Method for preparing basic magnesium chloride and magnesium oxide by pyrolyzing bischofite
A technology of using hydrochlorite to heat and magnesium chloride, which is applied in the preparation of chloride, magnesium oxide, magnesium chloride, etc., can solve the problems of poor filtering performance of magnesium hydroxide, generating a large amount of carbon dioxide, and being difficult to reach 99%, and achieving low cost. , the effect of slowing corrosion and high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
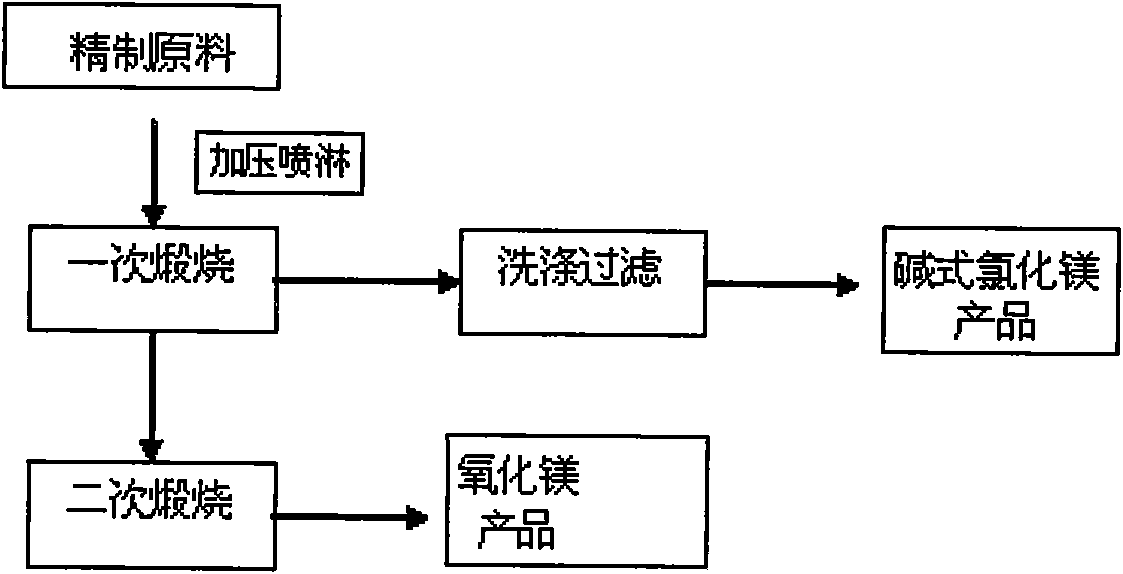
[0037] Spray the refined bischofite hot solution into the reaction kettle under pressure to obtain solid hydrate. The temperature of the first calcination is 250°C, and the calcination time is 3 hours. After the calcination, the product is washed with methanol, filtered and dried MgOHCl with a purity of 96% is obtained, and the decomposition rate of bischofite is 96%. The tail gas produced is absorbed and concentrated to obtain hydrochloric acid with a concentration greater than 28%, and the methanol is recycled; at the same time, the unwashed product is sent to the secondary calciner , heat up to 450°C, and calcined for 3 hours to obtain magnesium oxide with a purity of 99.5%. The HCl produced by the second calcining is condensed and recovered to obtain hydrochloric acid with a concentration greater than 28%. The remaining gas meets the emission standard and is discharged.
Embodiment 2
[0039] The same operating conditions as in Example 1, the first calcining temperature is 280°C, the calcining time is 2 hours, the bischofite decomposition rate is 96.5%, the MgOHCl purity after washing with methanol is 97%, and the hydrochloric acid concentration is greater than 28%; the second The secondary calcination temperature is 480° C., and the calcination time is 2 hours to obtain magnesium oxide with a purity of 99.2%, and a concentration of hydrochloric acid greater than 28%.
Embodiment 3
[0041] The same operating conditions as in Example 1, the first calcining temperature is 300°C, the calcining time is 1.5 hours, the bischofite decomposition rate is 96.2%, the MgOHCl purity after washing with methanol is 96.5%, and the hydrochloric acid concentration is greater than 28%; the second The secondary calcination temperature is 500° C., and the calcination time is 2 hours to obtain magnesium oxide with a purity of 99.6%, and a concentration of hydrochloric acid greater than 28%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com