preparation method has the advantages of simple operation, repeatability, low cost and easy realization of large scale production.
Owner:清创人和生态工程技术有限公司
Direct hydrogen storage alloy anode catalyst for borohydride fuel battery
InactiveCN101465430AImprove catalytic performanceHigh catalytic activityCell electrodesMetal/metal-oxides/metal-hydroxide catalystsElectricityDirect borohydride fuel cell
The invention provides a hydrogen storage alloy anode catalyst of direct hydroboron fuel batteries; transition metal oxide is added in the hydrogen storage alloy anode catalyst, and the mass ratio of the adding quantity of the transition metal oxide and the hydrogen storage alloy is 5-10 : 95-5. The doping of the transition metal oxide provides a new activity position for hydrogen adsorption and dissociation, improves the catalytic activity of the hydrogen storage alloy and solves the problem that anode discharging current of the hydroboron fuel battery is low. The hydrogen storage alloy anode catalyst is characterized in that: the mixture of the transition metal oxide and the hydrogen storage alloy increases the electrical catalysis performance of the hydrogen storage alloy to the hydroboron. In the essence of the invention, on the basis of anode catalyst of the hydrogen storage alloy of the direct hydroboron fuel batteries, the electrochemical oxidation susceptibility of the hydrogen storage alloy to the hydroboron is increased by mixing the transition metal oxide, and the discharge performance of the hydroboron anode is improved.
Owner:HARBIN ENG UNIV
Anode catalytic material for direct borohydride fuel cell, anode material as well as preparation method and fuel cell
ActiveCN109309236AReduce usageInhibit sheddingMaterial nanotechnologyCell electrodesDischarge efficiencyFuel cells
The invention discloses an anode catalytic material for a direct borohydride fuel cell, an anode material as well as a preparation method thereof and a fuel cell. The anode catalytic material for thedirect borohydride fuel cell directly synthesizes a two-metal catalytic material CoSnx with no carrier and no binder on foam nickel by virtue of a chemical reduction method, and x is 0 to 1. Accordingto the anode catalytic material for the direct borohydride fuel cell, the composite material of Sn and Co is firstly used as the catalytic material for the direct borohydride fuel cell, the anode material with good catalytic activity is combined with a material with high hydrogen overpotential, the obtained CoSnx is high in catalytic performance, the hydrolytic reaction of the borohydride is inhibited, the absolute hydrogen evolution rate is reduced, so that the discharging efficiency of fuel is greatly improved, and the contradiction between the catalytic capacity and the discharging efficiency can be effectively solved. The anode catalytic material for the direct borohydride fuel cell can provide theoretical foundation for optimizing and perfecting the DBFC anode catalytic material.
Owner:BEIFANG UNIV OF NATITIES
Non-noble metal-catalyzed polymer fibrous membrane hydroborate fuel cell
InactiveCN102437348BIncrease output powerLower resistanceCell component detailsFuel cell detailsPolyvinyl alcoholInternal resistance
Owner:XI AN JIAOTONG UNIV
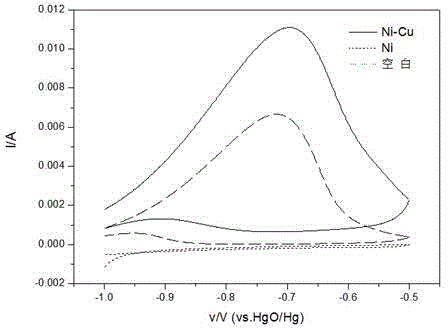
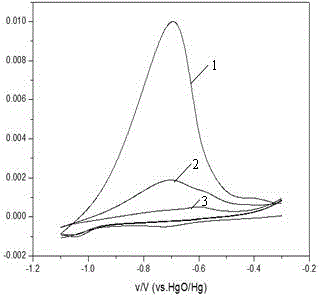
Ni-Cu binary catalyst for improving performance of direct borohydride fuel cell
InactiveCN102244276AEnhanced direct oxidation performanceReduce corrosionCell electrodesDischarge efficiencyElectrolytic agent
Provided is a Ni-Cu binary catalyst for improving performance of direct borohydride fuel cells. The catalyst is characterized by being prepared by the following steps: (1) preparing 0.25 mol / L of a CuSO4 solution in an acidic condition under normal pressure at a temperature in a range of 293.15 to 313.15K; (2) arranging a tri-electrode system, wherein 2 cm<2> of a Ni sheet used as a working electrode is put in the CuSO4 solution, a Cu rod is used as a counter electrode and a silver / silver chloride electrode is used as a reference electrode; (3) carrying out electrodeposition for 5 s by the potentiostatic method with the constant potential of -0.1V on an electrochemical work station so as to obtain the Ni-Cu binary catalyst. The Ni-Cu binary catalyst enhances direct oxidation performance of BH4<->, abates corrosion of an electrolyte to the Ni anode, reduces charge transfer resistance and improves discharge efficiency.
Owner:CHONGQING UNIV
Method for directly improving activity of borohydride fuel cell hydrogen storage alloy
InactiveCN101439304AIncrease contactImprove adsorption capacityCell electrodesCatalyst activation/preparationElectrochemical responseChemical reaction
The invention provides a method for improving the activity of a hydrogen storage alloy catalyst of a direct hydroboron fuel cell. According to the volume ration of 2: 1 between acid and the hydrogen storage alloy, the hydrogen storage alloy is dipped into the acid for 3 hours, and the concentration of alkali is 1 to 2M. The invention provides a method for improving the catalyzing performance of the anode of the hydrogen storage alloy catalyst of the direct hydroboron fuel cell by acid processing, the acid processing overcomes the defects of small specific area and low electric activity of the anode catalyst of the existing hydrogen storage alloy, and solves the problem of small discharge current of the anode of the hydrogen storage alloy catalyst. The method is characterized in that the hydrogen storage alloy is processed by acid before in use, thereby enlarging the electric oxidation performance of the hydrogen storage alloy catalyst to the hydroboron. The essential of the method is to enlarge the electric chemical oxidation performance of the hydrogen storage alloy catalyst to the hydroboron and to improve the electric chemical reaction performance of the hydroboron based on the anode catalyst of the hydrogen storage alloy of the direct hydroboron fuel cell by carrying out acid processing on the hydrogen storage alloy in ahead.
Owner:长春长光宇航复合材料有限公司
Anode catalyst of direct borohydride fuel cell and preparation method thereof
The invention relates to an anode catalyst of a direct borohydride fuel cell, a preparation method of the anode catalyst, the direct borohydride fuel cell and the anode catalyst of the direct borohydride fuel cell, the anode catalyst of the direct borohydride fuel cell is LaMgNi series A2B7 type hydrogen storage alloy, the molecular formula of the hydrogen storage alloy is (Mm<1-x>Mgx)Ni2.485Co0.525Mn0.28Al0.21, wherein x=0.1-0.5, and Mm is misch metal which is composed of, by weight, 63.1%of La, 26.0% of Ce, 2.7% of Pr and 8.2% of Nd. The anode catalyst is a La-Mg-Ni series A2B7 type hydrogenstorage alloy, which not only has direct catalytic capability, but also can adsorb (or store) generated hydrogen and release the hydrogen in an electric energy form; the fuel utilization rate is improved through dual action, and the problems that in the prior art, precious metal serving as the anode catalyst is high in price and high in cost performance are solved; and a common hydrogen storage alloy is poor in catalytic activity.
Owner:INNER MONGOLIA NORMAL UNIVERSITY
Preparation method and application of anode catalyst of direct borohydride fuel cell
InactiveCN105655601AImprove discharge efficiencyCell electrodesFinal product manufactureDischarge efficiencyHydrogen
The invention relates to the technical field of borohydride fuel cell production, and providing a preparation method of an anode catalyst of a direct borohydride fuel cell. The method comprises the following steps that a Co<2+> solution is taken; ammonia water is dripped into the solution; stirring is performed until the pH value is 8.5 to 9; a mixed solution is obtained; the mixed solution is subjected to solid-liquid separation; solid phases after separation are taken and are sequentially subjected to cleaning, drying, grinding and roasting; the anode catalyst of the direct borohydride fuel cell is obtained. The anode catalyst obtained through preparation has the advantages that the side reaction of hydrogen gas generation through anode hydrolysis in the direct borohydride fuel cell can be inhibited, and the discharging efficiency of anode fuels is improved.
Owner:BEIFANG UNIV OF NATITIES
Preparation method of carbon surface modified hydrogen storage alloy and method for improving anode catalytic performance of direct borohydride fuel cell
The invention provides a preparation method of a carbon surface modified hydrogen storage alloy and a method for improving the anode catalytic performance of a direct borohydride fuel cell. The preparation method includes: mixing hydrogen storage alloy powder and carbon evenly, then placing the mixture into a ball milling tank, conducting ball milling for 1-2h at a speed of 3000-5000rpm under the protection of nitrogen, then taking the mixture out, gradually adding a 60wt.% polytetrafluoroethylene solution and a 1.5wt.% sodium carboxymethylcellulose colloid that are in a mass ratio of 1:1, keeping the mass ratio of the hydrogen storage alloy powder and the carbon quality to the polytetrafluoroethylene solution and the sodium carboxymethyl cellulose at 9-10:1, and stirring them uniformly to prepare a slurry containing the hydrogen storage alloy and carbon, coating foamed nickel with the slurry, and carrying out drying for 2.5h at 70DEG C, thus obtaining the carbon surface modified hydrogen storage alloy, which can be used for improving the anode catalytic performance of a direct borohydride fuel cell.
Owner:HARBIN ENG UNIV
Anode catalyst for direct borohydride fuel cell, anode material, preparation method of anode material, and fuel cell
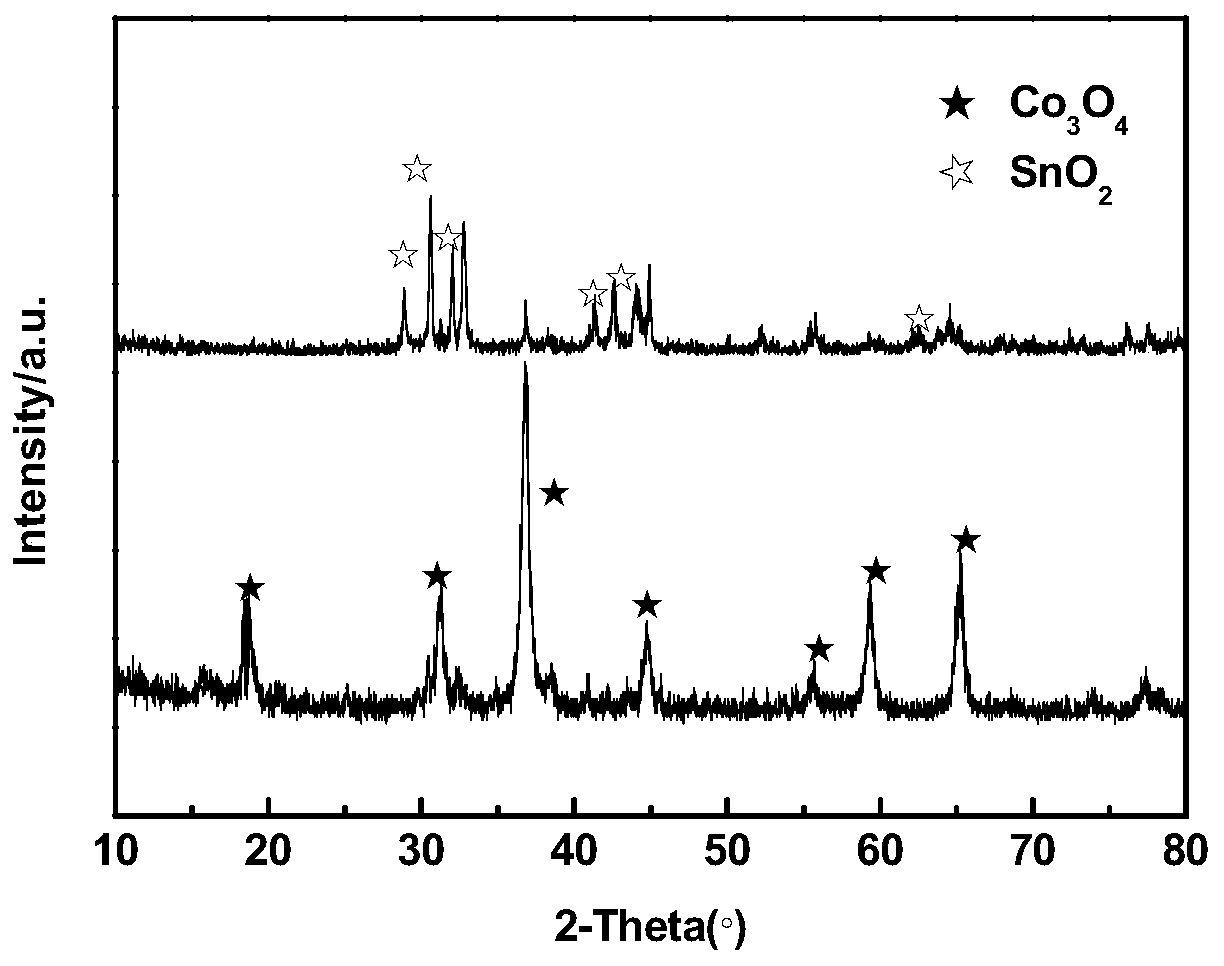
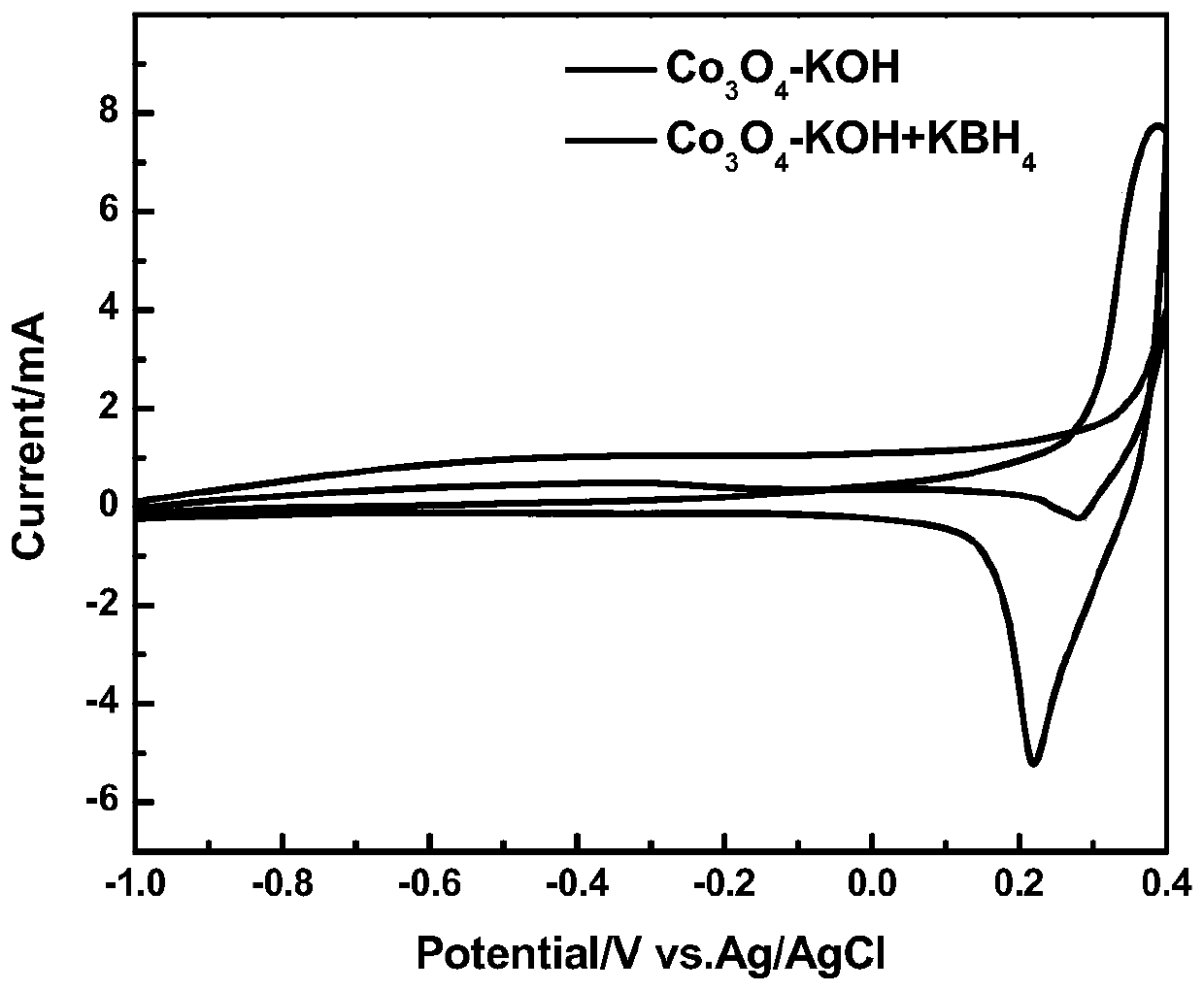
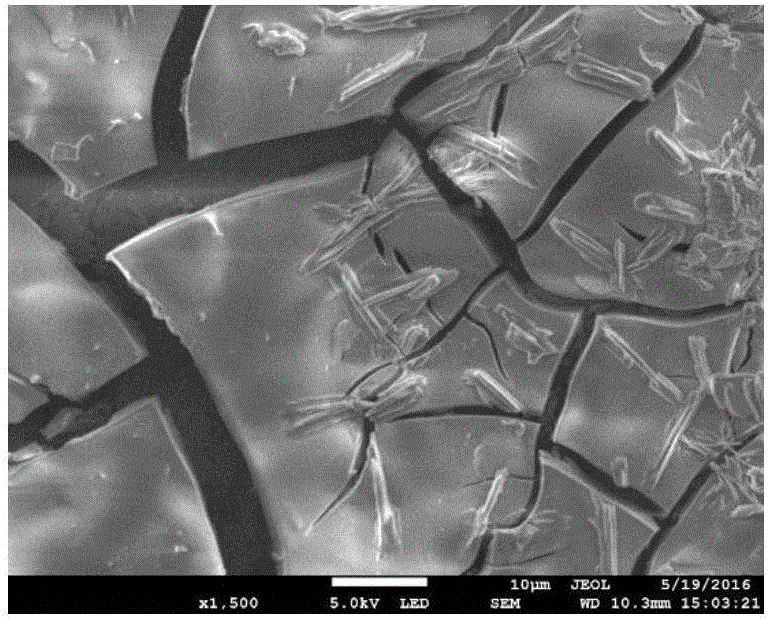
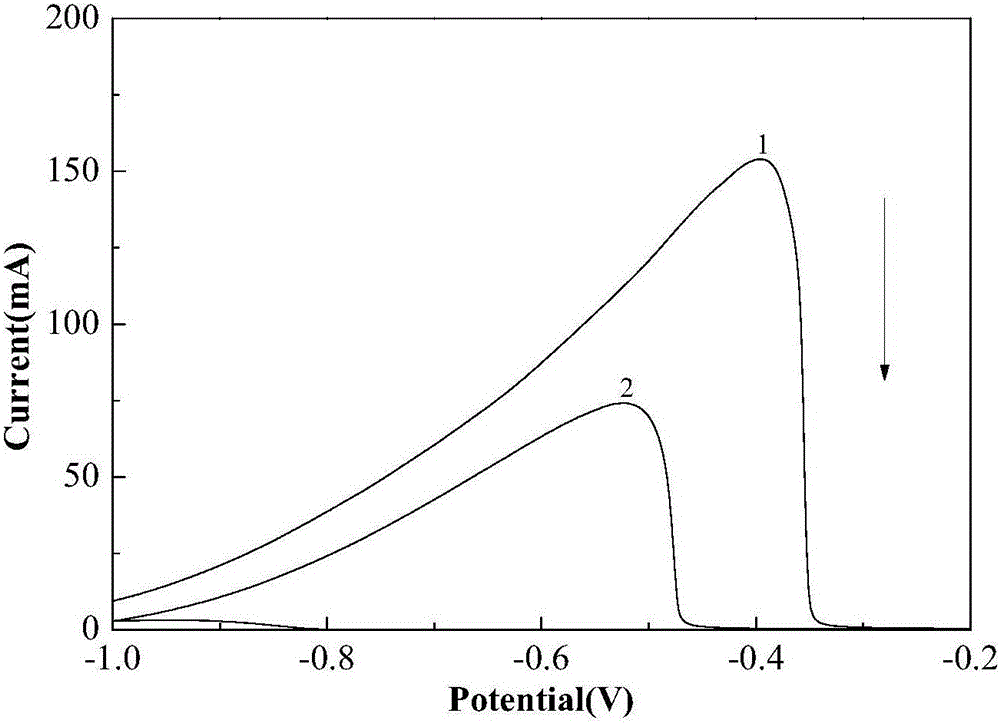
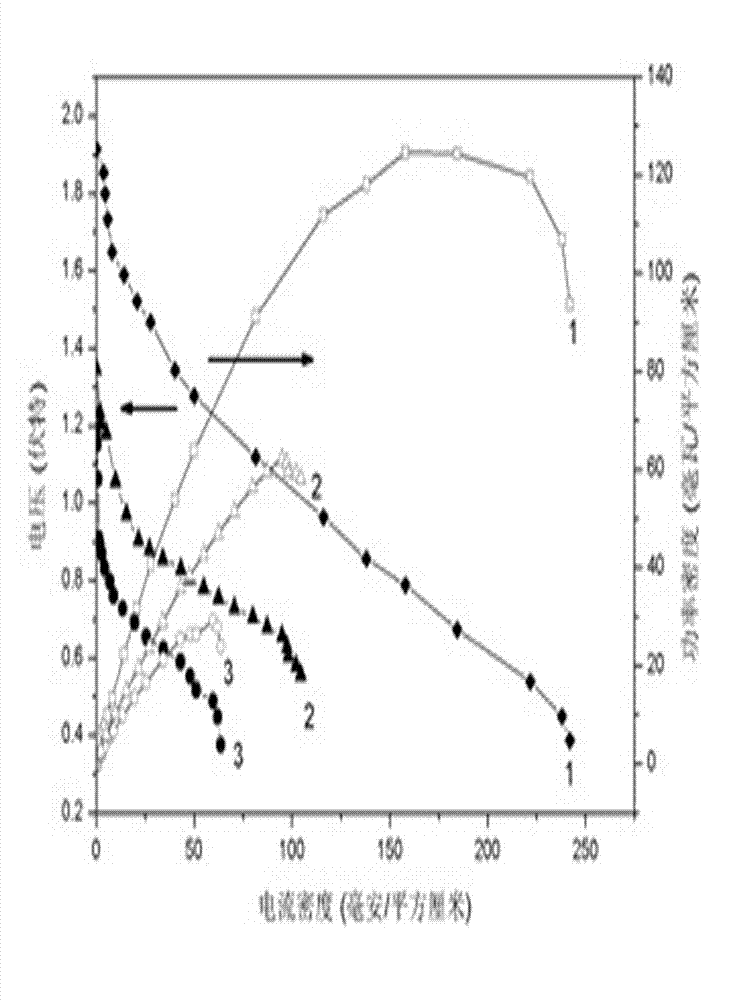
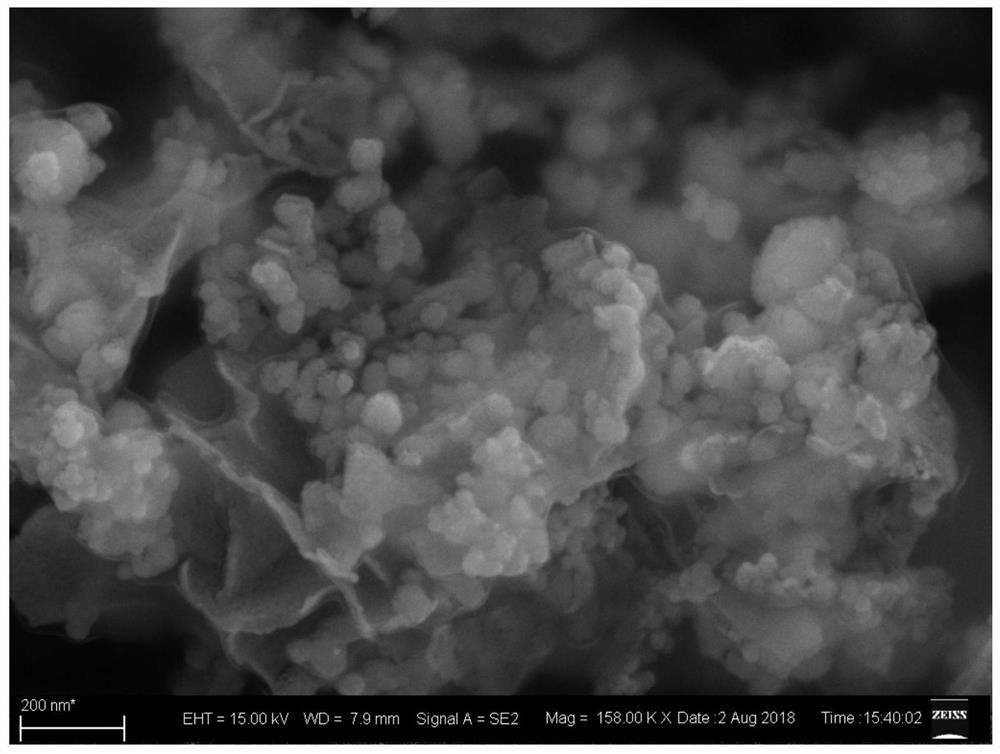
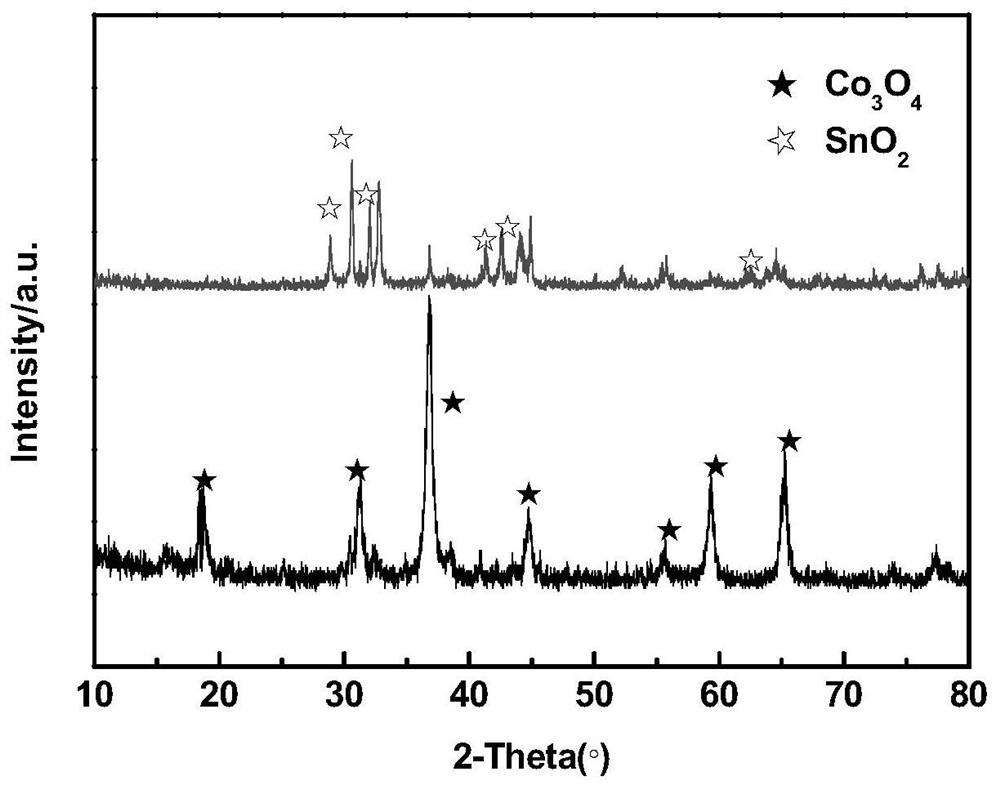
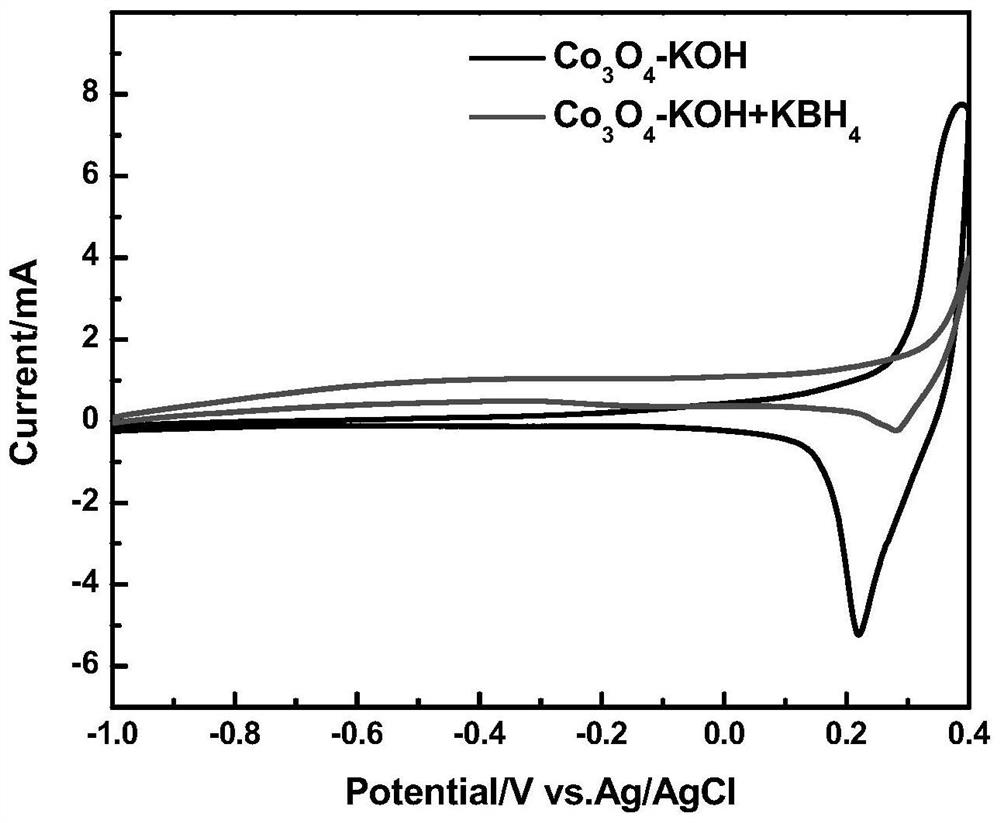
ActiveCN110364742AEasy transferStable supportMaterial nanotechnologyCell electrodesFuel cellsEngineering
The invention discloses an anode catalyst for a direct borohydride fuel cell, an anode material, a preparation method of the anode material and the fuel cell. The anode catalyst for the direct borohydride fuel cell adopts Co3O4 / SnO2. Co3O4 / SnO2 is attached to the surface of lamellar SnO2 through granular Co3O4, and the lamellar SnO2 is interwoven to form a 3D structure. According to the invention,the granular Co3O4 is attached to the surface of lamellar SnO2 and the lamellar SnO2 is interwoven, so a good supporting effect is achieved for the Co3O4 and the 3D structure similar to a pistil structure is formed; and through the Co3O4 / SnO2 structure, electron transmission is facilitated and more reaction space is provided for the electrolyte, thereby facilitating improvement of catalytic performance.
Owner:BEIFANG UNIV OF NATITIES
Additive for improving nickel anode catalyst performance of direct borohydride fuel cell
InactiveCN105826576AImprove electrochemical oxidation efficiencyImprove discharge efficiencyCell electrodesDischarge efficiencyFuel cells
The invention relates to a thiourea (TU) additive for improving the nickel anode catalyst performance of a direct borohydride fuel cell .A preparation method of the additive comprises the following steps that at atmospheric pressure, on the condition that the temperature ranges from 293.15 K to 313.15 K, metal nickel is deposited on a Ni piece electrode through a constant electric potential (-0.8 V) method, and a prepared nickel-based catalyst serves as a nickel anode catalyst; a thiourea (TU) solution of 0.045 mol / L is prepared, 1 mL of the well-prepared thiourea solution is taken and diluted by five times to enable the concentration of the thiourea solution to be 0.009 mol / L, and the solution serves as an electrolyte additive .A hydrophobic thin film is formed on the surface of the nickel-based catalyst by means of thiourea (TU), the thin film changes distribution of BH4<-> on the surface of the nickel-based catalyst, and the BH4<-> electrochemical oxidation efficiency is improved .Shift of BH4<-> electrochemical oxidation peak potential is caused by addition of thiourea (TU), the electrochemical impedance of the system becomes smaller, the discharging efficiency becomes higher, the discharge potential is more negative, and the discharging time is longer.
Owner:CHONGQING UNIV
Core-shell structural anode catalyst for direct borohydride fuel cells and preparation method thereof
InactiveCN102380400BGood anodic oxidation performanceIncrease profitCell electrodesMetal/metal-oxides/metal-hydroxide catalystsNano catalystNitrogen gas
Owner:TAIYUAN UNIV OF TECH
Preparation method of carbon surface modified hydrogen storage alloy and method for improving anode catalytic performance of direct borohydride fuel cell
The invention provides a preparation method of a carbon surface modified hydrogen storage alloy and a method for improving the anode catalytic performance of a direct borohydride fuel cell. The preparation method includes: mixing hydrogen storage alloy powder and carbon evenly, then placing the mixture into a ball milling tank, conducting ball milling for 1-2h at a speed of 3000-5000rpm under the protection of nitrogen, then taking the mixture out, gradually adding a 60wt.% polytetrafluoroethylene solution and a 1.5wt.% sodium carboxymethylcellulose colloid that are in a mass ratio of 1:1, keeping the mass ratio of the hydrogen storage alloy powder and the carbon quality to the polytetrafluoroethylene solution and the sodium carboxymethyl cellulose at 9-10:1, and stirring them uniformly to prepare a slurry containing the hydrogen storage alloy and carbon, coating foamed nickel with the slurry, and carrying out drying for 2.5h at 70DEG C, thus obtaining the carbon surface modified hydrogen storage alloy, which can be used for improving the anode catalytic performance of a direct borohydride fuel cell.
Owner:HARBIN ENG UNIV
Anode catalyst for direct borohydride fuel cell, anode material and preparation method thereof, and fuel cell
ActiveCN110364742BEasy transferStable supportMaterial nanotechnologyCell electrodesPtru catalystFuel cells
Owner:BEIFANG UNIV OF NATITIES
Anode catalyst material for direct borohydride fuel cell, anode material and preparation method thereof, and fuel cell
ActiveCN109309236BReduce usageInhibit sheddingMaterial nanotechnologyCell electrodesFuel cellsPhysical chemistry
Owner:BEIFANG UNIV OF NATITIES
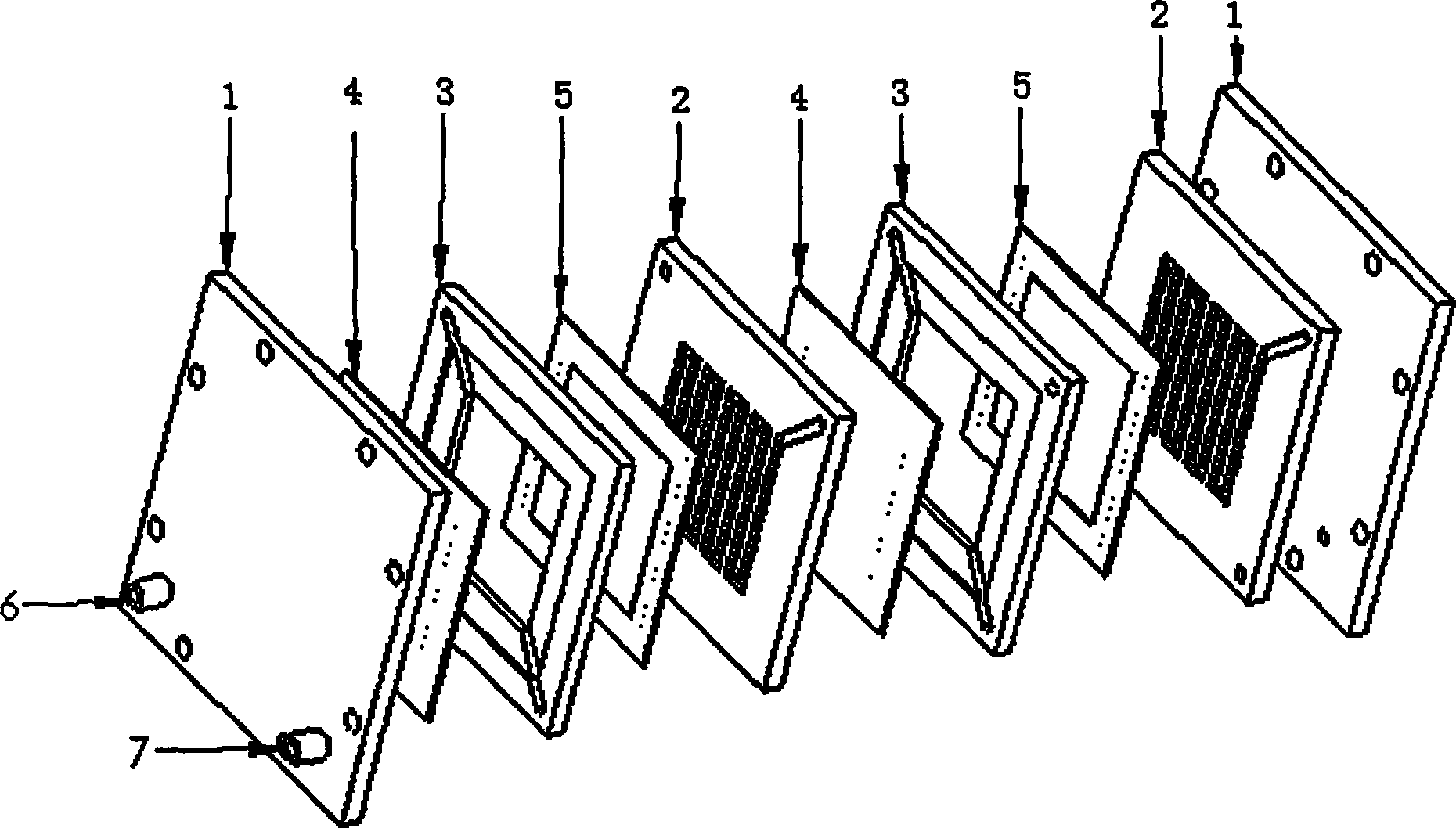
Non-film type direct borohydride fuel cell pack
InactiveCN101388468BLow costSimple structureFuel cells groupingCell electrodesElectrochemical responsePeristaltic pump
The invention relates to a leptomonasform direct borohydride fuel cell stack, which comprises an electrode, a bipolar plate, fuel cavities, end plates and a fastening device, wherein the fuel cavities are arranged between the cathodes and the anodes of monocells, any proton exchange membranes are not adopted. The cell stack is formed through connecting the monocells in series by the bipolar plate. Fuel and oxygen (air) respectively enter through a fuel hole and a gas hole on one of the end plates, fuel enters the fuel cavity of each monocell, oxygen (air) enters each monocell in turn along a gas flow field on the bipolar plate after entering, and fuel solution and gas after reacting respectively flow out from the fuel hole and the gas hole on the other end plate. The continuous supplies of fuel and oxygen (air) are achieved through other accessory equipment (such as a peristaltic pump, a peristaltic pump and the like). Since the leptomonasform direct borohydride fuel cell stack directly adopts cathode catalyst with high selectivity to achieve a leptomonasform fuel cell structure, the production cost is lowered in a greater degree, and meanwhile, the speed of electrochemical reaction is further improved, thereby improving the property of a cell.
Owner:XI AN JIAOTONG UNIV
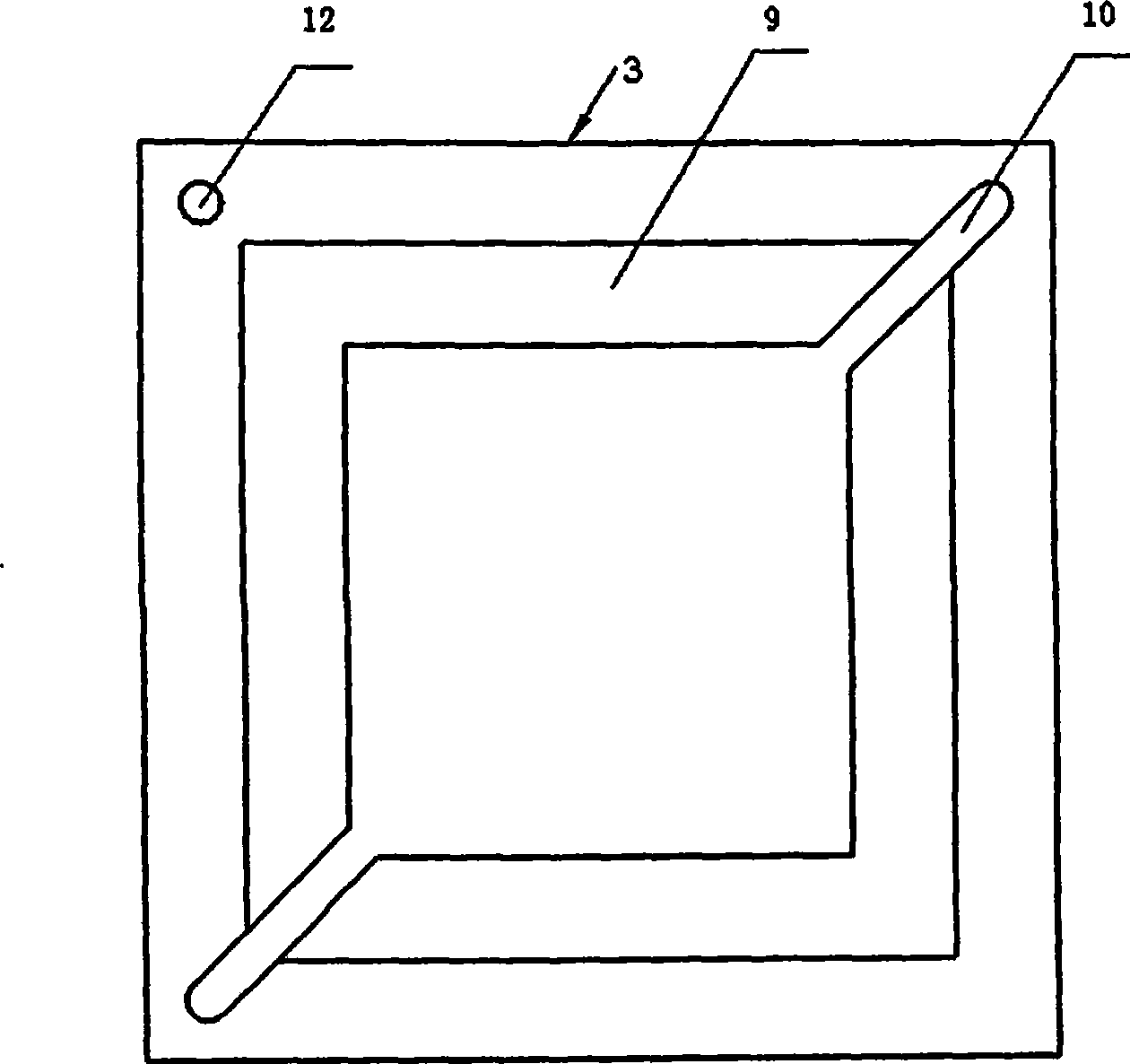
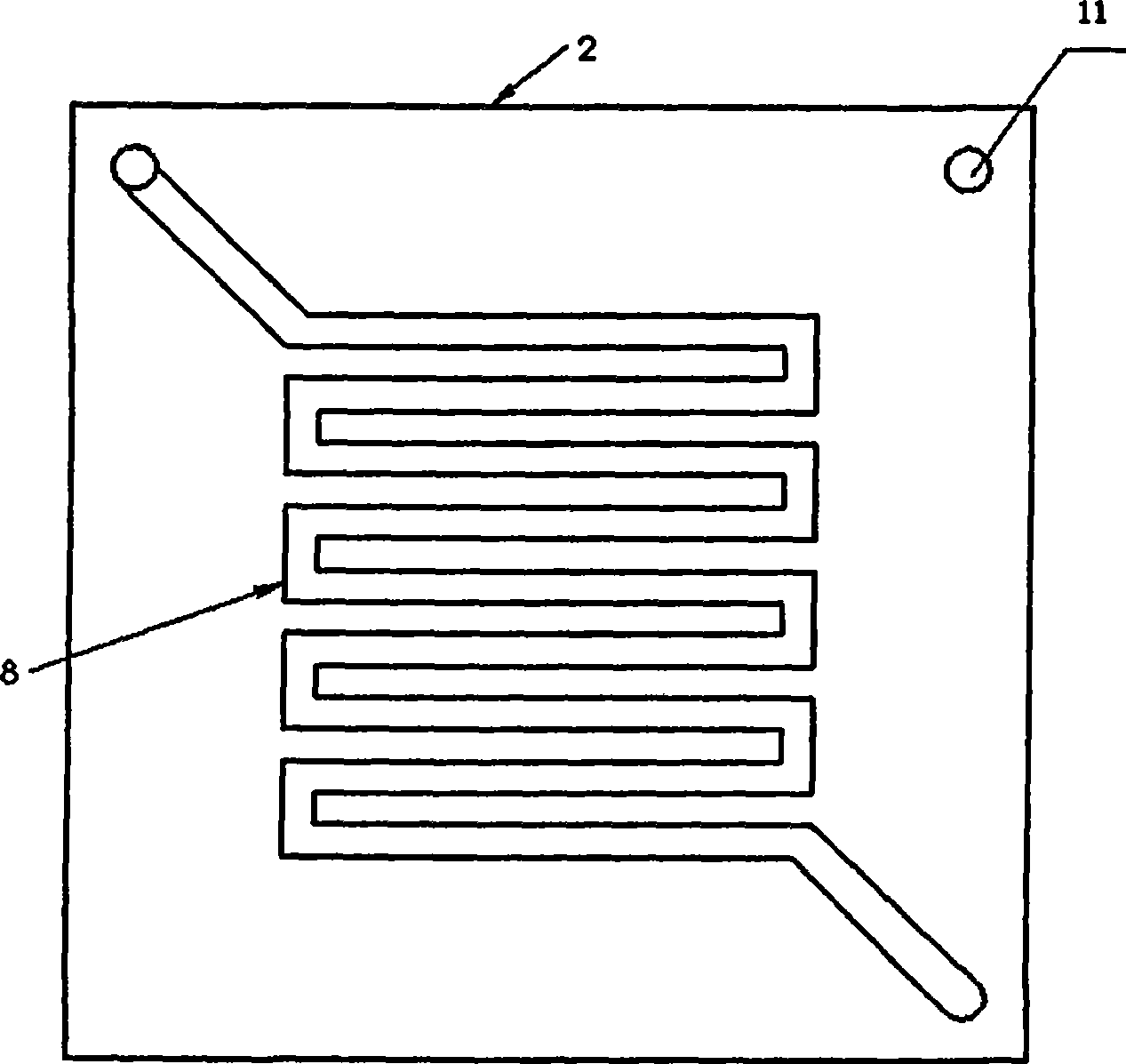
Composite membrane electrode of direct borohydride fuel cell
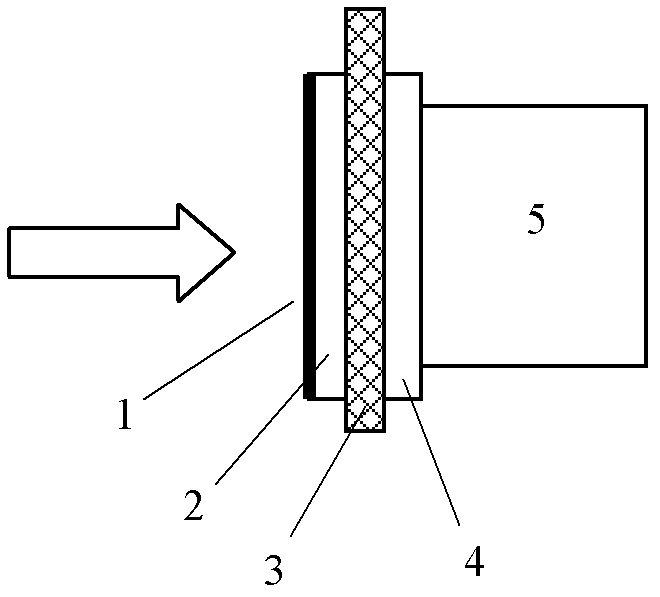
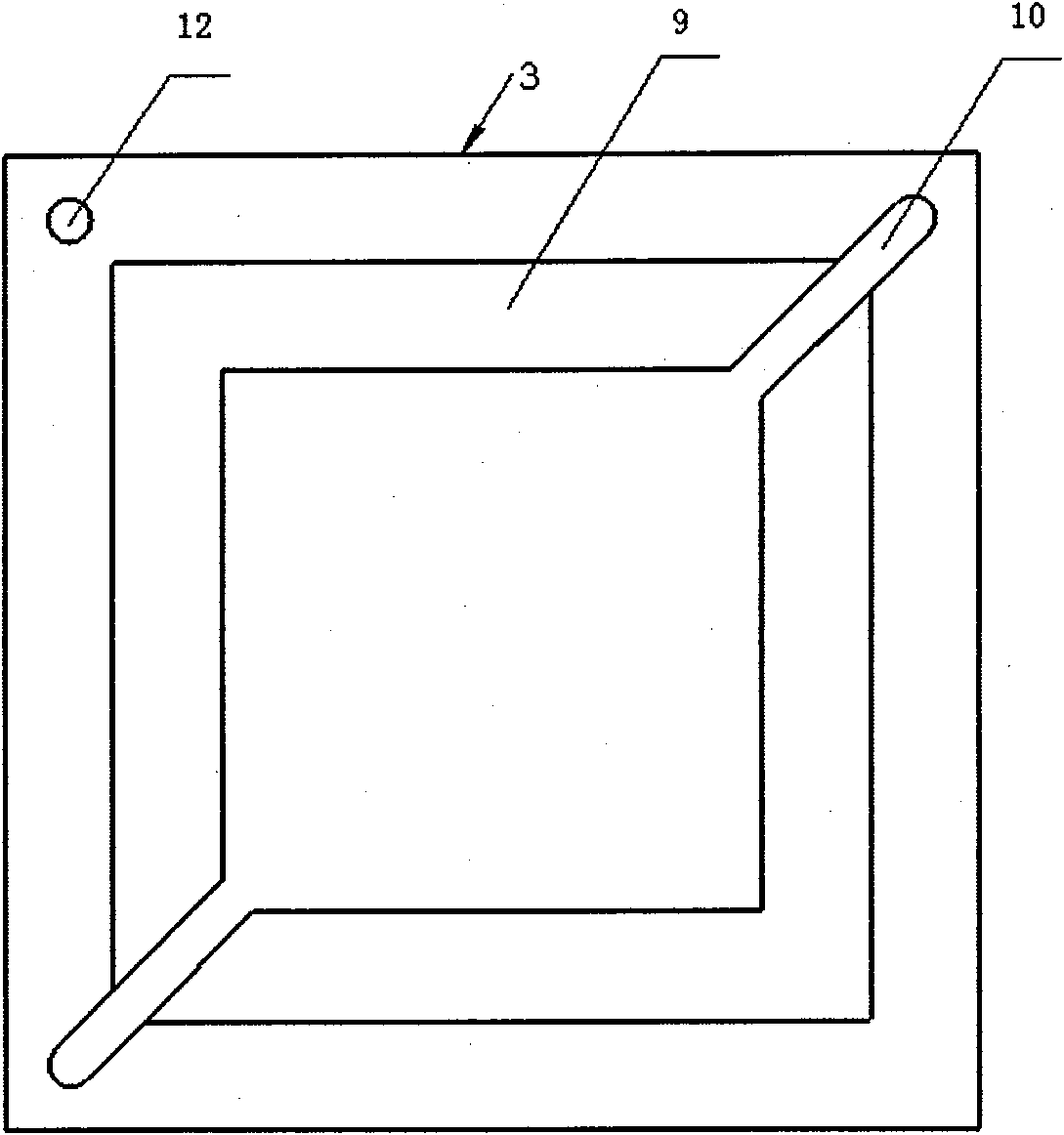
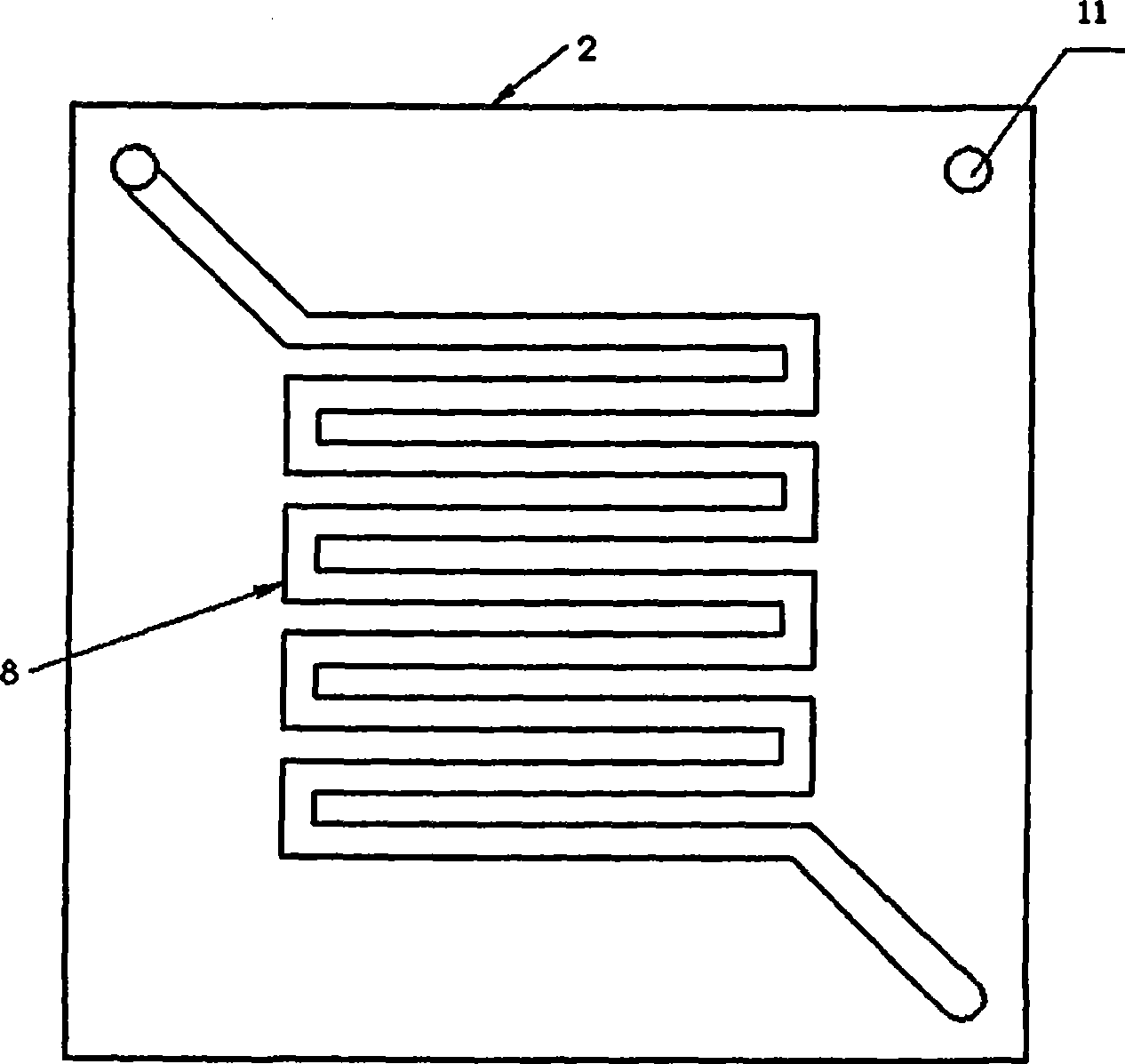
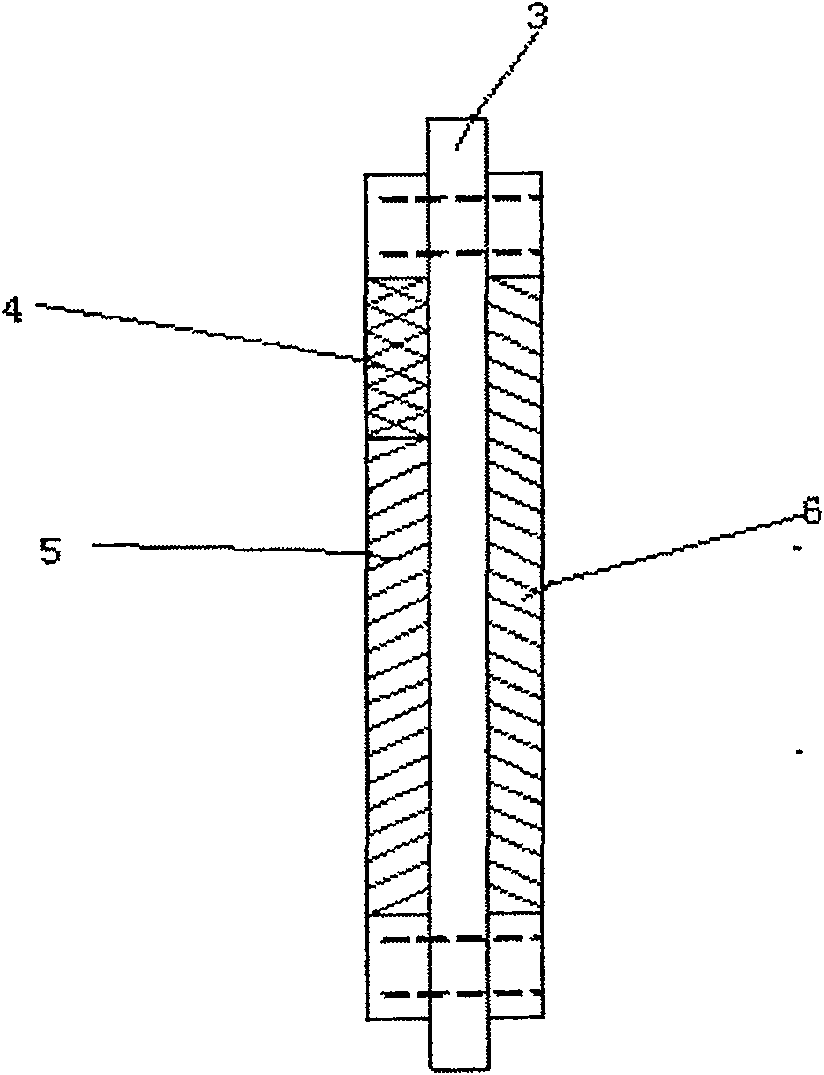
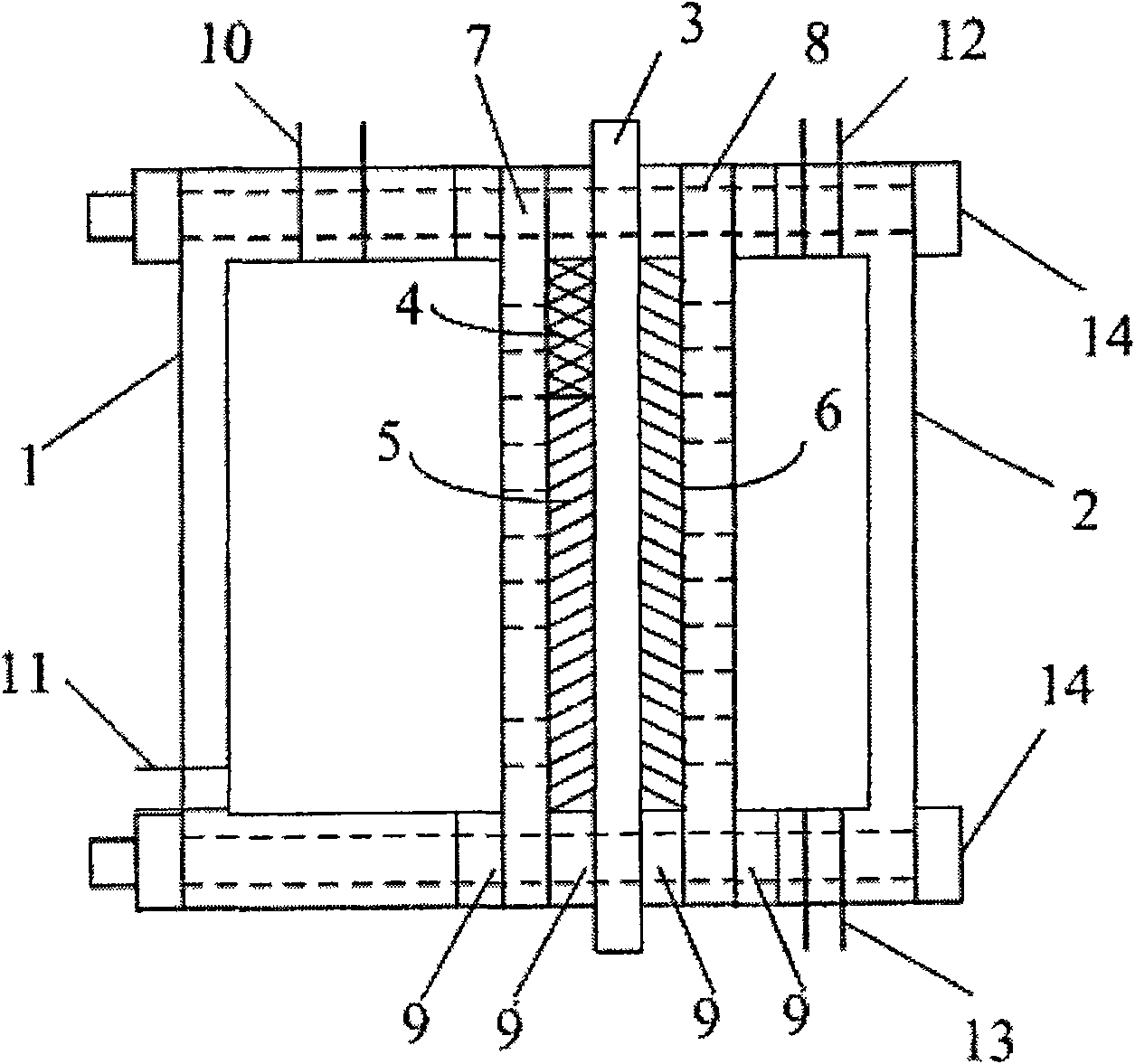
InactiveCN100593877CImprove overall utilizationLow densityCell electrodesSolid electrolyte fuel cellsCatalytic oxidationSide reaction
A composite membrane electrode of a direct borohydride fuel cell relates to a fuel cell membrane electrode. The composite membrane electrode solves the problems that the hydrogen produced by hydrolysis of the direct borohydride fuel cell can not be directly used and the overall utilization rate of the cell fuel is low. The composite membrane electrode of the direct borohydride fuel cell is composed of an anode, a cathode (6) and an electrolyte membrane (3). The anode and the cathode (6) are respectively arranged on two sides of the electrolyte membrane and parallel to the electrolyte membrane;the anode, the cathode and the electrolyte membrane are hot pressed to a membrane electrode; the anode is composed of a borohydride-radical catalytic oxide anode (5) which is arranged on the lower part of the anode and a hydrogen catalytic oxide anode (4) which is arranged on an upper part of the anode. The side reaction of the borohydride radical is unavoidable on the borohydride-radical catalytic oxide anode, and the hydrogen is produced and can continue to react as the fuel on the hydrogen catalytic oxide anode, which can improve the overall utilization rate of the fuel and causing the structure of the whole membrane electrode system to be more compact and safer.
Owner:HARBIN INST OF TECH
Method for improving hydrogen storage alloy anode catalyse performance of borohydride fuel battery directly
InactiveCN101465429AImprove performanceHigh catalytic activityCell electrodesMetal/metal-oxides/metal-hydroxide catalystsDirect borohydride fuel cellDischarge current
The invention provides a method for improving catalysis performance of hydrogen storage alloy anode of direct hydroboron fuel batteries; according to the volume ratio of alkali and hydrogen storage alloy with 2:1, the hydrogen storage alloy is soaked in alkali liquid with 80 DEG.C for five hours, the alkali density is 5-8M. The invention proposes a method for improving catalysis performance of hydrogen storage alloy anode of direct hydroboron fuel batteries with alkali treatment; the alkali treatment overcomes the disadvantages that the existing hydrogen storage alloy anode catalyst has small specific surface, low electrical activity and the like and solves the problem that the discharge current of the hydroboron fuel batteries is small. The method is characterized in that: before being used, the hydrogen storage alloy is treated by the alkali, thus increasing the catalysis performance of the hydrogen storage alloy to the hydroboron. In the essence of the invention, on the basis of anode catalyst of the hydrogen storage alloy of the direct hydroboron fuel batteries, the electrochemical catalysis performance of the hydrogen storage alloy to the hydroboron is increased by the alkali treatment to the hydrogen storage alloy in advance, and the discharge performance of the hydroboron anode is improved.
Owner:HARBIN ENG UNIV
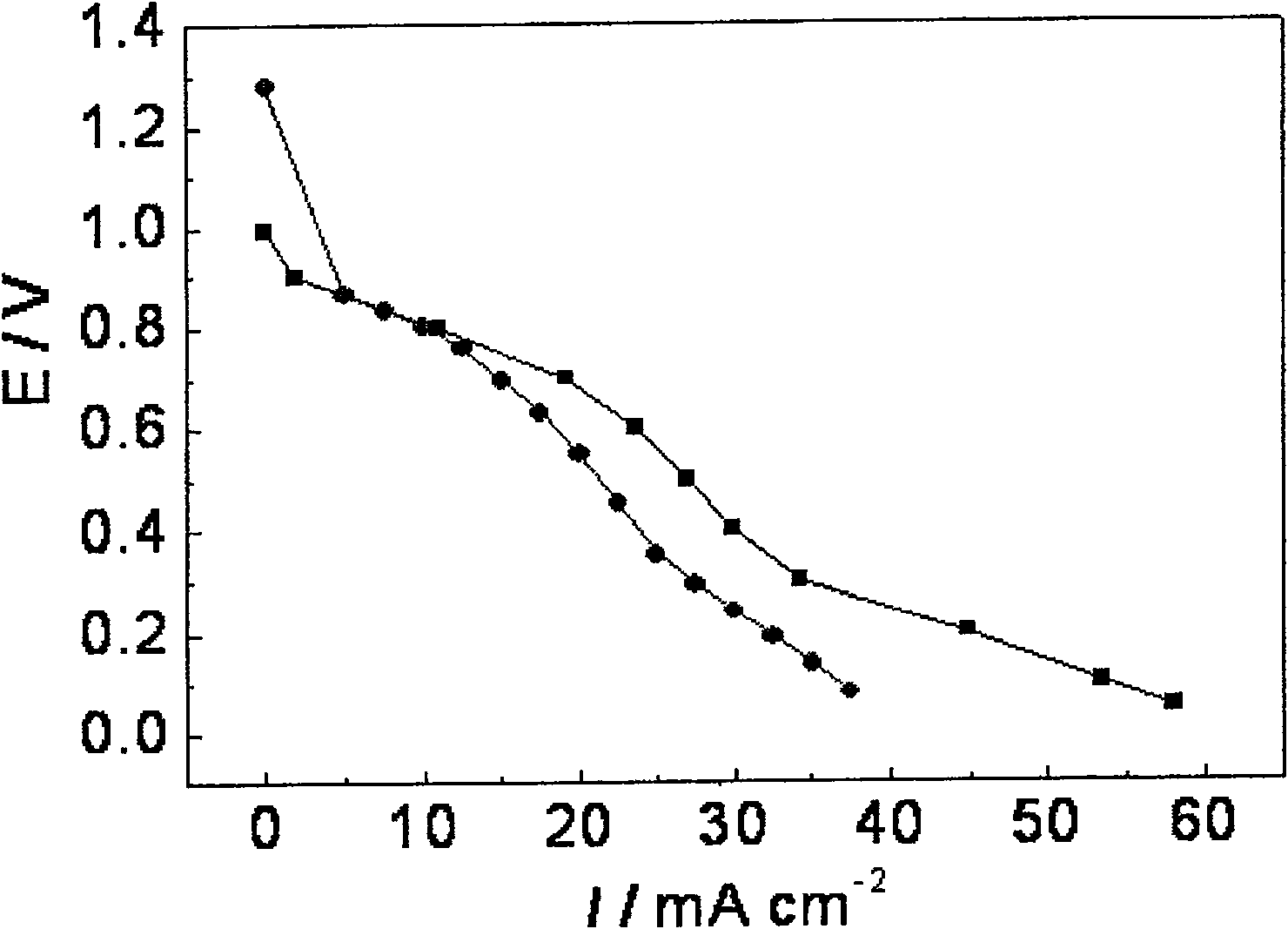
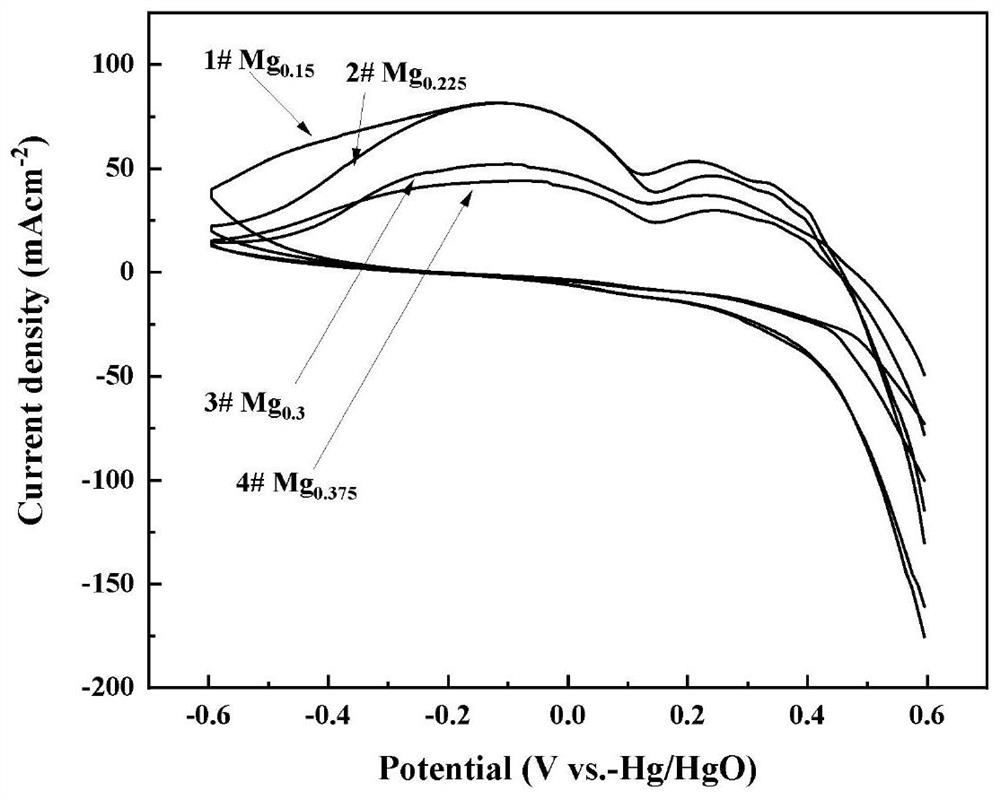
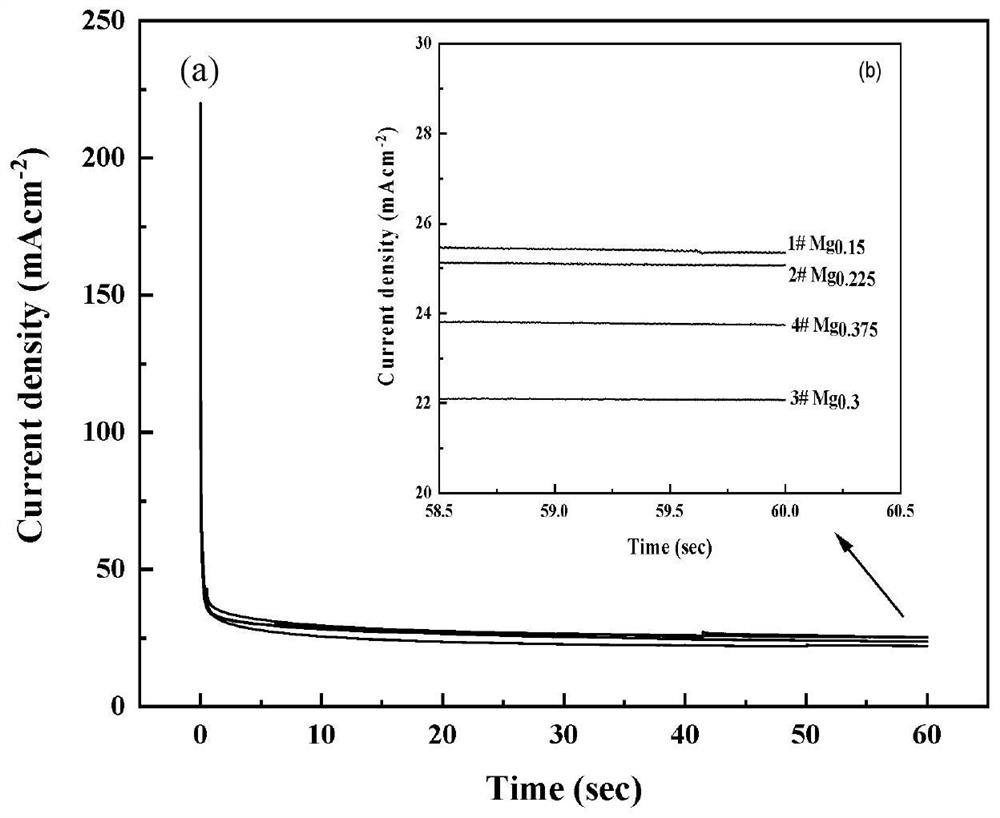
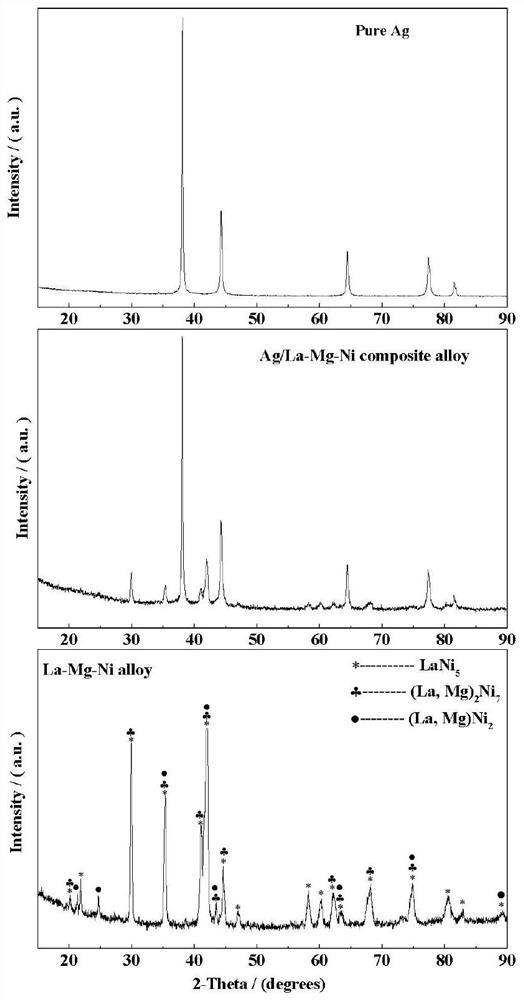
Preparation method of Ag-modified La-Mg-Ni type hydrogen storage alloy and application of Ag-modified La-Mg-Ni type hydrogen storage alloy as DBFC anode catalyst
ActiveCN113410476APrevent surface oxidationAvoid formingCell electrodesLiquid/solution decomposition chemical coatingPtru catalystFuel cells
The invention relates to a preparation method of an Ag-modified La-Mg-Ni type hydrogen storage alloy and an application of the Ag-modified La-Mg-Ni type hydrogen storage alloy as an anode catalyst of a direct borohydride fuel cell (DBFC), and the Ag-modified La-Mg-Ni type hydrogen storage alloy is obtained by placing a prepared PVP + AgNO3 / La-Mg-Ni type hydrogen storage alloy mixture under an ultraviolet xenon lamp for irradiation. The alloy prepared by the method is used as an anode catalyst of a direct hydroboron fuel cell. The noble metal Ag is adopted for surface modification of the La-Mg-Ni hydrogen storage alloy, surface oxidation of the hydrogen storage alloy in the electrochemical reaction process can be effectively inhibited, and good catalytic performance is embodied; the target product Ag / La-Mg-Ni type hydrogen storage alloy powder is obtained by adopting an ultraviolet irradiation method; the method is simple and convenient to operate, time-saving and pollution-free, and compared with a conventional mechanical ball milling method, repeated vacuumizing and repeated gas flushing are not needed, so the preparation time is greatly shortened, and Fe impurities are also prevented from being mixed into the alloy powder; the direct hydroboron fuel cell prepared by taking the alloy as an anode catalyst can achieve better discharge voltage and relatively long discharge time.
Owner:INNER MONGOLIA NORMAL UNIVERSITY
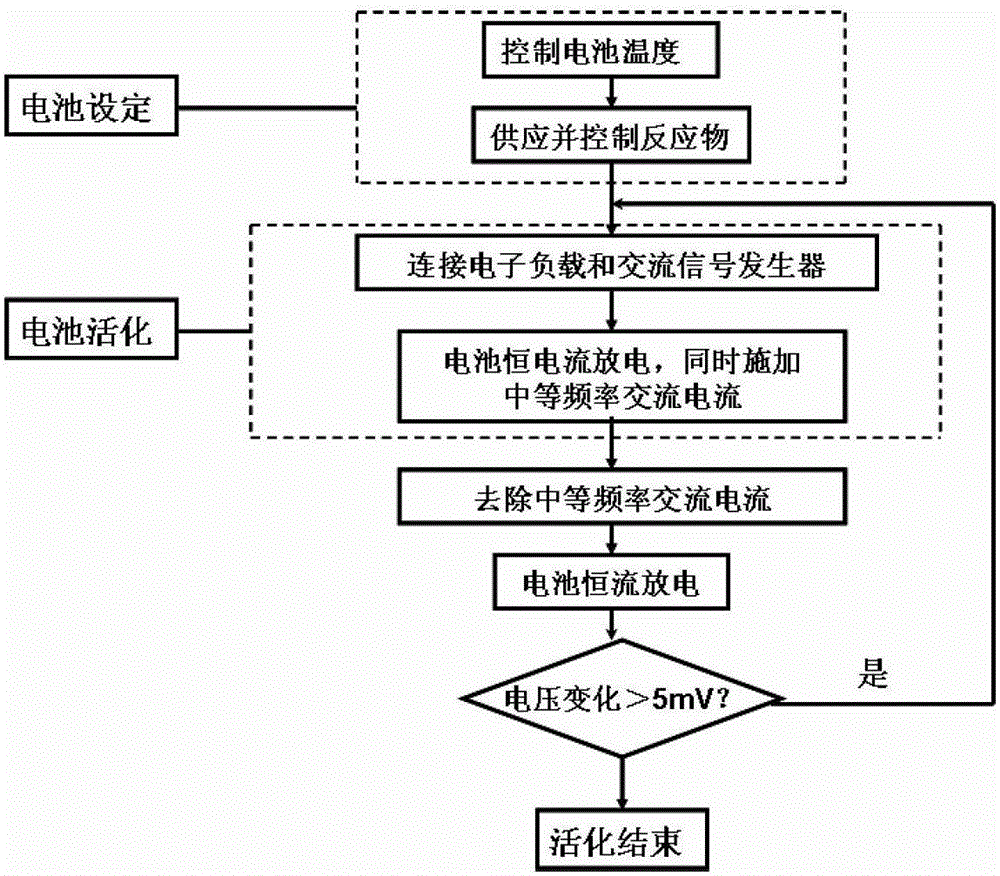
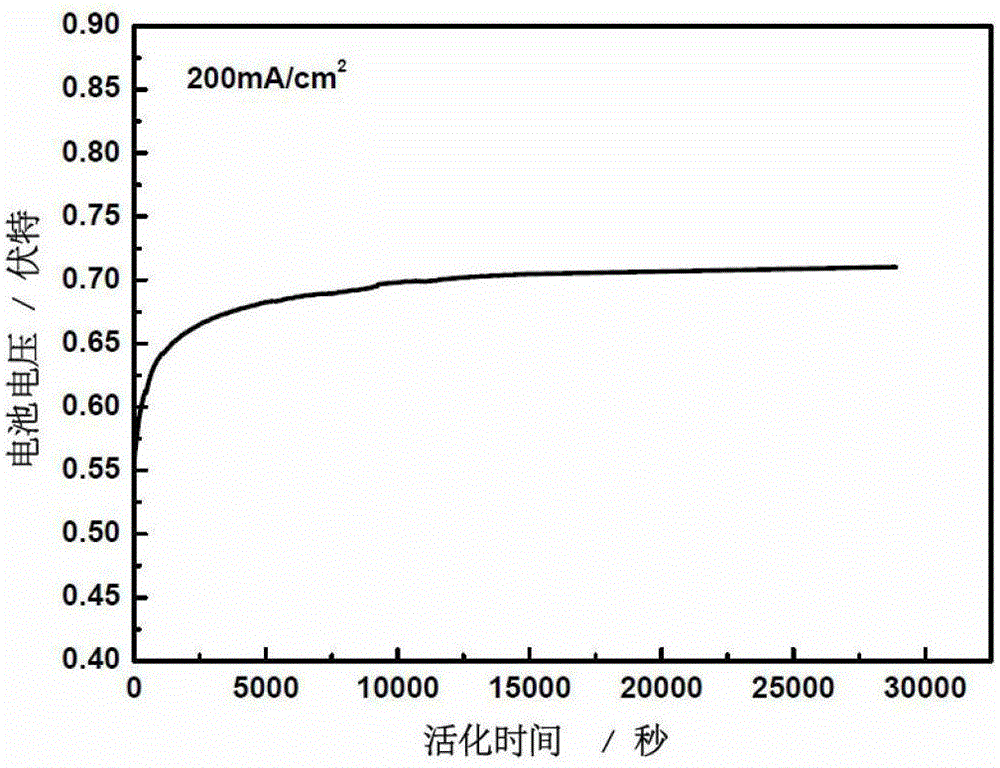
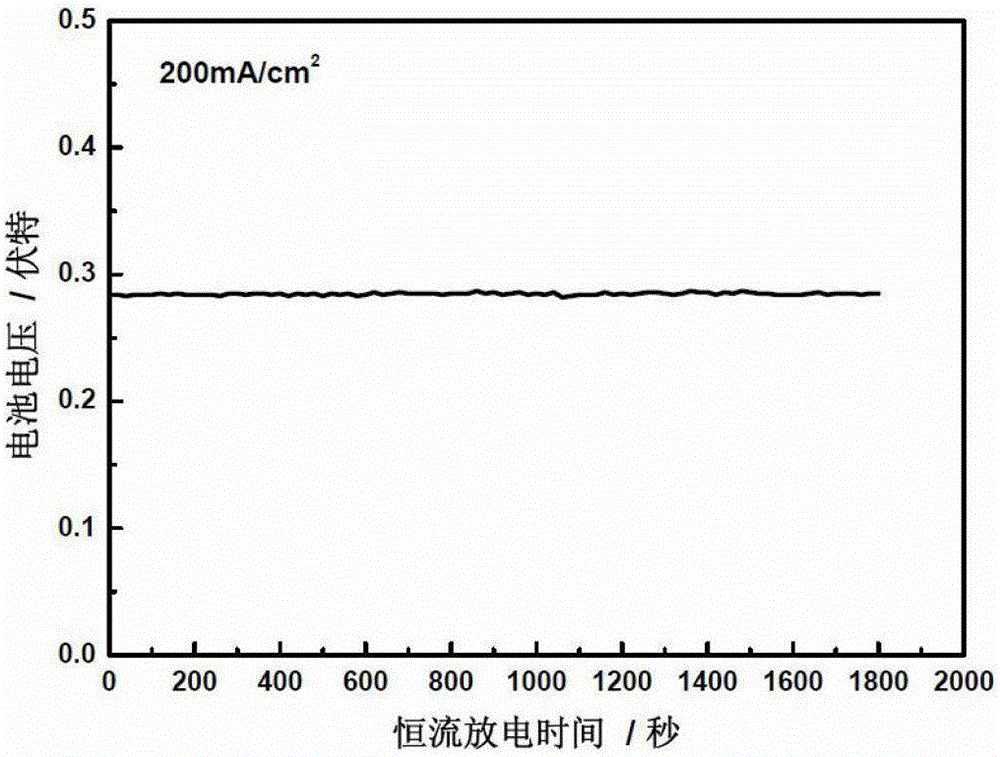
A kind of direct borohydride fuel cell cell activation method
ActiveCN103840184BPromote activationQuick buildFuel cell controlAqueous electrolyte fuel cellsElectrochemical responseCell activation
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
A nickel-based catalyst for improving the performance of direct borohydride fuel cells
InactiveCN105070926BImprove discharge efficiencyEnhanced direct oxidation performanceCell electrodesPtru catalystHydrogen fuel cell
A nickel-based catalyst for improving the performance of a direct borohydride fuel cell is characterized in that the catalyst is prepared according to the following simple method of: (1) preparing a NiSO4 solution of 0.2 mol / dm<3> at a normal pressure and a temperature ranging from 293.15K to 313.15K; (2) assembling a three-electrode system, namely placing a smooth Ni sheet (serving as a working electrode) of 2 square meters in the above solution, wherein the Ni sheet is taken as a counter electrode, and a silver / silver chloride electrode is taken as a reference electrode; and (3) depositing Ni on a metal nickel sheet to prepare the nickel-based catalyst by using a constant potential (-0.8V) method. The surface morphology of the catalyst is changed due to deposition of the Ni, the specific surface area is obviously increased, catalytic active sites are greatly increased, and thus, the direct oxidizability of BH4<-> is enhanced; and meanwhile, the charge transfer resistance of electrode reaction is further reduced due to the deposition of the Ni, and fuel discharging efficiency is obviously improved.
Owner:CHONGQING UNIV
Ni-Cu binary catalyst for improving performance of direct borohydride fuel cell
InactiveCN102244276BEnhanced direct oxidation performanceReduce corrosionCell electrodesElectrolytic agentDischarge efficiency
A Ni-Cu binary catalyst for improving the performance of a direct boron-hydrogen fuel cell, characterized in that the catalyst is prepared by the following method: (1) under normal pressure, the temperature is in the range of 293.15-313.15K, and the acidic condition is configured at 0.25 mol / L CuSO4 solution; (2) Device three-electrode system: place a 2cm2 Ni sheet as a working electrode in the above solution, use a Cu rod as a counter electrode, and a silver / silver chloride electrode as a reference electrode; (3) On the electrochemical workstation, Ni-Cu binary catalyst was prepared by electrodeposition at -0.1V for 5s by constant potential method. The catalyst enhances the direct oxidation performance of BH4-, weakens the corrosion of the electrolyte on the Ni anode, reduces the charge transfer resistance, and improves the discharge efficiency.
Owner:CHONGQING UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com