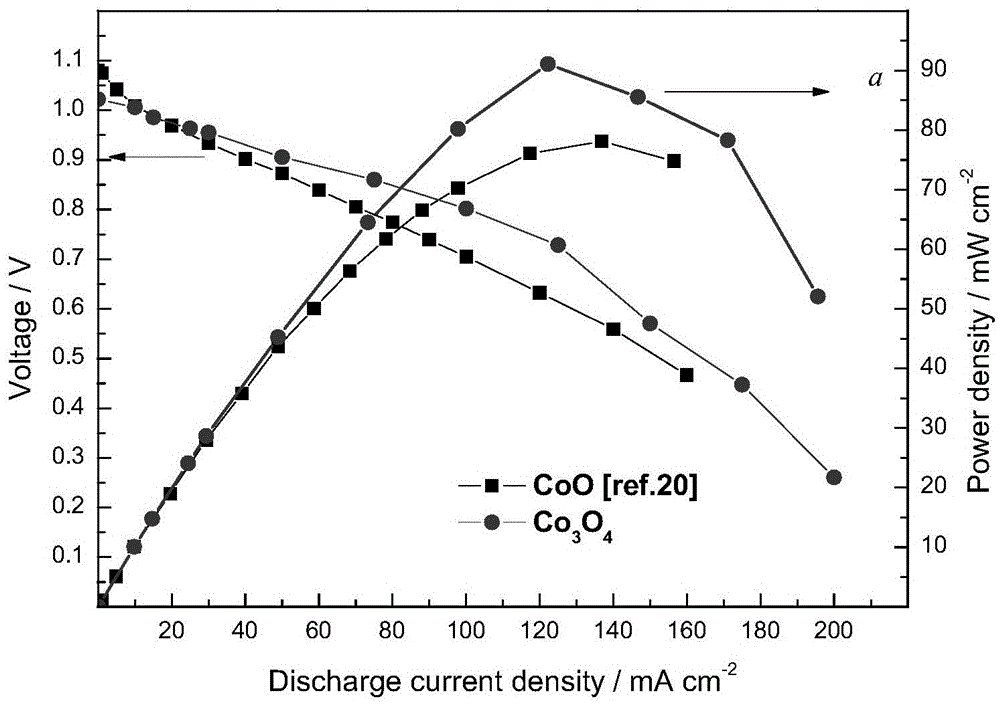
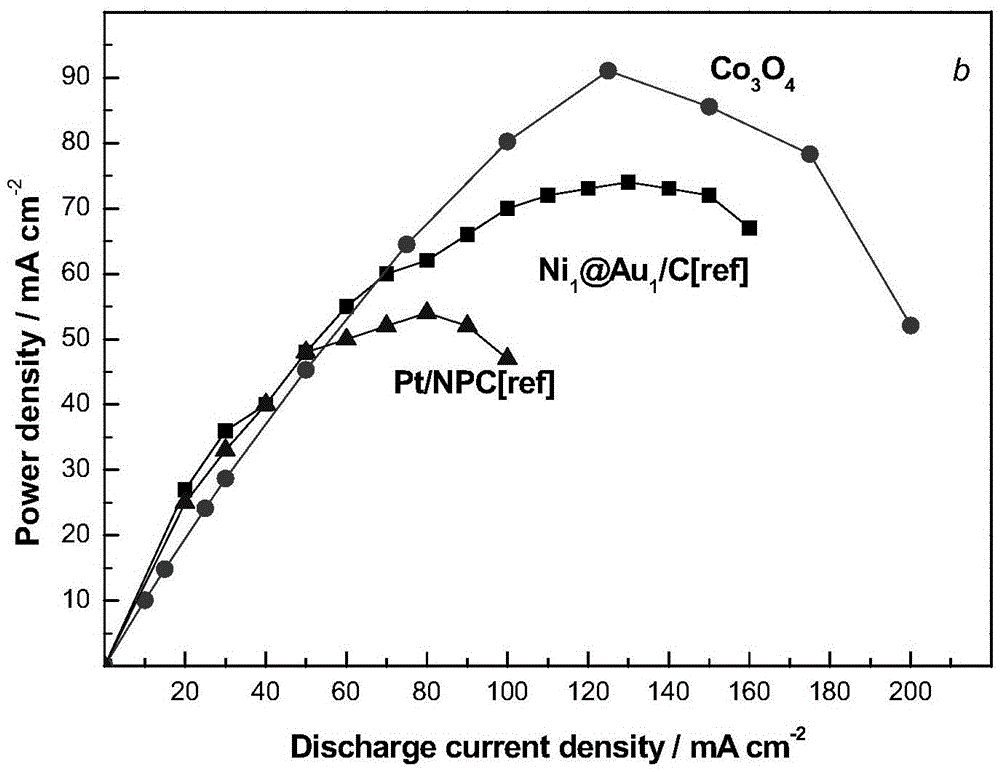
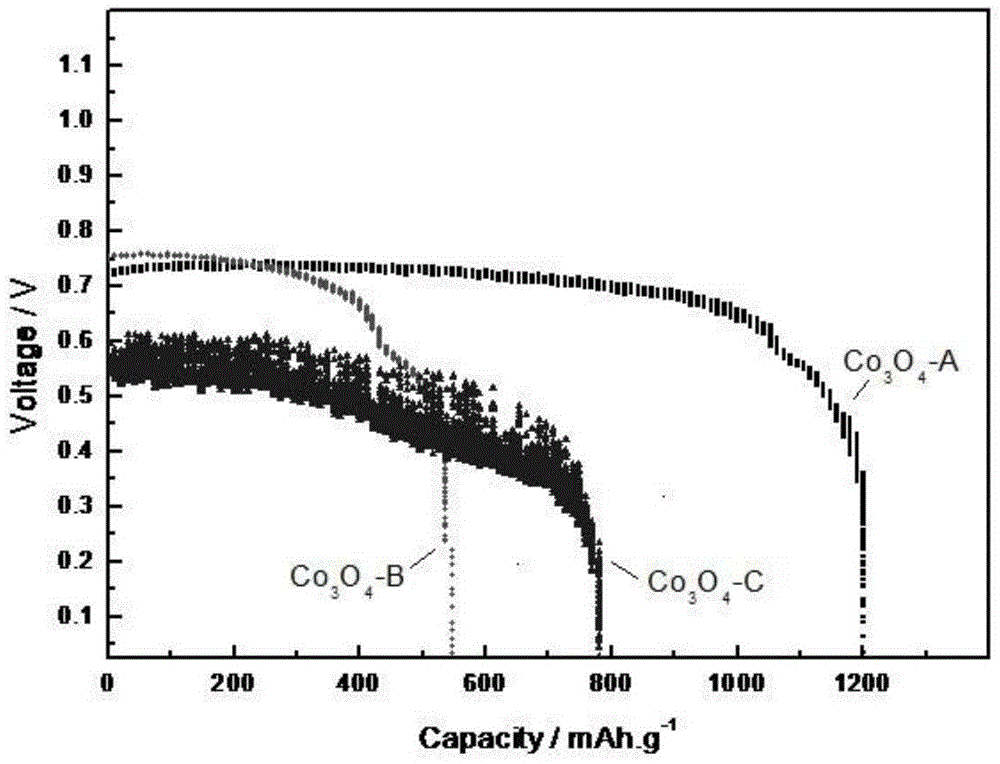
Preparation method and application of anode catalyst of direct borohydride fuel cell
A borohydride, fuel cell technology, applied in fuel cells, battery electrodes, circuits, etc., can solve problems such as problems that cannot be completely solved, and achieve the effect of improving discharge efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0025] The preparation method of direct borohydride fuel cell anode catalyst provided by the invention comprises the following steps:
[0026] (1) Take Co 2+ solution, drop ammonia water therein, and stir until the pH value is 8.5 to 9 to obtain a mixed solution, which is subjected to solid-liquid separation;
[0027] (2) Taking the separated solid phase, washing, drying, grinding and roasting in sequence to obtain the direct borohydride fuel cell anode catalyst.
[0028] Specifically, in step (1), Co 2+ The solution is one of cobalt chloride, cobalt nitrate or cobalt acetate.
[0029] It has been found through experiments that the discharge specific capacity of the anode catalysts prepared with different Co sources is different, among which, the discharge specific capacity of the anode catalyst prepared by using cobalt chloride as the Co source is the largest.
[0030] As preferably, in step (1), Co 2+ The solution is cobalt chloride.
[0031] The drying temperature and ...
Embodiment 1
[0039] 1. Direct borohydride fuel cell anode catalyst Co 3 o 4 Synthesis
[0040] The specific method is as follows:
[0041] (1) Prepare a cobalt chloride solution with a concentration of 1mol L-1, add ammonia water drop by drop to the solution and stir until the color of the mixed solution turns green and its pH value is measured to be in the range of 8.5 to 9, then stop dripping Add ammonia water, stir the mixture for 30 minutes, and then filter it with suction.
[0042] (2) Suction filtration At the same time, the solid phase obtained during the suction filtration was washed successively with deionized water and alcohol, 3 times with deionized water and 2 times with alcohol; dry the washed solid phase at 80 °C for 24 hours; Grind the dried solid phase and put it in a muffle furnace for calcination; the calcination temperature control process is as follows: firstly raise the temperature to 275°C for 2 hours, then keep it warm for 2 hours, and after cooling to room temper...
Embodiment 2
[0051] 1. Direct borohydride fuel cell anode catalyst Co 3 o 4 Synthesis
[0052] The specific method is as follows:
[0053] (1) Prepare a cobalt nitrate solution with a concentration of 1mol L-1, add ammonia water drop by drop to the solution and stir until the color of the mixed solution turns green and the measured pH value is in the range of 8.5 to 9, then stop adding Ammonia, after stirring the mixture for 30 min, it was suction filtered.
[0054] (2) Suction filtration At the same time, the solid phase obtained during the suction filtration was washed successively with deionized water and alcohol, 3 times with deionized water and 2 times with alcohol; dry the washed solid phase at 80 °C for 24 hours; Grind the dried solid phase and put it in a muffle furnace for calcination; the calcination temperature control process is as follows: firstly raise the temperature to 275°C for 2 hours, then keep it warm for 2 hours, and after cooling to room temperature with the furnac...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com