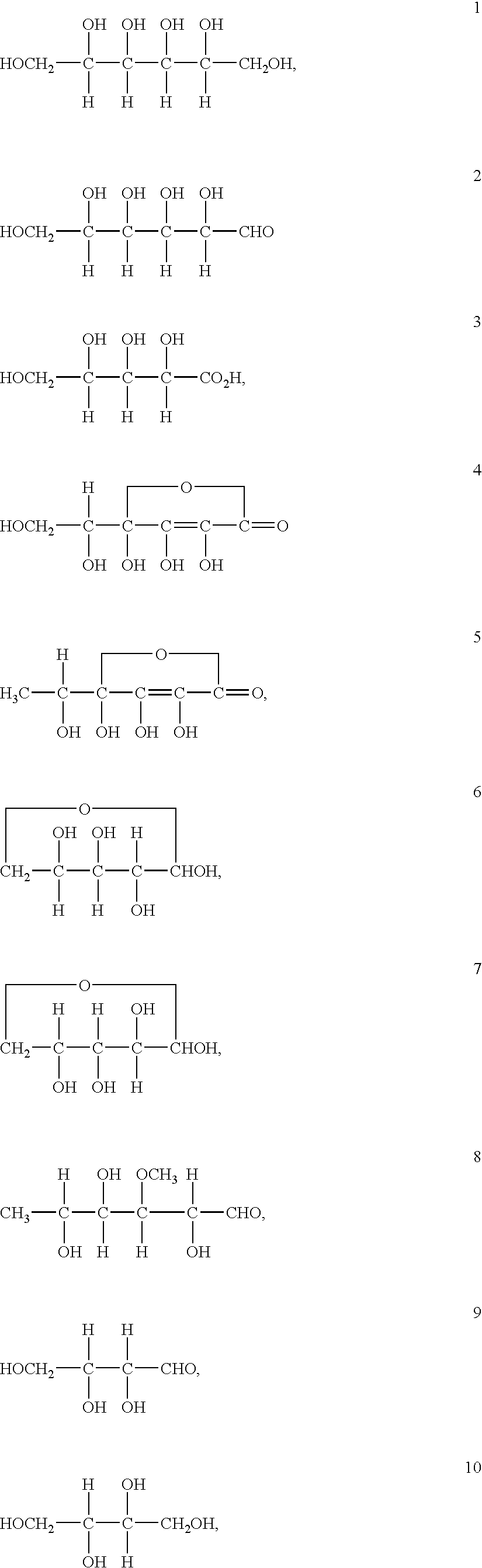
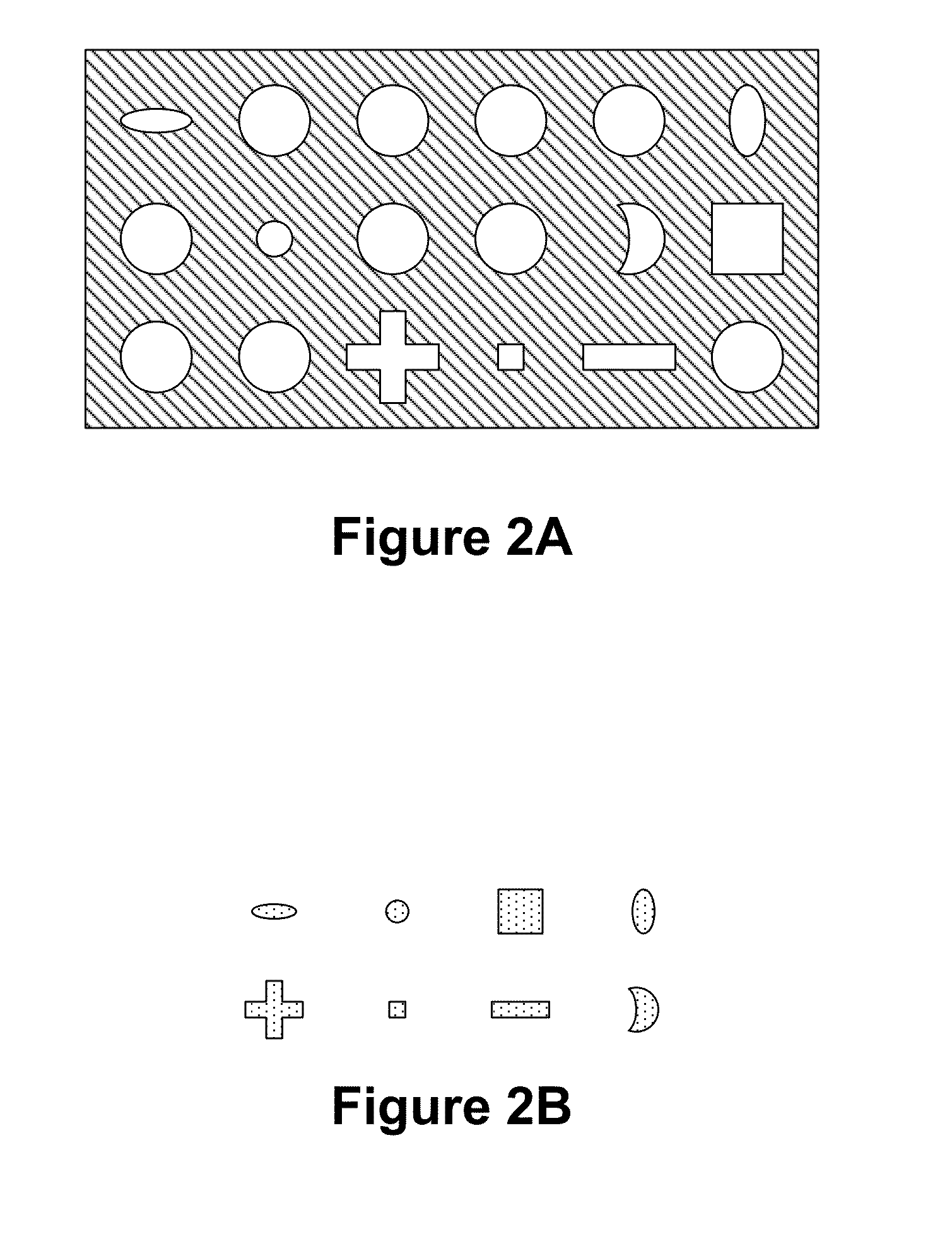
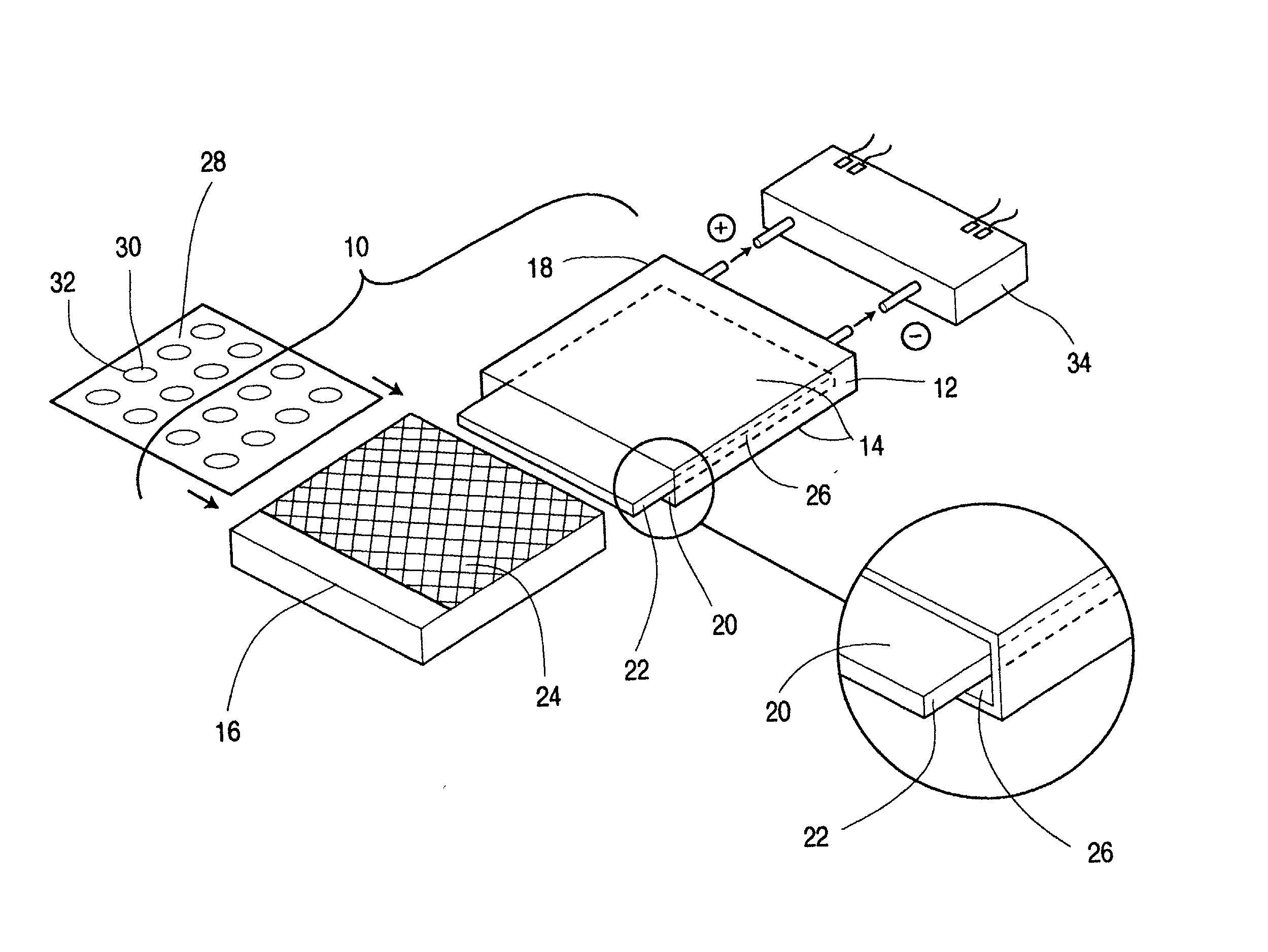
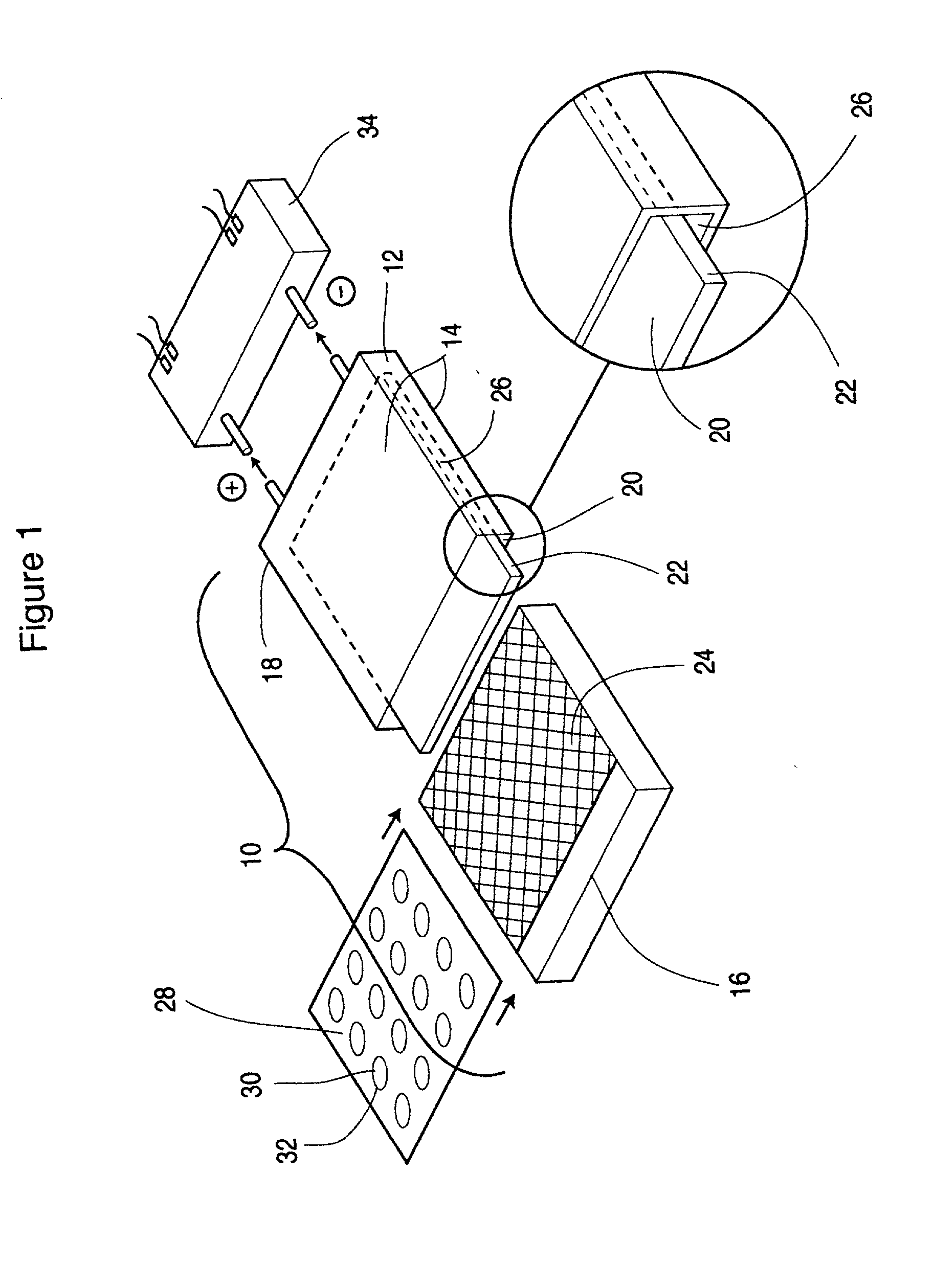
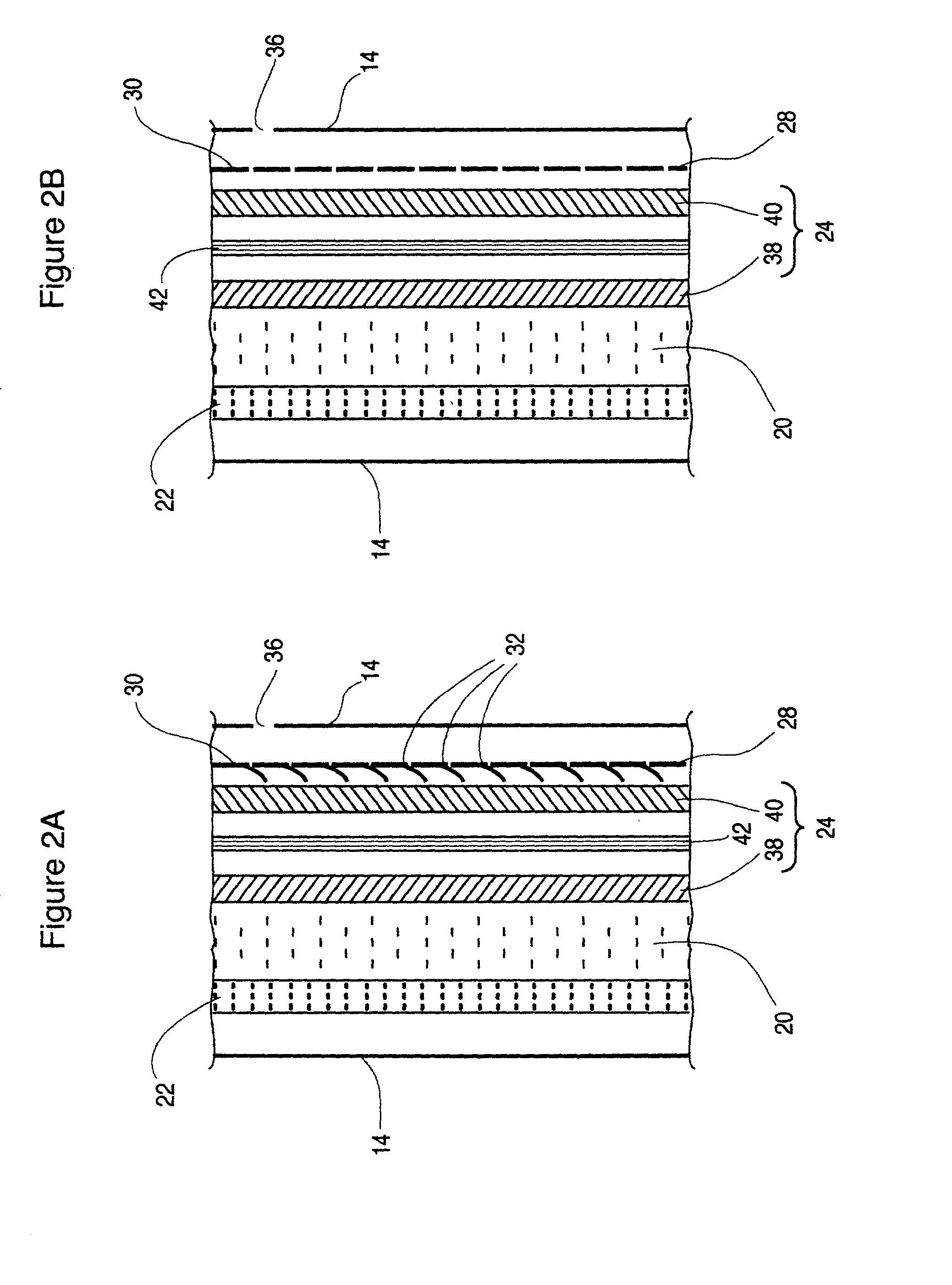
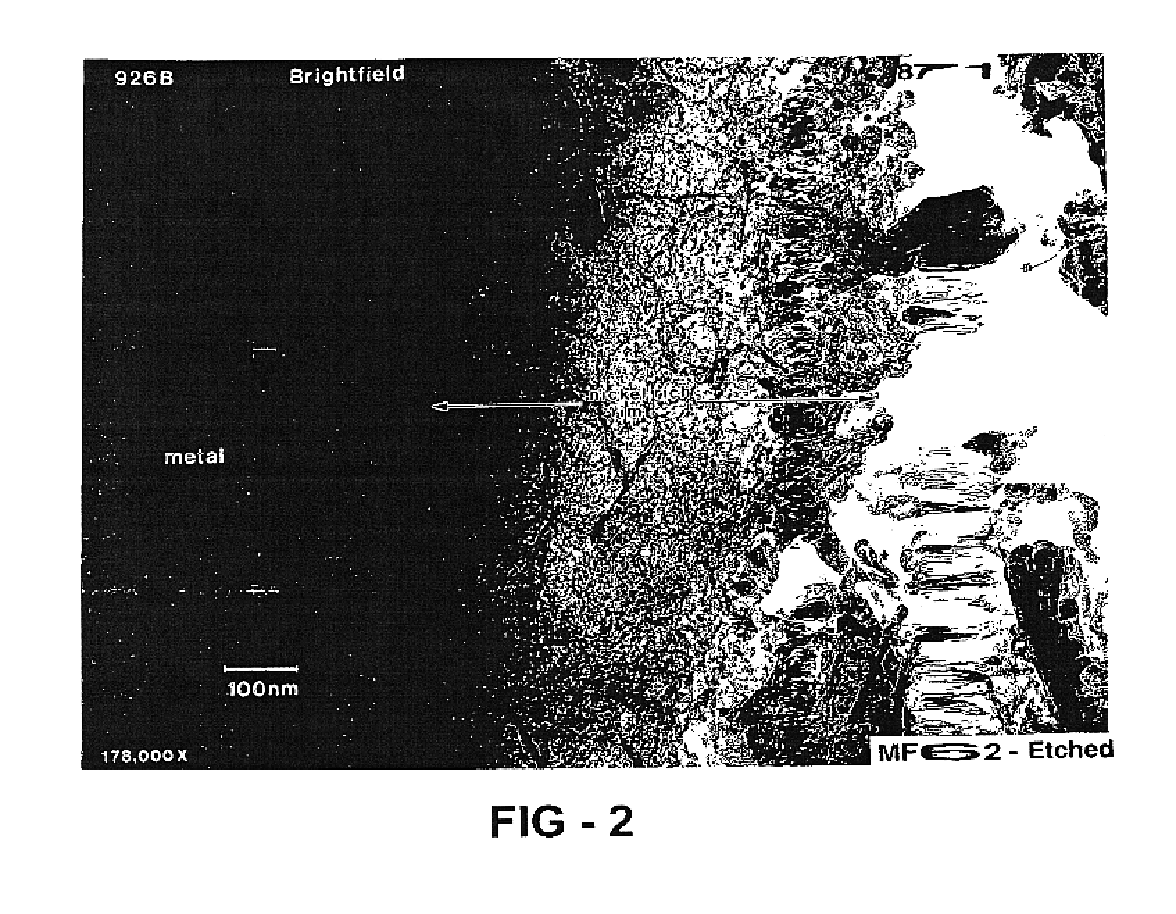
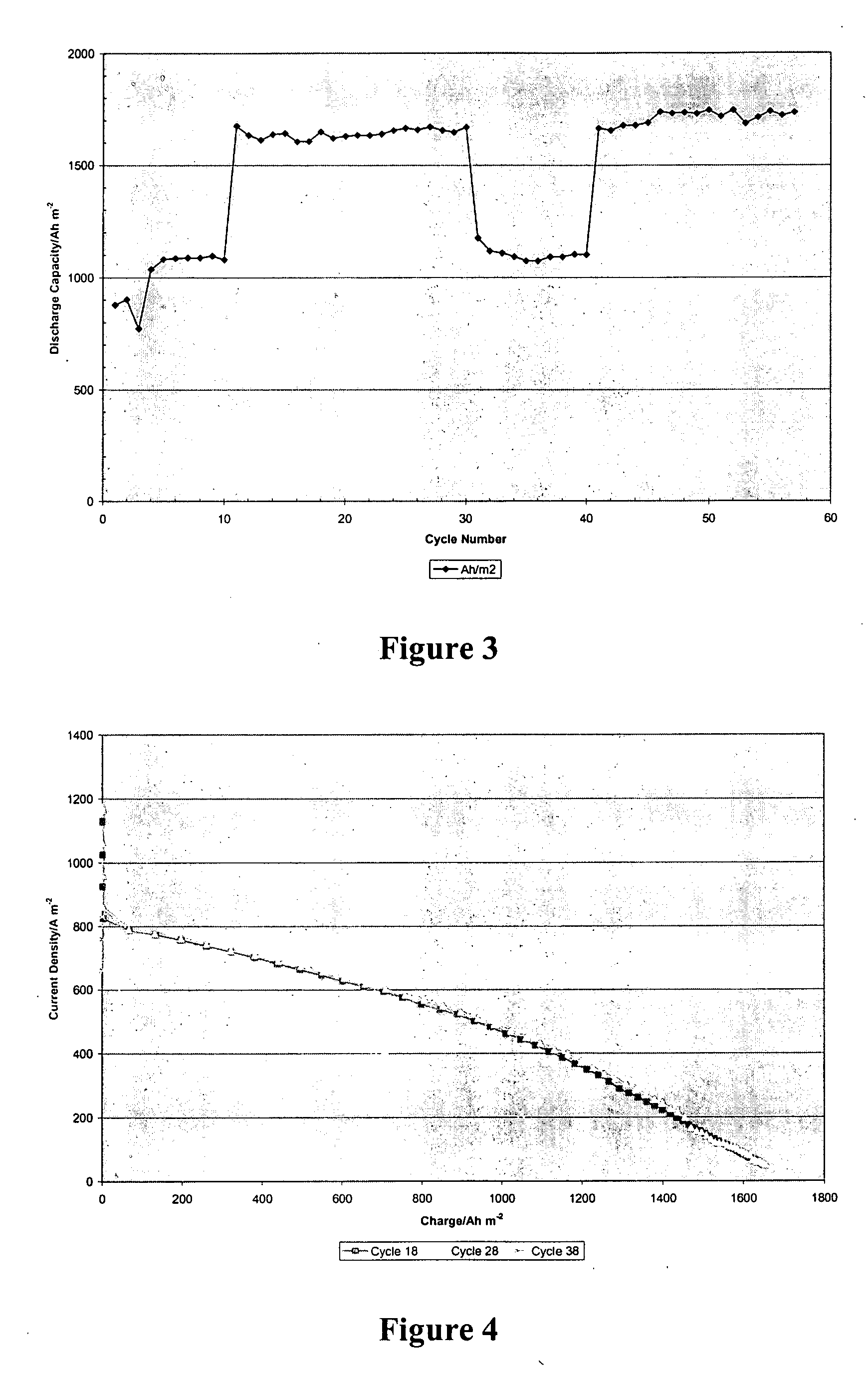
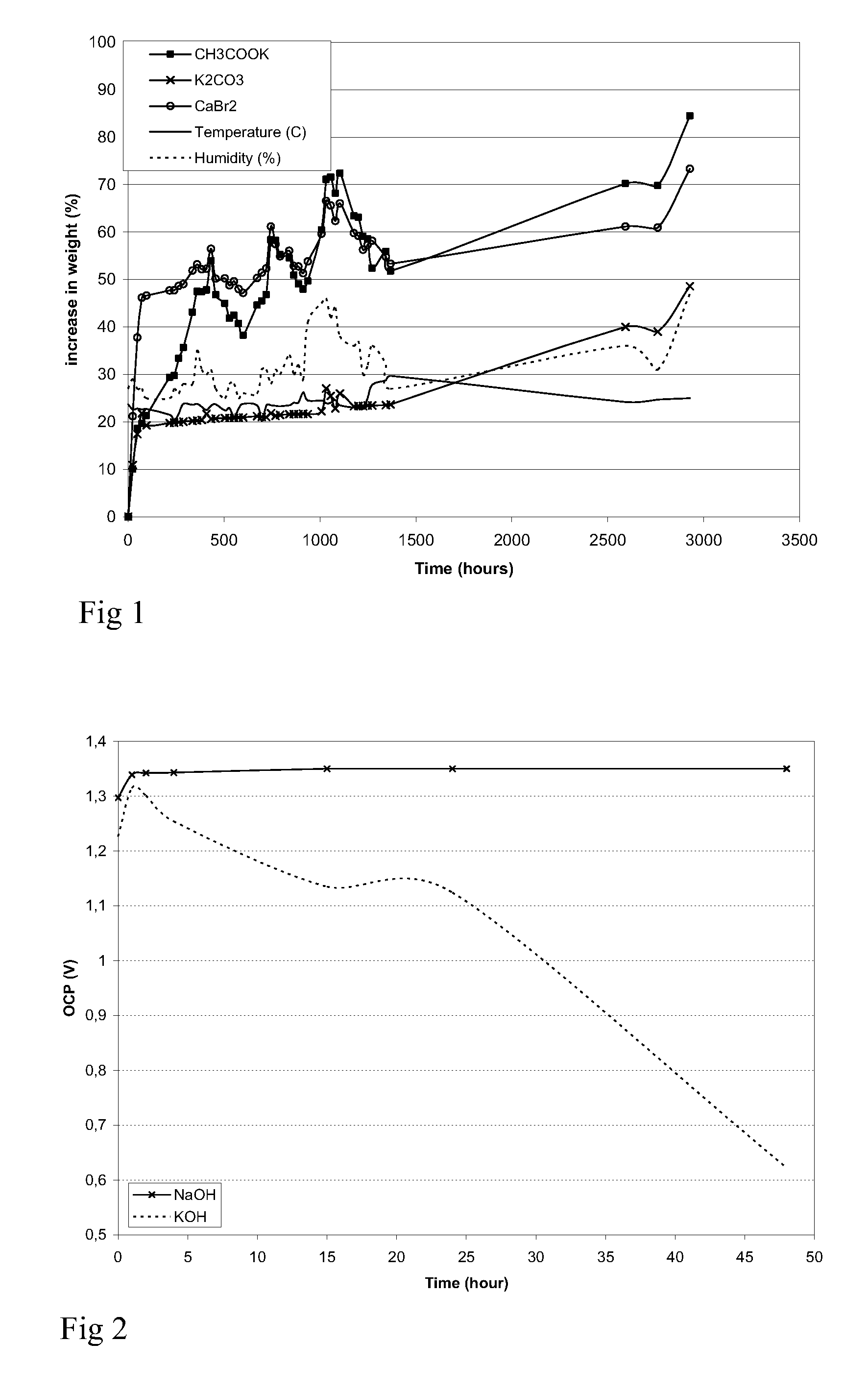
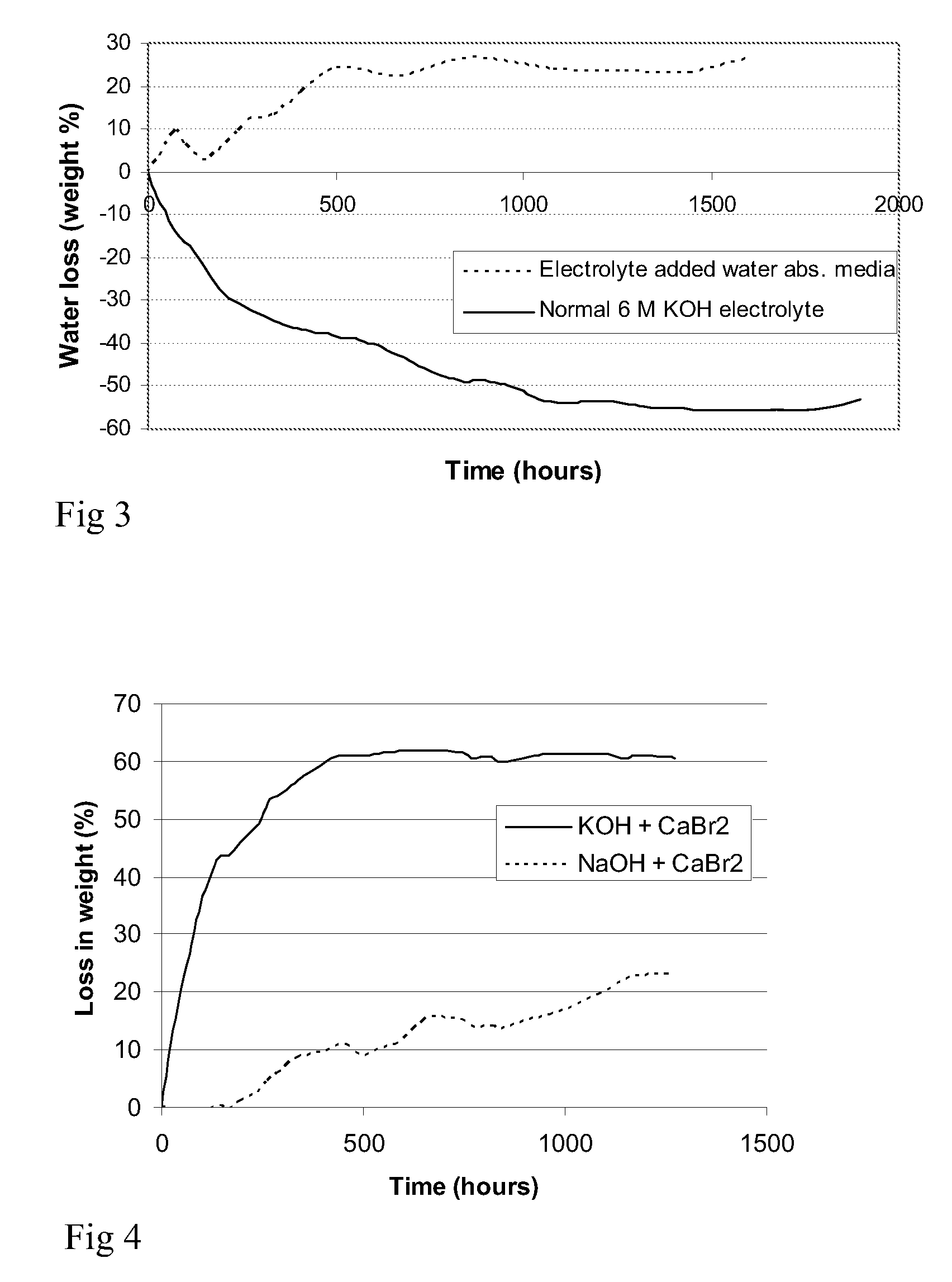
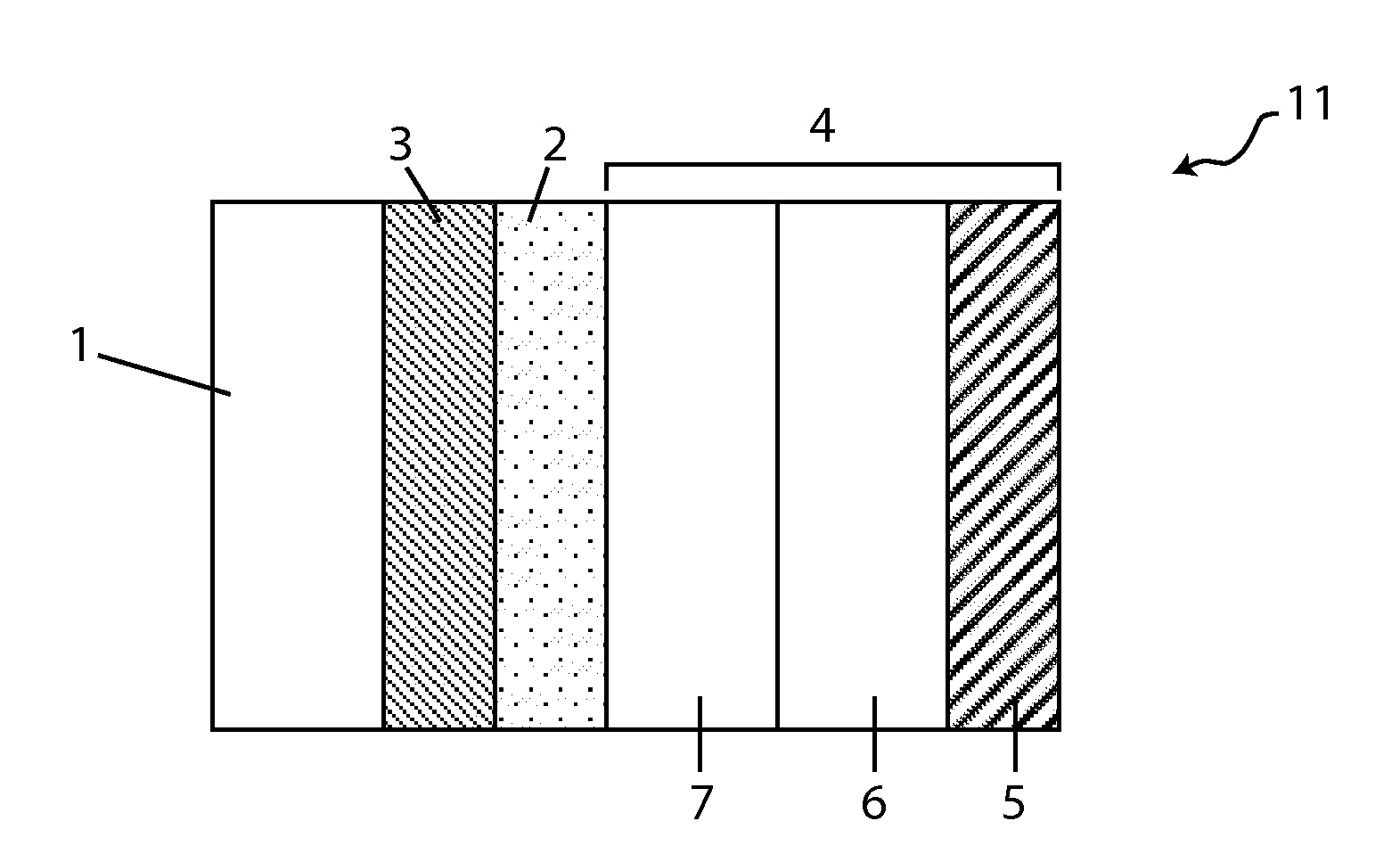
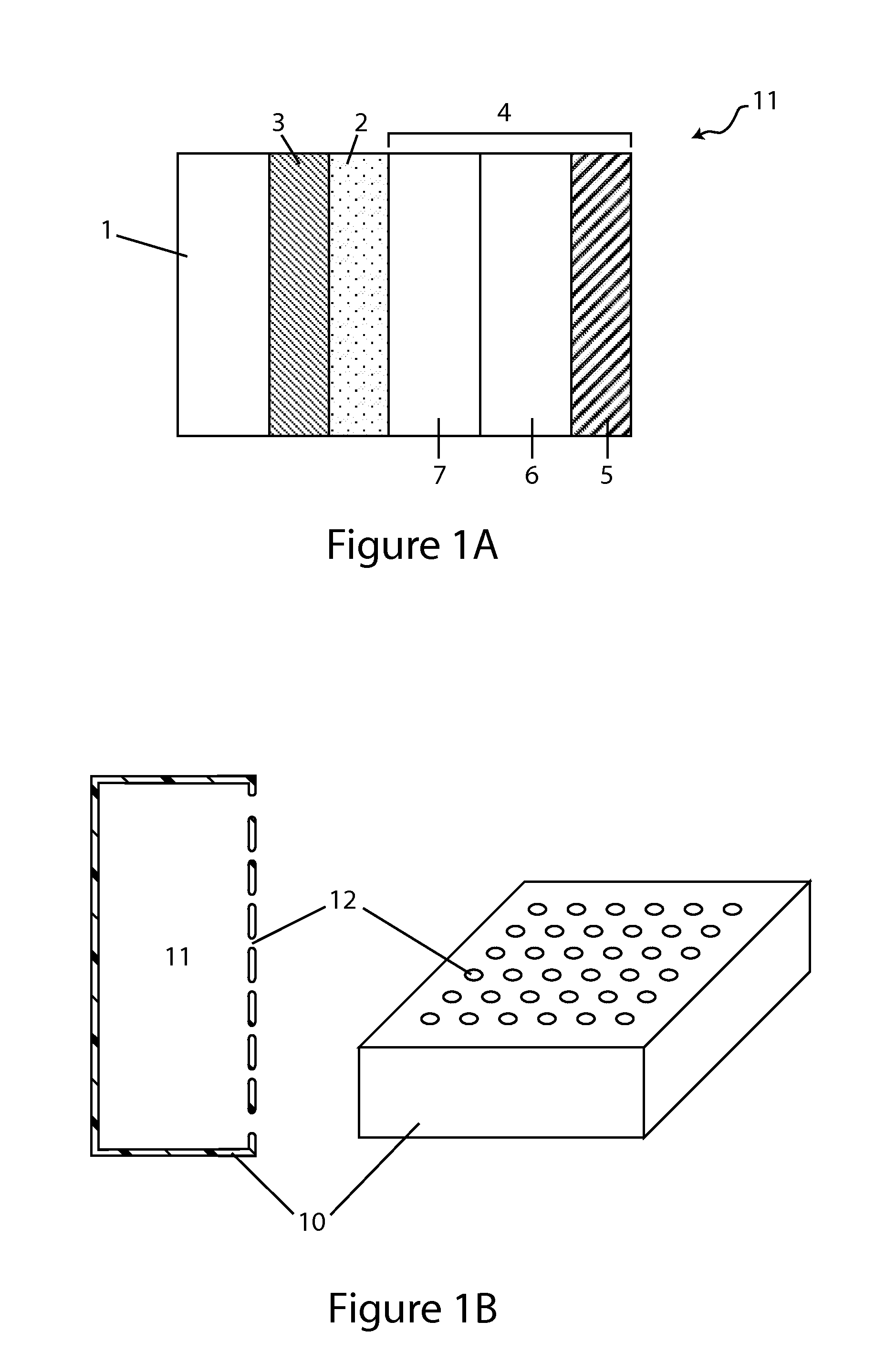
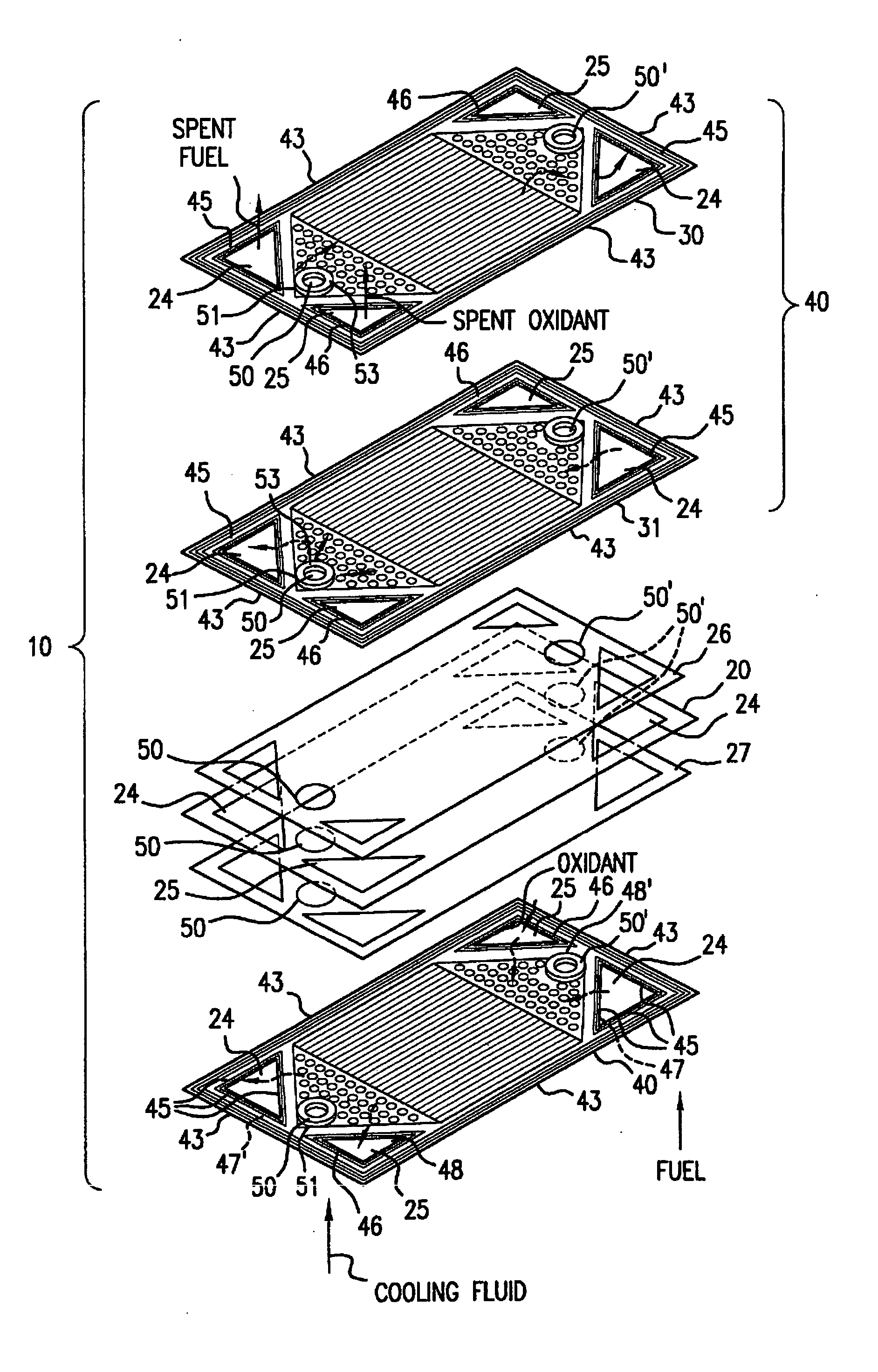
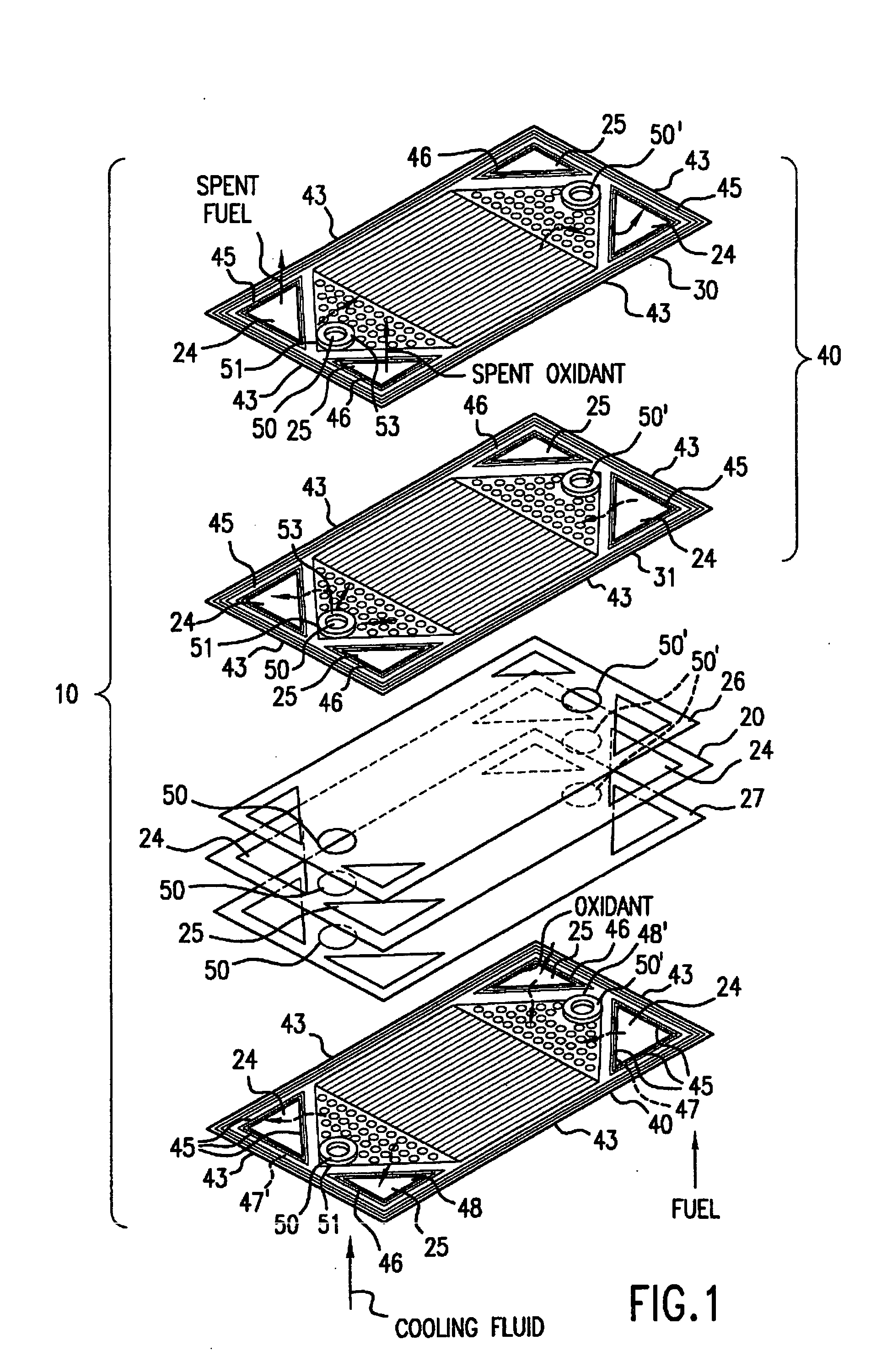
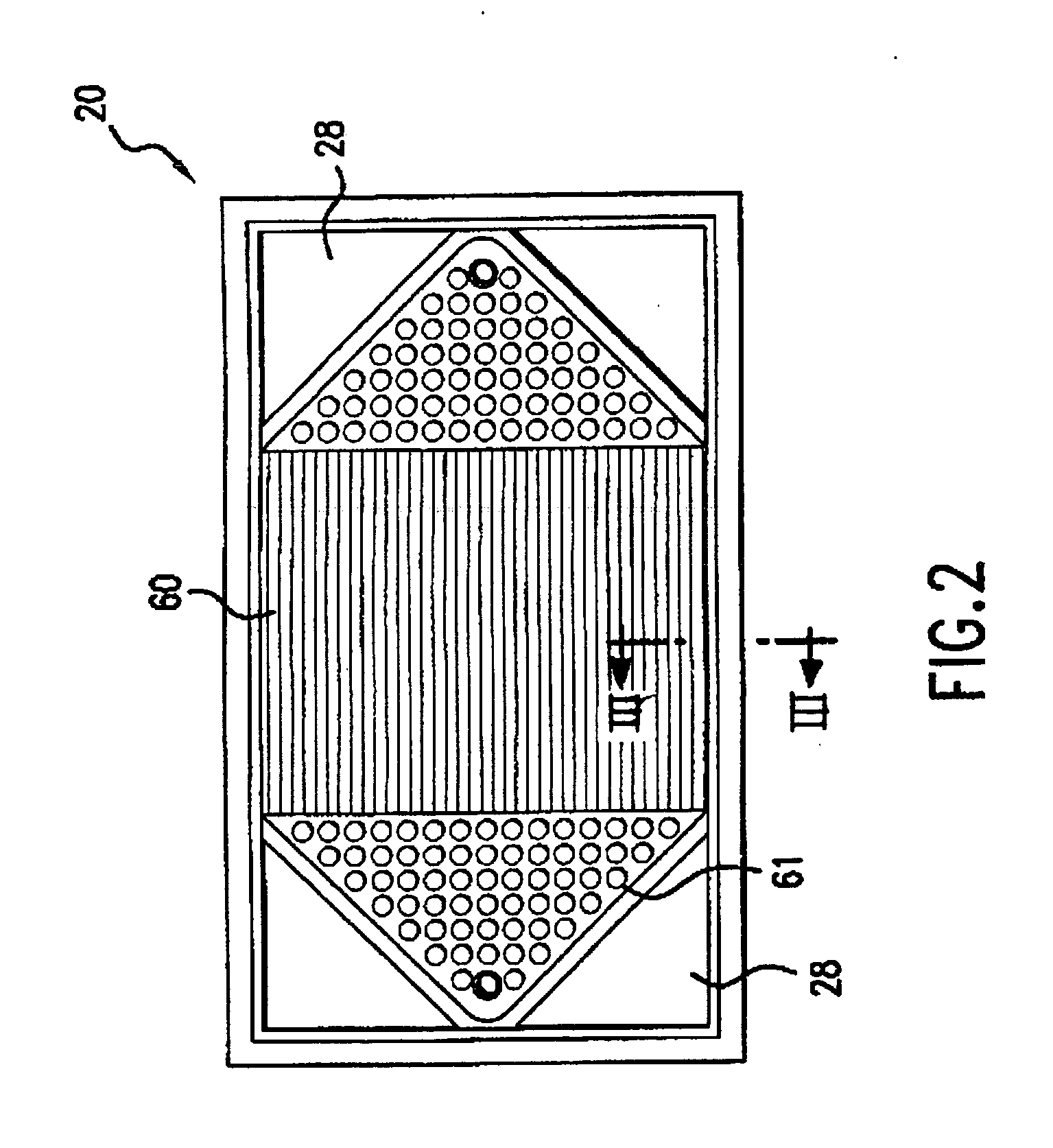
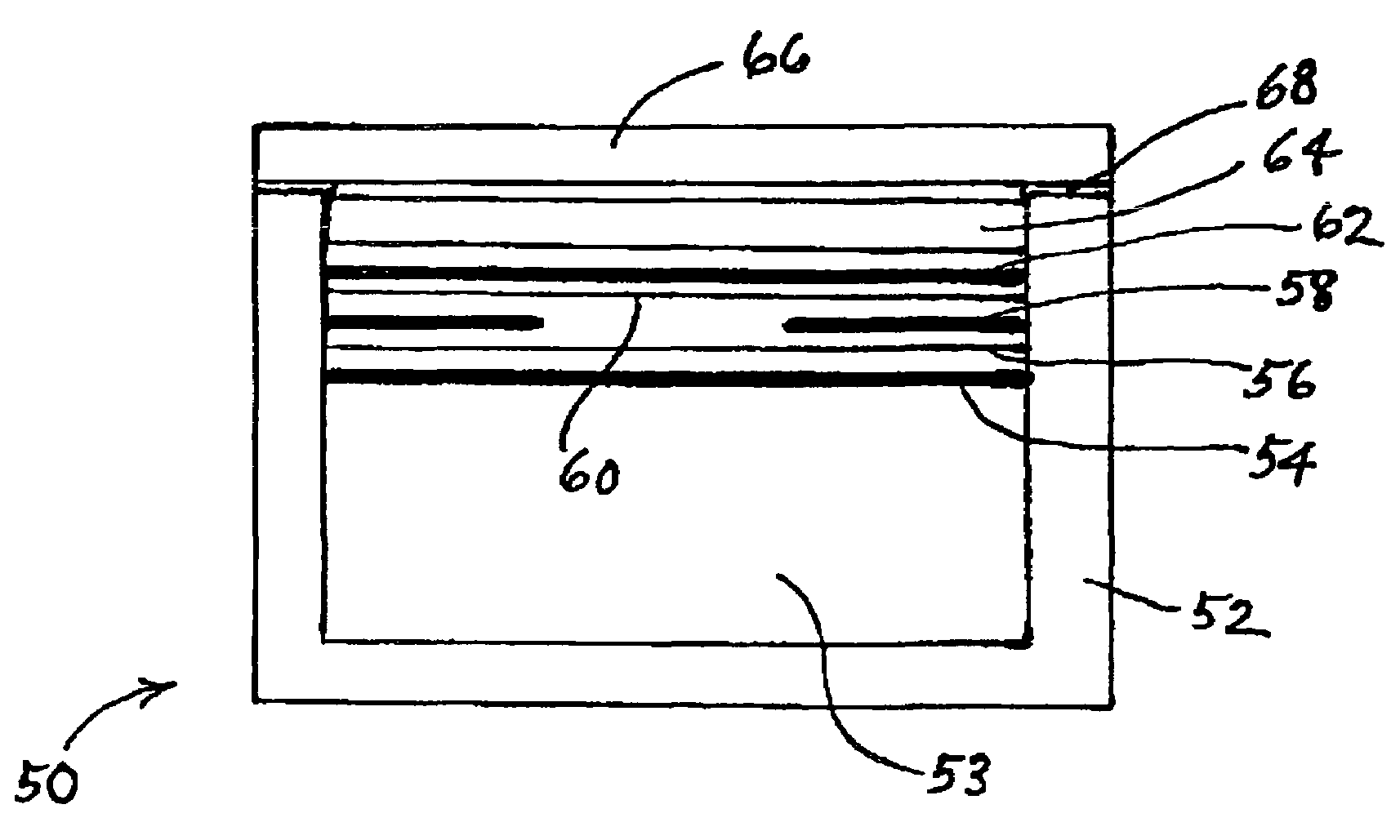
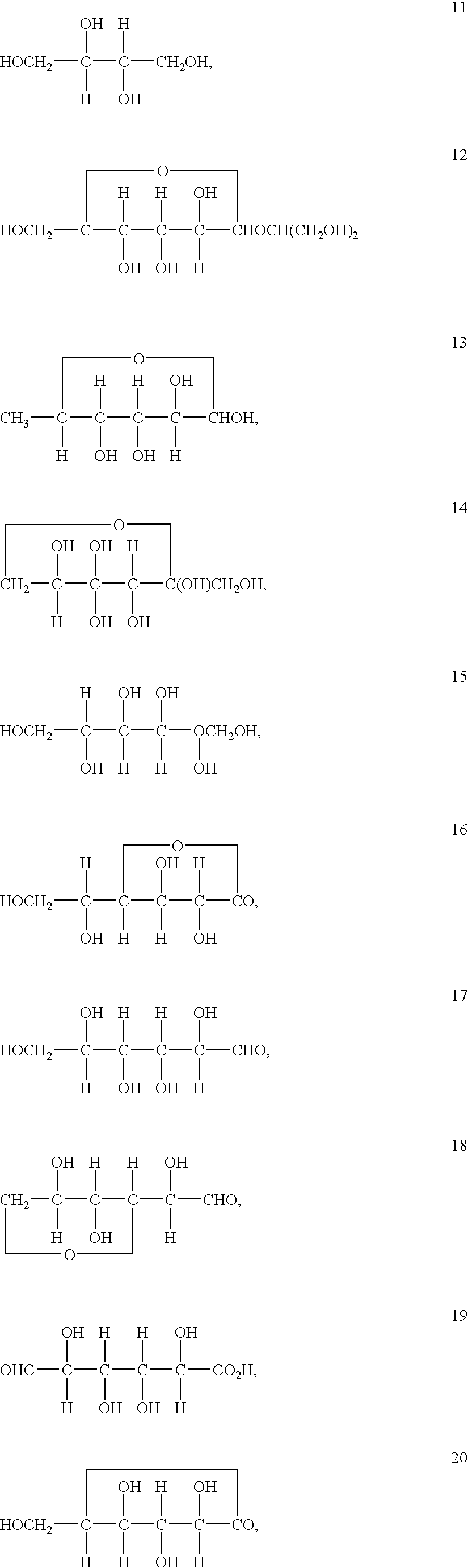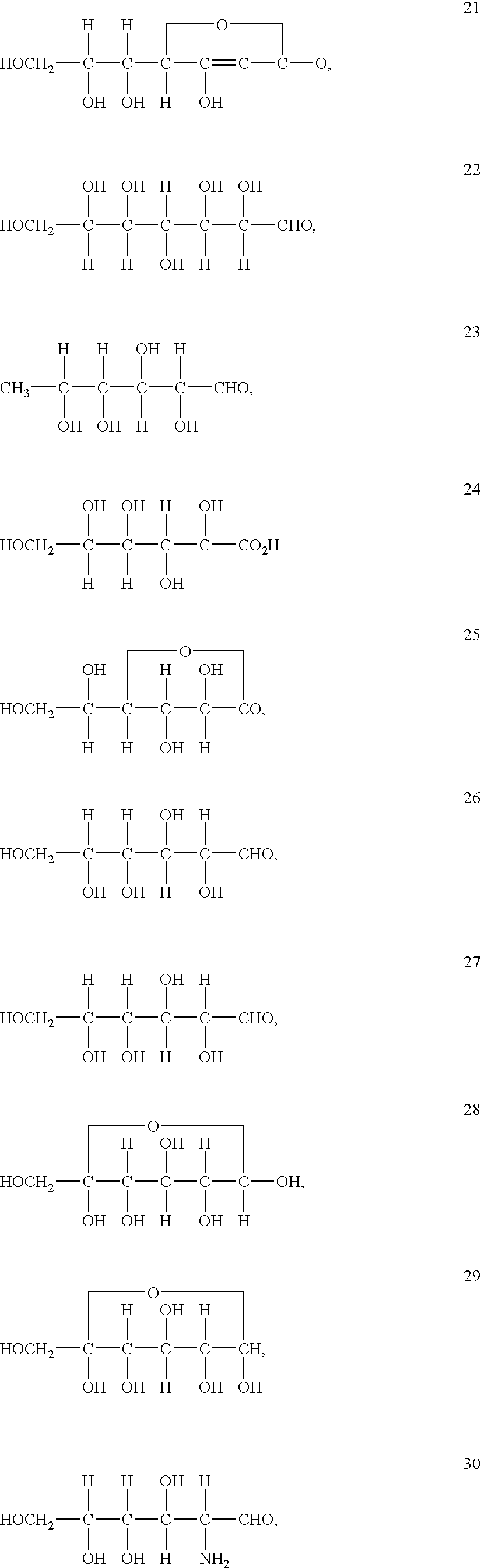Patents
Literature
740results about "Aqueous electrolyte fuel cells" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
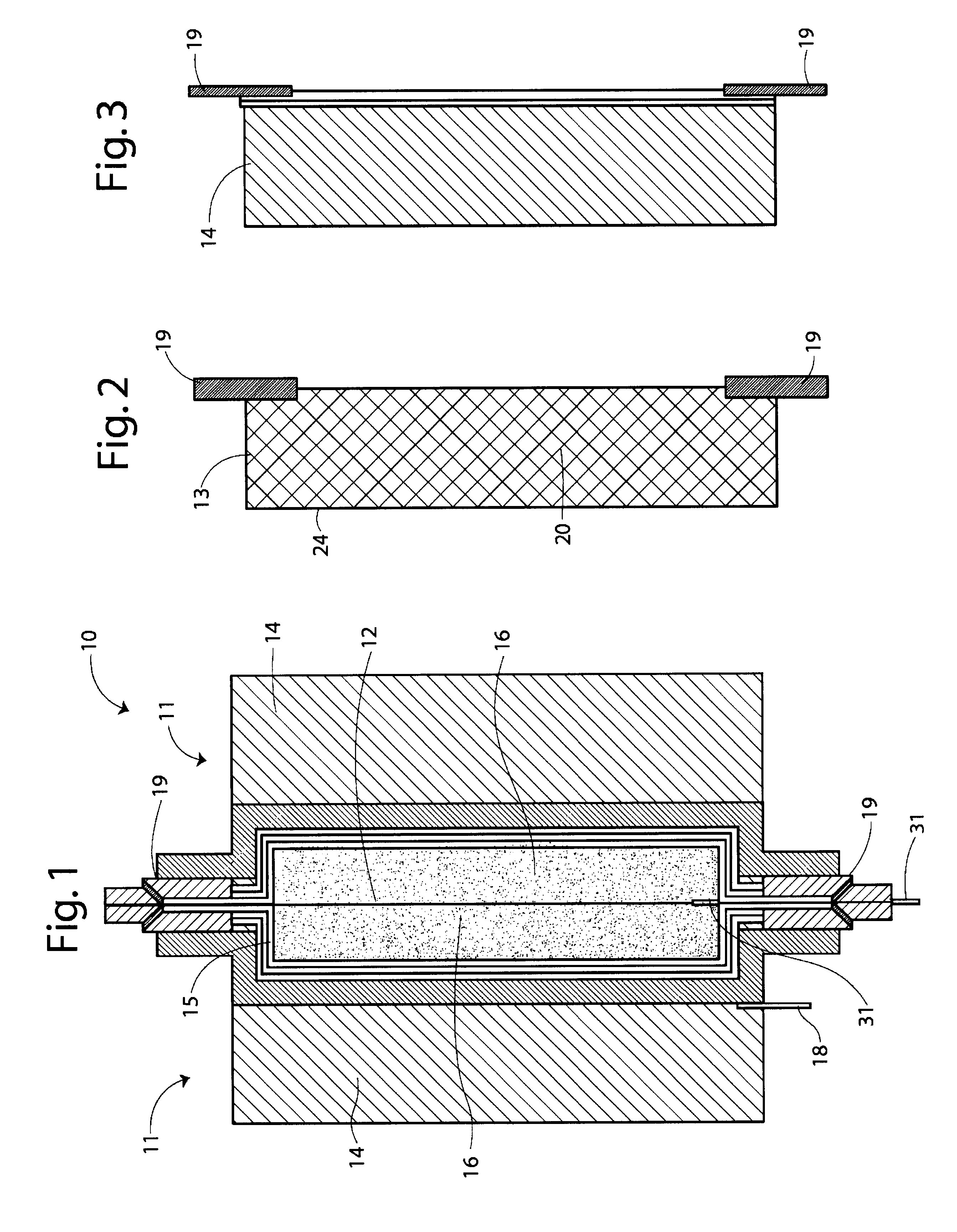
Stabilized vanadium electrolyte solutions for all-vanadium redox cells and batteries
InactiveUS6562514B1Effective amountEasy to modifyFinal product manufactureRegenerative fuel cellsRedoxPhysical chemistry
Owner:JD HLDG INC
Redox flow cell
InactiveUS20100003586A1Facilitate ion exchangeCell electrodesFuel cell auxillariesFlow cellPorous membrane
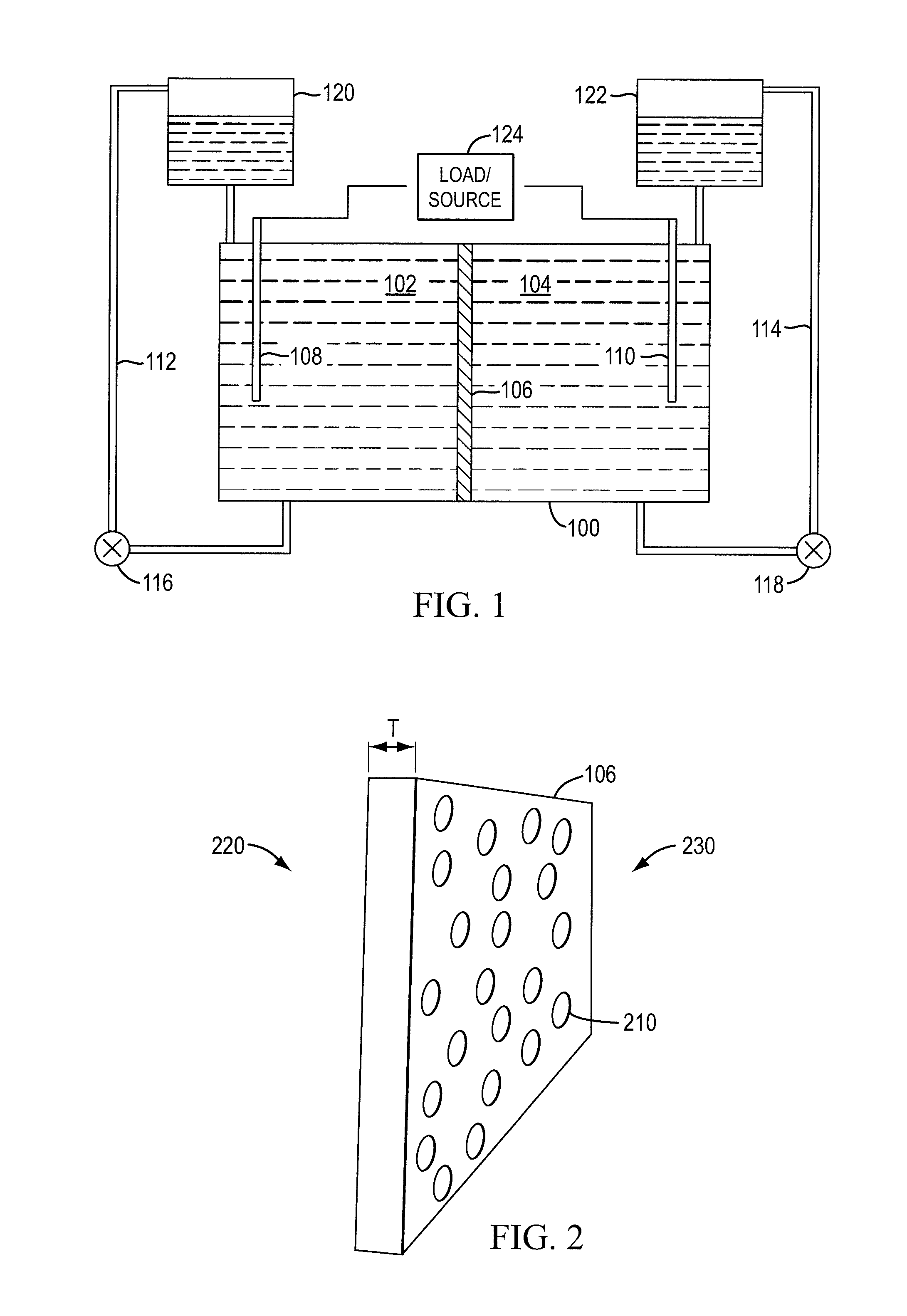
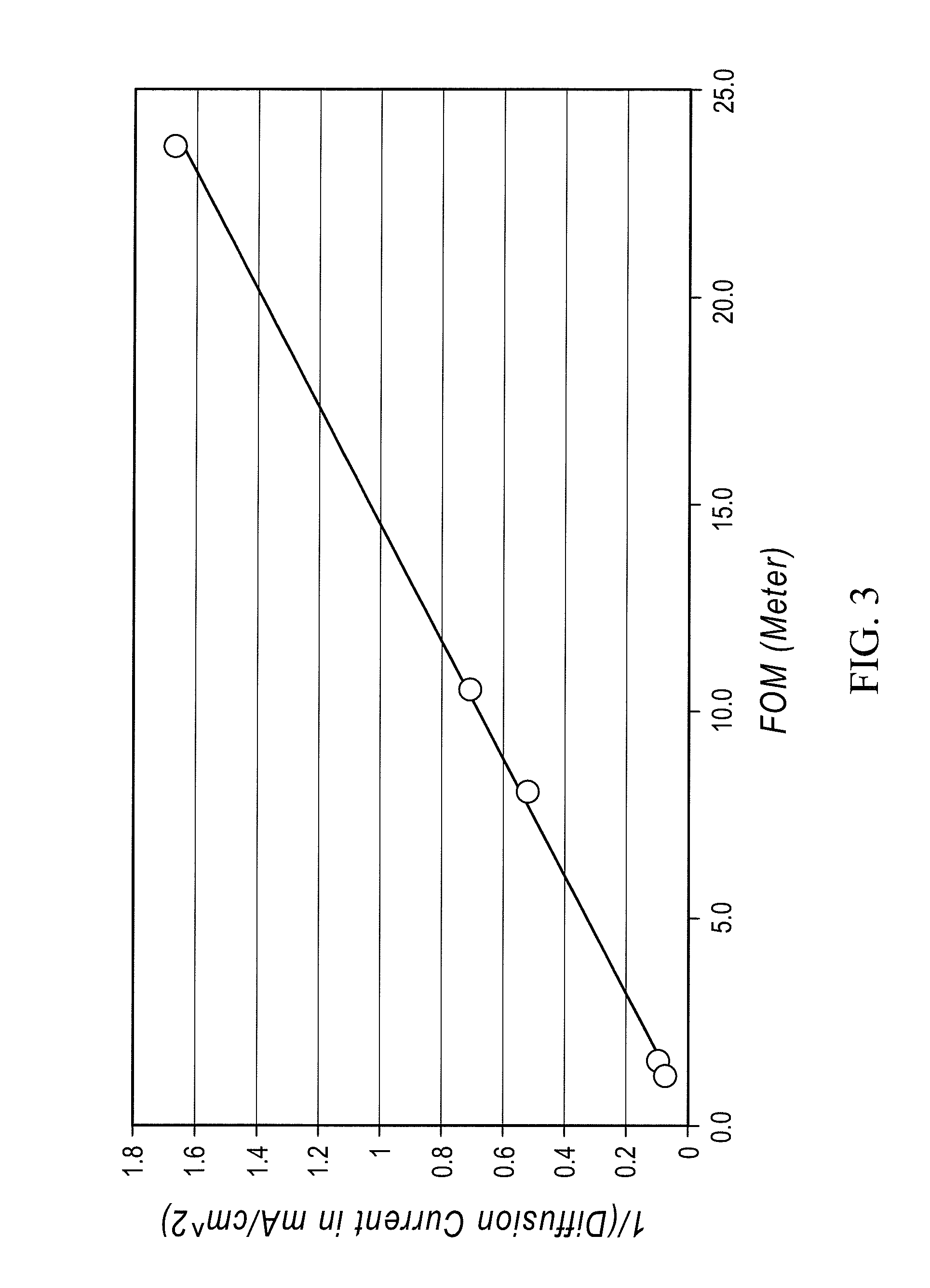
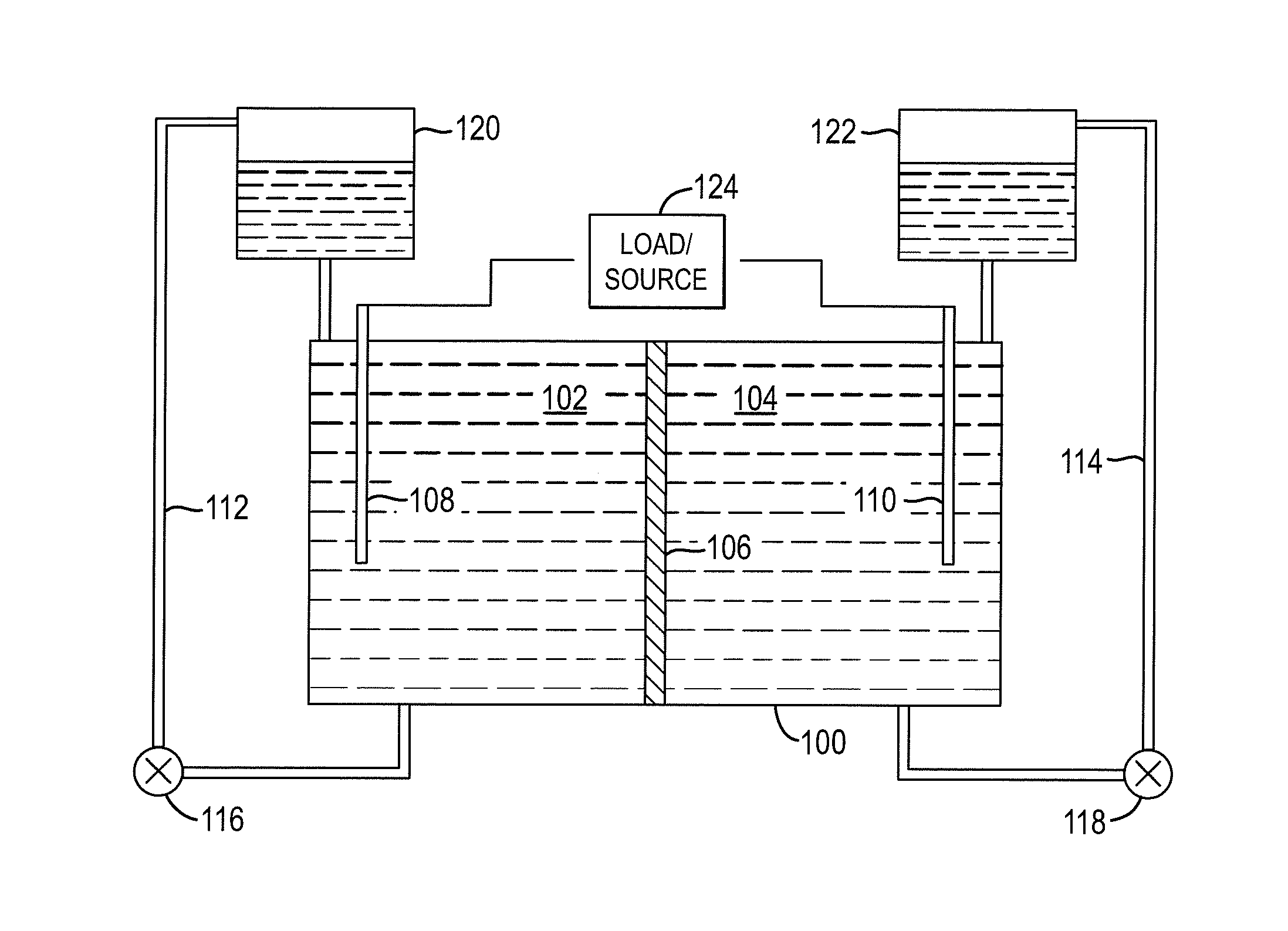
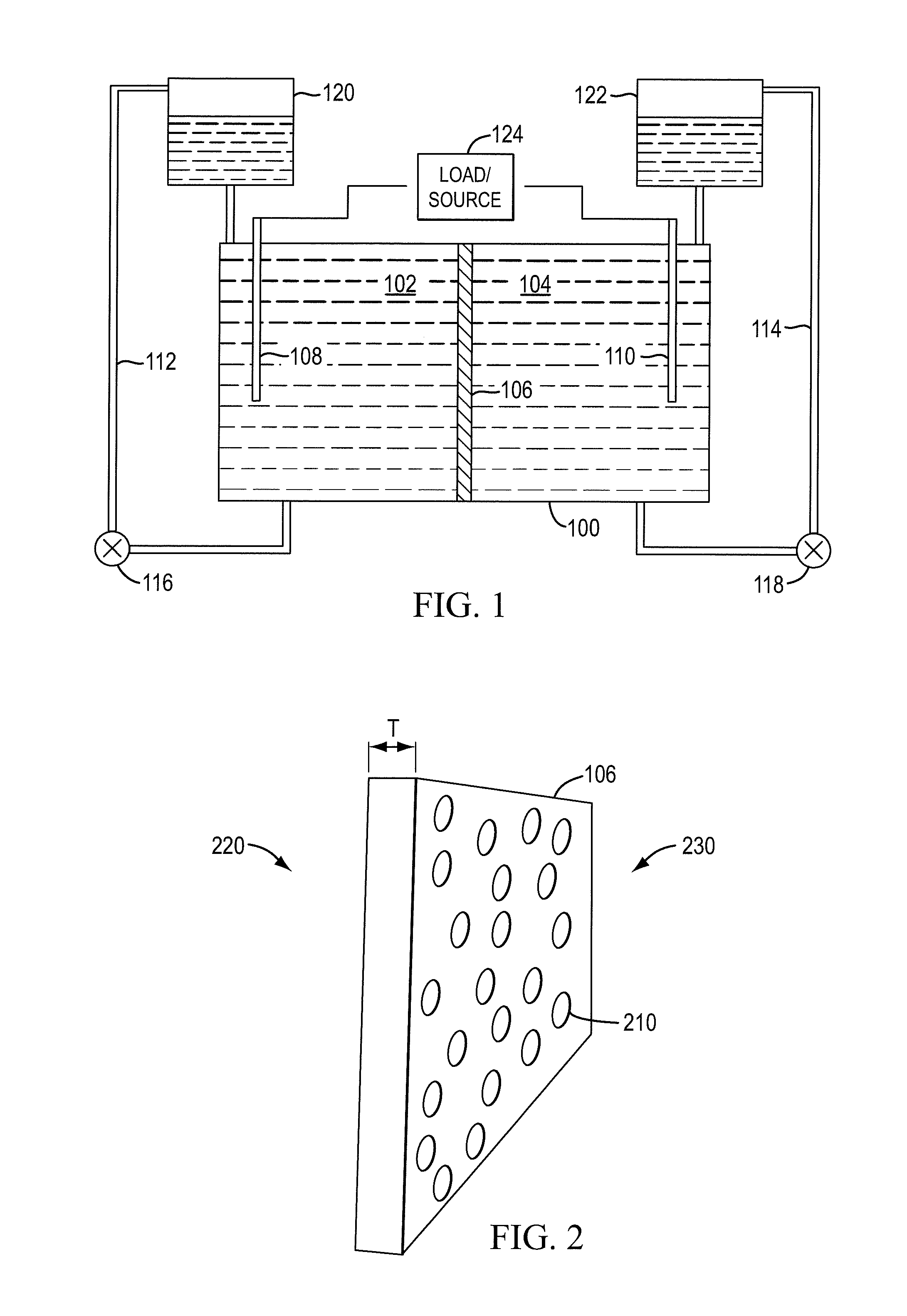
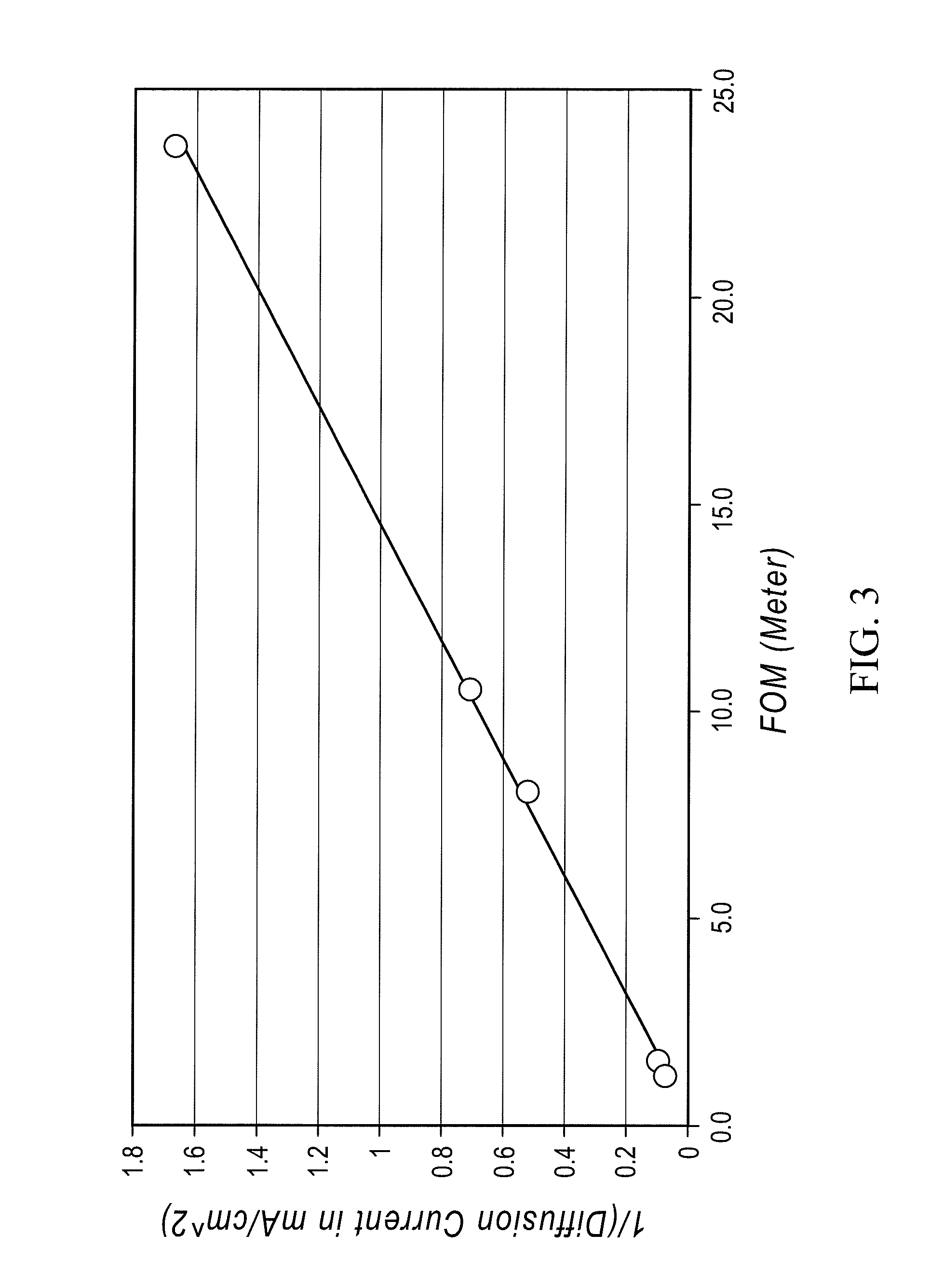
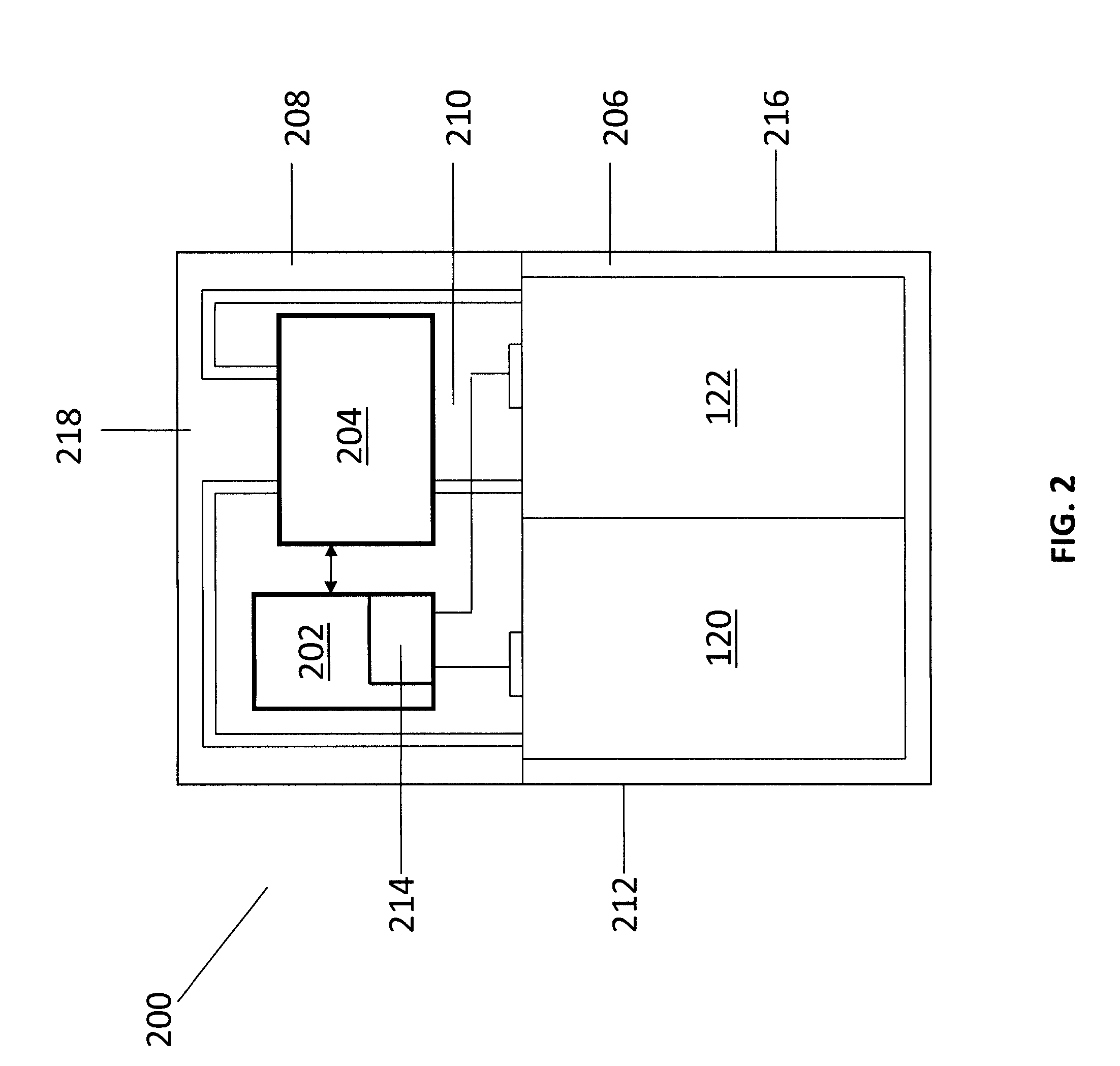
A redox flow cell is presented that utilizes a porous membrane separating a first half cell and a second half cell. The porous membrane is chosen to have a figure of merit (FOM) is at least a minimum FOM. A method of providing a porous membrane for a flow cell can include determining a figure of merit; determining a first parameter from a pore size or a thickness for the porous membrane; determining a second parameter from the pore size or the thickness that is not the first parameter for the porous membrane, based on the figure of merit; and constructing a porous membrane having the pore size and the thickness.
Owner:IMERGY POWER SYST
Part solid, part fluid and flow electrochemical cells including metal-air and li-air battery systems
PendingUS20130189592A1Provide integrityAvoid shortingPrimary cell to battery groupingFuel and primary cellsLithium–air batteryEngineering
Owner:CALIFORNIA INST OF TECH
Metal gas batteries
InactiveUS20030049508A1Reduce passageEffectively block and closeFuel and primary cellsPrimary cell maintainance/servicingLiquid waterWater vapor
An improved gas-diffusion cathode for use in an electrochemical cell comprising an electrically conductive cathode member having a first side communicable with an aqueous electrolyte and a second side communicable with a gaseous medium; and a water-impermeable membrane adjacent said cathode member second side to reduce passage of liquid water between said cathode member and said gaseous medium and having a membrane first side and a membrane second side wherein said membrane first side faces said cathode member and wherein said water-impermeable membrane comprises one or more portions defining one or more openable and closeable apertures the improvement wherein said apertures are associated with one or more integrally-formed resiliently flexible flaps on said membrane first side to effect said opening and closing. The batteries have reduced unwanted water vapour ingress and egress characteristics in its no-load mode.
Owner:ALUMINUM POWER
Integrated oxygen generation and carbon dioxide absorption method apparatus and systems
InactiveUS20050031522A1Improvement in mission durationLower acquisition costsFuel cell auxillariesMachines/enginesChemical speciesCo2 absorption
A method for producing oxygen and absorbing carbon dioxide in a single operation using a solution that contains an oxygen source and a redox partner that can react to form oxygen and a chemical species that can form an insoluble carbonate to precipitate and chemically store carbon dioxide. Carbon dioxide is introduced into the solution and the carbonate precipitates as the oxygen is generated. In particular, the invention uses an aqueous solution of permanganate and hydrogen peroxide that react in the presence of a catalyst to produce oxygen and manganese (II) ions. Carbon dioxide gas introduced into the solution reacts with the manganese (II) ions to precipitate manganese carbonate. Other cations capable of reacting with carbon dioxide to form an insoluble carbonate, for example calcium, barium and magnesium, may also be added to the solution to precipitate carbonate salts. Calcium permanganate may used as a source of both calcium and permanganate.
Owner:CAPITAL MANAGEMENT +1
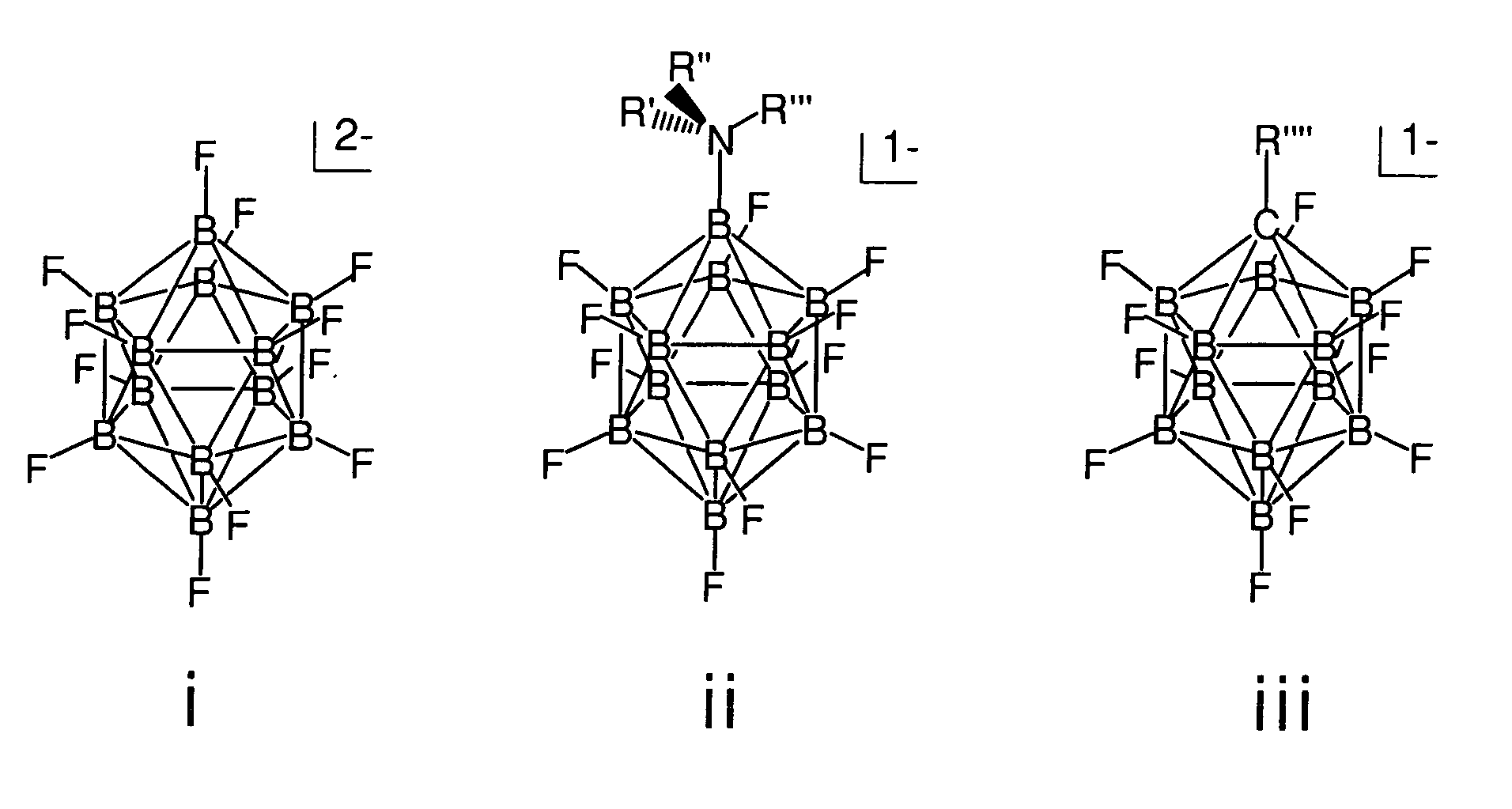
Stable electrolyte counteranions for electrochemical devices
InactiveUS20070048605A1Desirable chemical stabilityDesirable thermal stabilityConductive materialOrganic electrolyte cellsArylHydrogen
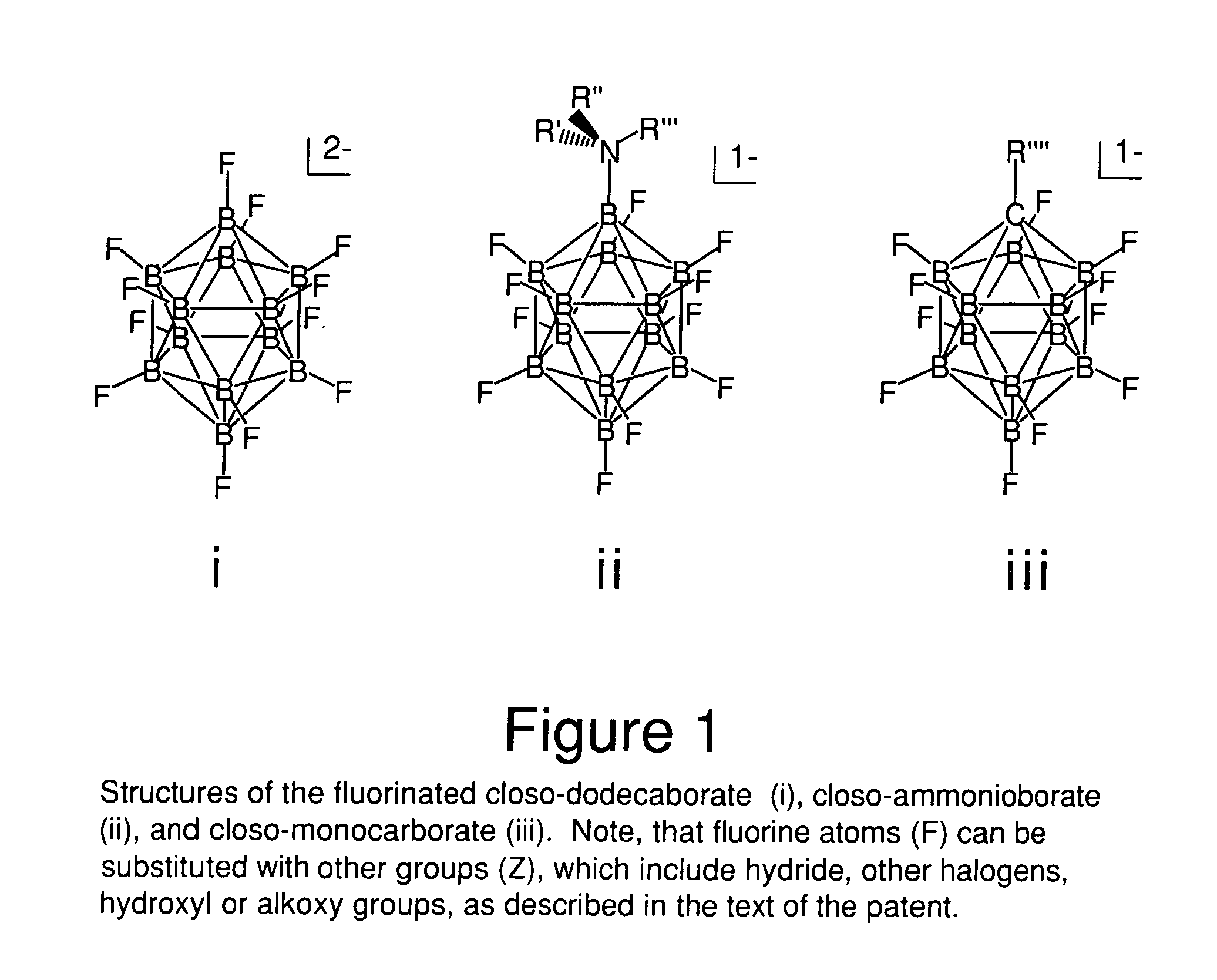
The invention relates to electrolyte salts for electrochemical devices of improved physical, chemical and electrochemical stability. The improvement resides in the use of anions of salts of the formula comprising: i) (B12FxZ12-x)2− wherein Z comprises at least one of H, Cl, Br or OR; R comprises at least one of H, alkyl or fluoroalkyl, or at least one polymer and x is at least 3 on an average basis but not more than 12; ii) ((R′R″R′″)NB12FxZ(11-x))−, wherein N is bonded to B and each of R′, R″, R′″ comprise a member independently selected from the group consisting of hydrogen, alkyl, cycloalkyl, aryl and a polymer; Z comprises H, Cl, Br, or OR, where R comprises H, alkyl or perfluoroalkyl or a polymer, and x is an integer from 0 to 11; or iii) (R″″CB11FxZ(11-x))−, wherein R″″ is bonded to C and comprises a member selected from the group consisting of hydrogen, alkyl, cycloalkyl, aryl, and a polymer, Z comprises H, Cl, Br, or OR, wherein R comprises H, alkyl or perfluoroalkyl or a polymer, and x is an integer from 0 to 11.
Owner:AIR PROD & CHEM INC
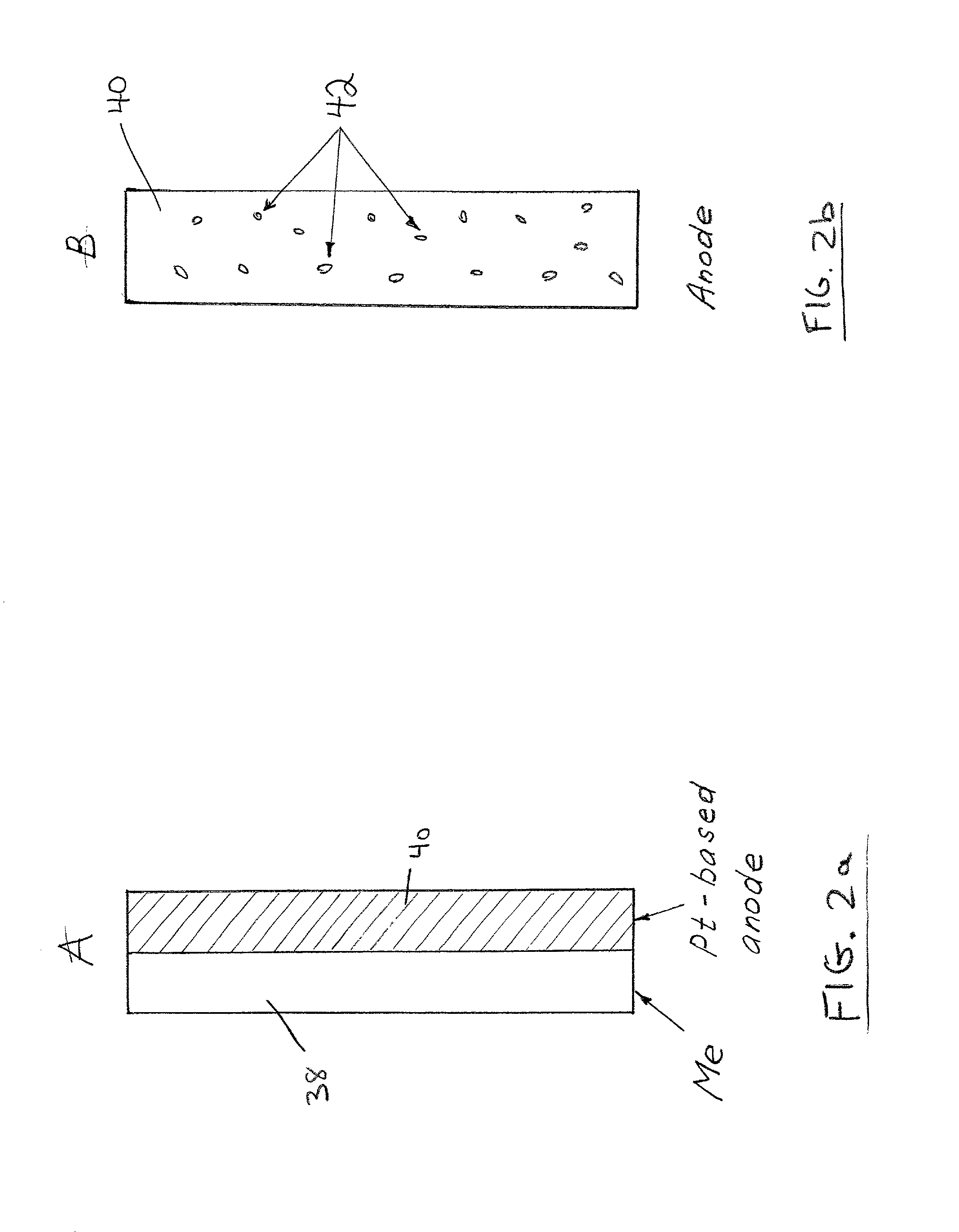
Direct liquid fuel cell and a novel binary electrode therefor
A fuel cell comprising: (a) a binary anode, (b) a cathode, and (c) a liquid electrolyte disposed between and interacting with the binary anode and the cathode, wherein the binary anode includes at least one liquid fuel and at least one solid fuel. Preferably, the electrolyte includes an alcohol such as methanol, and the solid fuel includes aluminum, magnesium and / or zinc.
Owner:MORE ENERGY
Liquid fuel compositions for electrochemical fuel cells
A new fuel composition useful for catalytic fuel cells is made up of at least two components. The primary fuel component is a surface active compound, such as methanol, that is a source of and acts to prevent unwanted decomposition of the auxiliary fuel. The auxiliary fuel is a hydrogen-containing inorganic compound with a high reduction potential, such as NaBH4, which acts as a highly reactive source of energy and serves to catalyze the catalytic oxidation of the primary fuel.
Owner:MORE ENERGY
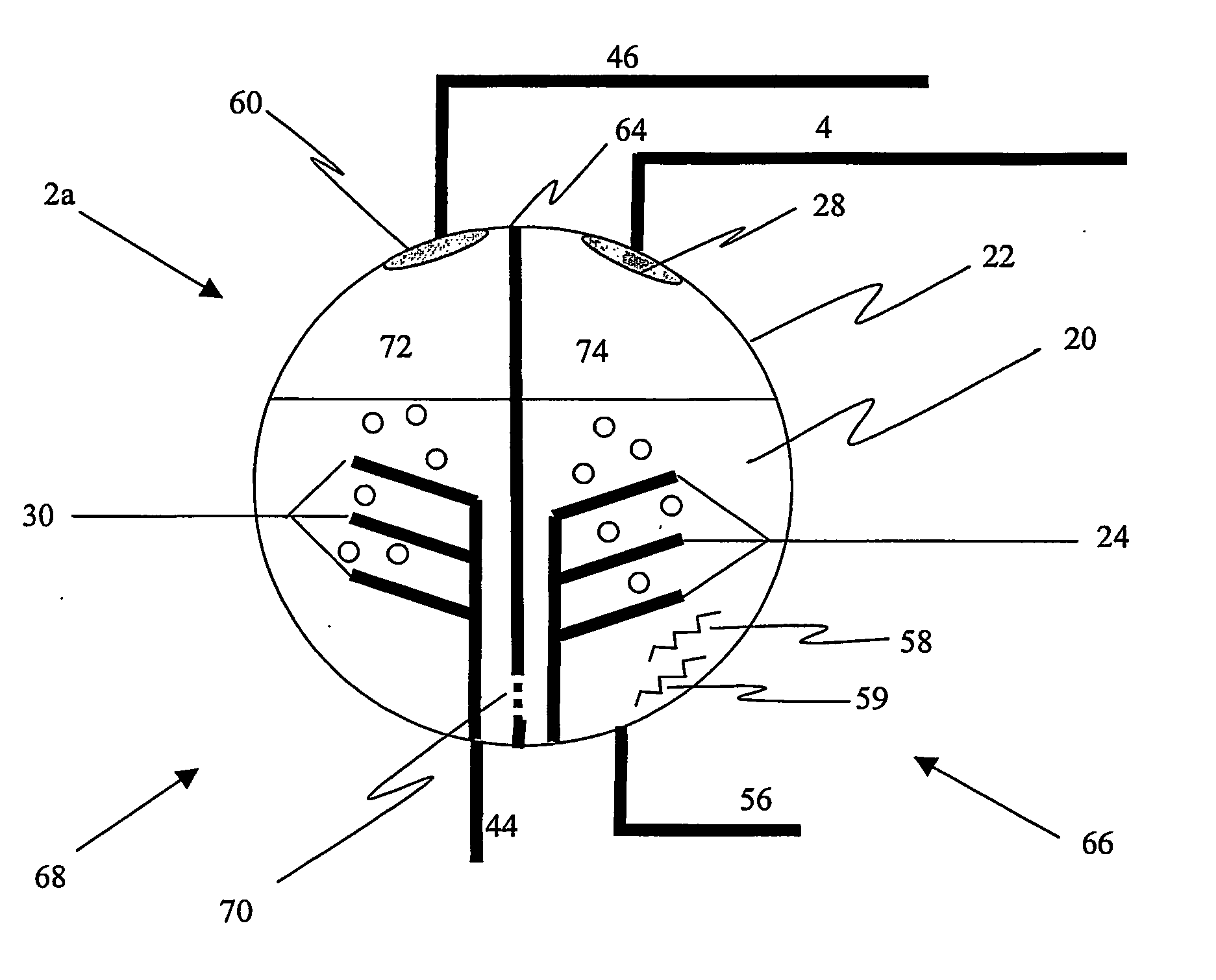
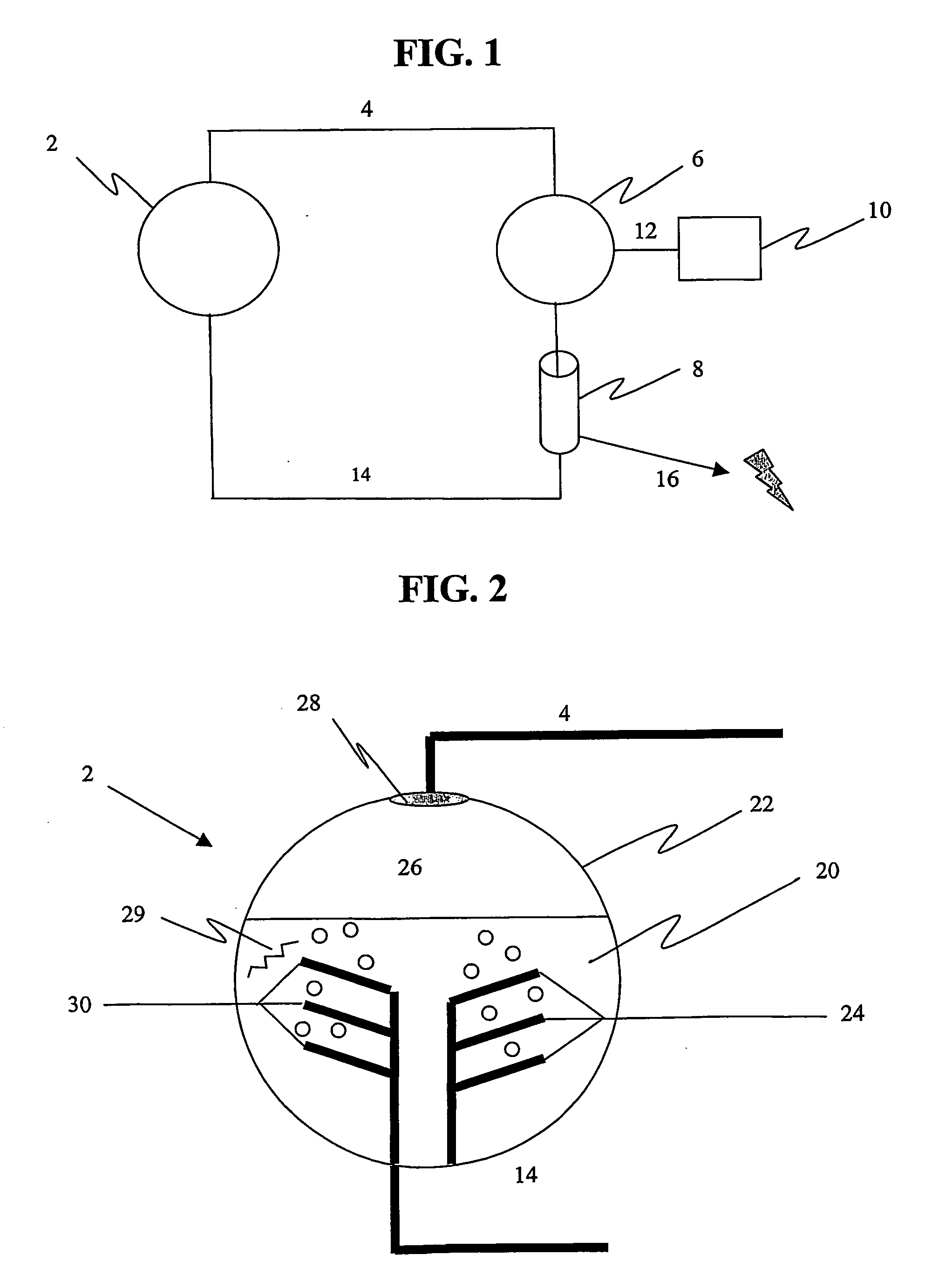
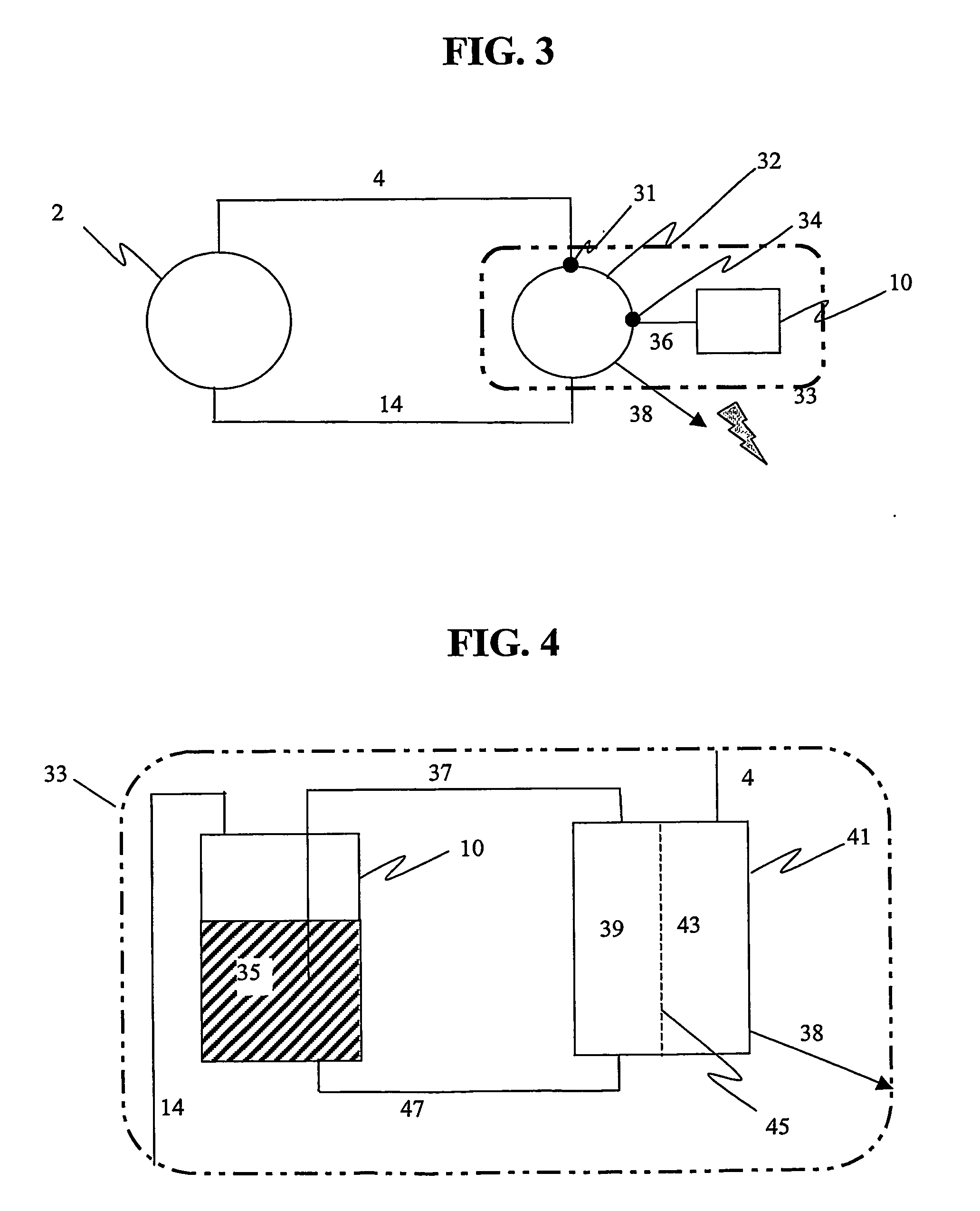
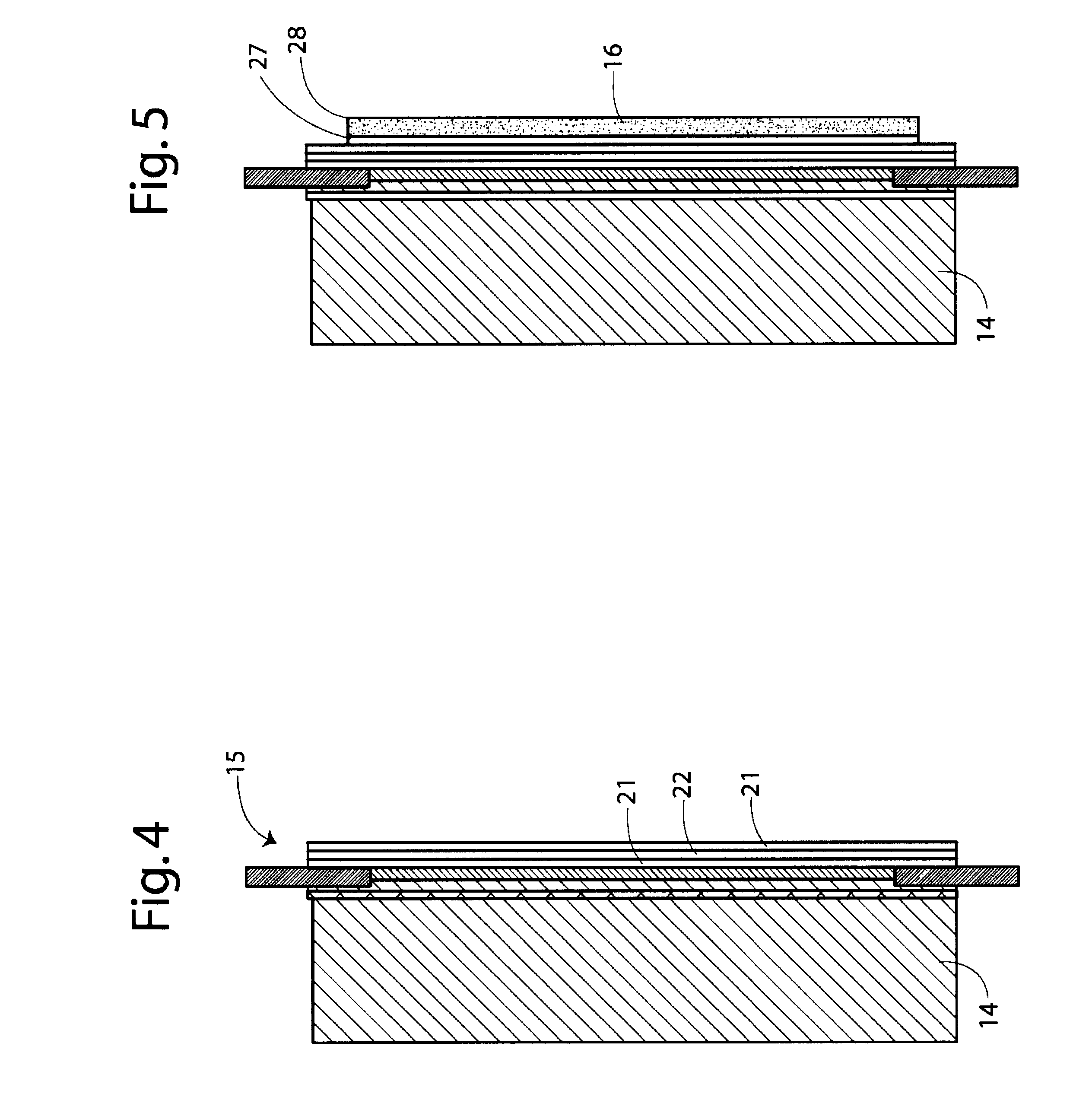
Non-volatile cathodes for lithium oxygen batteries and method of producing same
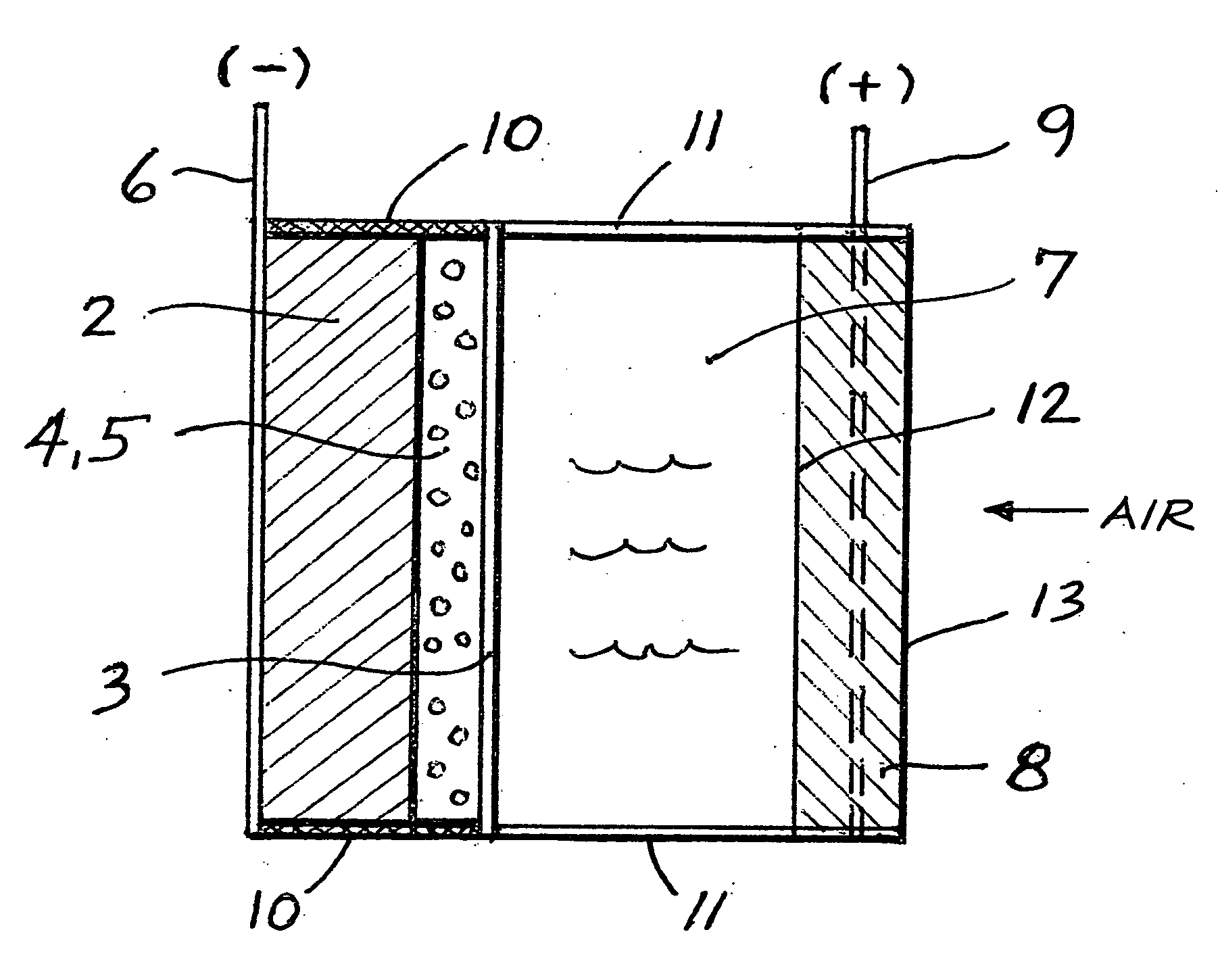
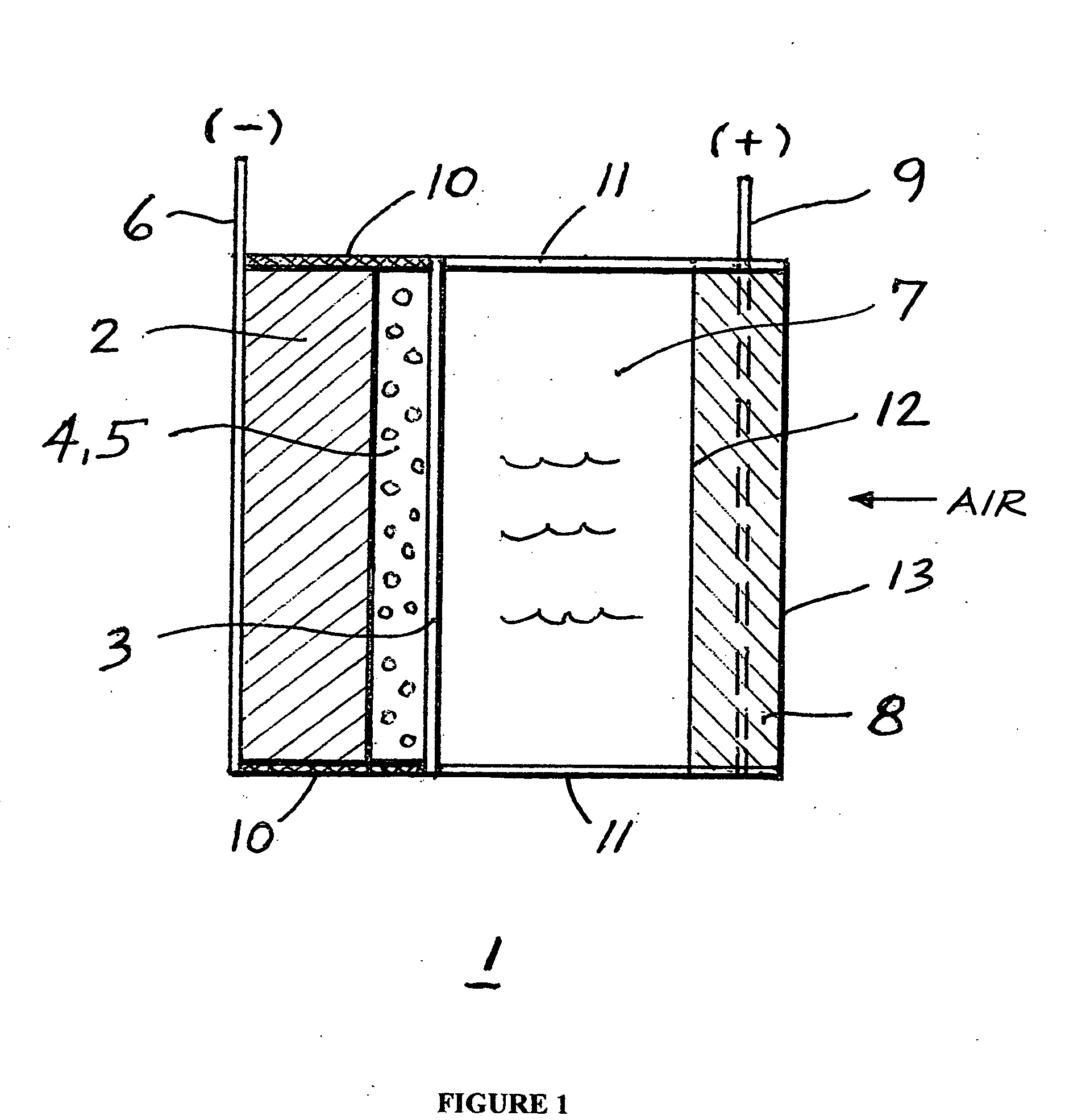
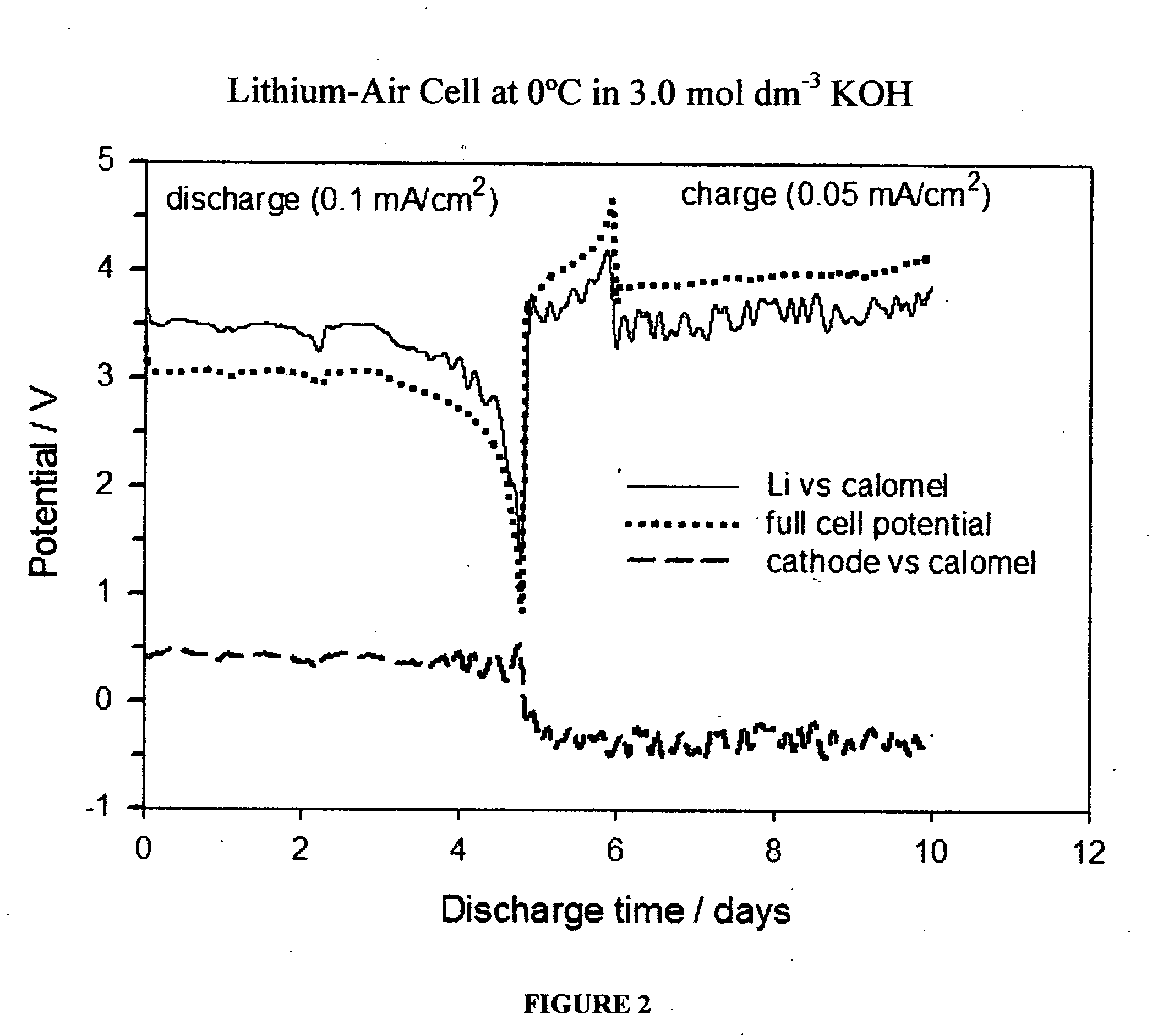
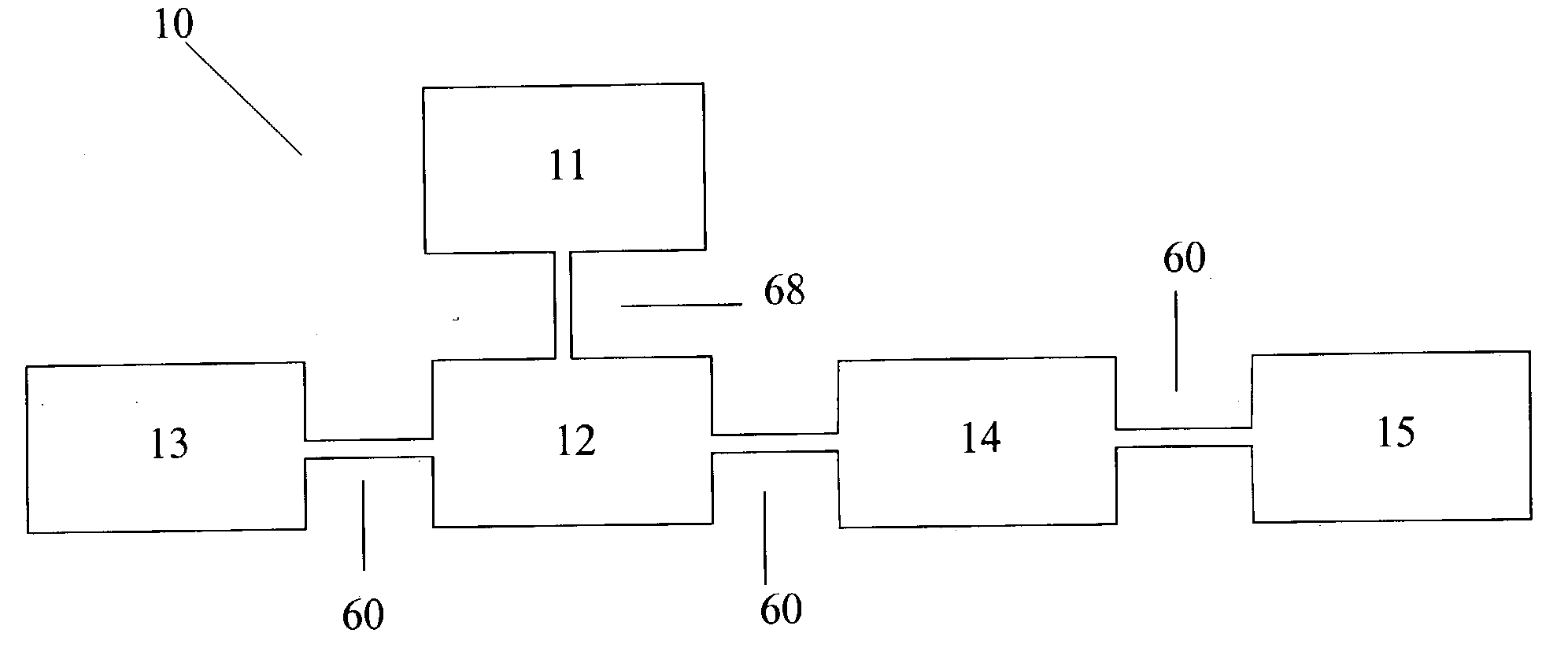
An air lithium battery is provided having two equal halves (60, 69) that are joined together along a centerline. Each half includes a porous substrate (64), an oxygen cathode (67) having a non-volatile lithium ion conductive electrolyte cathode, a non-volatile electrolyte (66), and an anode (65). The electrolyte may include alternating layers of ion conductive glass or ceramic layer and ion conductive polymer layer.
Owner:JOHNSON IP HLDG LLC
Manclaw-Harrison fuel cell
The Manclaw-Harrison Fuel Cell is a new Environmentally SAFE Fuel Cell (Lead Free, Acid Free, Mercury Free and has No Heavy Metals), and as such, it sets the definition of a new Class of Fuel Cell device because it has been verified to be a Non-Faraday device, making it the first such device discovered in the past 160+ years. See the “Preamble” for a more technical explanation.
Owner:MANCLAW RONALD R +1
Finely divided metal catalyst and method for making same
InactiveUS6841512B1Facilitating hydrogen consumptionSmall particle sizeReactant parameters controlReversible hydrogen uptakeParticulatesMetal catalyst
An inexpensive, highly catalytic material preferably formed by a leaching process. The catalyst comprises a finely divided metal particulate and a support. The active material may be a nickel and / or nickel nickel alloy particulate having a particle size less than about 100 Angstroms. The support may be one or more metal oxides.
Owner:CHEVRONTEXACO TECH VENTURES
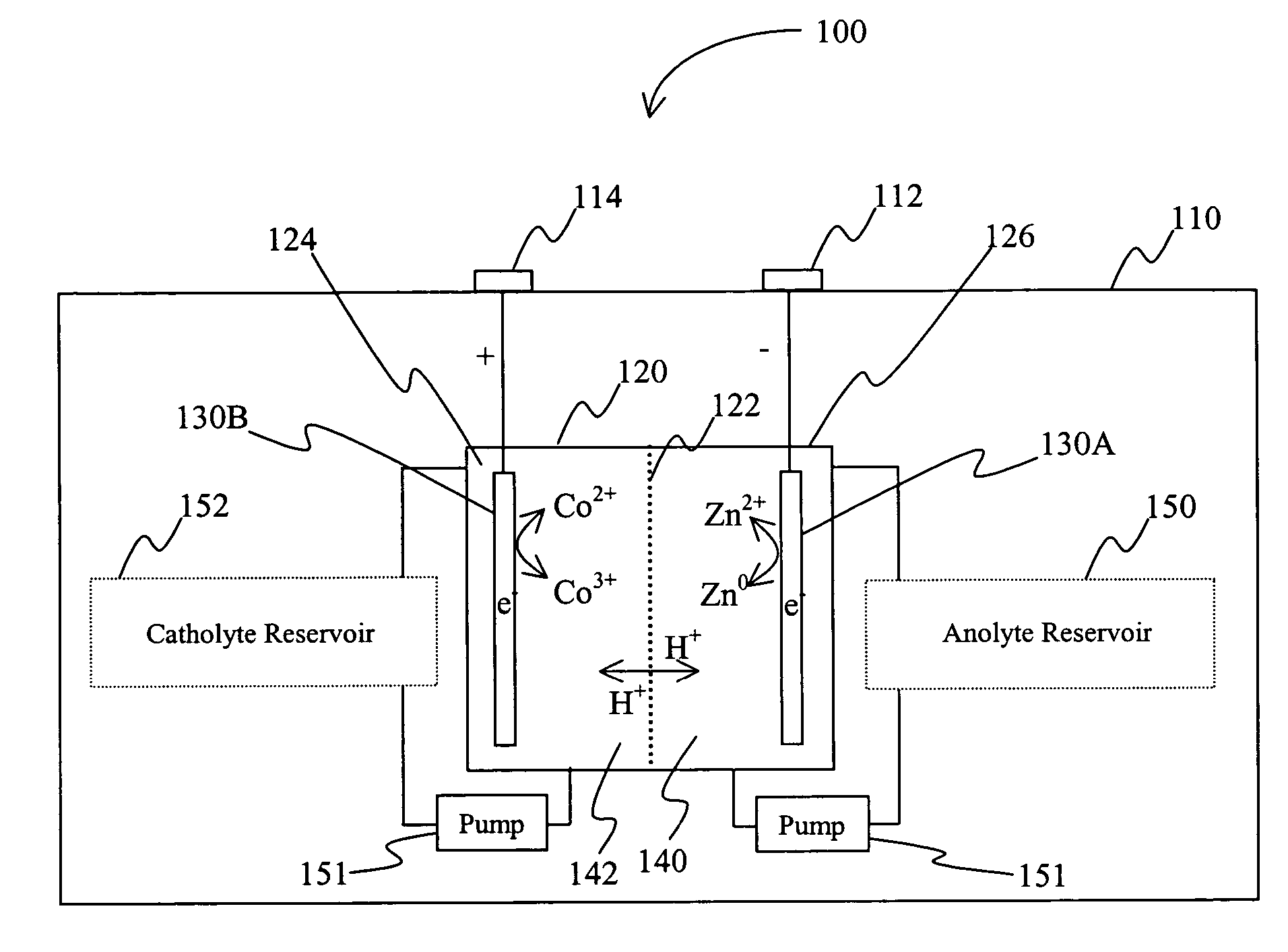
Battery with bifunctional electrolyte
InactiveUS20060063065A1Improve solubilityCell electrodesRegenerative fuel cellsSolubilityOxidation-Reduction Agent
A battery comprises an acid electrolyte in which a compound provides acidity to the electrolyte and further increases solubility of at least one metal in the redox pair. Especially preferred compounds include alkyl sulfonic acids, amine sulfonic acids, and alkyl phosphonic acids, and particularly preferred redox coupled include Co3+ / Zn0, Mn3+ / Zn0, Ce4+ / V2+, Ce4+ / Ti3+, Ce4+ / Zn0, and Pb4+ / Pb0.
Owner:PLURION LTD
Metal-air cell with performance enhancing additive
ActiveUS20110281184A1Improves oxygen reduction thermodynamicsImproved kineticsFuel and primary cellsFuel and secondary cellsOxygenElectrochemical cell
Systems and methods drawn to an electrochemical cell comprising a low temperature ionic liquid comprising positive ions and negative ions and a performance enhancing additive added to the low temperature ionic liquid. The additive dissolves in the ionic liquid to form cations, which are coordinated with one or more negative ions forming ion complexes. The electrochemical cell also includes an air electrode configured to absorb and reduce oxygen. The ion complexes improve oxygen reduction thermodynamics and / or kinetics relative to the ionic liquid without the additive.
Owner:ARIZONA STATE UNIVERSITY
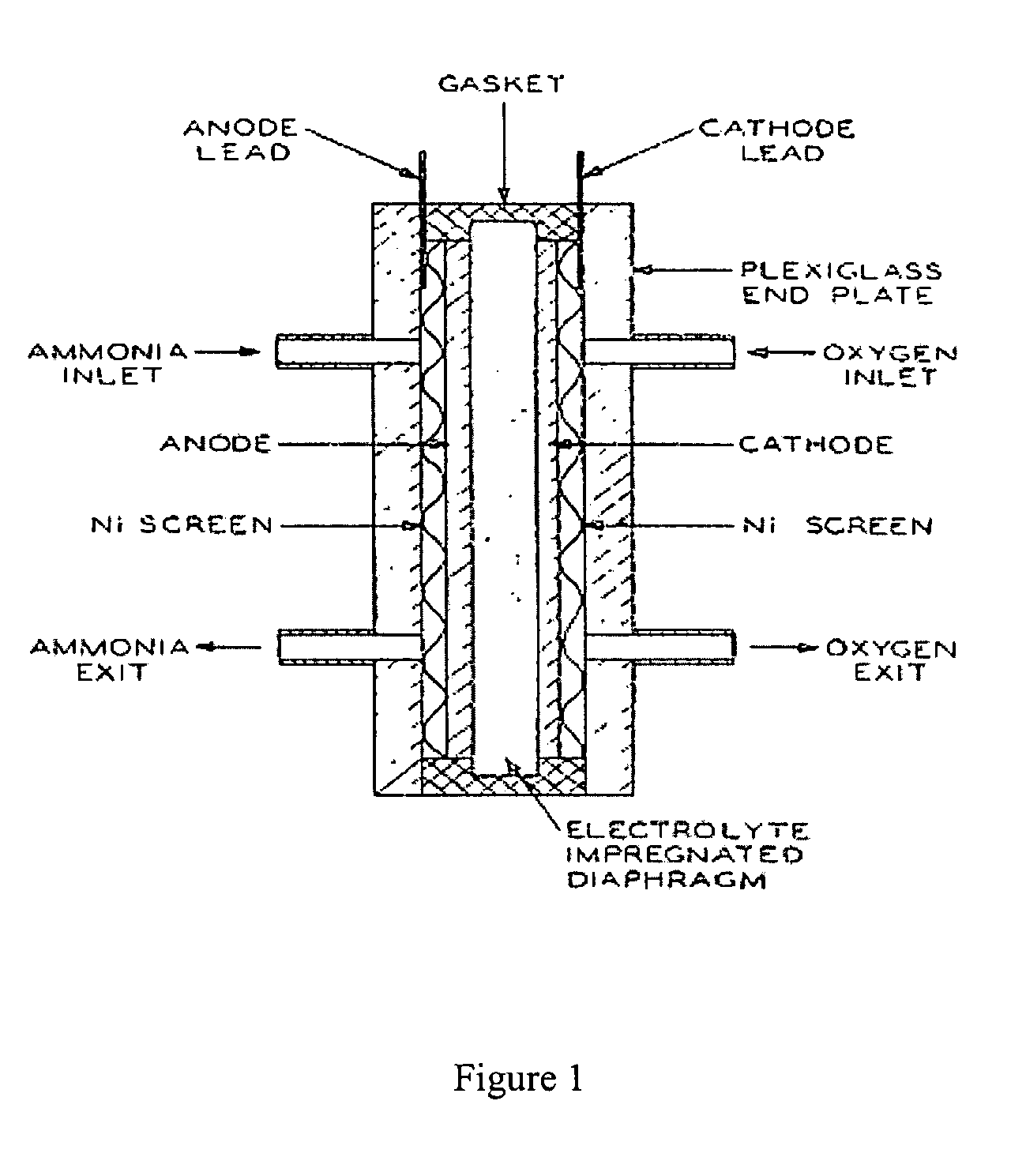
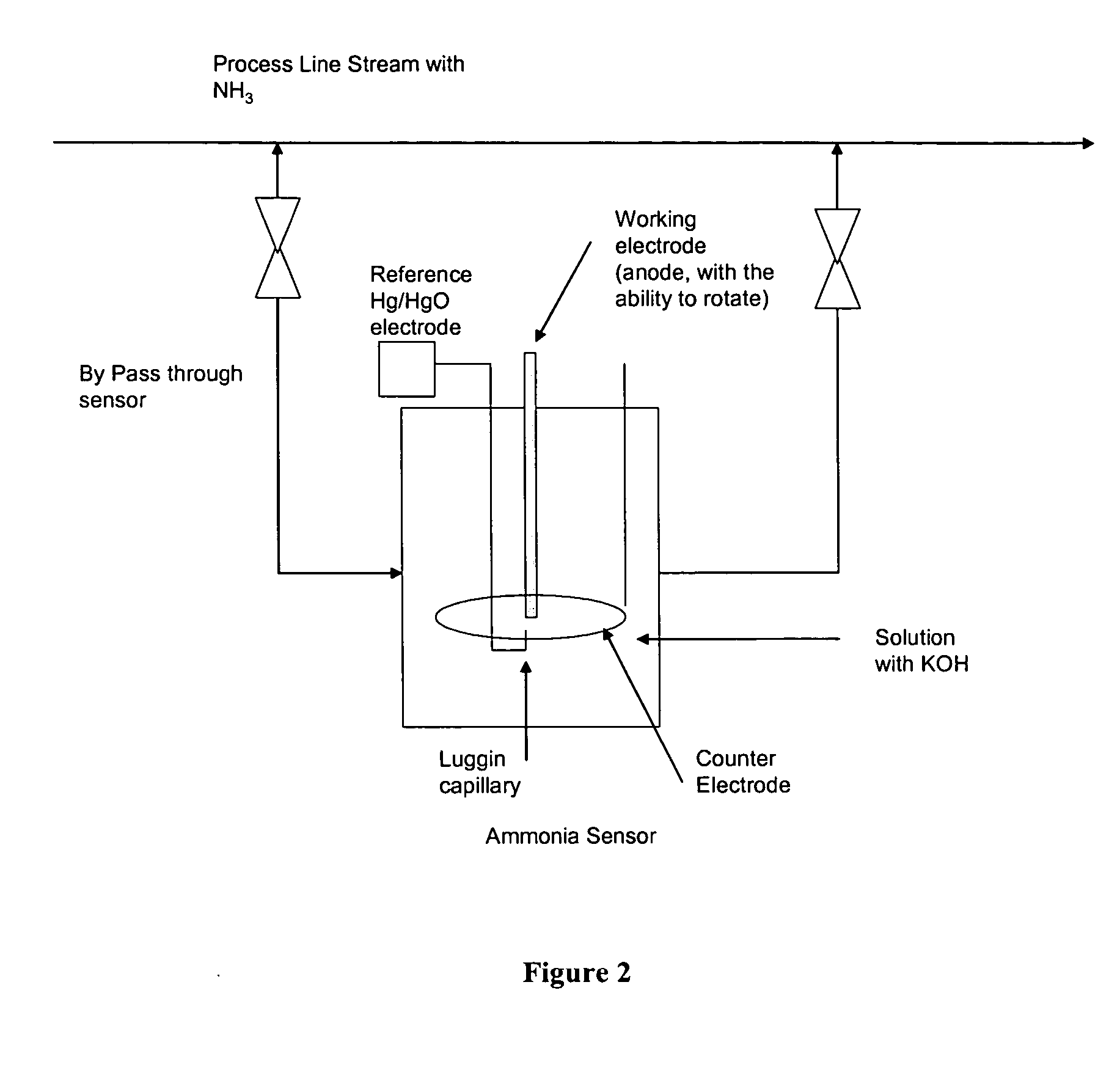
Electro-catalysts for the oxidation of ammonia in alkaline media
InactiveUS20050211569A1From normal temperature solutionsLiquid separation by electricityChemistryIridium
An electro-catalyst for the oxidation of ammonia in alkaline media; the electrocatalyst being a noble metal co-deposited on a support with one or more other metals that are active to ammonia oxidation. In some embodiments, the support is platinum, gold, tantalum, or iridium. In some embodiments, the support has a layer of Raney metal deposited thereon prior to the deposition of the catalyst. Also provided are electrodes having the electro-catalyst deposited thereon, ammonia electrolytic cells, ammonia fuel cells, ammonia sensors, and a method for removing ammonia contaminants from a contaminated effluent.
Owner:OHIO UNIV
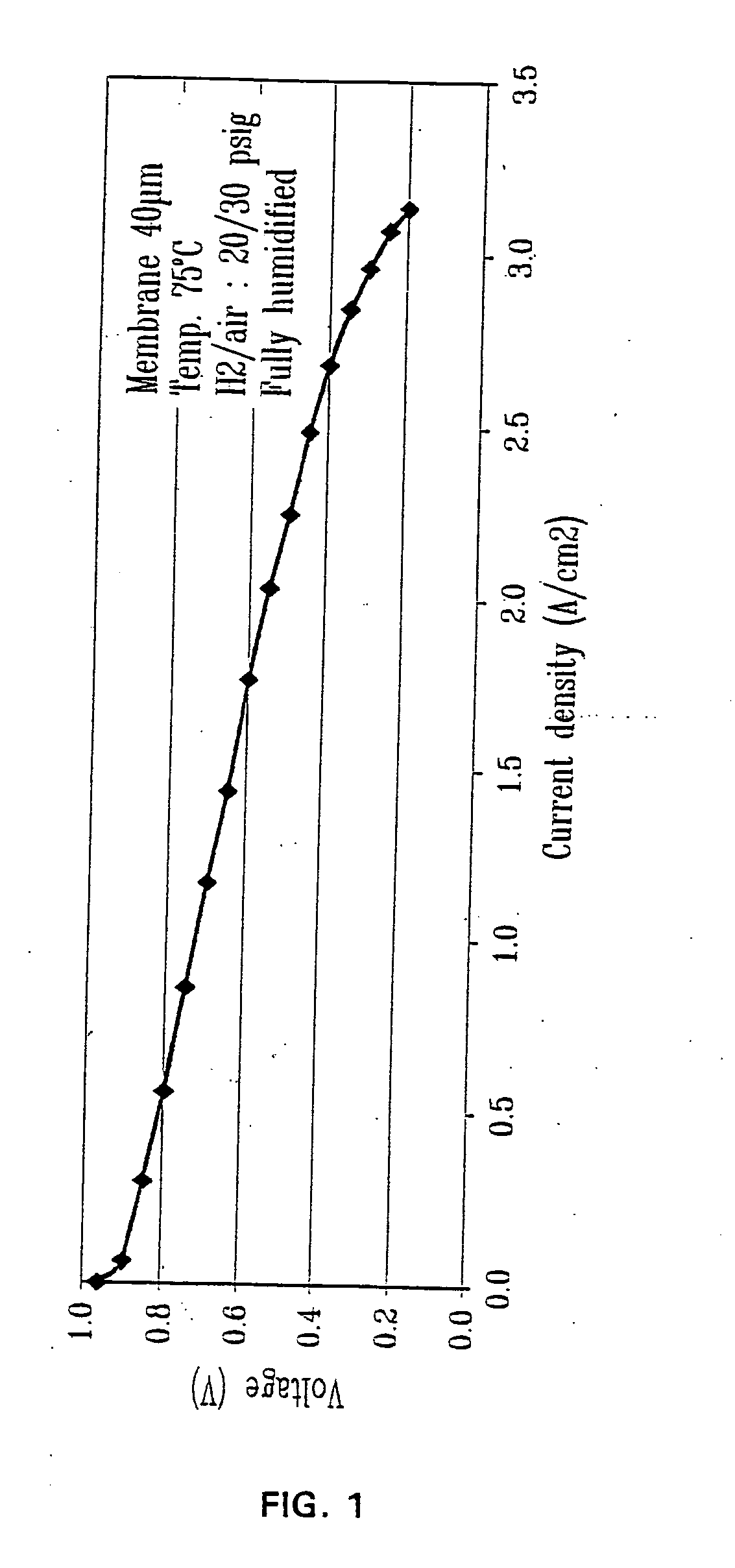
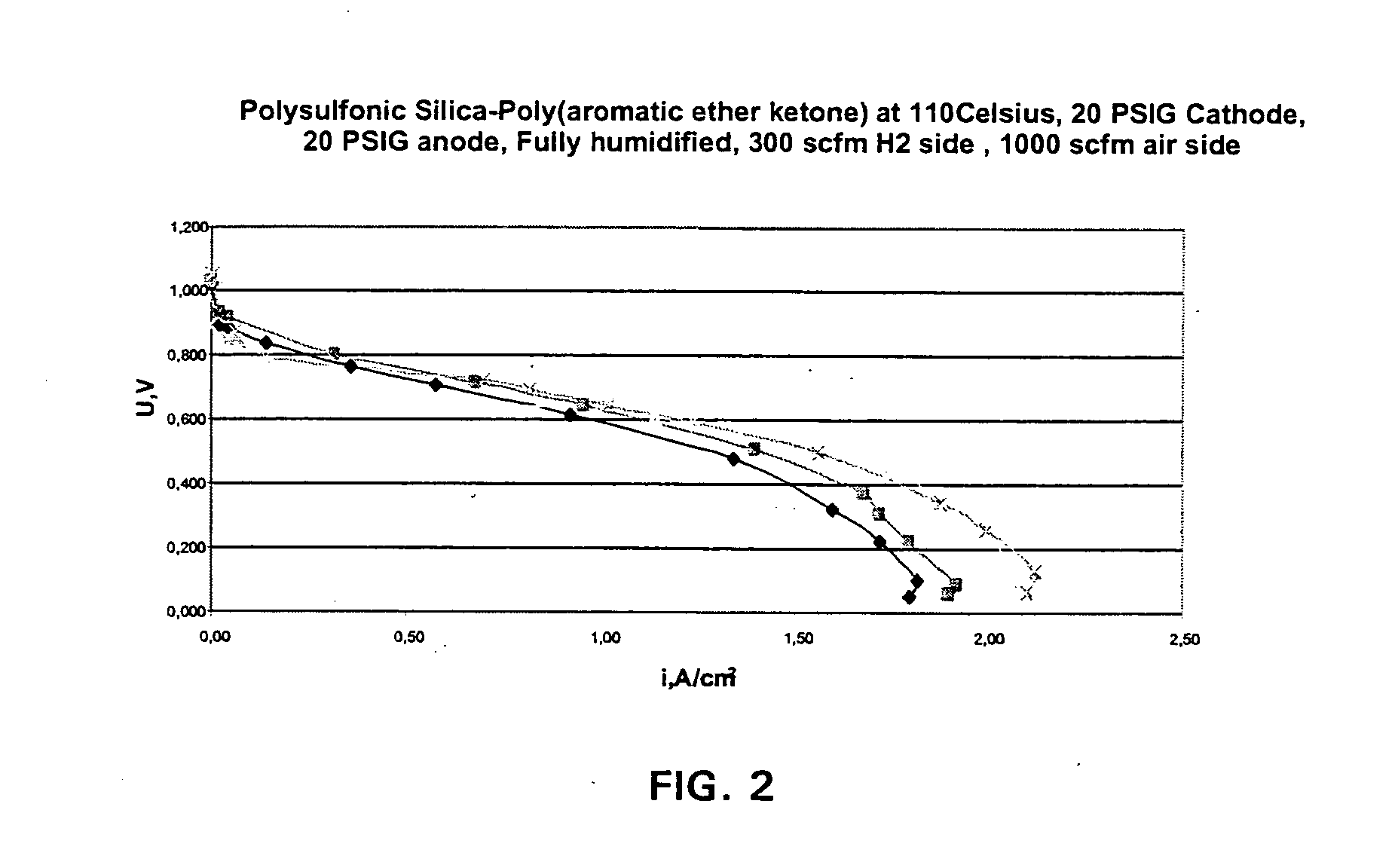
Ion exchange composite material based on proton conductive functionalized inorganic support compounds in a polymer matrix
InactiveUS20050053818A1Improve mechanical propertiesImprove impermeabilitySemi-permeable membranesSolid electrolytesIon exchangeLiquid fuel
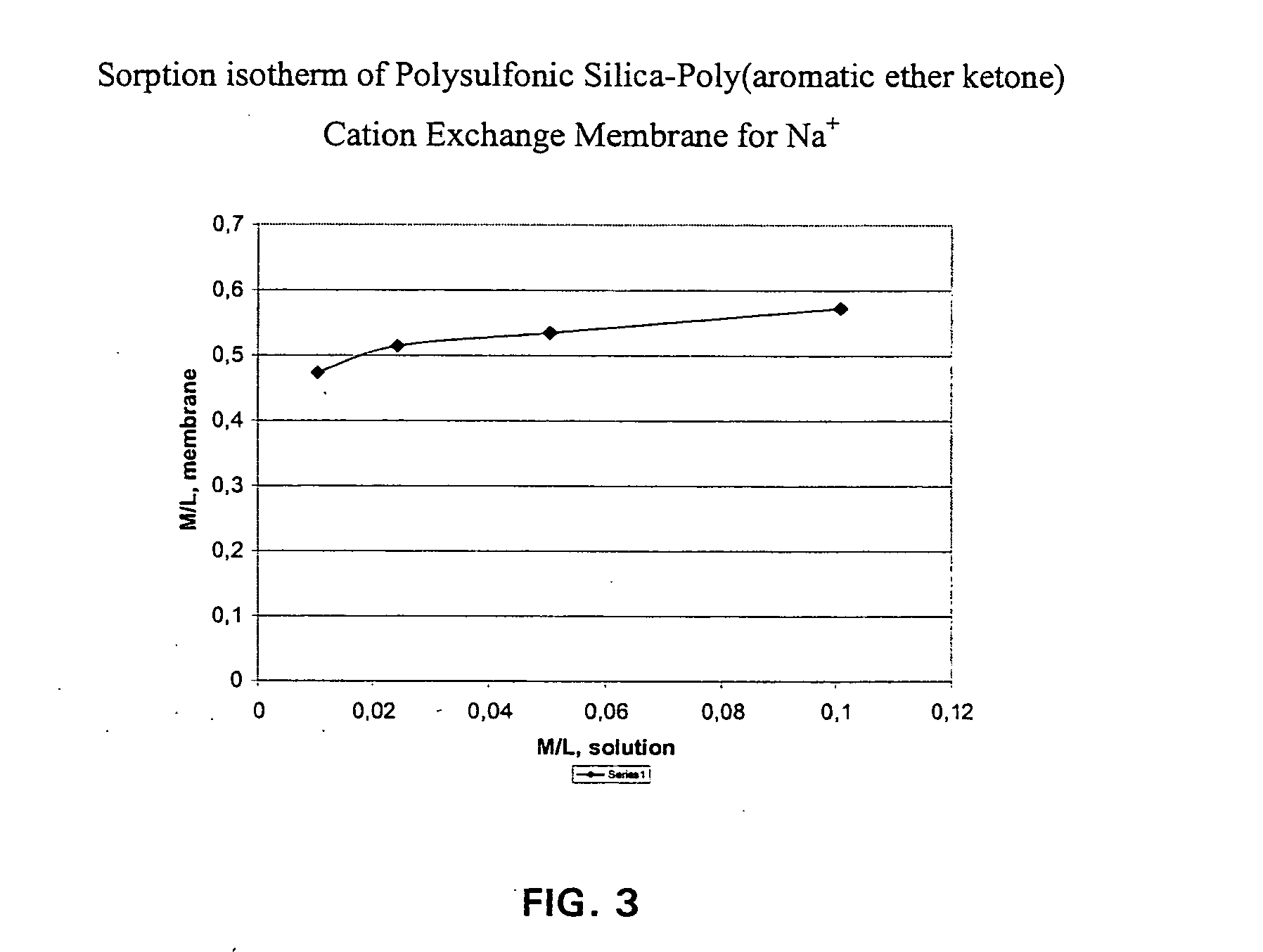
The composite material comprise acid functionalized inorganic supports such as silica dispersed in a functionalized and / or non-functionalized polymer matrix that is based on numerous polymers such as poly(aromatic ether ketones), or poly(benzoyl phenylene), or derivatives thereof. The composite material is characterized by good water retention capabilities due to the acidic functions and the hydrophilicity of the silica particles. Moreover, a good impermeability to gas and liquid fuels commonly used in fuel cell technology, like hydrogen gas or methanol solution, is also obtained due to the presence of silica particles. Good mechanical properties of the composite material let the material to be formed easily in thin film or membrane form. In that form, the composite material is usable for proton exchange membrane for fuel cells, for drying or humidifying membrane for gas or solvent conditioning, or as acid catalytic membrane.
Owner:SIM COMPOSITES INC
Electrocatalyst for oxygen reduction with reduced platinum oxidation and dissolution rates
InactiveUS20060263675A1Low platinum loadingImprove stabilityMaterial nanotechnologyConductive materialOxide compositeDissolution
The invention relates to platinum-metal oxide composite particles and their use as electrocatalysts in oxygen-reducing cathodes and fuel cells. The invention particularly relates to methods for preventing the oxidation of the platinum electrocatalyst in the cathodes of fuel cells by use of these platinum-metal oxide composite particles. The invention additionally relates to methods for producing electrical energy by supplying such a fuel cell with an oxidant, such as oxygen, and a fuel source, such as hydrogen.
Owner:BROOKHAVEN SCI ASSOCS
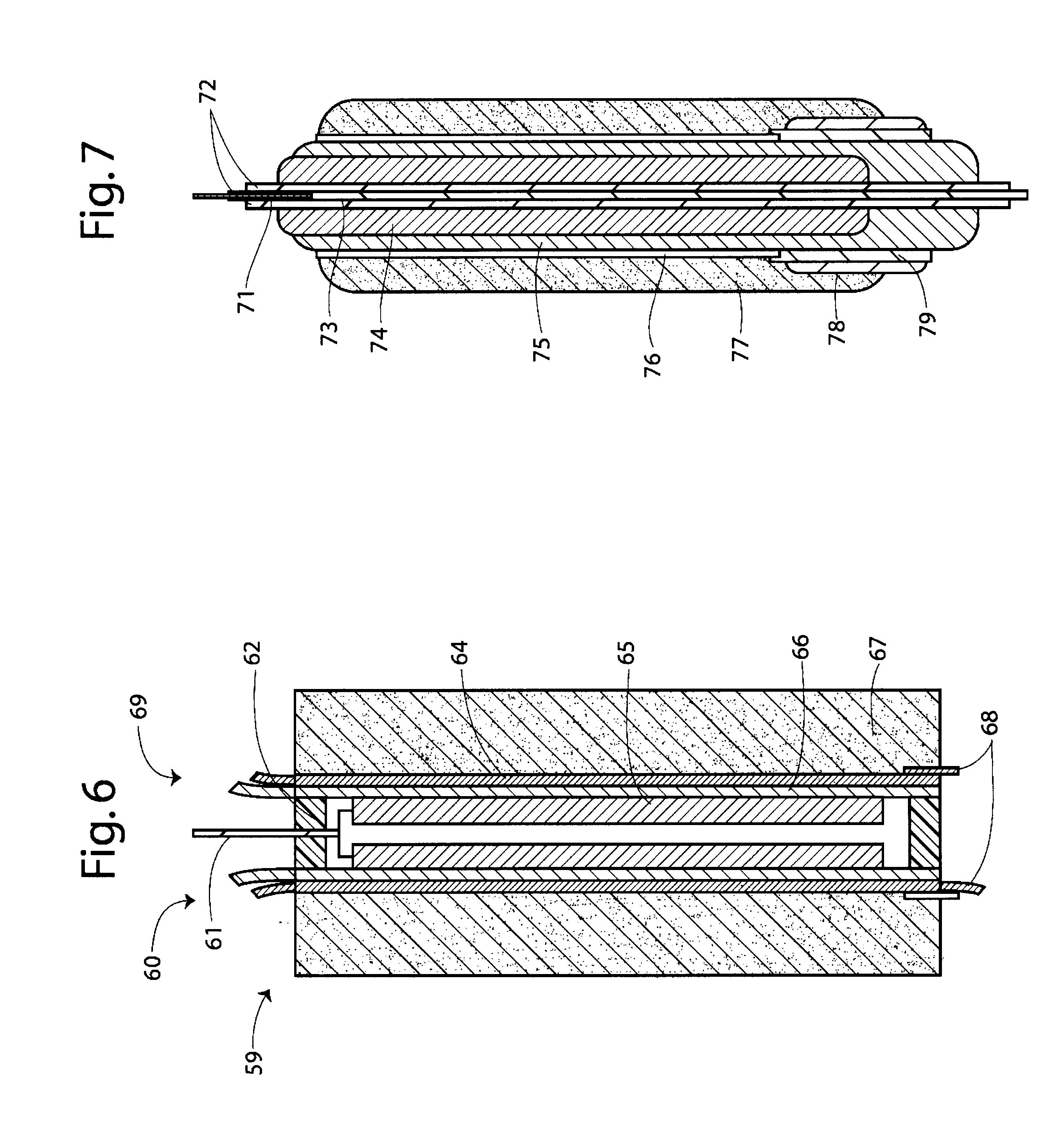
Metal-air semi-fuel cell with an aqueous acid based cathode
InactiveUS20070259234A1Long time operationCell energy increasedFuel and primary cellsFuel and secondary cellsFuel cellsOxygen
A metal-air semi-fuel cell is provided, preferably based on lithium anode and a fuel cell type air / oxygen electrode immersed in an aqueous neutral, alkali or acid solution. The lithium anode is comprised of the active metal and one or more separators protecting the anode from reacting with an aqueous solution. The outermost layer on the lithium electrode is a solid-state lithium-ion conducting glass-ceramic which is impervious to and stable towards aqueous solutions. The cathode is comprised of an air or oxygen fuel cell type electrode in contact with the aqueous solution. The lithium anode of this invention also can be replaced by other electroactive metals which react with water and acids, bases and neutral solutions, such as metals from Groups 1 and 2 of the Periodic Table of Elements in addition to Zn, Mg, and Al.
Owner:CHUA DAVID +2
Redox flow cell
A redox flow cell is presented that utilizes a porous membrane separating a first half cell and a second half cell. The porous membrane is chosen to have a figure of merit (FOM) is at least a minimum FOM. A method of providing a porous membrane for a flow cell can include determining a figure of merit; determining a first parameter from a pore size or a thickness for the porous membrane; determining a second parameter from the pore size or the thickness that is not the first parameter for the porous membrane, based on the figure of merit; and constructing a porous membrane having the pore size and the thickness.
Owner:IMERGY POWER SYST
Controlled activation ingestible identifier
Controlled activation identifiers for use in ingestible compositions, such as pharma-informatics enabled compositions, are provided. The identifiers include a controlled activation element that provides for activation of the identifier in response to the presence of a predetermined stimulus at a target site of interest. The invention finds use in a variety of different applications, including but not limited to, monitoring of therapeutic regimen compliance, tracking the history of pharmaceutical agents, etc.
Owner:OTSUKA PHARM CO LTD
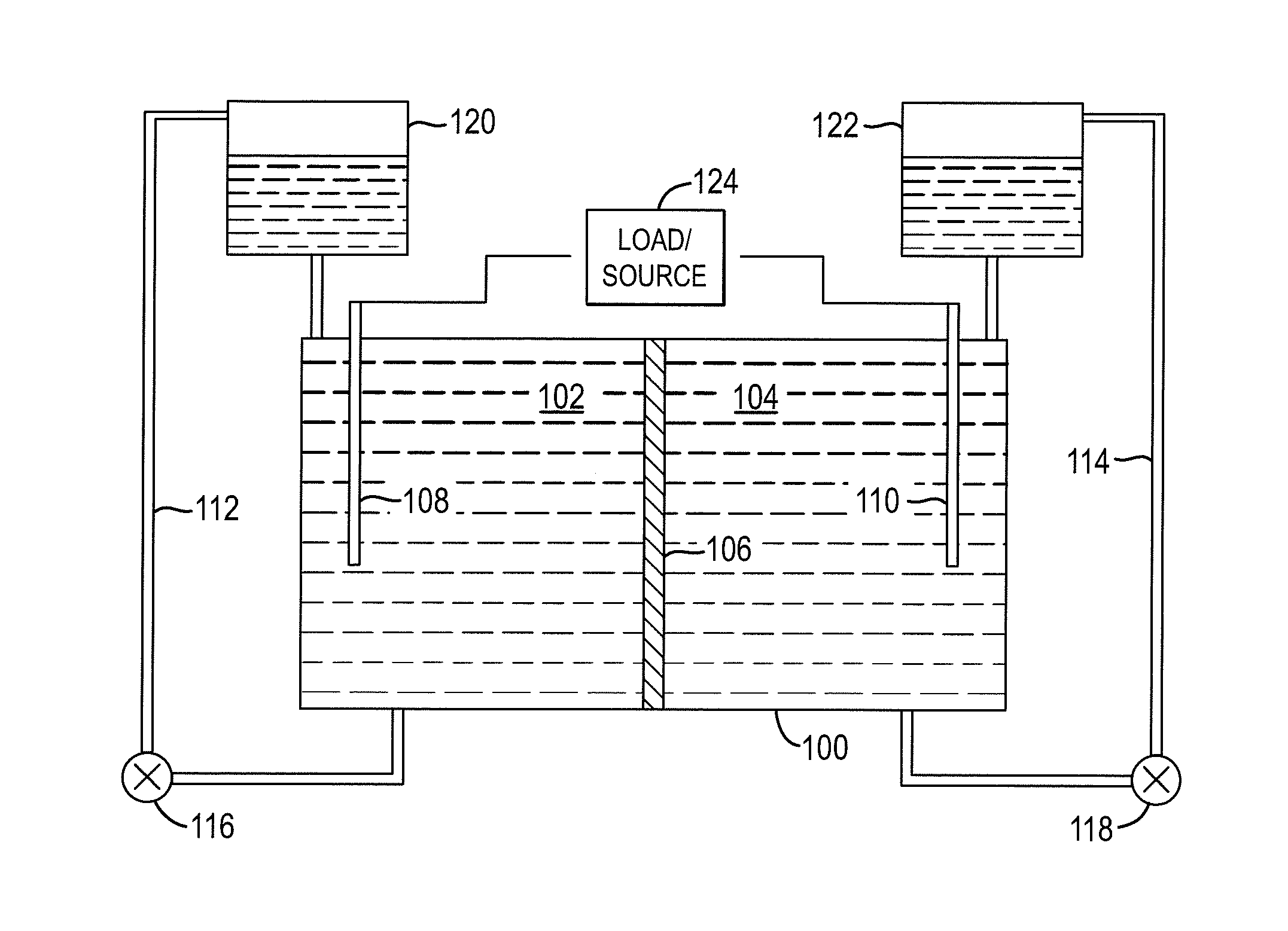
Thermal Control of a Flow Cell Battery
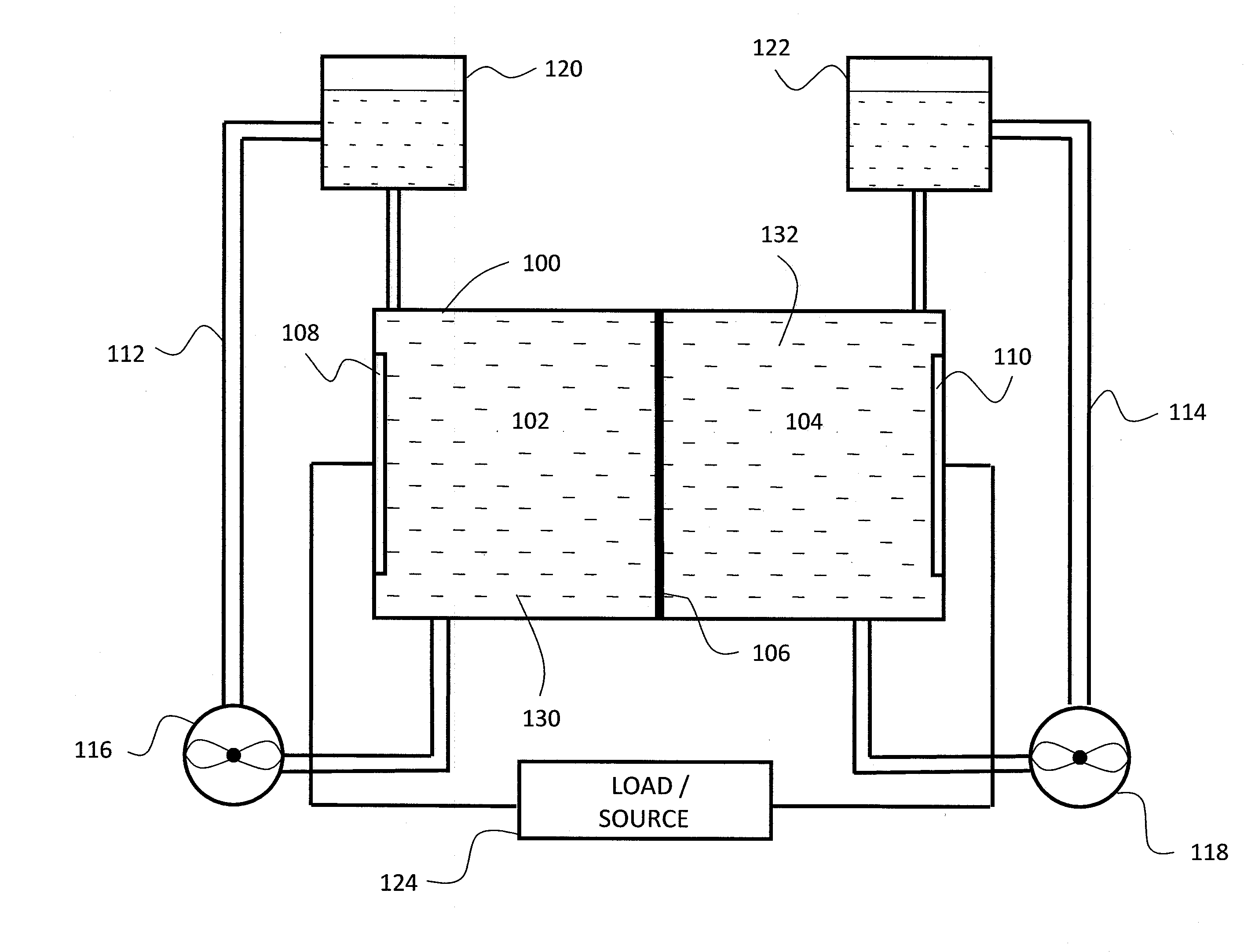
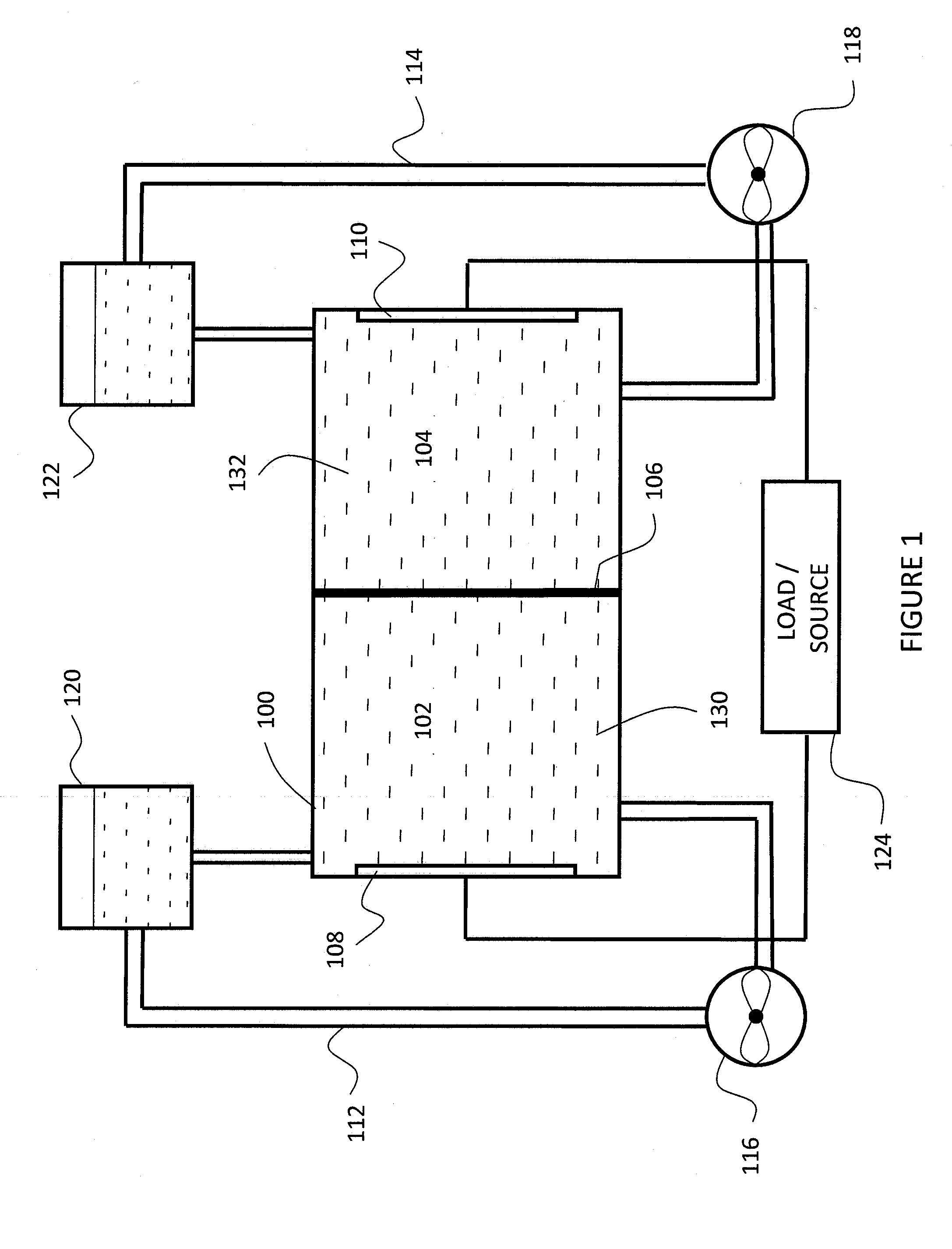
A flow battery with thermal management is presented. The flow battery is housed in an enclosure where fluid is uniformly circulated about holding tanks of electrolyte to control the temperature inside the enclosure.
Owner:IMERGY POWER SYST
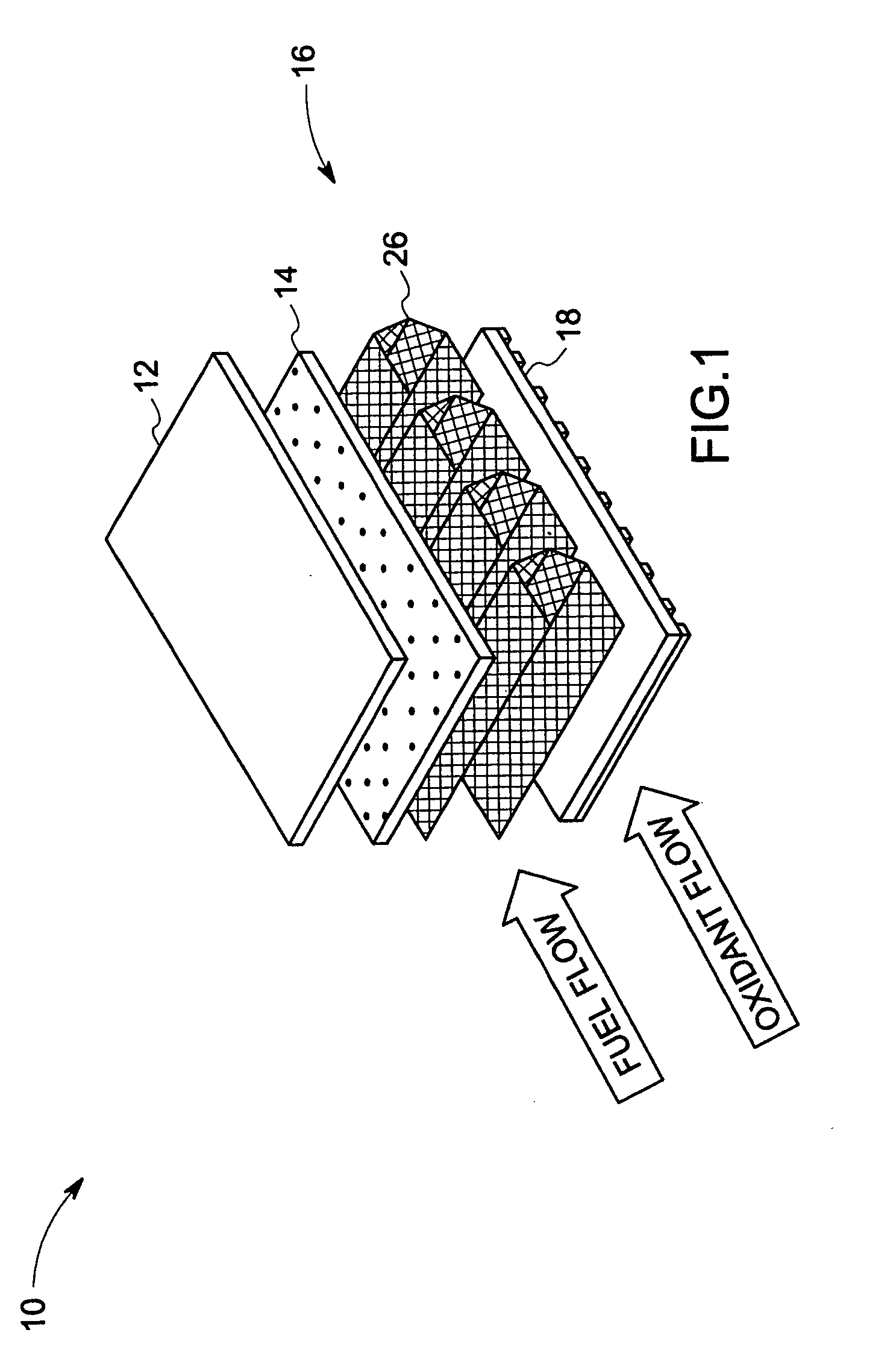
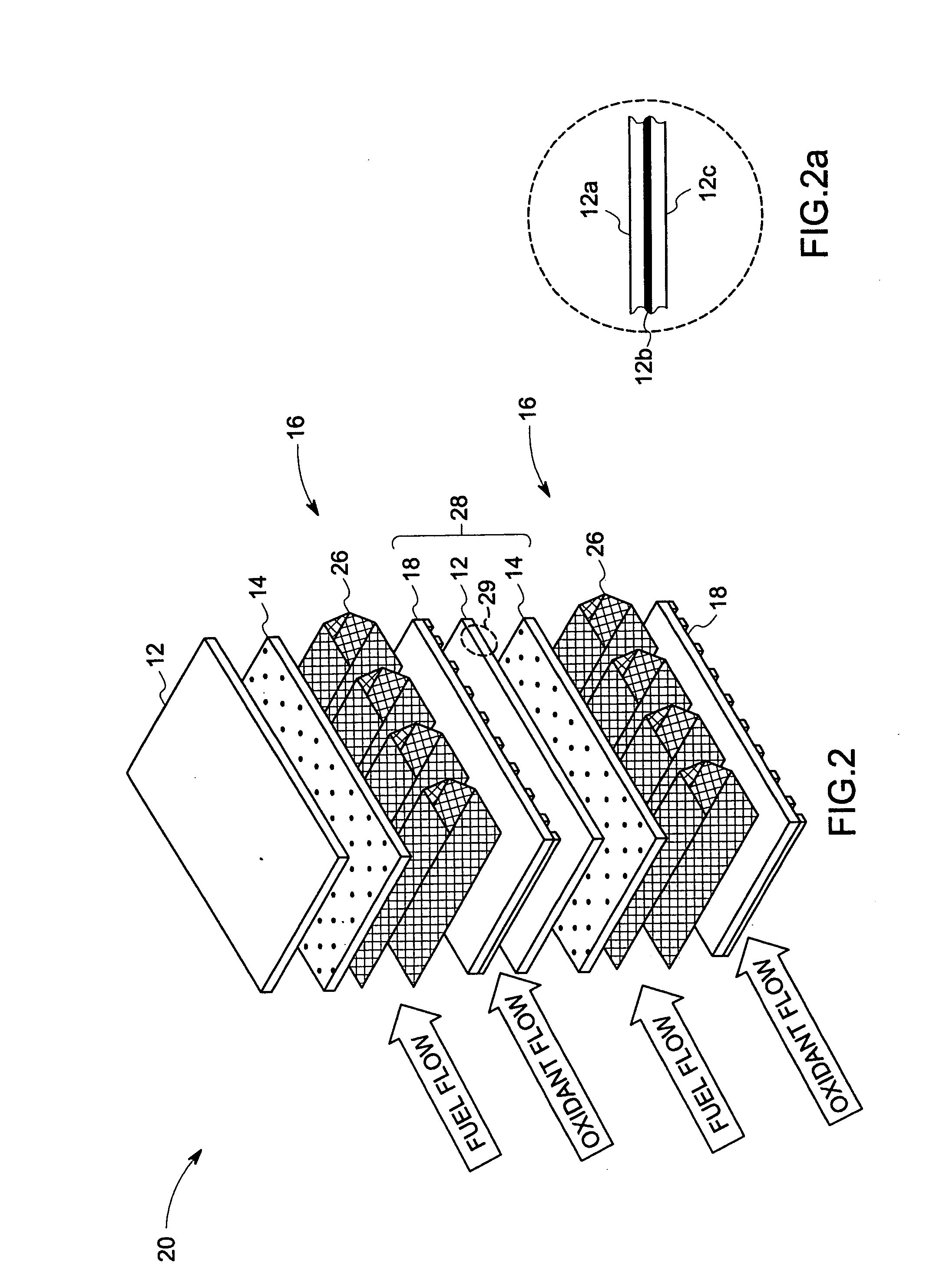
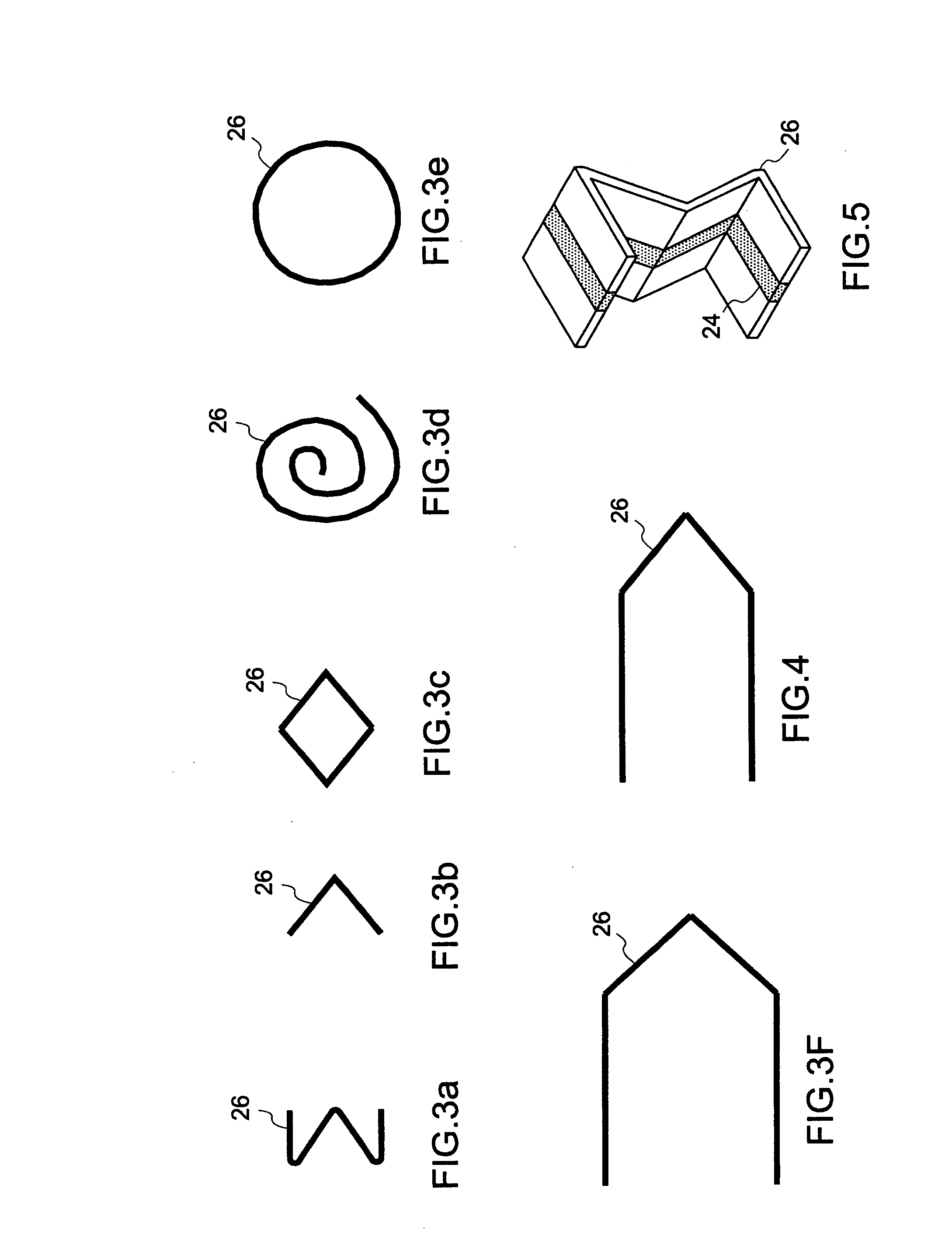
Compliant fuel cell system
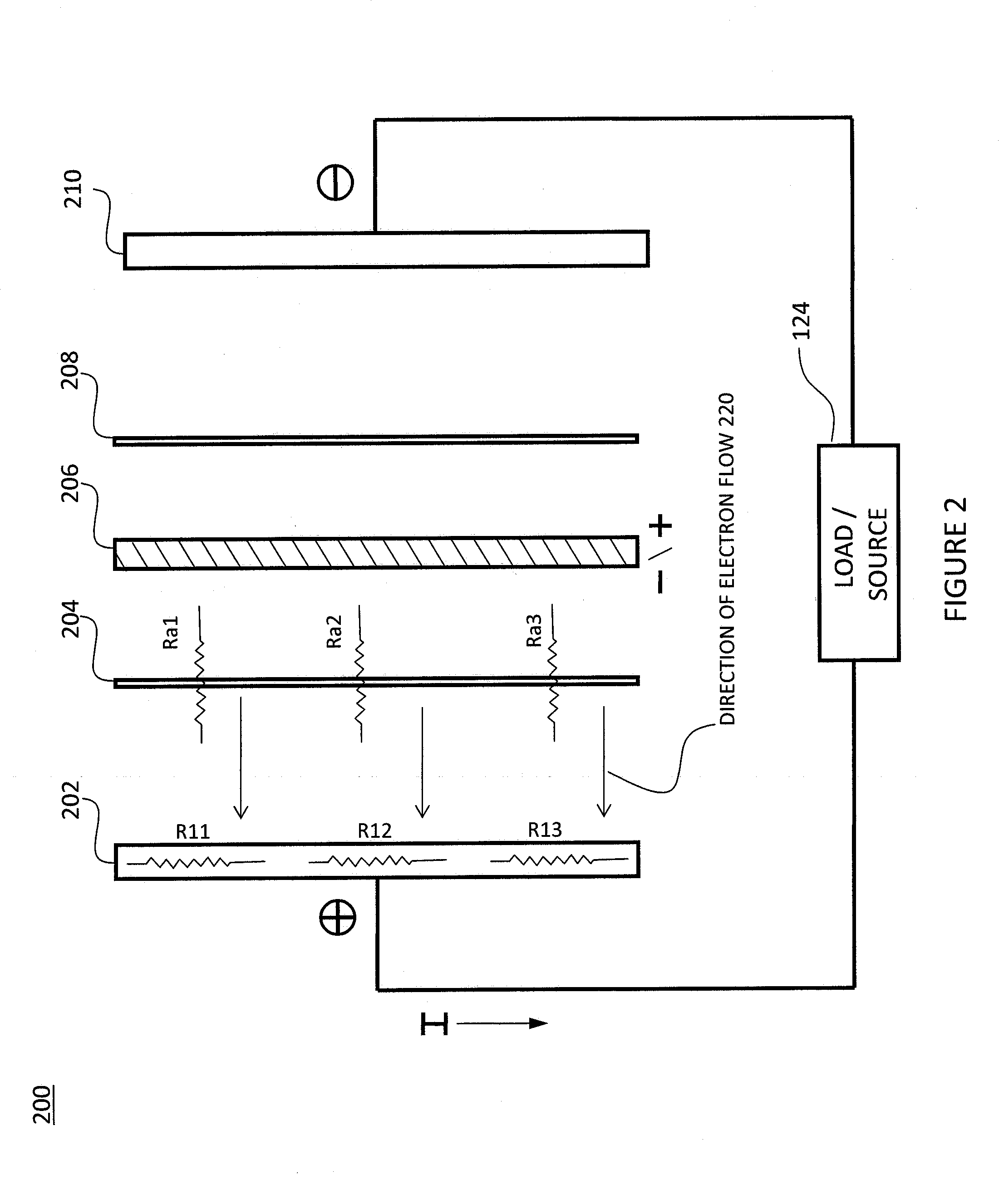
A fuel cell, comprising a first electrode layer, a second electrode layer and an electrolyte interposed therebetween. The fuel cell further comprises a first electrode interconnect for supporting the first electrode layer. The first electrode interconnect is in intimate contact with the first electrode layer. The fuel cell also comprises a separator plate incorporating a second electrode interconnect for supporting the second electrode layer, which second electrode interconnect is in intimate contact with the second electrode layer, and at least one compliant structure disposed between the first electrode interconnect and the separator plate. In operation, the compliant structure deforms to accommodate motion in the fuel cell.
Owner:GENERAL ELECTRIC CO
Metal-Air Battery or Fuel Cell
InactiveUS20080096061A1Improve conductivityStabilises itFuel and primary cellsFuel and secondary cellsFuel cellsWater vapor
A metal-air battery or fuel cell comprising a metal or metal hydride anode, an aqueous liquid electrolyte containing an ion conducting material, and an air electrode which allows ingress and egress of oxygen and which contains one or more catalysts capable of evolution and / or reduction of oxygen, wherein the air electrode has both hydrophobic and hydrophilic pores, the hydrophilic pores are at least partially filled with aqueous liquid electrolyte and the air electrode and / or the electrolyte comprises hygroscopic material and OH− ions, whereby water vapour exchange with the environment is limited. The hygroscopic material is used to control the humidity of the system.
Owner:REVOLT TECH LTD
Catholytes for aqueous lithium/air battery cells
ActiveUS20130122380A1Prevent drynessFacilitate numberFuel and primary cellsHybrid cell detailsHigh energyLithium metal
Owner:POLYPLUS BATTERY CO INC
Processing techniques for the fabrication of solid acid fuel cell membrane electrode assemblies
ActiveUS20060014068A1Improve proton conductivityMaterial nanotechnologyElectrolyte holding meansFuel cellsSolid acid
Processes, techniques, and compositions used to fabricate high performance solid acid fuel cell membrane electrode assemblies are disclosed. The techniques include preparing the solid acid electrolyte material, depositing the electrolyte membrane, depositing the electrocatalyst layer, preparing the electrodes, fabricating the gas seals, and constructing the membrane electrode assembly.
Owner:CALIFORNIA INST OF TECH
Hydrogen generation and storage method for personal transportation applications
InactiveUS20080138675A1Improve the heating effectElectrolysis componentsReactant parameters controlThermal energyFuel cells
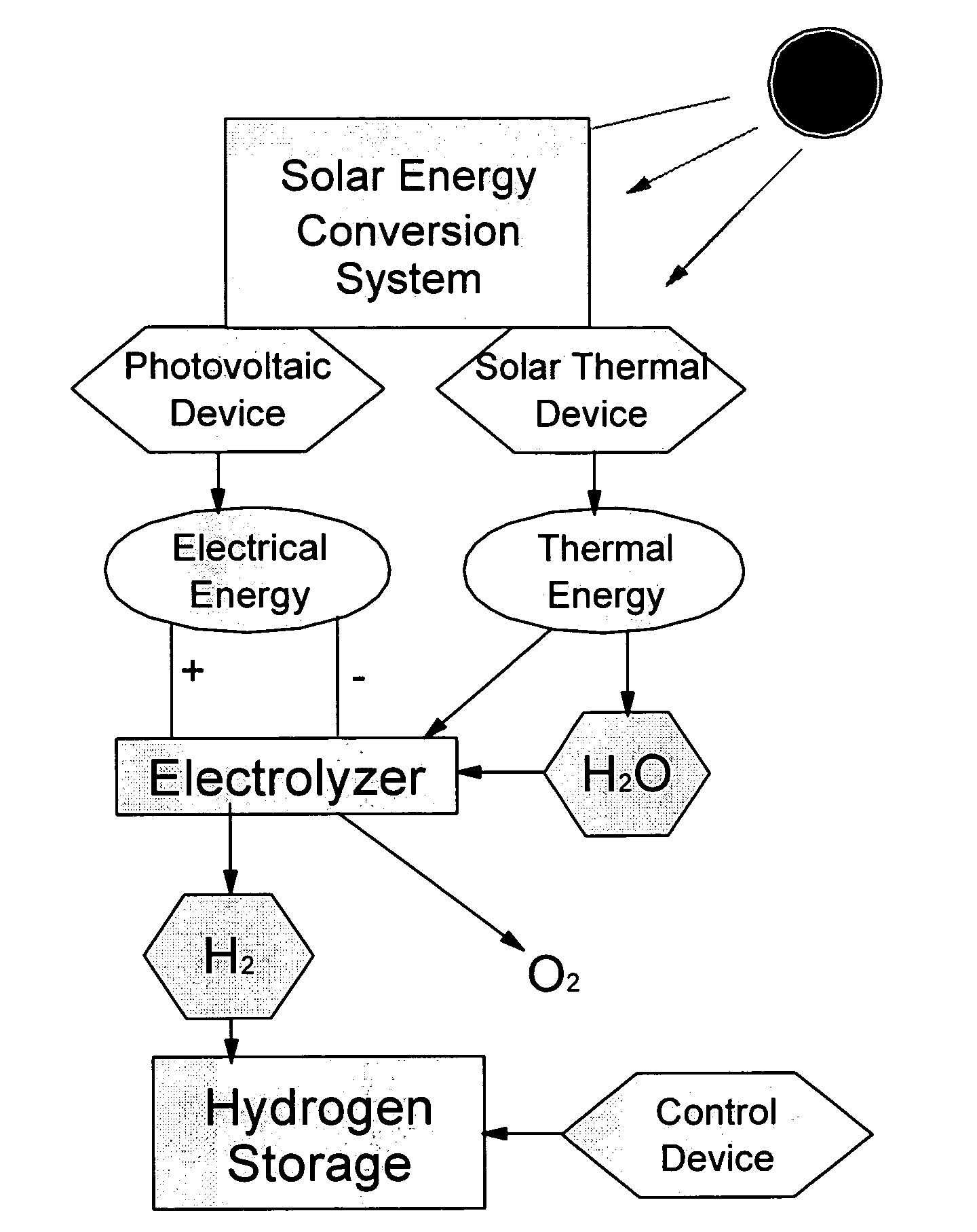
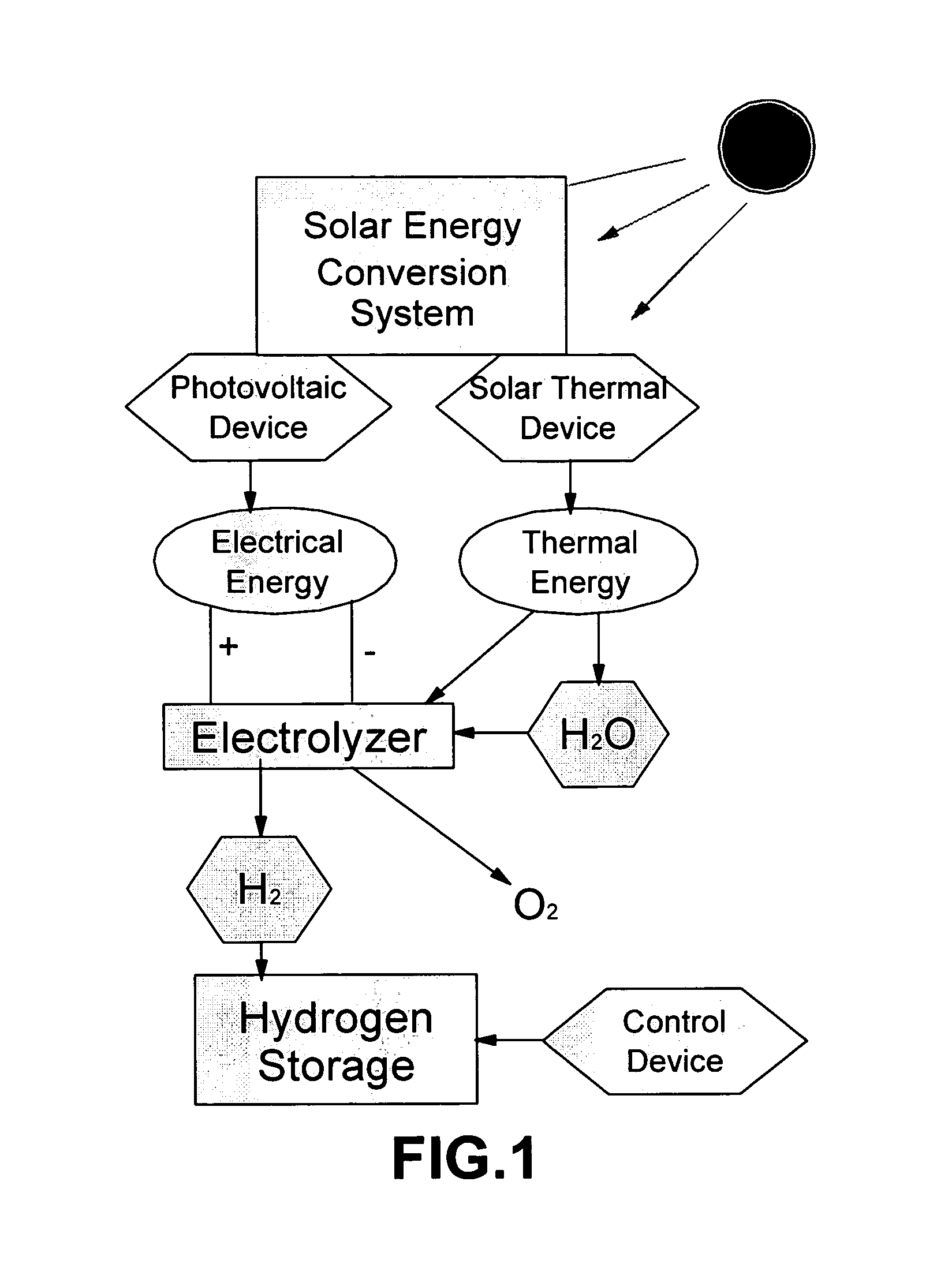
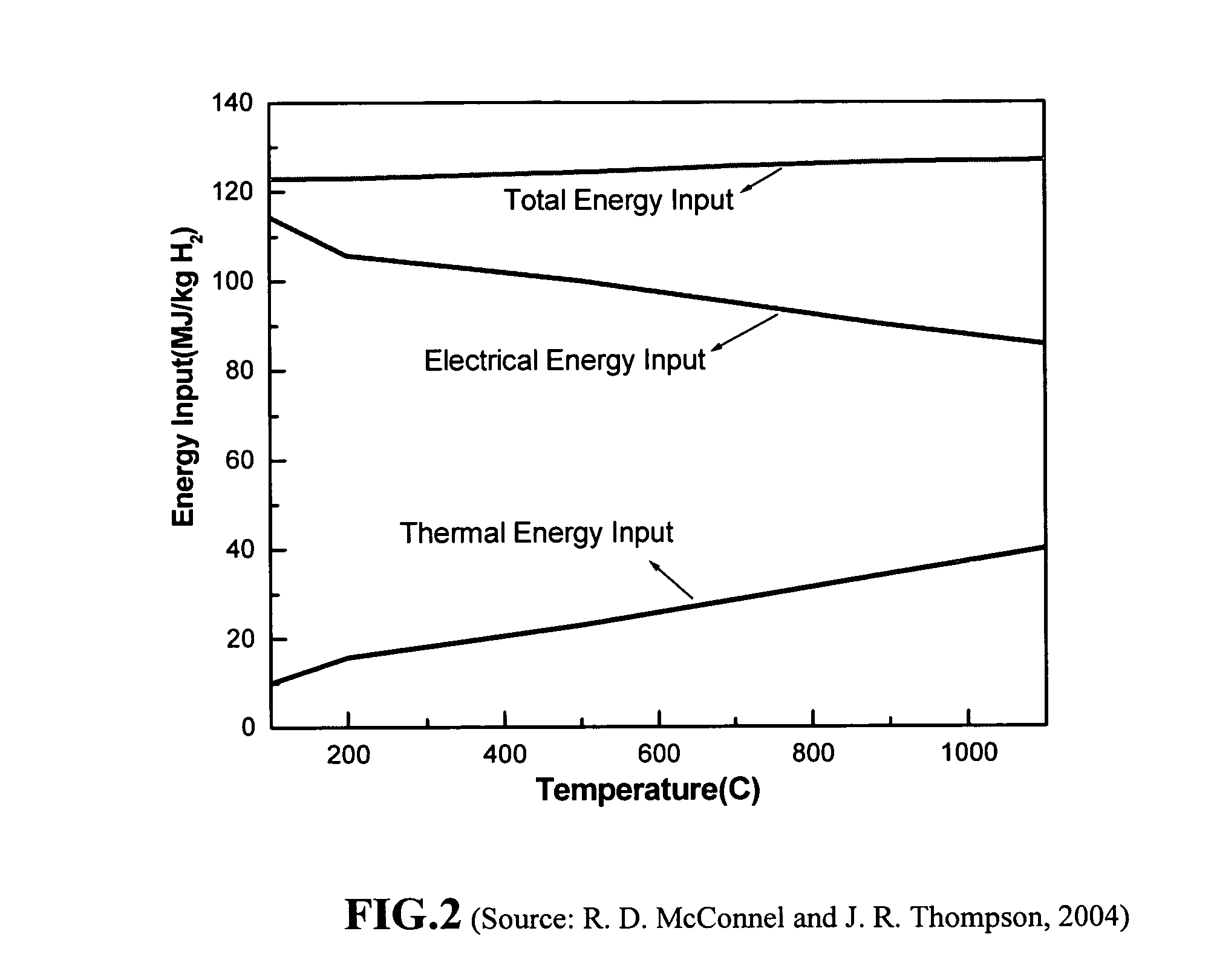
A hydrogen generation and storage method for producing and storing hydrogen at a home or business site that enables refueling hydrogen to a personal vehicle at a convenient location. The method comprises (a) operating a solar energy conversion subsystem to capture and convert solar radiation into both electrical energy and thermal energy; (b) operating a fuel cell electrolyzer that uses the converted electrical energy and thermal energy to split water into hydrogen and oxygen wherein the fuel cell electrolyzer operates at a temperature between 80° C. and 300° C.; and (c) operating a hydrogen storage means to store the generated hydrogen. The hydrogen storage means preferably comprises (1) a hydrogen storage container comprising a metal hydride, chemical hydride, or other solid or liquid phase material as a storage medium to capture and store the generated hydrogen; and (2) control means to regulate the uptake of hydrogen in the storage container.
Owner:NANOTEK INSTR
Urea based composition and system for same
InactiveUS20030219371A1Safe handlingLow degreeGas turbine plantsFused electrolyte fuel cellsNitrogenNitrogen gas
A method and apparatus for generating energy from a composition comprising urea and water are described. The method in one embodiment includes: (a) reacting the urea with water to form ammonia; and (b) oxidizing the ammonia formed in step (a) to form water and nitrogen generating energy. The apparatus in one embodiment contains: (a) a first container for providing the composition; (b) a second container for reacting the urea with water to form ammonia, wherein the second container is connected to the first container by means for delivering the composition from the first container to the second container; (c) a third container for providing ammonia, wherein the third container is connected to the second container by means for delivering ammonia from the third container to the second container; and (d) a fourth container for oxidizing ammonia to form water and nitrogen generating energy, wherein the fourth container is connected to the second container by means for delivering ammonia from the second container to the fourth container. The method and apparatus are used to generate energy for use in stationary and mobile applications.
Owner:AMENDOLA STEVEN C
Method and apparatus for generating power from voltage gradients at sediment-water interfaces
A method and apparatus for generating power from voltage gradients at sediment-water interfaces or within stratified euxinic water-columns is provided. Natural voltage gradients typically exist at and about sediment-water interfaces or in isolated water bodies. One electrode (anode) is positioned in the sediment or water just below the redox boundary and the other electrode (cathode) is positioned in the water above the redox boundary over the first electrode. The anode is lower in voltage than the cathode. Current will flow when the electrodes are connected through a load, and near-perpetual generating of worthwhile power may be sustained by the net oxidation of organic matter catalyzed by microorganisms.
Owner:NAVY SEC OF THE GOVERNMENT OF THE UNITED STATES +1
Fuel cell bipolar separator plate
InactiveUS20050064270A1Increase the number ofImprove conductivityFuel cells groupingFuel cell auxillariesFuel cellsCoolant flow
A bipolar separator plate for a fuel cell stack constructed of at least two coextensive sheet metal elements shaped to promote the distribution of reactant gases to the electrodes of the fuel cell units of the fuel cell stack. The coextensive sheet metal elements are corrugated and fitted together to form at least one coolant flow channel between corresponding peaks, or valleys, of the corrugations of the sheet metal elements.
Owner:GAS TECH INST
Electrochemical cell for gas sensor
InactiveUS7060169B2Improve electrochemical performanceOrganic chemistryWeather/light/corrosion resistanceElectrolysisFuel cells
An electrochemical cell for applications such as electrochemical fuel cells, or electrochemical cell gas sensors used for detection of target gas species in environments containing or susceptible to presence of same. The electrochemical cell utilizes an ionic liquid as an electrolyte medium, thereby achieving a broader range of operational temperatures and conditions, relative to electrochemical cells utilizing propylene carbonate or other conventional electrolytic media.
Owner:HONEYWELL INT INC
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com