Preparation method of reagent for quantitatively determining Helicobacter pylori antigen in excrement
A technology for Helicobacter pylori and anti-Helicobacter pylori is applied in the field of medical testing, which can solve the problems of application limitation, cannot truly reflect the Helicobacter pylori in the stomach, etc., and achieves the effects of no potential safety hazard, not easy to interfere, and high detection efficiency.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] This implementation provides a method for preparing a reagent for the quantitative determination of Helicobacter pylori antigen in feces, the reagent includes R1 reagent and R2 reagent, wherein, in terms of mass percentage, the R1 reagent contains 0.1-10% electrolyte, 0.1-10% stabilizer, 0.1-10% surfactant, 0.1-5% preservative, and the balance is 1-100mmol / L MES buffer.
[0036] 1. Preparation of R2 reagent
[0037] The R2 reagent includes latex particles coated with anti-Helicobacter pylori antigen (urease) polyclonal antibody, electrolyte, stabilizer, surfactant, preservative and buffer, and the R2 reagent is prepared by the following process:
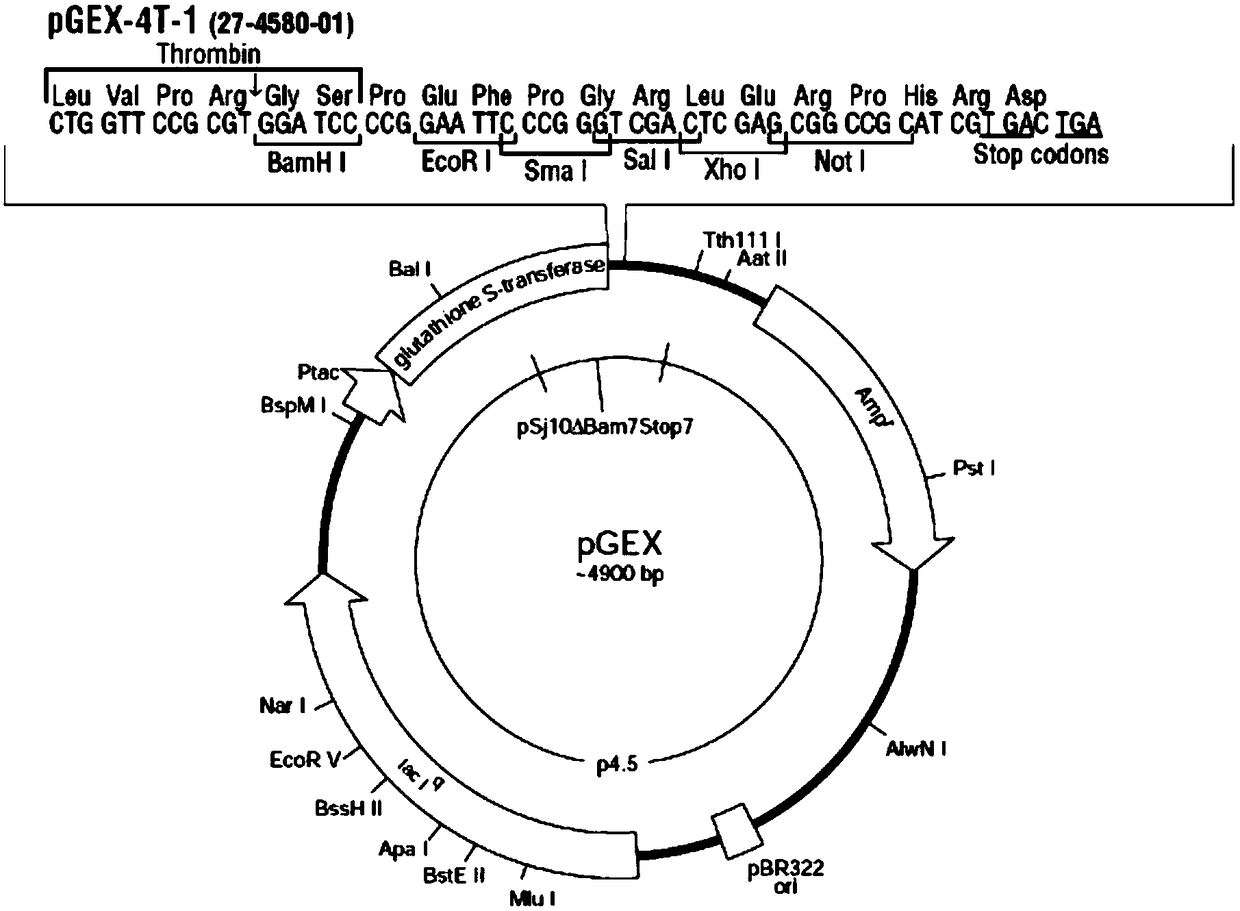
[0038] S1. Select any protein subunit of urease as the Helicobacter pylori antigen. Urease is composed of two protein subunits, namely UreA and UreB. Cloning the UreA or UreB gene into the pGEX-4T-1 plasmid, The cloning sites are BamHI and EcoRI in the pGEX-4T-1 plasmid (such as figure 1shown), the pGEX-4T-1 plasmid contains...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com