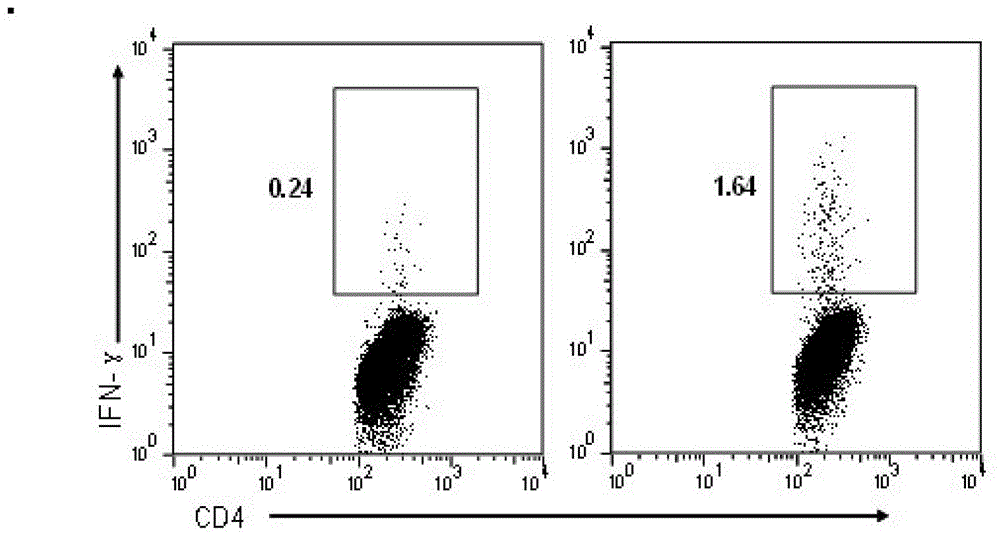
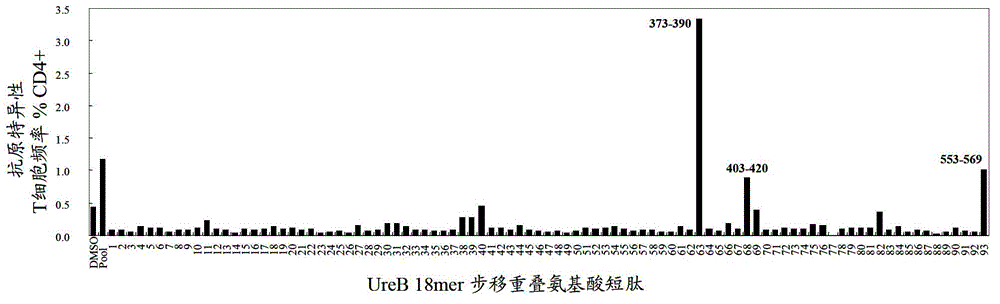
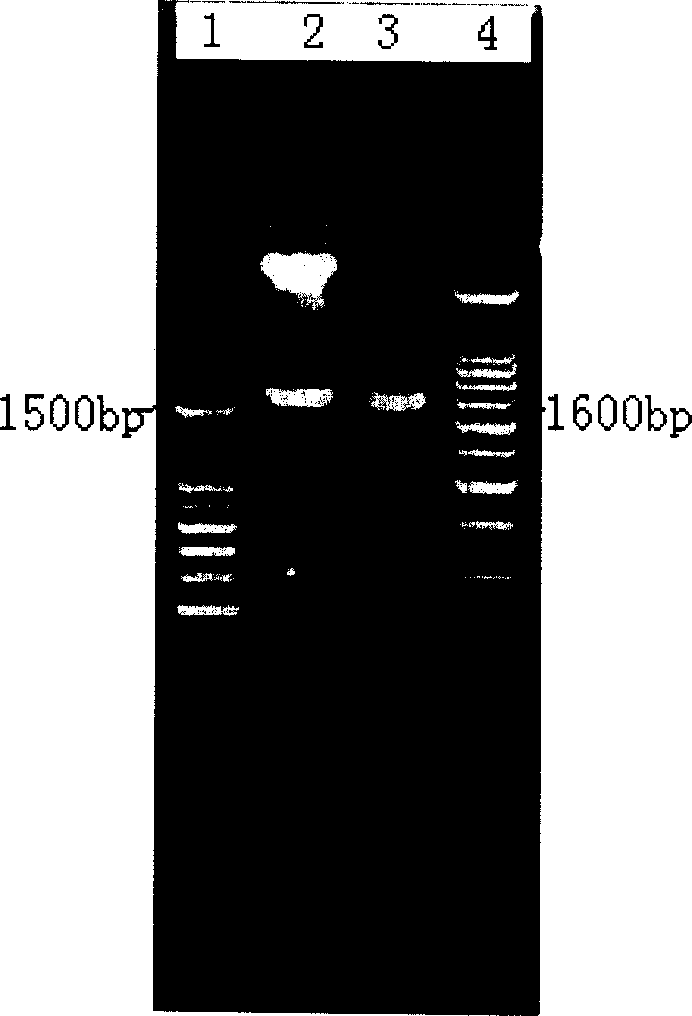
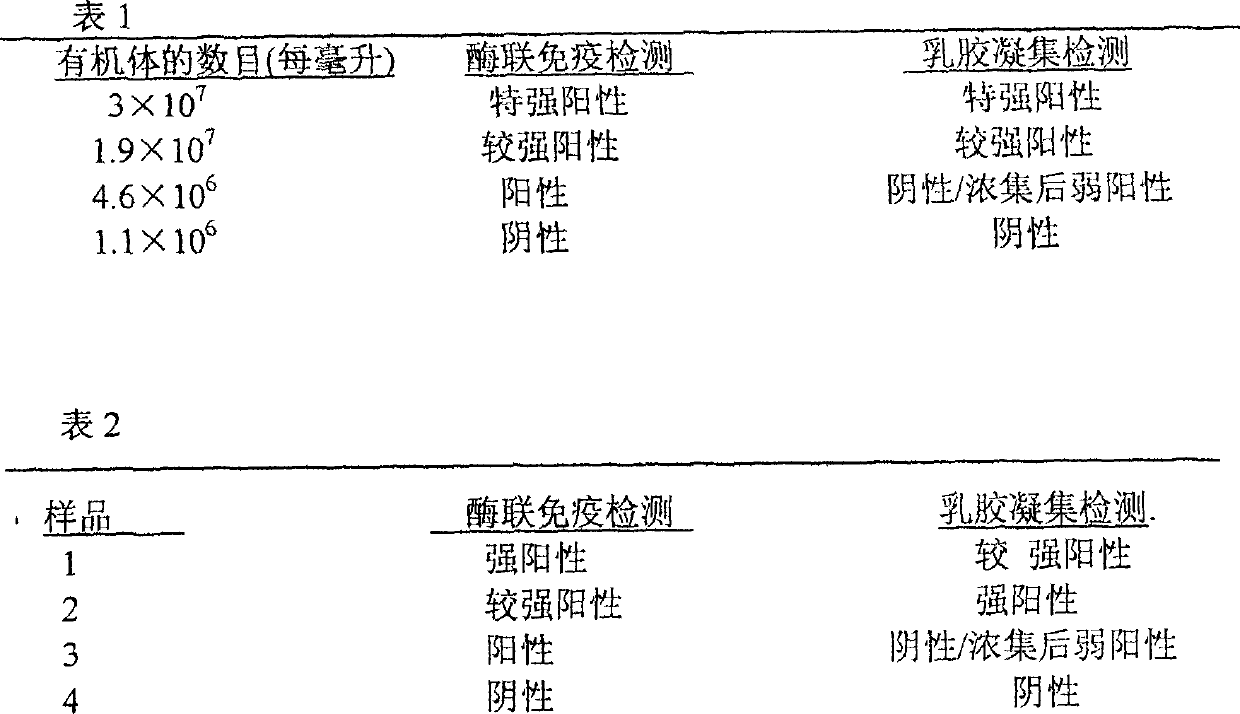
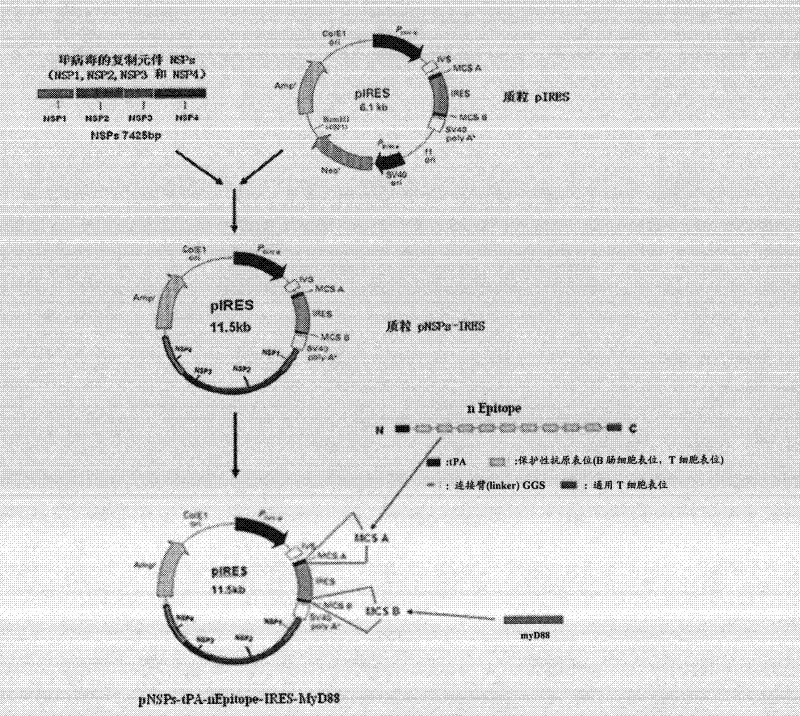
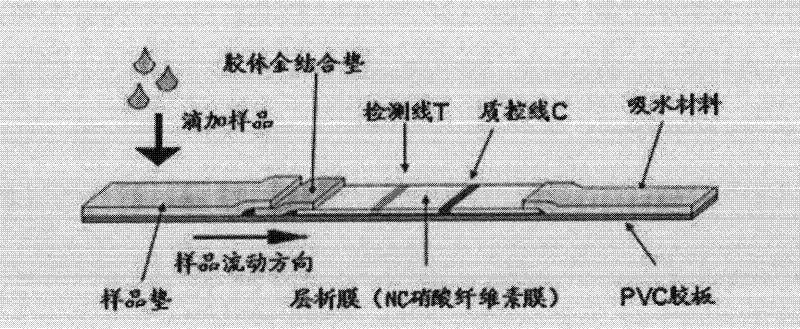
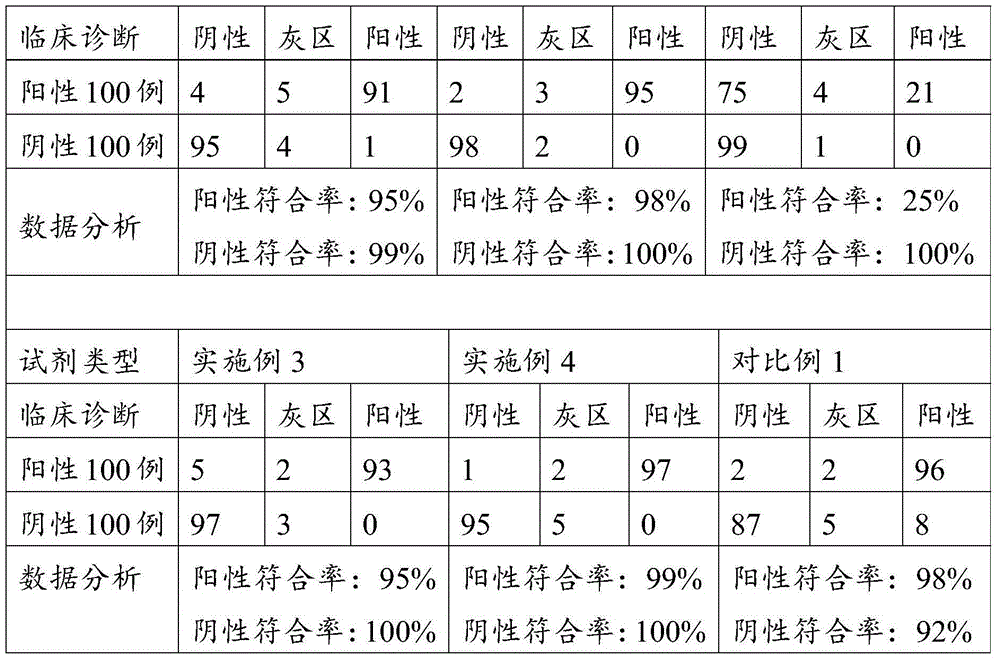
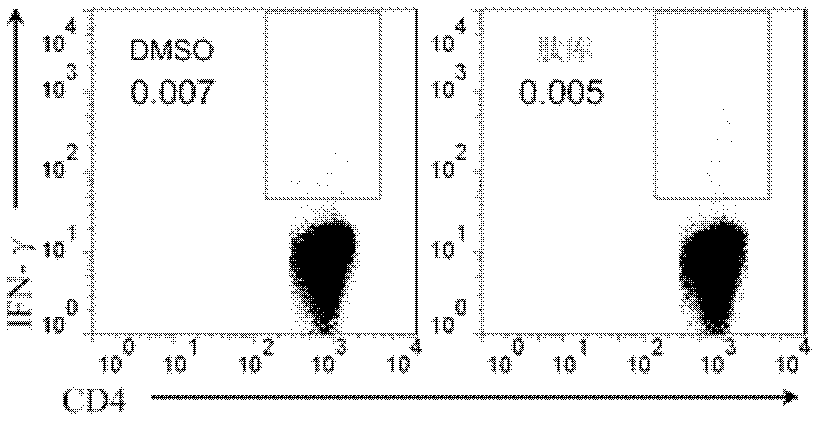
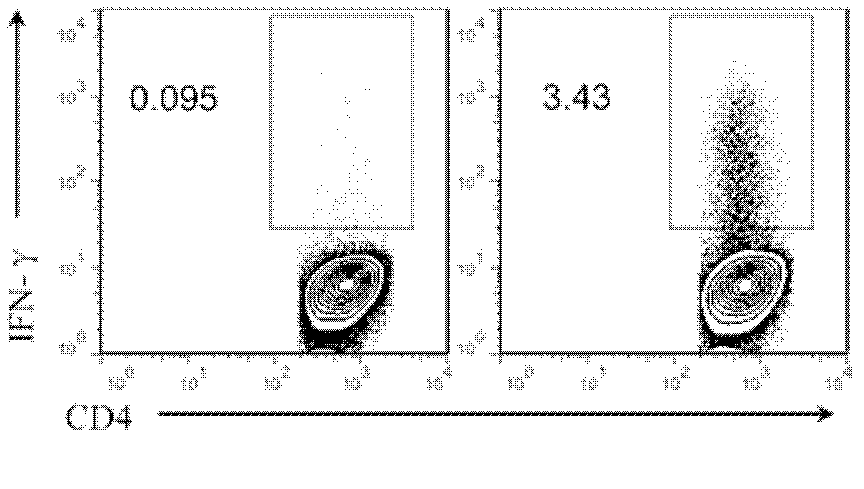
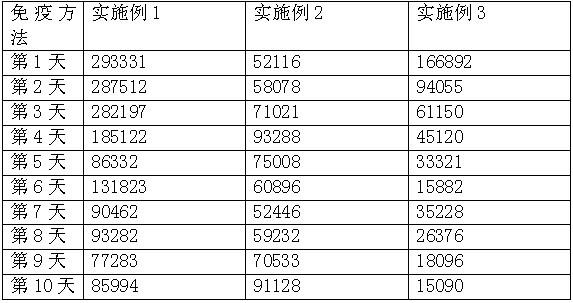
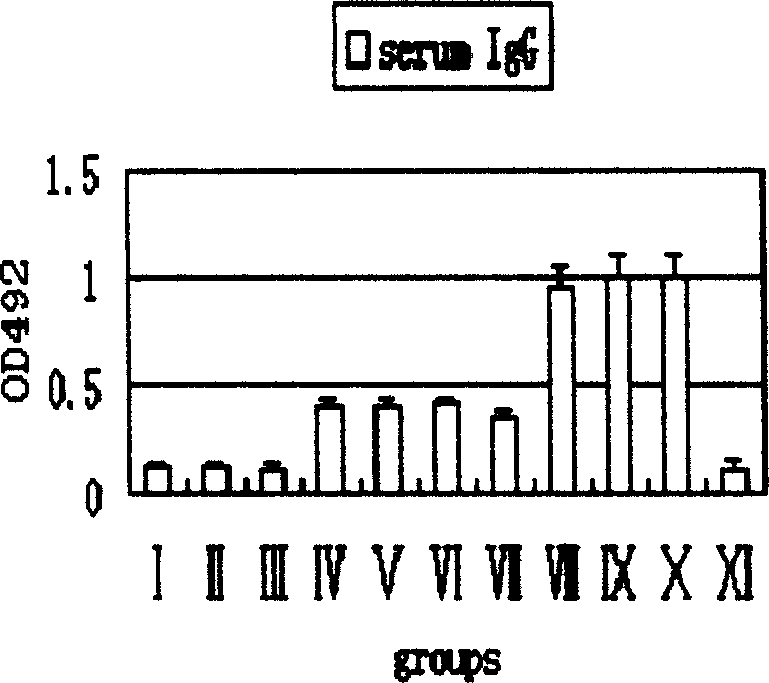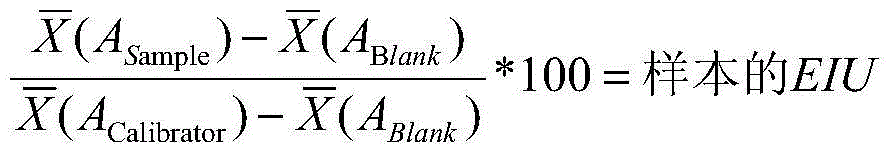Patents
Literature
65 results about "Helicobacter pylori Antigen" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A Helicobacter pylori antigen test is used to determine if a patient is infected with Helicobacter pylori (H. pylori). H. pylori is a bacteria that grows in the digestive tract and attacks the stomach walls causing ulcers in the stomach and in the intestines.
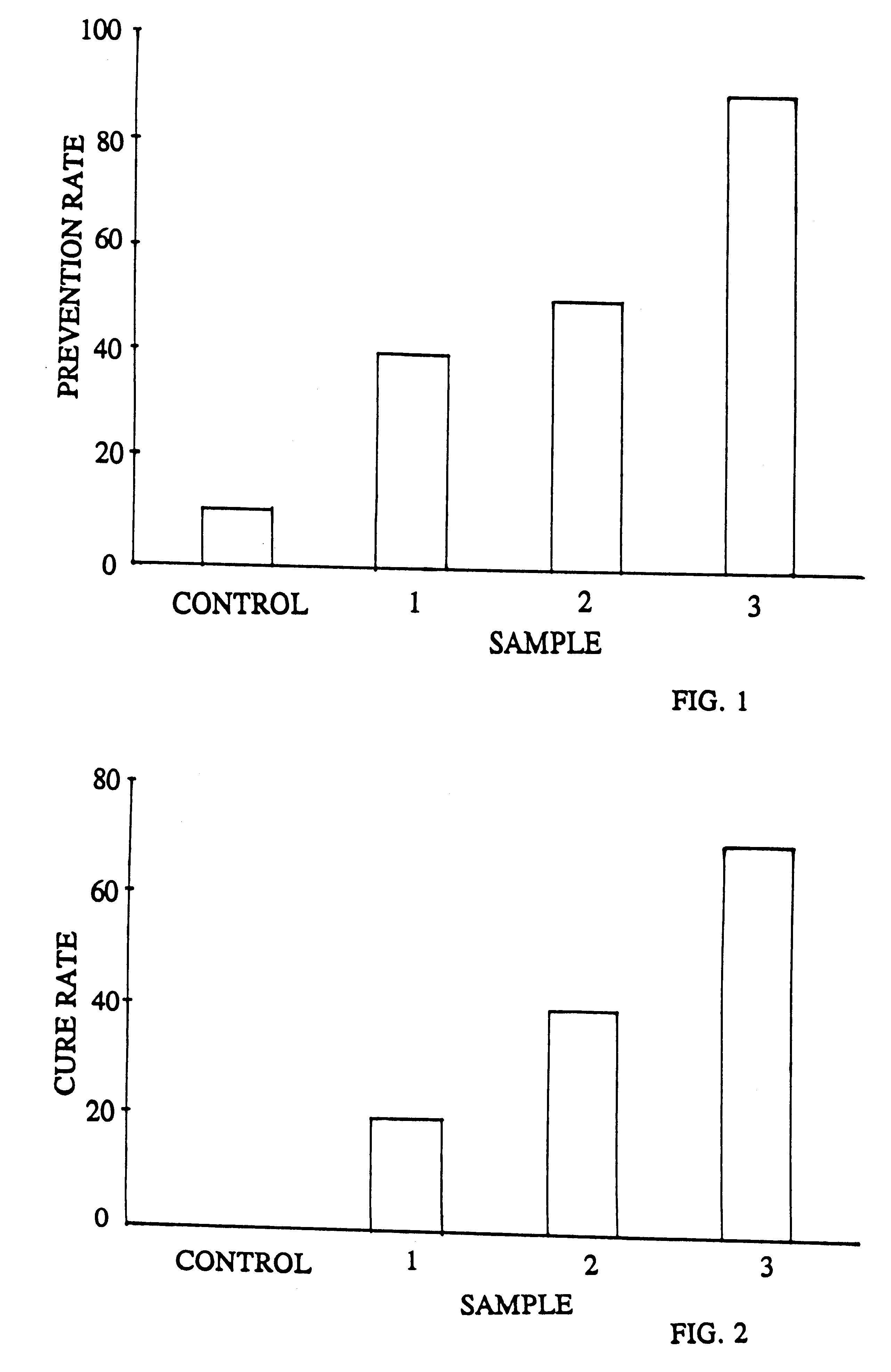
Food for inhibiting infection and treating gastritis, gastric and duodenal ulcers
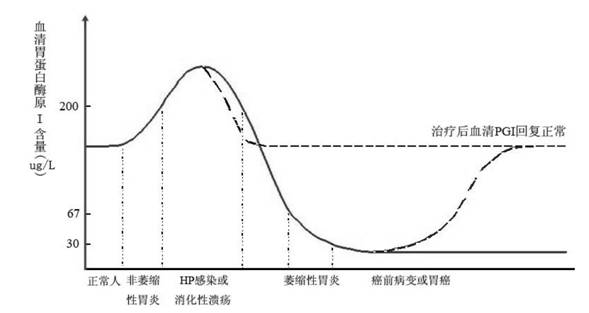
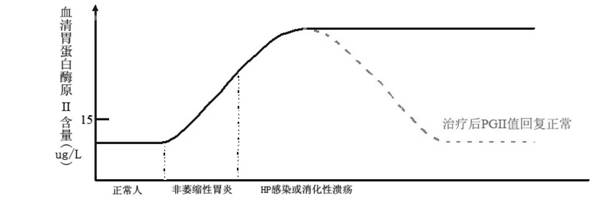
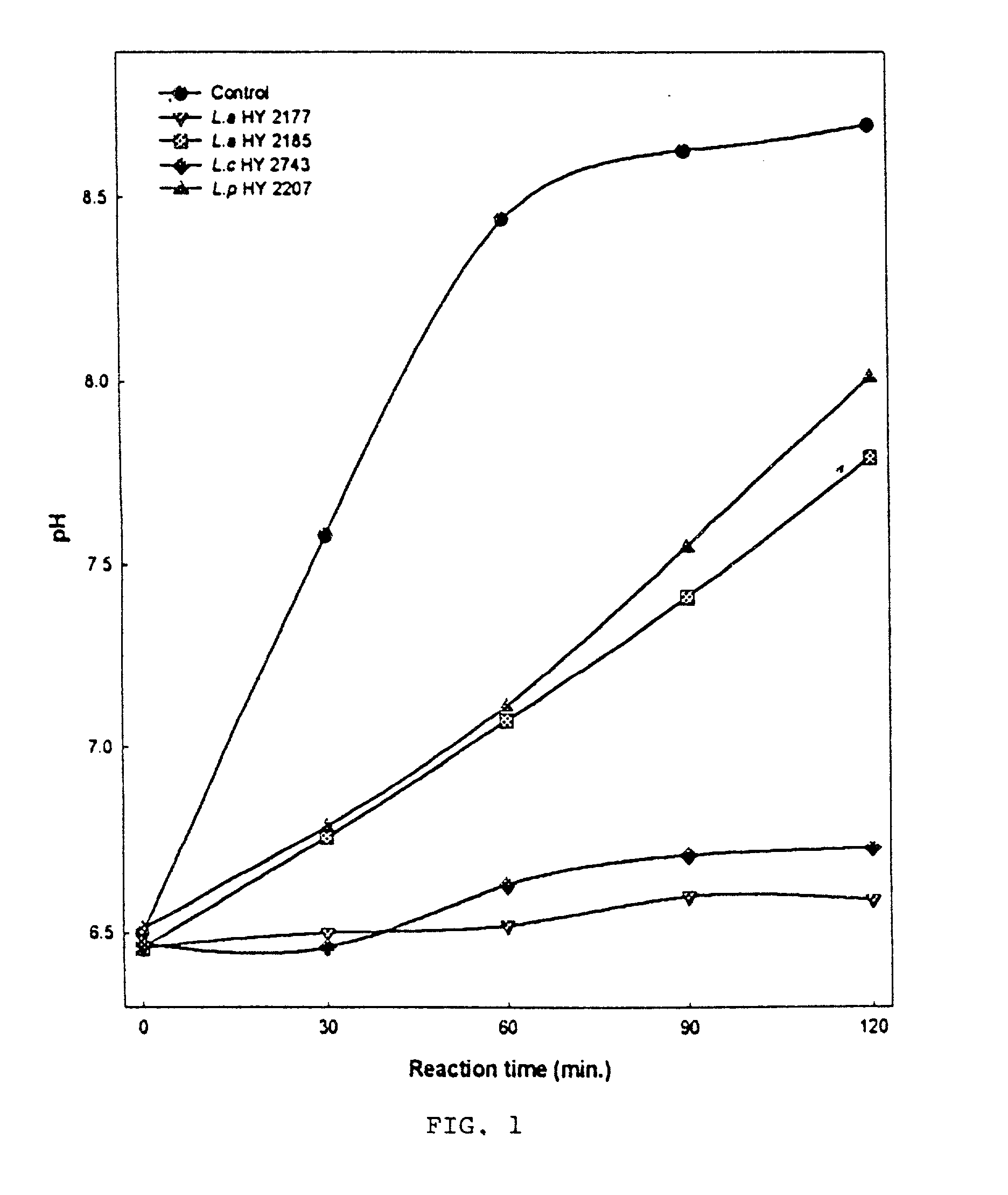
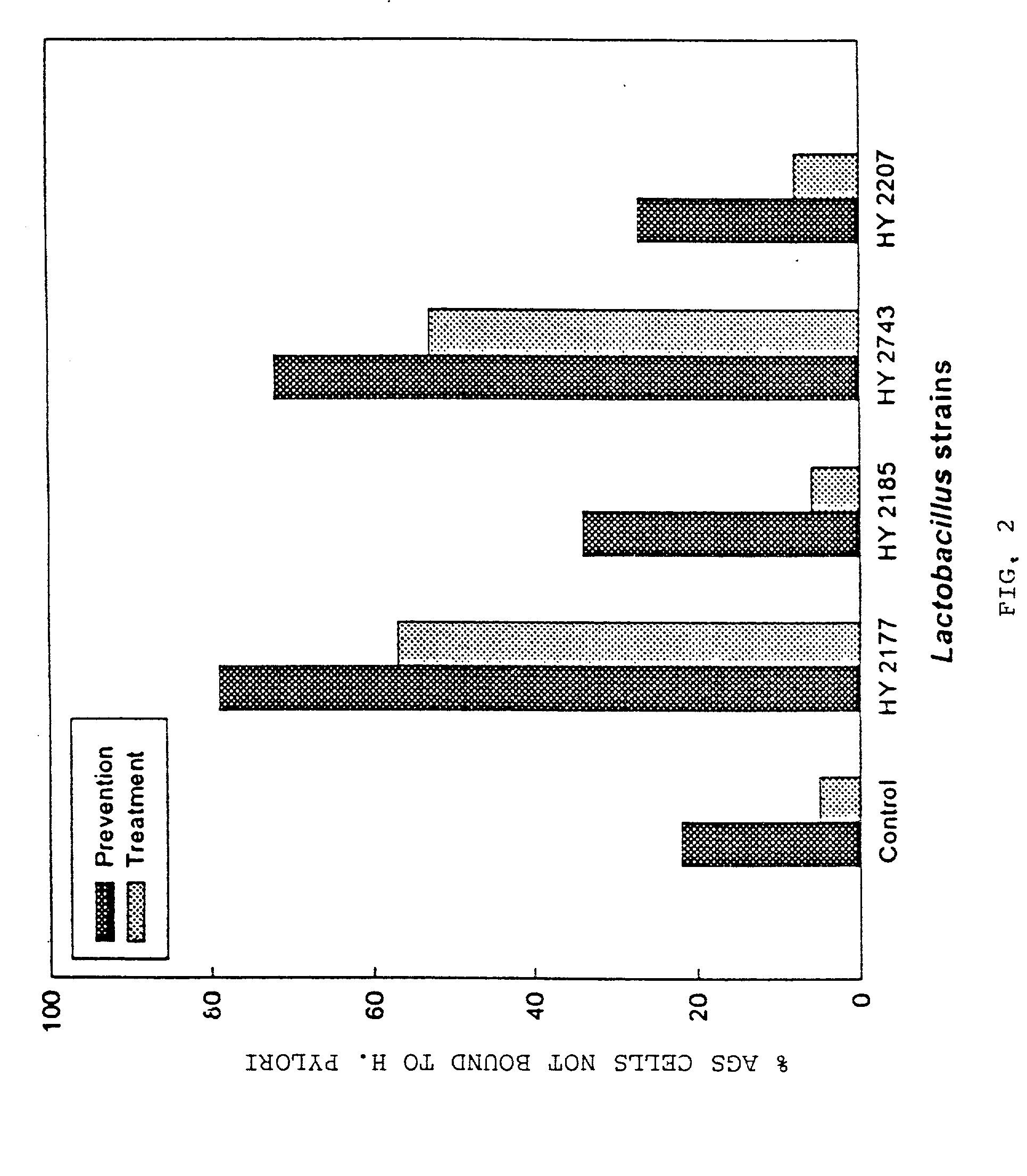
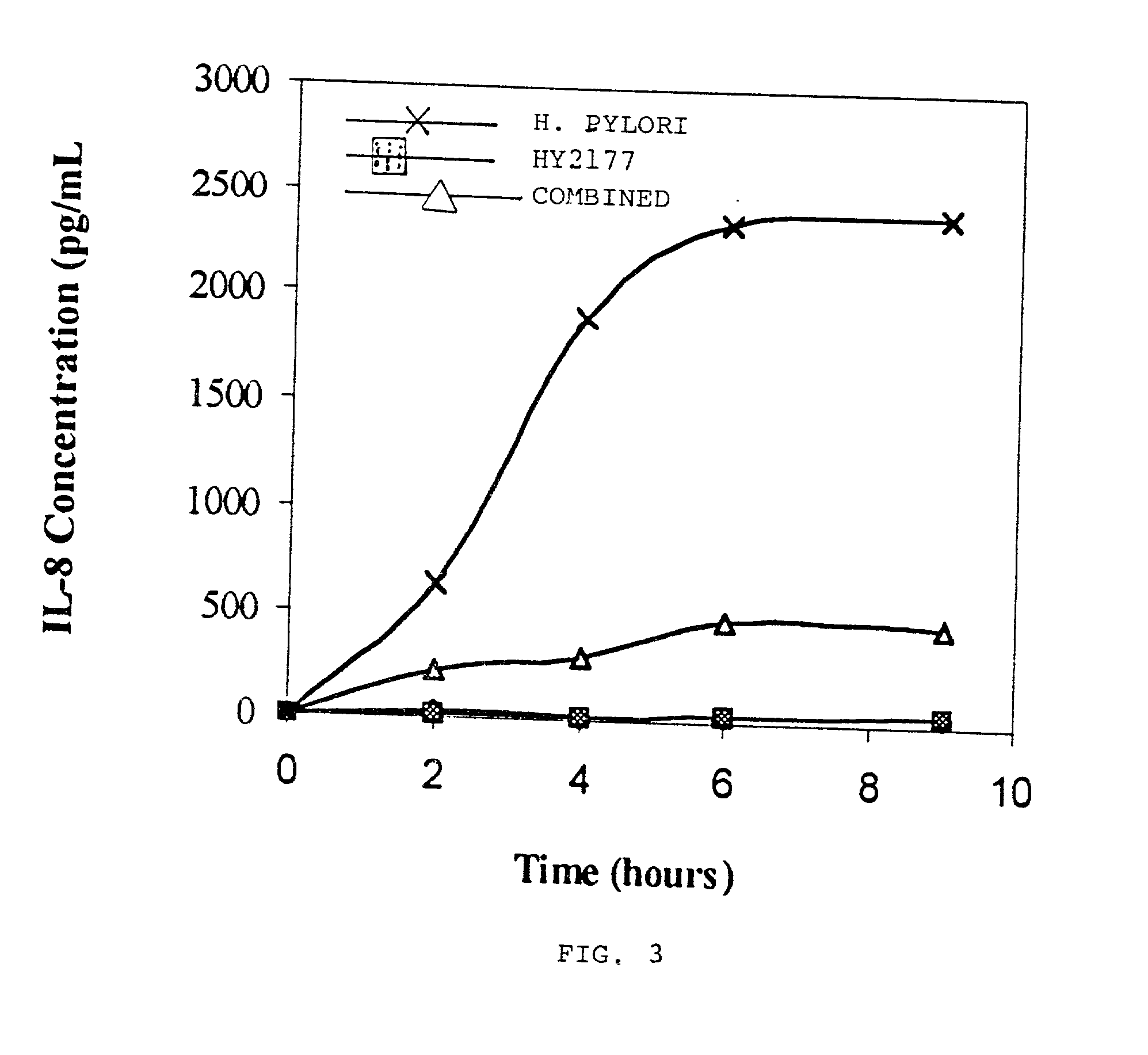
Live strains of Lactococcus sp. HY 49, Lactobacillus casei HY 2782, and Bifidobacterium longum HY 8001 maintained in nutritious foods, such as yogurt, imbue them with prophylactic and / or therapeutic properties. Such foods are beneficial in the prevention and / or treatment of gastritis, duodenal and gastric ulcers caused by infection from Helicobacter pylori (also referred to as H. pylori). The properties of these bacteria are boosted by the addition of egg yolk containing antibodies specific to H. pylori antigen derived from "fractionated H. pylori".
Owner:KOREA YAKULT
Helicobacter pylori diagnostics
InactiveUS6902903B1Simple and extremely accurate and efficient methodMonitoring usingSugar derivativesBacteriaImmunodiagnosticsAssay
Novel methods, membrane supports and immunodiagnostic test kits for diagnosing Helicobacter pylori infection, are disclosed. The methods can also be used to monitor the progress of treatment of an infection. The methods, supports and kits employ both type-common and type-specific H. pylori antigens and can conveniently be performed in a single-step assay format. The methods provide for highly accurate results and discriminate between H. pylori Type I and H. pylori Type II infection so that an accurate diagnosis can be accomplished.
Owner:CHIRON CORP
Latex-enhanced immunoturbidimetry kit for measuring Helicobacter pylori antibody as well as preparation method and application thereof
ActiveCN102662059AHigh sensitivityWide linear rangeMaterial analysisHelicobacter pylori AntibodyBiology
The invention discloses a latex-enhanced immunoturbidimetry kit for measuring a Helicobacter pylori antibody as well as a preparation method and application thereof. The kit comprises a diluent, latex reagent and blank liquid of Helicobacter pylori antigen, a standard product and a quality control product, wherein the Helicobacter pylori antigen can be one or more of full-tropina antigen of Helicobacter pylori, urease antigen of Helicobacter pylori, cytotoxin-related protein A antigen of Helicobacter pylori or cell vacuole toxin A antigen of Helicobacter pylori; the latex reagent is a mixture of two latex granules which have different particle sizes and are adsorbed by Helicobacter pylori; and the Helicobacter pylori antibody for preparing the standard product and the quality control product is derived from IgM (immunoglobulin M) and / or IgG (immunoglobulin G) in serum of a patient infected by Helicobacter pylori. The kit is suitable for various full-automatic biochemical analyzers and semiautomatic biochemical analyzers, and has the advantages of rapid detection, high sensitivity, strong specifically, good accuracy and the like.
Owner:BEIJING MOKOBIO LIFE SCI CO LTD
Food containing active strains for inhibiting infection and treating gastritis, gastric and duodenal ulcers
Live strains of Lactobacillus acidophilus HY2177 and Lactobacillus casei HY2743 maintained in nutritious foods, such as yogurt, imbue them with prophylactic and / or therapeutic properties. Such foods are beneficial in the prevention and / or treatment of gastritis, duodenal and gastric ulcers caused by infection from Helicobacter pylori (also referred to as H. pylori). The properties of these bacteria are boosted by the addition of egg yolk containing antibodies specific to H. pylori antigen derived from "fractionated H. pylori" and may be administered as active strains alone in a food supplement, or the active strains may be combined with H. pylori-antibodies (IgY).
Owner:KOREA YAKULT
Immunization against and treatment for infection by H. pylori
InactiveUS6841155B1Good effectAntibacterial agentsBacterial antigen ingredientsHelicobacter pyloriHp - Helicobacter pylori
Owner:NOVARTIS AG
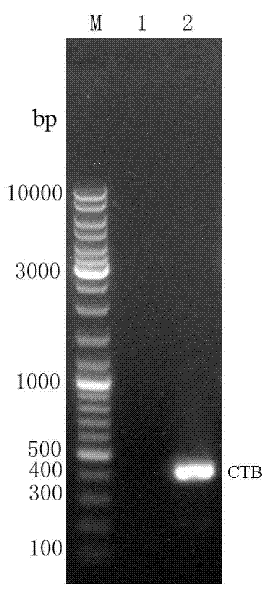
Recombination antigen composition, vaccine and carrier and method for preparing antigen composition
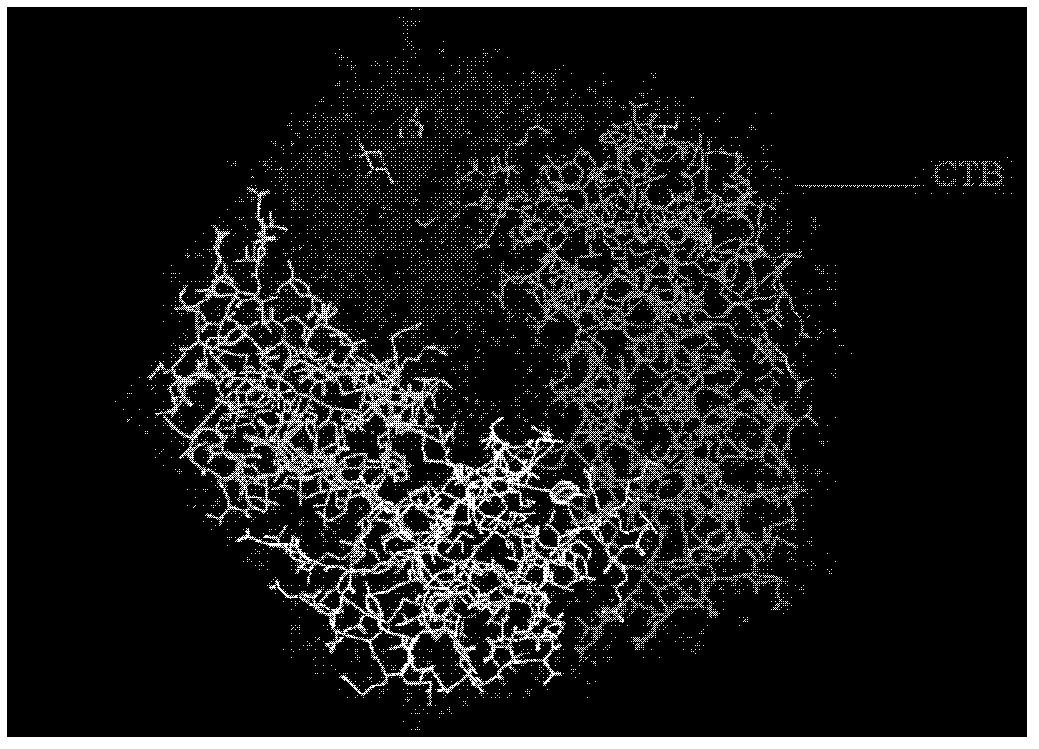
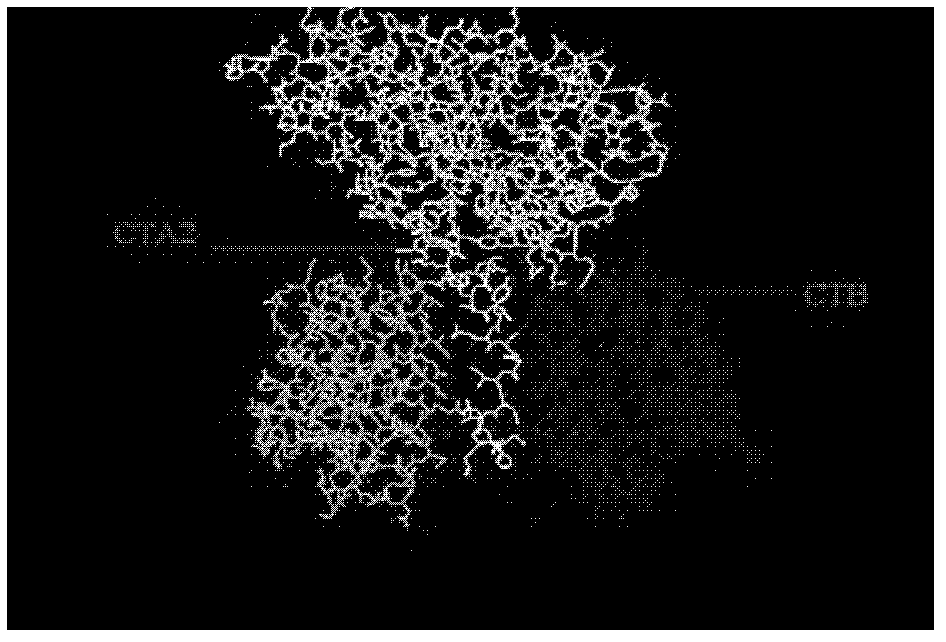
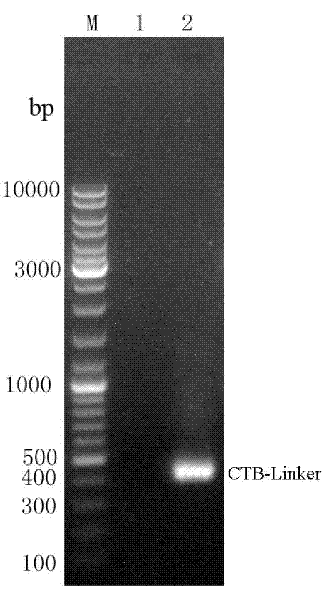
The invention provides a recombination antigen composition, a vaccine and a carrier and a method for preparing the antigen composition. A recombination helicobacter pylori antigen which comprises a helicobacter pylori antigen, the fusion proteins of a cholera toxin CT-A2 subunit and cholera toxin CT-B proteins is optimized. The compatibility of the CT-A2 and the CT-B proteins is used, a chimeric structure of CT-A2-5CT-B is formed, and accordingly an antigen with higher titer is formed through the helicobacter pylori antigen and the fusion proteins of the cholera toxin CT-A2 subunit. Meanwhile, the effect that the CT-B enhances immunization is used, and the effect that the antigen immunogenicity of the recombination antigen composition is enhanced is achieved. In addition, the chimeric protein constituted by the recombination antigen stimulates a mucous membrane to generate secreting type IgA immunization.
Owner:SHANGHAI UNITED CELL BIOTECH
Pepsinogen, helicobacter pylori antibody and gastrin 17 detection method and kit thereof
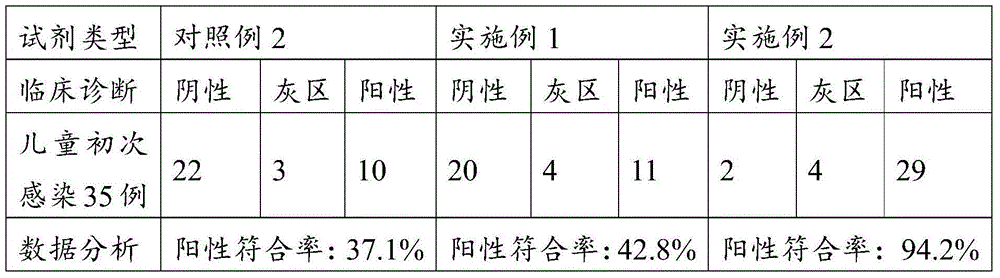
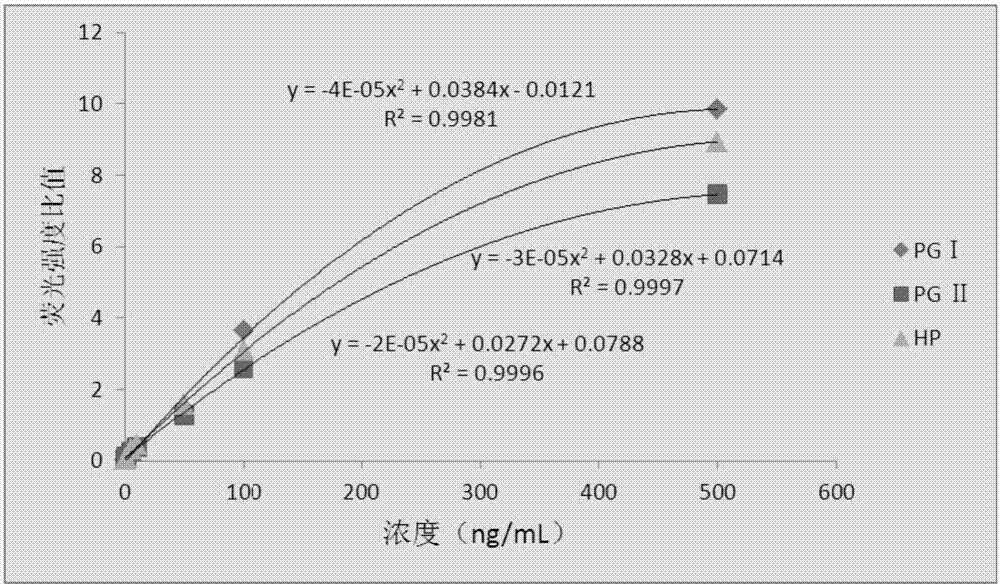
InactiveCN107271669AQualitative and quantitative detectionFast and highly sensitive assayDisease diagnosisBiological testingPepsinogen IHelicobacter pylori Antibody
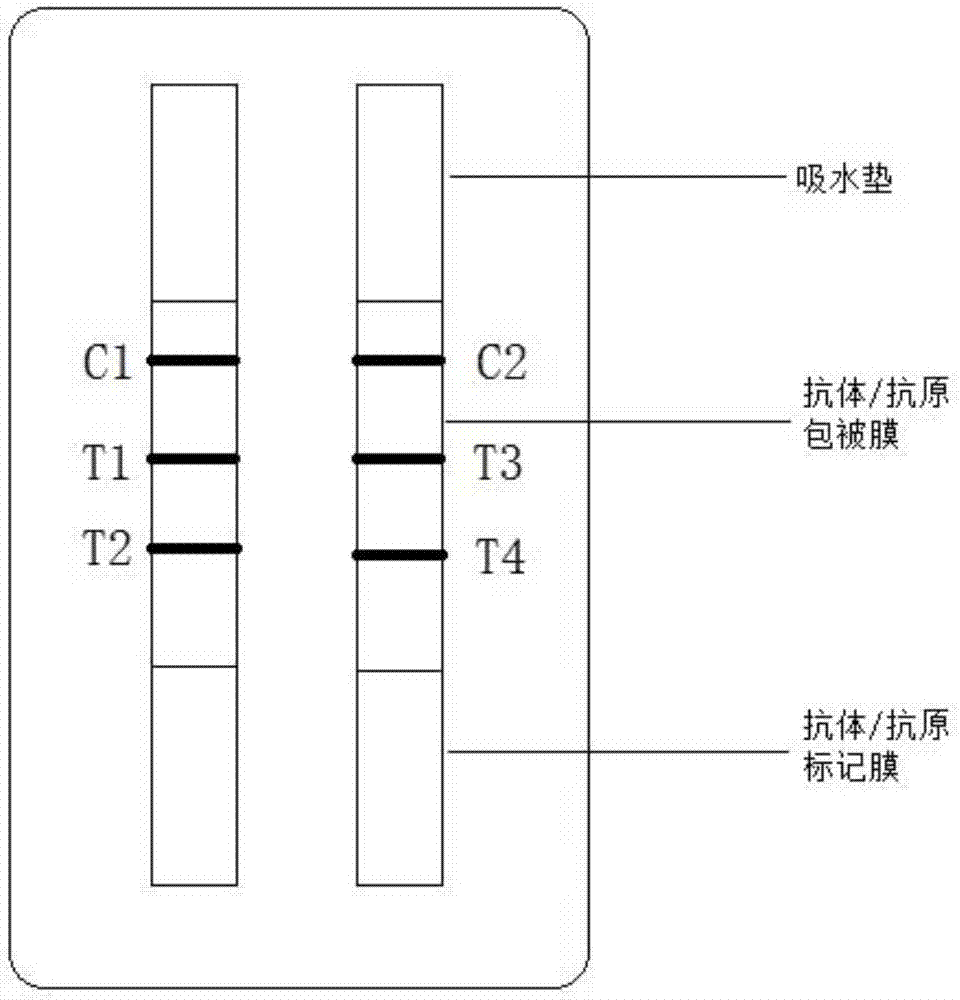
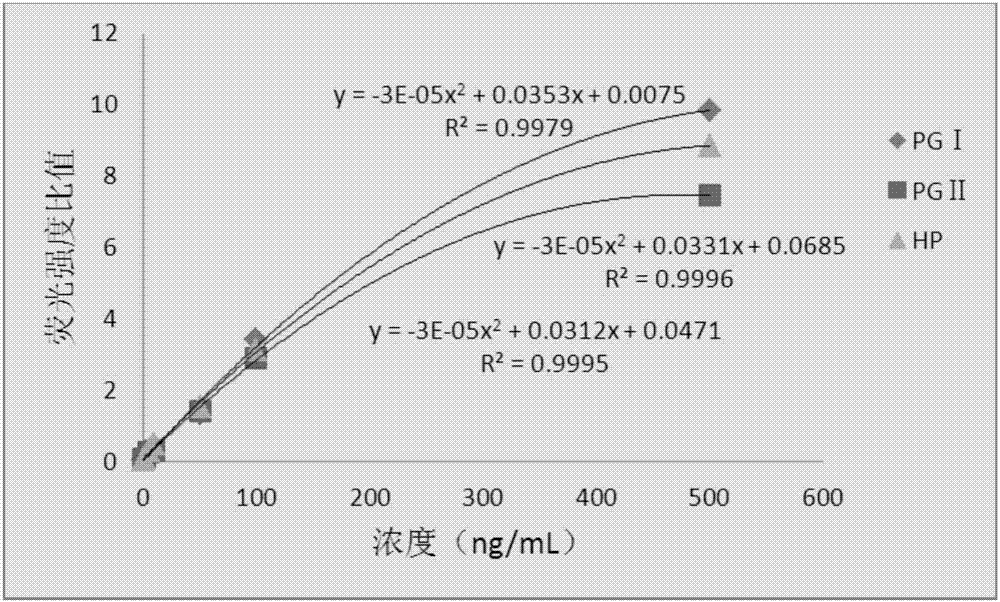
The invention provides a kit, application of the kit in detection of pepsinogen I, pepsinogen II, a helicobacter pylori antibody and gastrin 17 and a method for detecting the pepsinogen I, the pepsinogen II, the helicobacter pylori antibody and the gastrin 17 by using the kit. The kit comprises a first coating film and a second coating film, wherein at least one region in the first coating film is coated with a fluorescent microsphere-labeled pepsinogen I antibody, a fluorescent microsphere-labeled pepsinogen II antibody, a first fluorescent microsphere-labeled helicobacter pylori antigen and a fluorescent microsphere-labeled gastrin 17 antibody; the second coating film comprises a first region, a second region, a third region, a fourth region and a fifth region which are separated; a formed fluorescent microsphere material comprises a polystyrene-methyl methacrylate copolymer. The kit and the method which are provided by the invention have the advantages of high sensitivity, high specificity, quickness, simplicity, capability of realizing objective measurement and the like.
Owner:SHENZHEN GRADUATE SCHOOL TSINGHUA UNIV
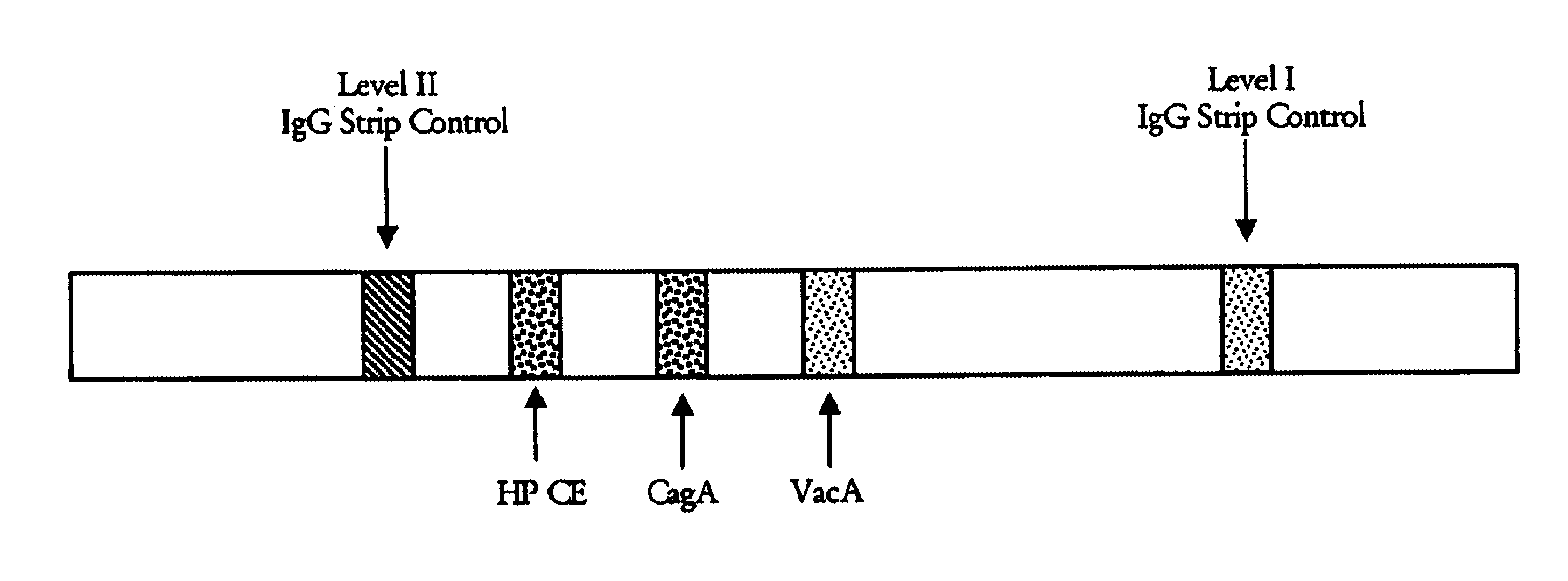
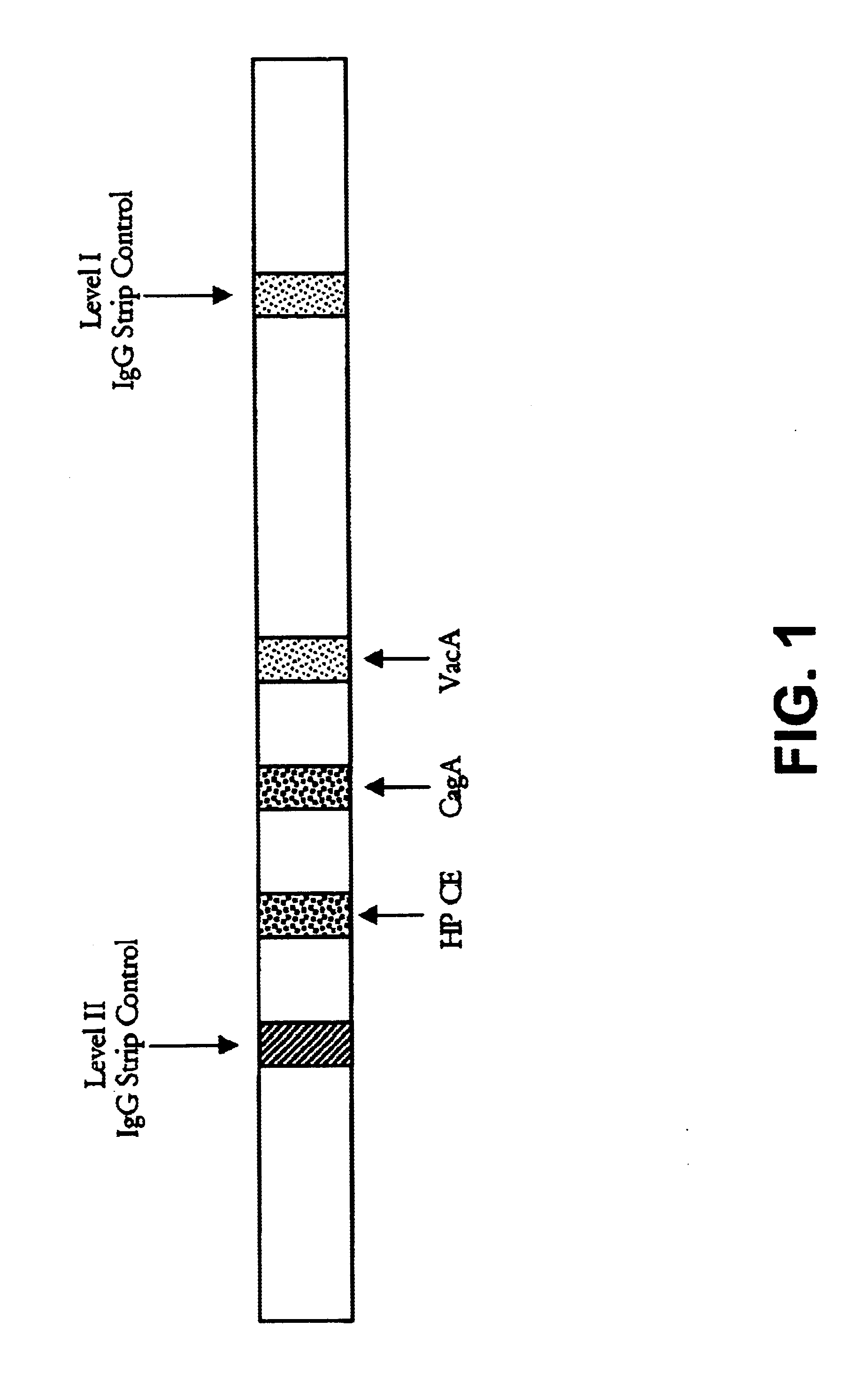
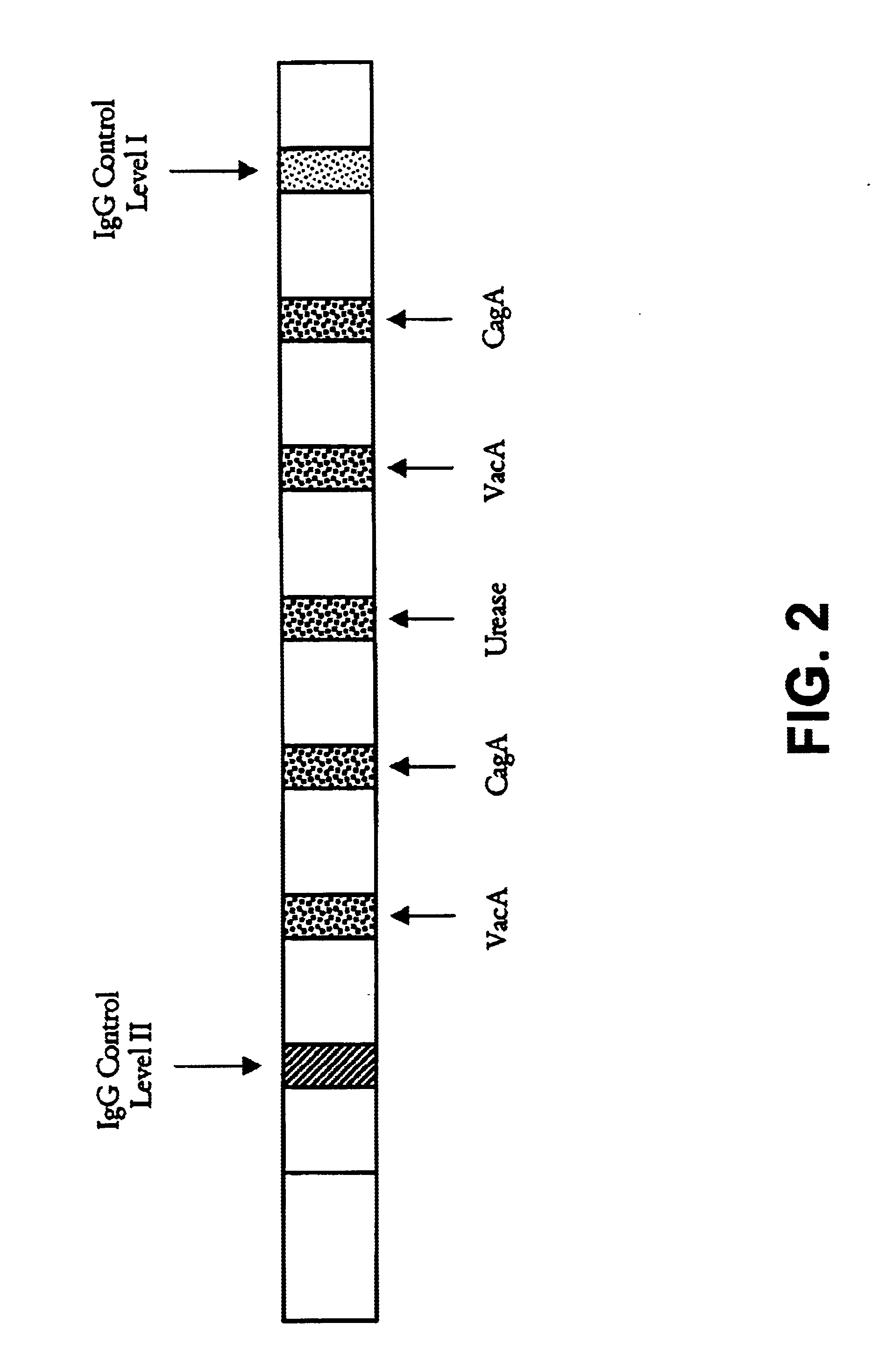
Immuo-colloidal gold test paper for detecting pyloric helicobacter antigen and its prepn process
The present invention relates to one kind of immuno-colloidal gold test paper for detecting pyloric helicobacter antigen and its preparation process. The test paper has coated urease monoclonal antibody, CagA or VacA monoclonal antibody and anti-mouse polyclonal antibody. It is used in detecting pyloric helicobacter related antigen existing in saliva, gastric juice, vomited matter, dental plaque and excrement of mammal so as to monitor the infection status of pyloric helicobacter, assist the diagnosis of gastritis, peptic ulcer and other diseases and predict gastric cancer probability. The present invention simple operation, high specificity and high sensitivity.
Owner:LANZHOU UNIVERSITY
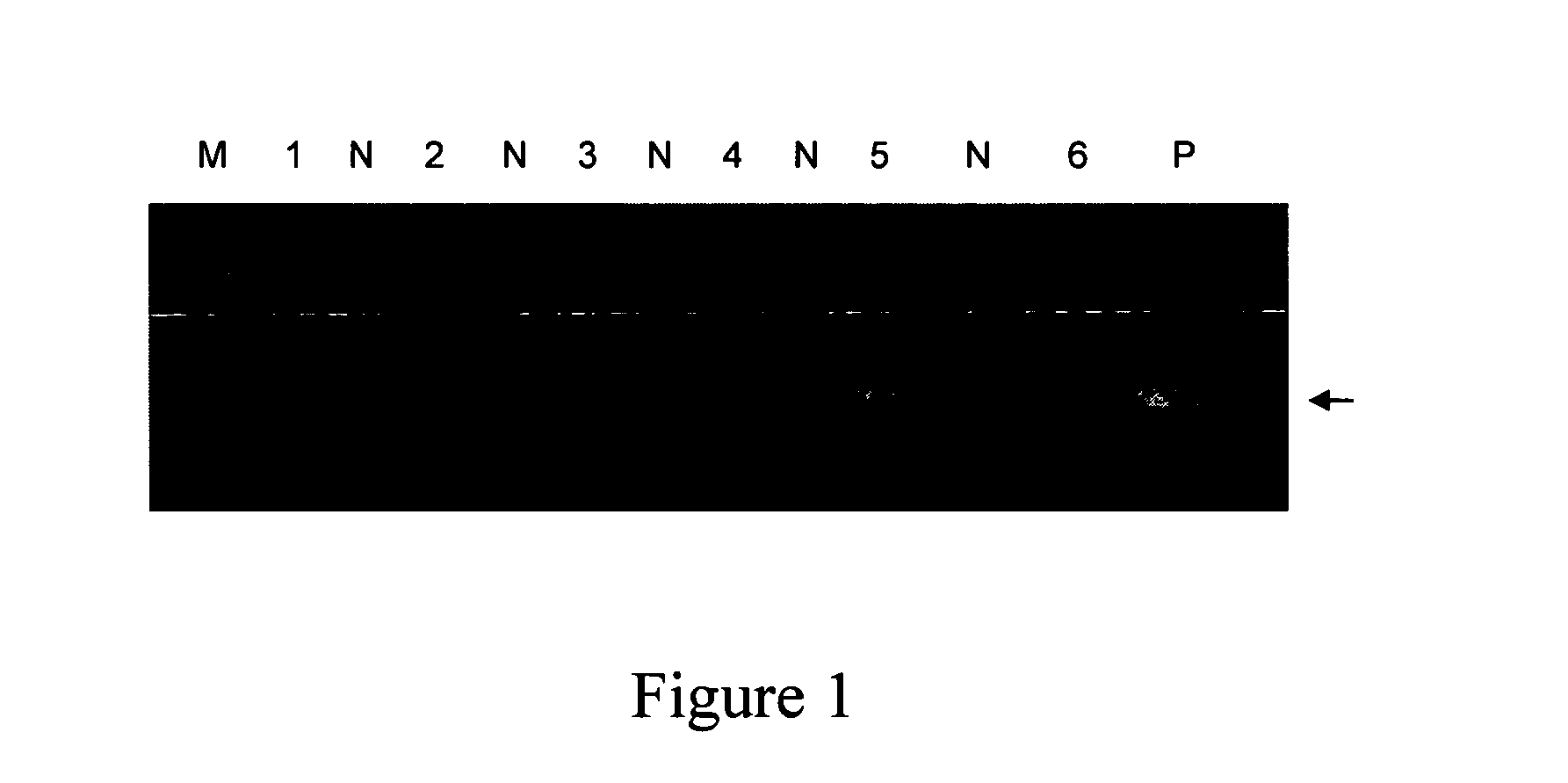
Helicobacter pylori antigen epitope polypeptide and application thereof
ActiveCN101863963AInfection Prevention and ControlImproving immunogenicityAntibacterial agentsPeptide/protein ingredientsOrganismAmino acid
The invention discloses a helicobacter pylori urease B antigen epitope polypeptide and application thereof. The amino acid sequence of the antigen epitope polypeptide is the sequence 34 in a sequence table, and the coding gene sequence of the antigen epitope polypeptide is the sequence 25 in the sequence table. The antigen epitope polypeptide provided by the invention can stimulate an organism togenerate helicobacter pylori resistance protective immunity reaction, thereby enhancing the effect of inhibiting helicobacter pylori infection, improving germ eliminating capacity of the organism, and playing an improving role in corresponding researches on development of vaccine and pathogenic mechanism and clinical diagnosis.
Owner:INST OF BIOENG ACAD OF MILITARY MEDICAL SCI OF THE CHINESE
Helicobacter pylori antigen HLA restrictive immunodominance epitope peptide and preparation method and application thereof
ActiveCN102746381AImproving immunogenicityReduce the risk of useAntibacterial agentsBacterial antigen ingredientsImmunodominant EpitopesImmunodominance
The invention relates to Helicobacter pylori antigen HLA restrictive immunodominance epitope peptide as well as a preparation method and application thereof. The dominance epitope peptide has the amino acid sequences shown in SEQ ID NO:63, 74, 95 and 105. The invention also provides a preparation method of the epitope peptide, and further provides application of the epitope peptide to preparation for preventing or treating Helicobacter pylori infection.
Owner:ARMY MEDICAL UNIV
Pyloric spiral bacillus antigen recombinant vaccine
InactiveCN1899610AHigh yieldHigh yield for high purityAntibacterial agentsBacterial antigen ingredientsAntigenAdjuvant
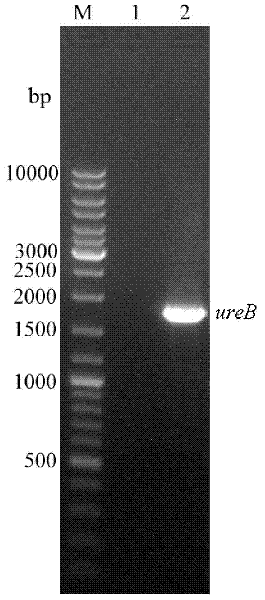
The present invention discloses recombinant vaccine based on the Helicobacter pylori neutral granulocyte activated protein A (NapA) antigen and its preparation process and application in inducing the protecting immune reaction resisting Helicobacter pylori infection. The vaccine consists of independent Helicobacter pylori NapA as the basic active component, or the fusion protein comprising Helicobacter pylori NapA, adhesion HpaA and urase B subunit active segment (UreB414), and one or several kinds of pharmaceutically acceptable adjuvant and excipient.
Owner:ARMY MEDICAL UNIV
Immunological microball method for detecting pyloric helicobacterium in stool sample
The immunological microsphere method of detecting pyloric helicobacterium in stool sample includes labeling microsphere to form immunological microsphere; suspending the tested stool sample containing pyloric helicobacterium in buffering liquid; mixing the immunological microsphere and the sample buffering liquid or mixing pyloric helicobacterium resisting antibody with the buffering liquid before adding pyloric helicobacterium resisting antigen labeled microsphere; and detecting pyloric helicobacterium in the stool sample. The method has the advantages of low cost, fast and convenient operation and reliable result.
Owner:王滔
Helicobacter pylori antigenic peptide and its application
The invention relates to antigen peptide containing Helicobacter pylori protective epitope, polypeptide containing the antigen peptide, nucleic acid for encoding the polypeptide, antibody for the antigen peptide, and related vaccine, composition and kit for treating, preventing and diagnosing the Helicobacter pylori.
Owner:SHENZHEN GENEBIOHEALTH
Helicobacter pylori antibody detection kit, detection method and application
InactiveCN104614522AAccurate detectionHigh detection sensitivityChemiluminescene/bioluminescenceFluorescence/phosphorescencePre-DilutionMicrosphere
The invention discloses a helicobacter pylori antibody detection kit, comprising the following constituents: a magnetic microsphere solution directly coating or indirectly connecting helicobacter pylori antigen; and a marking tracer solution directly coating or indirectly connecting anti-human IgG antibody; and / or a marking tracer solution directly coating or indirectly connecting anti-human IgM antibody; and / or a marking tracer solution directly coating or indirectly connecting anti-human IgA antibody; and / or a marking tracer solution directly coating or indirectly connecting staphylococcus aureus A protein. The invention further discloses a corresponding helicobacter pylori antibody detection method and application thereof. The kit, the method and the application provided by the invention can detect various antibodies, including helicobacter pylori IgG antibody, helicobacter pylori IgM, helicobacter pylori IgA and total helicobacter pylori antibody, are applicable to complex conditions of helicobacter pylori infected person better, moreover, can accurately detect the helicobacter pylori antibody without pre-dilution to samples.
Owner:SHENZHEN NEW INDS BIOMEDICAL ENG
PG (pepsinogen) and H.pylori antibody detection method and kit
InactiveCN107328938AQualitative and quantitative detectionRealize objective measurementMaterial analysisPepsinogen APolystyrene
The present invention proposes a kit, the use of the kit in detecting pepsinogen I, pepsinogen II and anti-Helicobacter pylori antibodies, and the method of using the kit to detect pepsinogen I, pepsinogen II and anti-Helicobacter pylori antibodies method. The kit comprises: a first coating membrane; and a second coating membrane, one end of the first coating membrane is connected to one end of the second coating membrane; at least one area of the first coating membrane is coated with pepsinogen labeled with fluorescent microspheres Ⅰ antibody pepsinogen Ⅰ antibody, fluorescent microsphere-labeled pepsinogen Ⅱ antibody and fluorescent microsphere-labeled first Helicobacter pylori antigen, the second coating membrane contains the separated first area, second area, third area and In the fourth area, the materials for forming fluorescent microspheres include: polystyrene-methyl methacrylate copolymer. The kit and method of the invention have the advantages of high sensitivity, strong specificity, rapidity, simplicity, objective determination and the like.
Owner:SHENZHEN GRADUATE SCHOOL TSINGHUA UNIV
Immunoassay for H. Pylori in fecal specimens
InactiveUSRE38088E1BacteriaMicrobiological testing/measurementPolyclonal antibodiesHelicobacter pylori DNA
A process for the determination of H. Pylori in a fecal specimen comprising (a) dispersing a fecal specimen suspected of carrying H. pylori in a sample diluent; (b) contacting the fecal specimen in the diluent with a first polyclonal antibody for H. pylori antigen to form a complex of the antibody and the antigen; (c) separating said specimen and said complex; (d) exposing the complex to a second polyclonal antibody for said antigen and a portion of the antibody reacting with said complex, one of said first and second antibody being bound to a solid carrier and the other being labeled with a detecting agent; and (e) determining the amount of the labeled antibody and in turn determining the presence of H. pylori antigen in said fecal specimen.
Owner:MERIDIAN BIOSCIENCE
Personal self-test chromatography reagent detection device
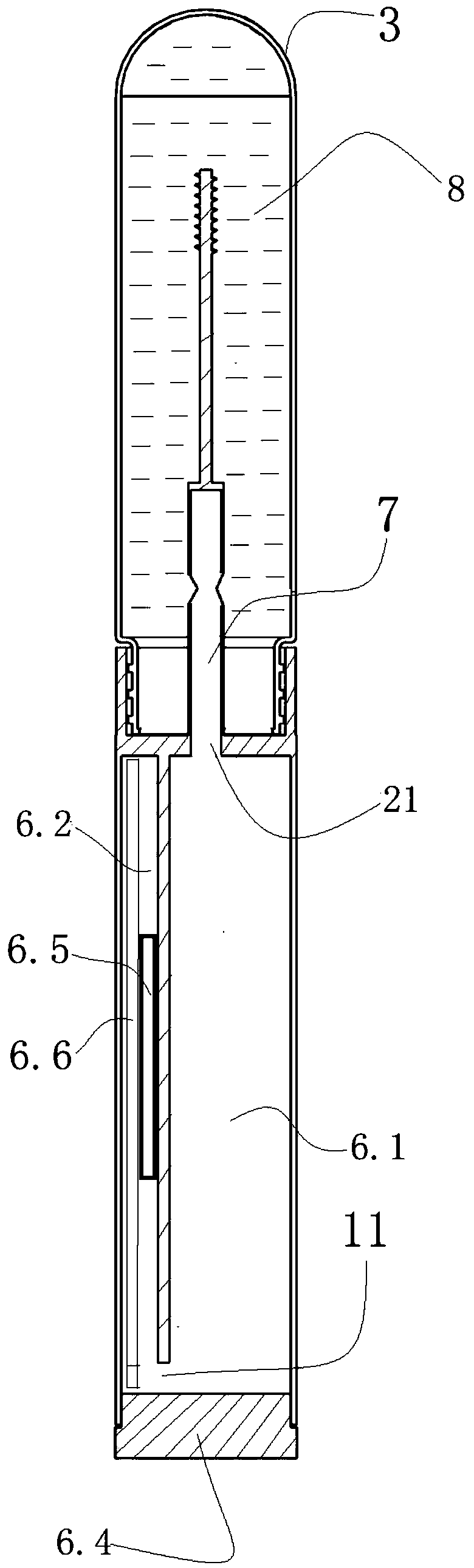
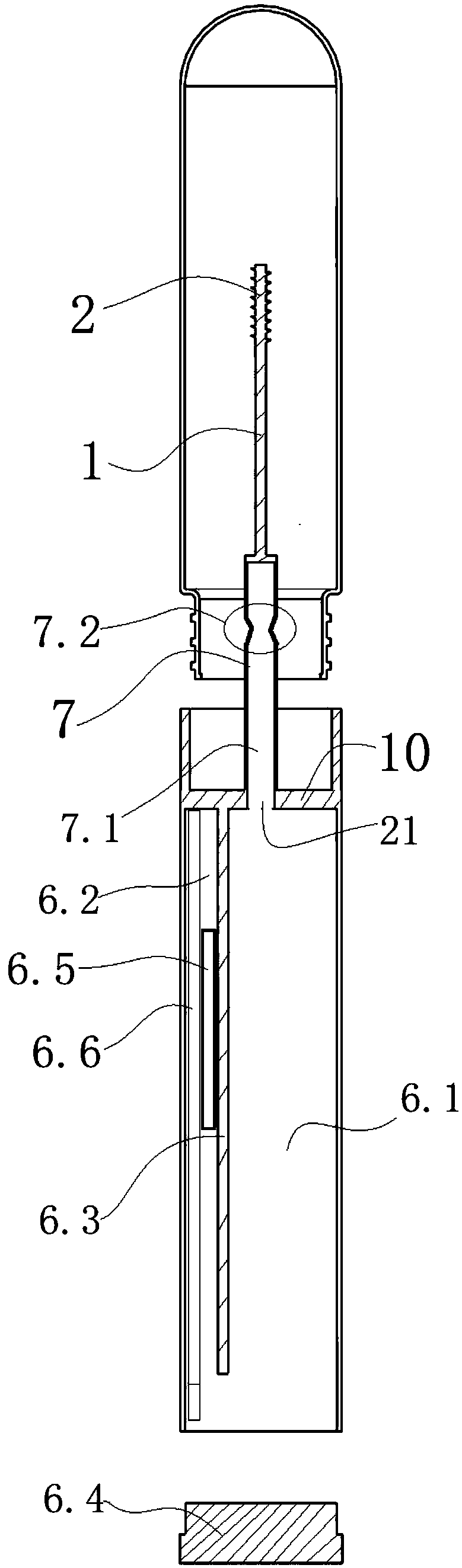
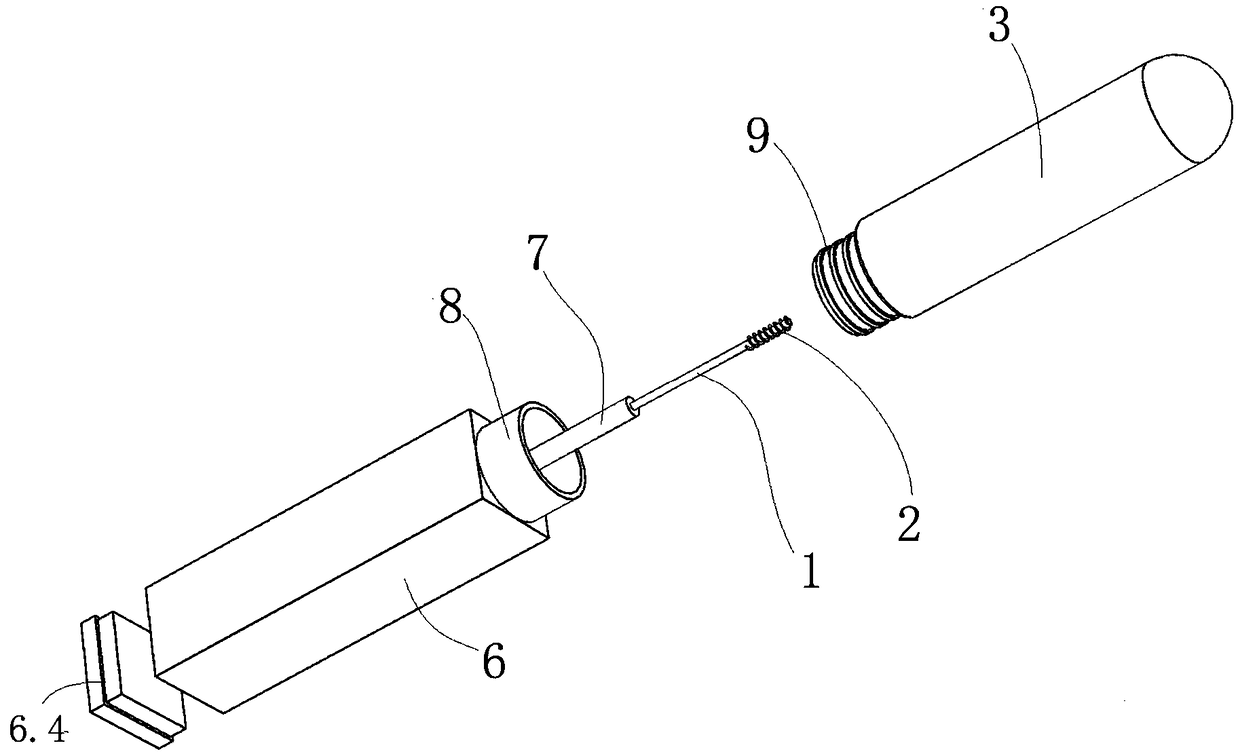
InactiveCN109187959AGuaranteed tightnessIngenious structureWithdrawing sample devicesPreparing sample for investigationIsolation layerDiluent
The invention discloses a personal self-test chromatography reagent detection device which comprises a liquid bottle and a detection cup, wherein the liquid outlet end of the liquid bottle is detachably connected with the opening of the detection cup; an isolated layer is arranged in the opening of the detection cup; a conduction pipe is arranged on the isolated layer; a hollow channel is formed in the conduction pipe; and the channel outlet of the hollow channel is communicated with the detection cup. According to the device disclosed by the invention, the sampling rod has a sampling functionand also has functions of conducting the liquid bottle with outside after rod breakage and enabling the liquid to flow to the connected detection cup. When the device is applied to detection of fecaloccult blood and helicobacter pylori antigen stool samples, operations of stool sample collection, dilution, uniform mixing, sample adding and detection can be integrated, so the high risk group withintestinal cancer and stomach cancer can be screened at home alone, and the social significance is great. The detection device is ingenious in structure and convenient to use, and due to the structure, the sealing property of diluent pre-filled in the liquid bottle is ensured.
Owner:刘小玲
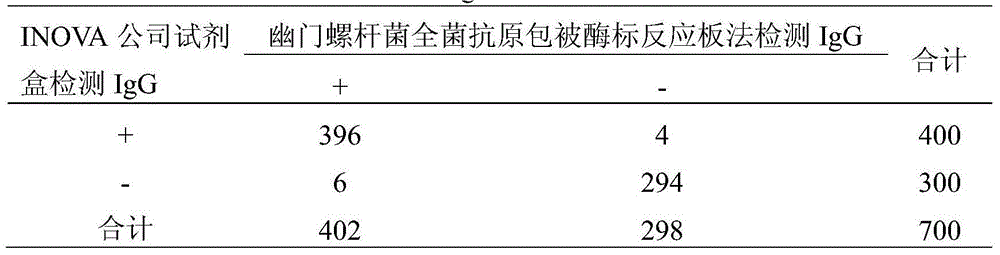
Helicobacter pylori IgG antibody ELISA semi-quantitative detection kit and application thereof
InactiveCN104833804AImprove consistencyImprove featuresBiological material analysisPositive controlSpecific igg
The invention provides a helicobacter pylori IgG antibody ELISA semi-quantitative detection kit. The kit is formed by a helicobacter pylori antigen coated enzyme labeled reaction plate, a sample diluting solution, a washing liquid, an enzyme labeled second antibody, a substrate solution, a stopping solution, a positive control solution, a negative control solution and a standard solution. A method for rapid and sensitive ELISA semi-quantitative detection of a helicobacter pylori specific IgG antibody in serum is established by using the kit. The helicobacter pylori IgG antibody standard solution is prepared, the helicobacter pylori antigen coated enzyme labeled reaction plate is prepared based on the standard solution, an indirect ELISA method is adopted to detect the specific helicobacter pylori IgG antibody in the serum, and the specific helicobacter pylori IgG antibody is quantified through calculation of an enzyme immunity unit (EIU). The kit has the advantages of high specificity and high sensitivity, and is mainly used for laboratory researches and clinic auxiliary diagnosis.
Owner:BIOHIT BIOTECH HEFEI
Helicobacter pylori antigens in blood
The present invention provides methods for detecting Helicobacter pylori (H. pylori) DNA and / or fragments thereof in blood. The first method involves extracting DNA from a blood sample, preferably plasma, by amplifying the DNA using a polymerase chain reaction (PCR) or a ligase chain reaction (LCR) method, and detecting a target DNA sequence in the amplified DNA. The preferred target DNA sequence comprises a Mr26 or a 16S rRNA gene or fragments thereof specific to H. pylori. The second method involves extracting DNA from a blood sample, preferably serum, by hybridizing the extracted DNA with a radioisotope or fluorescence labeled H. pylori DNA probe.
Owner:JOY BIOMEDICAL
Immunologic adjuvant-Helicobacter pylori antigen fused protein oral vaccine and preparation method thereof
ActiveCN102604993ASolve demand bottlenecksImprove immunityAntibacterial agentsBacterial antigen ingredientsProtein targetOrganism
The invention relates to an immunologic adjuvant-Helicobacter pylori antigen fused protein oral vaccine and a preparation method and product thereof, belonging to the technical field of biomedicine. A connecting peptide sequence section is connected with the downstream part of an oral immunologic adjuvant gene by using a PCR (Polymerase Chain Reaction) technology; the oral immunologic adjuvant gene connected with the connecting peptide sequence is fused with a protein antigen gene by using the overlap extension RCR technology; and then, a recombination silkworm baculovirus of the fused gene is established and a target protein is expressed by using a silkworm bioreactor. According to the fused protein oral vaccine provided by the invention, the immunologic adjuvant-Helicobacter pylori antigen fused protein is expressed by using the silkworm bioreactor, so that the protein is effectively expressed, and the protein fusion expression of the immunologic adjuvant and the Helicobacter pyloriantigen is used as an intra-molecular immunologic adjuvant to enhance the immune effects.
Owner:贵州贵安精准医学股份有限公司
Latex enhanced immunoturbidimetry kit for content of helicobacter pylori antibodies
The invention relates to a latex enhanced immunoturbidimetry kit for content of helicobacter pylori antibodies, in particular to a kit for measuring the content of helicobacter pylori antibodies in human serum and plasma samples by using latex enhanced immunoturbidimetry. The kit contains reaction buffer, latex particles crosslinked with helicobacter pylori antigens, and a calibrator. According to the kit, the helicobacter pylori antigens crosslinked with the surfaces of the latex particles and the helicobacter pylori antibodies in a sample are subjected to immunoreaction to form turbidity, and the content of the helicobacter pylori antibodies is detected through the rising degree of the turbidity. The kit can be used for a clinical frequently used biochemical analyzer, and is easy and convenient to operate, speedy and high in specificity. The stability of the kit is improved, and sensitivity and a detection range of the latex enhanced immunoturbidimetry kit can meet requirements of clinical application.
Owner:BEIJING STRONG BIOTECH INC
Recombinant influenza virus carrying Helicobacter pylori, host cells, and preparation method and application of recombinant influenza virus
ActiveCN111363727AImmunization method is convenientSimple immunizationSsRNA viruses negative-senseAntibacterial agentsEpitopeHelicobacter
The invention discloses a recombinant influenza virus carrying Helicobacter pylori, host cells, and a preparation method and application of the recombinant influenza virus. The recombinant influenza virus is obtained by integrating a Helicobacter pylori antigen or antigen-dominant epitope into a NS fragment of the influenza virus genome. The recombinant influenza virus carrying Helicobacter pyloriof the invention can be stably passaged in the host cells or chicken embryos, and can be used for the development of Helicobacter pylori vaccines, the development of related drugs, and the productionof Helicobacter pylori proteins by using chicken embryos or cells as a bioreactor.
Owner:WUHAN UNIV
Helicobacter pylori antigen colloidal gold detection kit
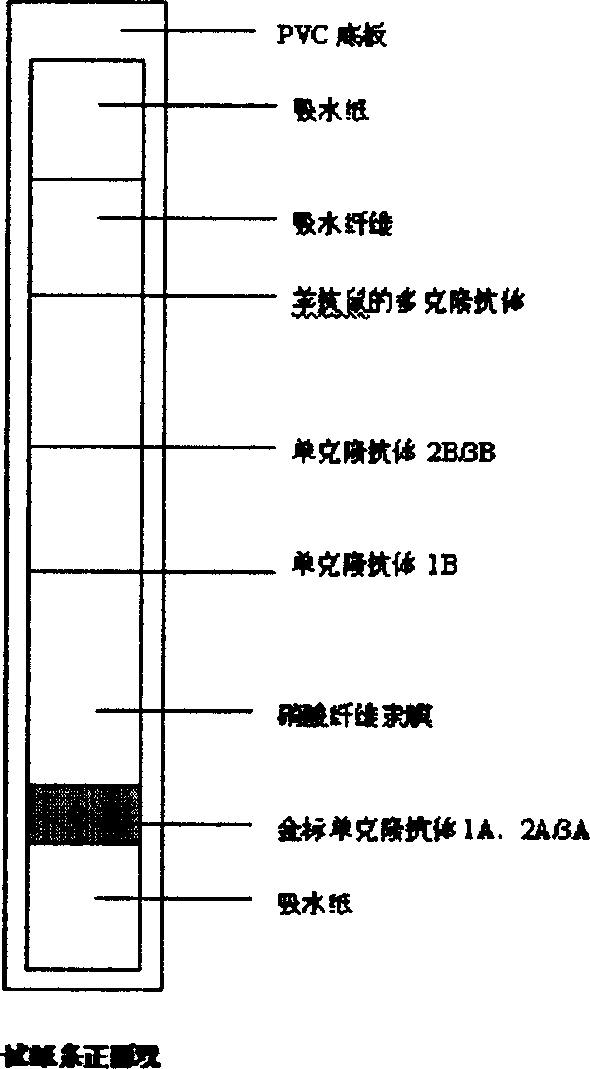
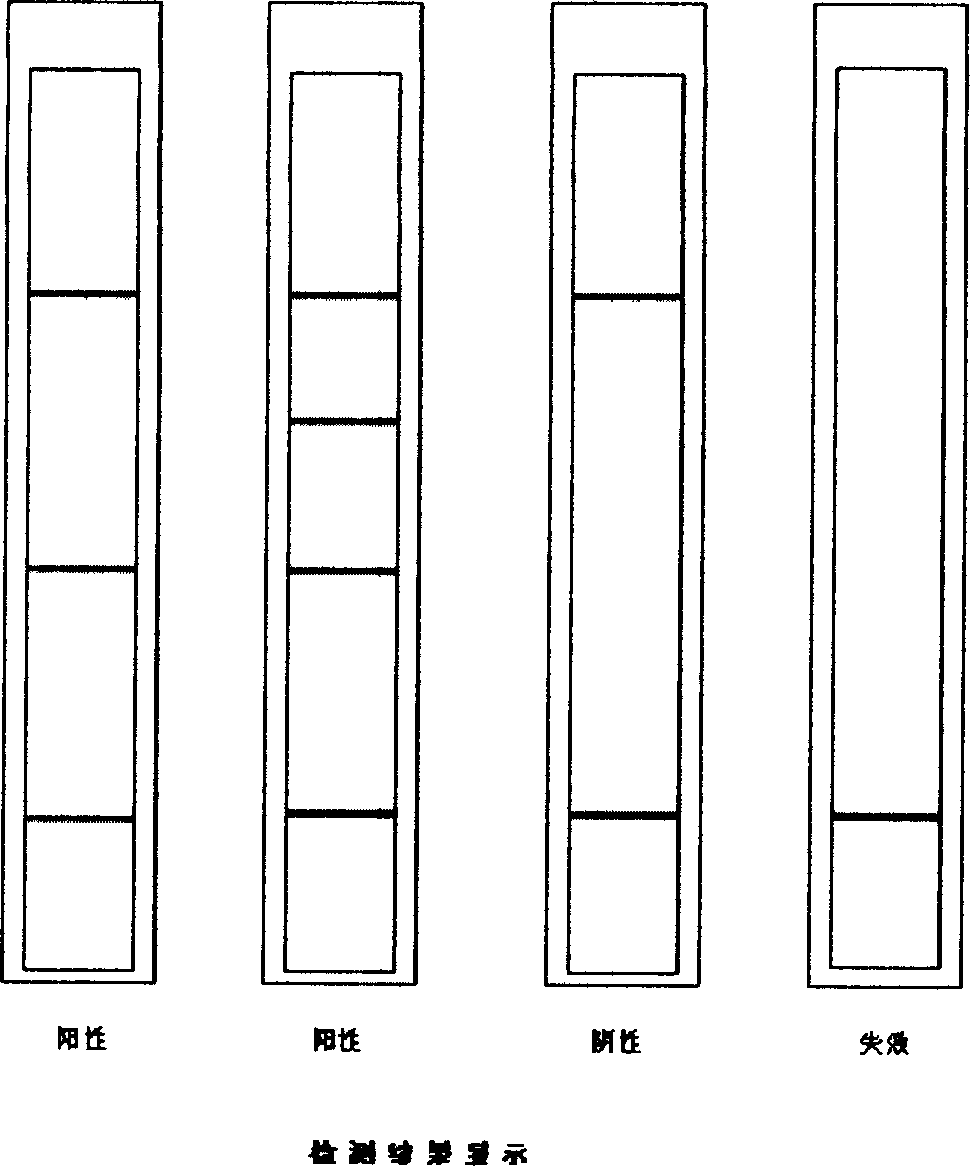
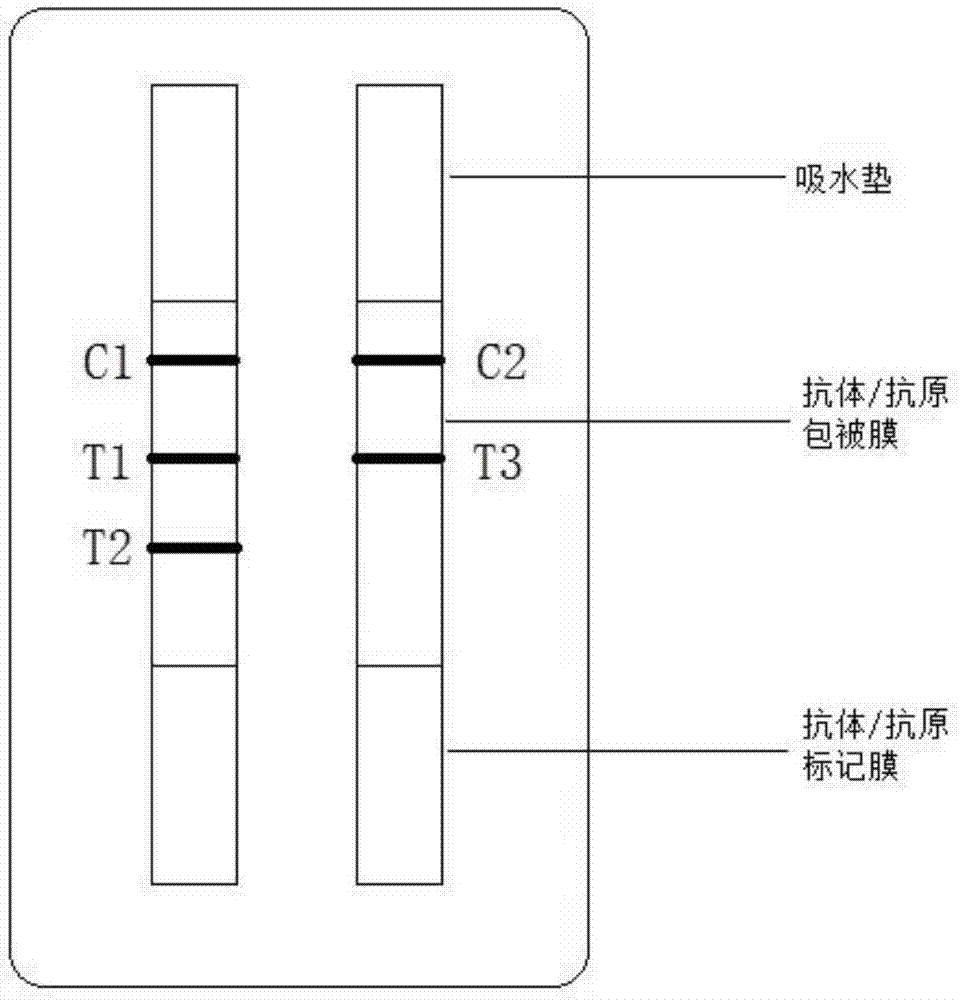
ActiveCN104897892AQuick checkStrong specificityBiological material analysisTrue positive rateMonoclonal antibody
The invention relates to the field of biological detection, in particular to a helicobacter pylori antigen colloidal gold detection kit, and a preparation method and an application thereof. The helicobacter pylori antigen colloidal gold detection kit provided by the invention comprises a test paper card, wherein the test paper card comprises a bottom plate, a sample pad, a gold mark pad, a nitrocellulose membrane and an absorbent pad; the sample pad, the gold mark pad, the nitrocellulose membrane and the absorbent pad are located on the surface of the bottom plate and are sequentially arranged from a sampling end; the gold mark pad comprises a colloidal gold-marked anti-helicobacter pylori monoclonal antibody-1; and the nitrocellulose membrane is coated with a detection line and a quality control line. The helicobacter pylori antigen colloidal gold detection kit provided by the invention has sensitivity and specificity and has the advantages of being fast, simple and convenient to operate, accurate in result, economical and practical.
Owner:SHANGHAI CHEMTRON BIOTECH
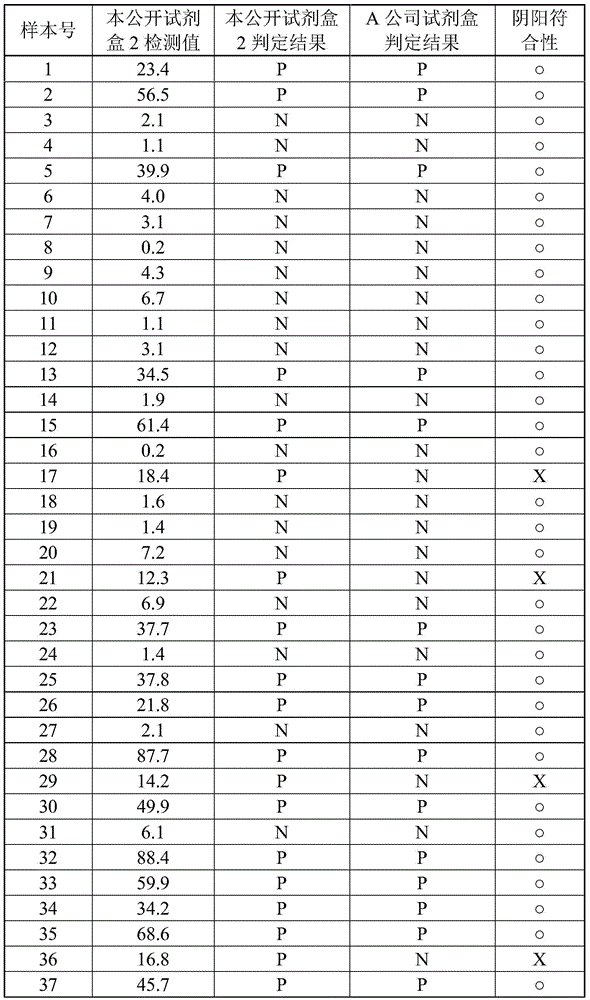
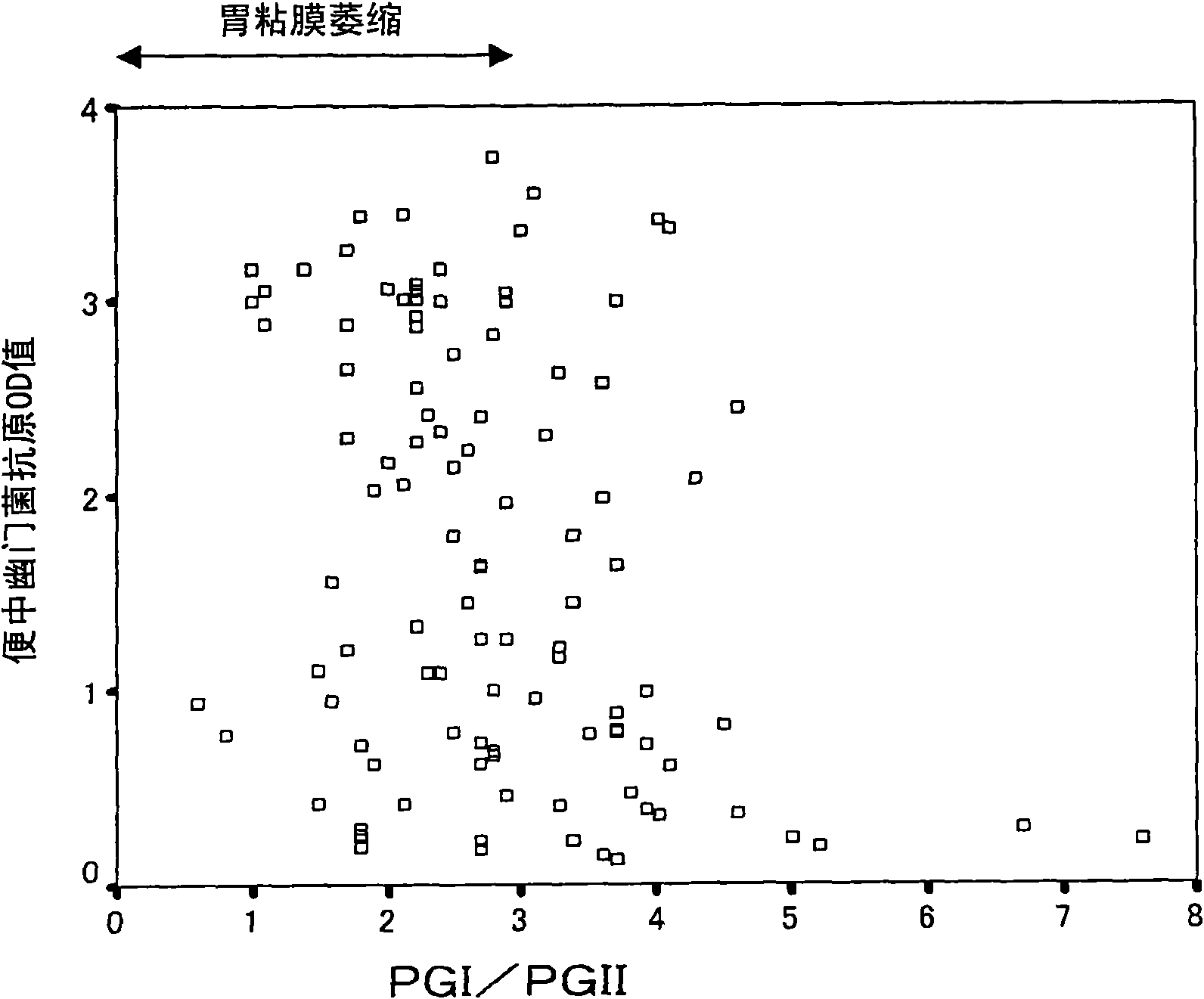
Method for evaluating gastric mucosal state by quantifying helicobacter pylori antigen in feces
It is intended to provide a method for evaluating the gastric mucosal state which can be conveniently carried out while loading little burden on a subject, shows missing cases at an extremely low ratio, and enables detailed classification of not only the gastric mucosal states with the pathological severity but also the risk of gastric cancer and the risk thereof in future. It is also intended toprovide a method for quantifying Helicobacter pylori cells occurring in the stomach in relation to the judgment of various pathological conditions. The above-described problems can be solved by a method for evaluating the gastric mucosal state of a subject by using a quantitative measurement value of Helicobacter pylori antigen occurring in the feces of the subject as an indication and a method ofquantifying Helicobacter pylori cells occurring in the stomach of the subject.
Owner:MEIJI CO LTD
Diagnostic methods
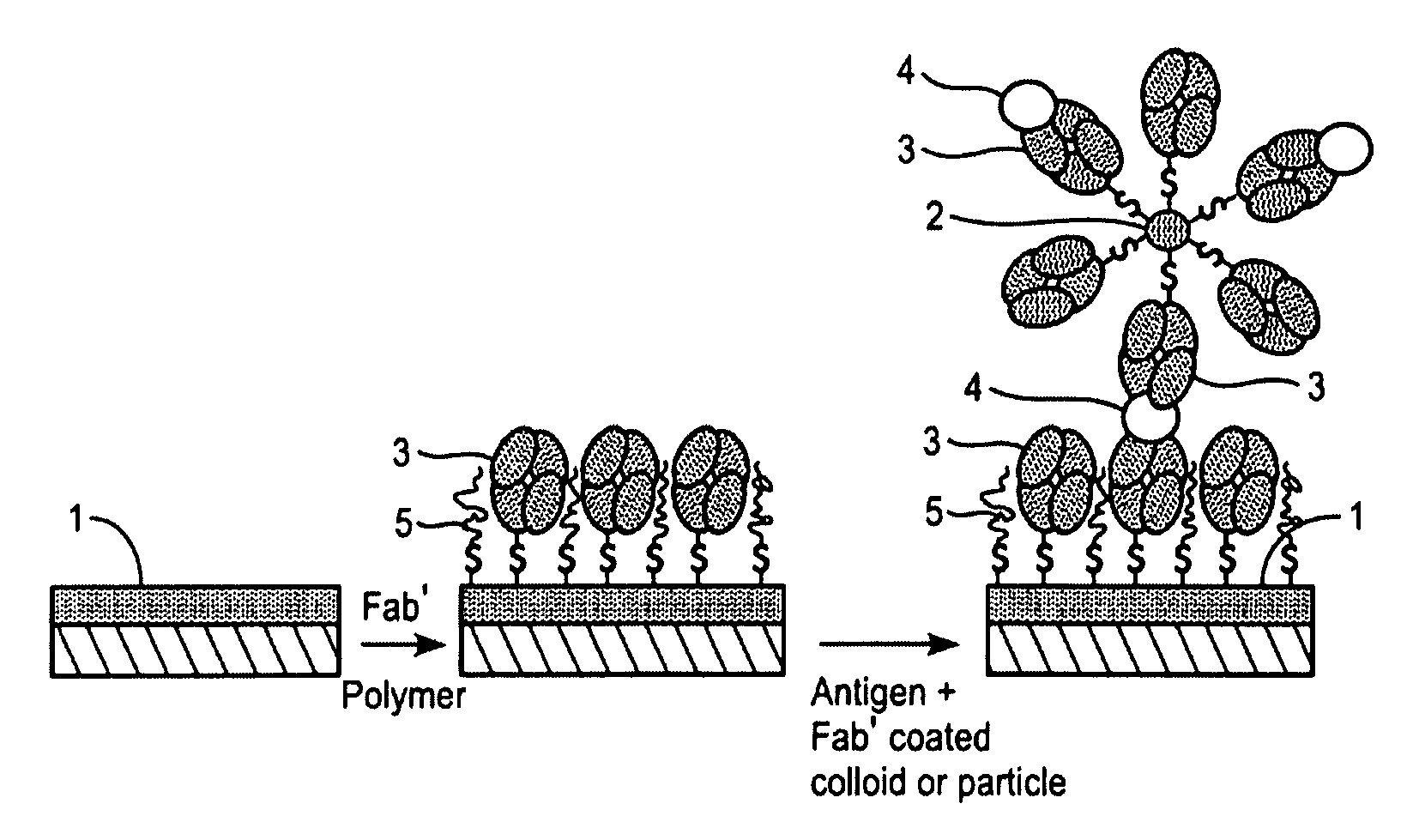
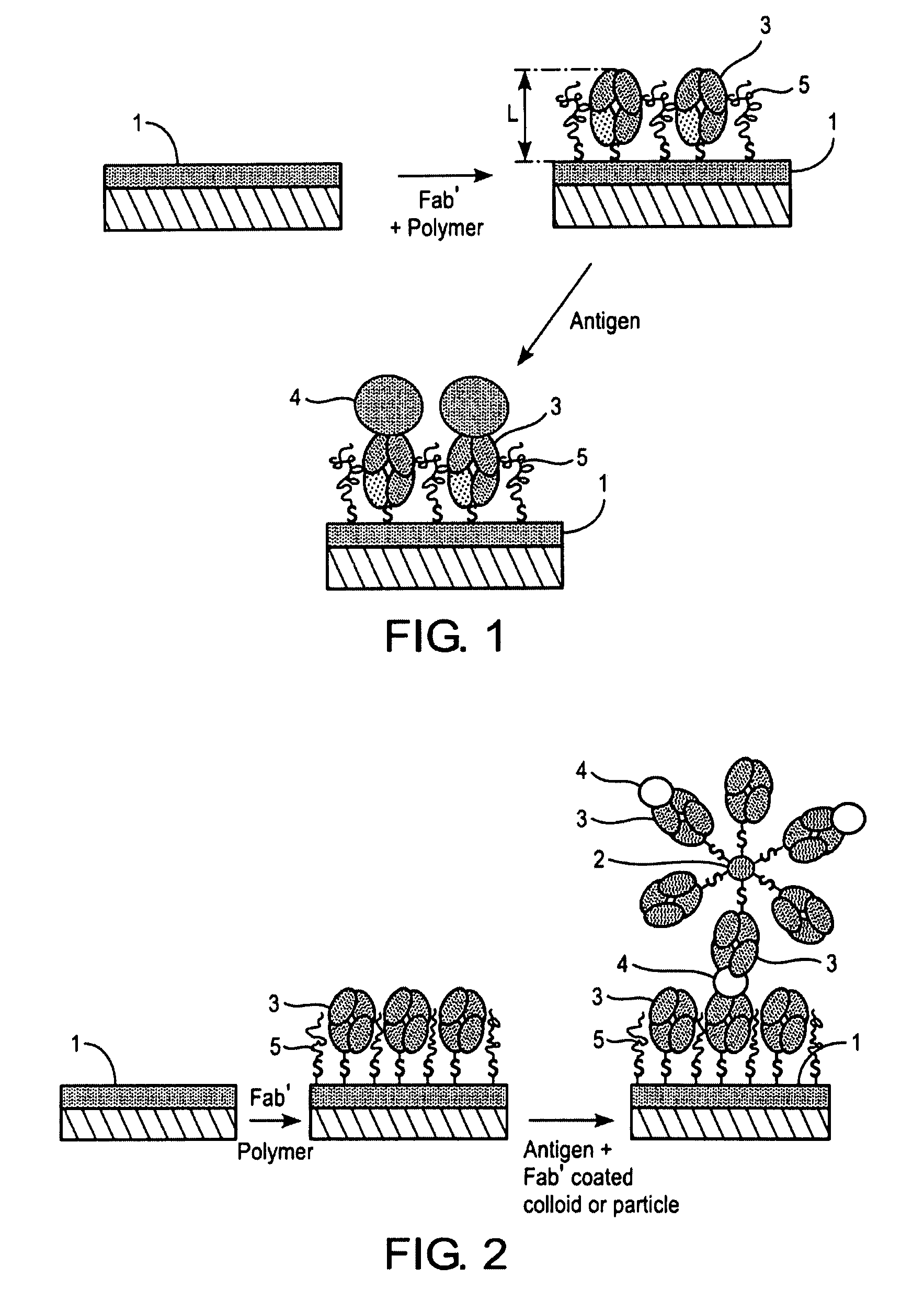
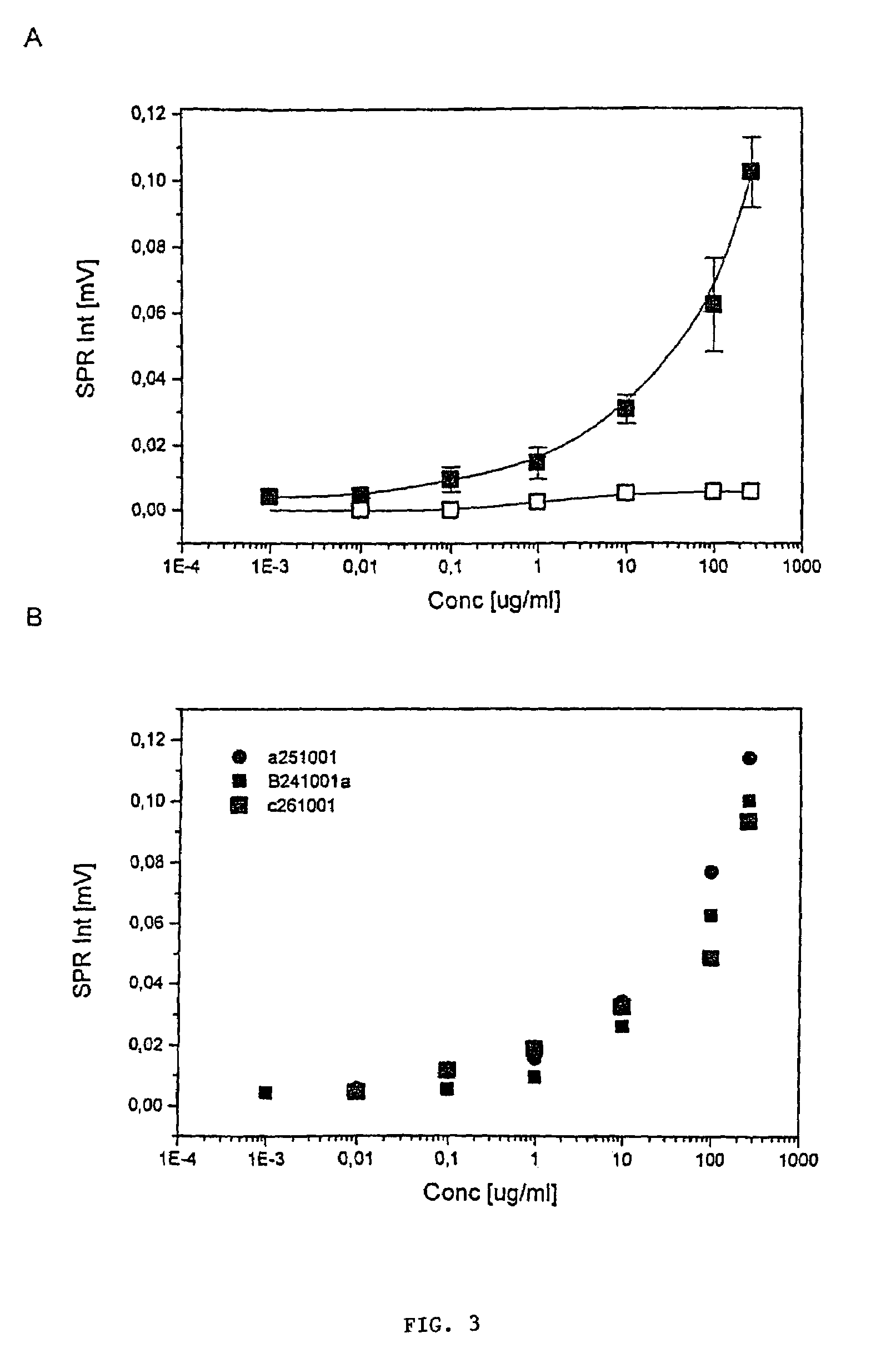
InactiveUS7544504B2High detection sensitivityReliable detectionBioreactor/fermenter combinationsBiological substance pretreatmentsBacteroidesMetabolite
The present invention relates to novel methods for the diagnosis of Helicobacter pylori infection. Specifically, the present invention relates to novel non-invasive methods for the detection of the presence or absence of a Helicobacter pylori antigen or a metabolite produced by the bacterium in a biological sample with a biosensor-based measurement. The present invention also related to the use of a biosensor containing specific antibodies against H. pylori or antigen-binding fragments thereof immobilized thereto together with biomolecule-repellent polymers preventing the non-specific binding. The invention also relates to test kits useful in the methods.
Owner:BIONAVIS
Helicobacter pylori antigen hla-restricted immunodominant epitope peptide and its application
ActiveCN102276697AImproving immunogenicityReduce the risk of useAntibacterial agentsBacterial antigen ingredientsImmunogenicityTGE VACCINE
The invention relates to a helicobacter pylori antigen HLA restricted immuno-dominant epitope peptide and an application thereof. The helicobacter pylori HpaA antigen immuno-dominant epitope polypeptide has amino acid sequences as shown in SEQ ID No:8, 22, 30 and 42. The polypeptide of the invention has high immunogenicity, and can initiate strong immune response. Additionally, the immuno-dominant epitope polypeptide does not contain unnecessary or even harmful parts, and thus application risk of vaccines prepared by the polypeptide is reduced. Superiority combination of the immuno-dominant epitope polypeptide with other vaccine components can be realized, which extends the width of immune response. Vaccines prepared by the immuno-dominant epitope polypeptide not only have prevention effect on helicobacter pylori infection, but also can be used as therapeutic vaccines.
Owner:ARMY MEDICAL UNIV
Immunoassay method for helicobacter pylori in gastric mucosa sample
An immunoassay method for Helicobacter pylori (H. pylori) in a gastric mucosa sample comprises (a) suspending the gastric mucosa sample doubted about containing the H. pylori into sample diluent; (b) enabling the gastric mucosa sample in the diluent to be contacted with a first polyclonal antibody of an antigen resisting to the H. pylori to form a compound of the antibody and the antigen; (c) separating the sample and the compound; (d) enabling the compound to be exposed on a second polyclonal antibody of the antigen, enabling one part of the antibody to react with the compound, combining one of the first polyclonal antibody and the second polyclonal antibody onto a solid carrier, and marking the other one of the first polyclonal antibody and the second polyclonal antibody through a detection agent; and (e) assaying volume of the marked antibody and sequentially assaying existence of H. pylori antibodies in the gastric mucosa sample. The immunoassay method for the H. pylori in the gastric mucosa sample has the advantages of being low in cost, visual and accurate in detection result and fast in reaction speed.
Owner:王滔
Anti-helicobacter pylori bovine colostrum product
The invention relates to an anti-helicobacter pylori bovine colostrum product which is realized by the following steps of: preparing helicobacter pylori antigens; immunizing the helicobacter pylori antigens; collecting immune milk; and preparing the immune milk into a milk product. The anti-helicobacter pylori bovine colostrum product prepared in the invention not only has the good effects of preventing and treating diseases caused by helicobacter pylori infection, but also has the advantages of good specificity, fewer side effects, difficulty in generating drug-resistant strains, simple and convenient preparation method, easiness for industrialization and the like.
Owner:江西英雄乳业股份有限公司
Helicobacter pylori vaccination
InactiveUS20050175629A1Longer timescale of immunotherapyAntibacterial agentsAmpoule syringesAdjuvantVaccination
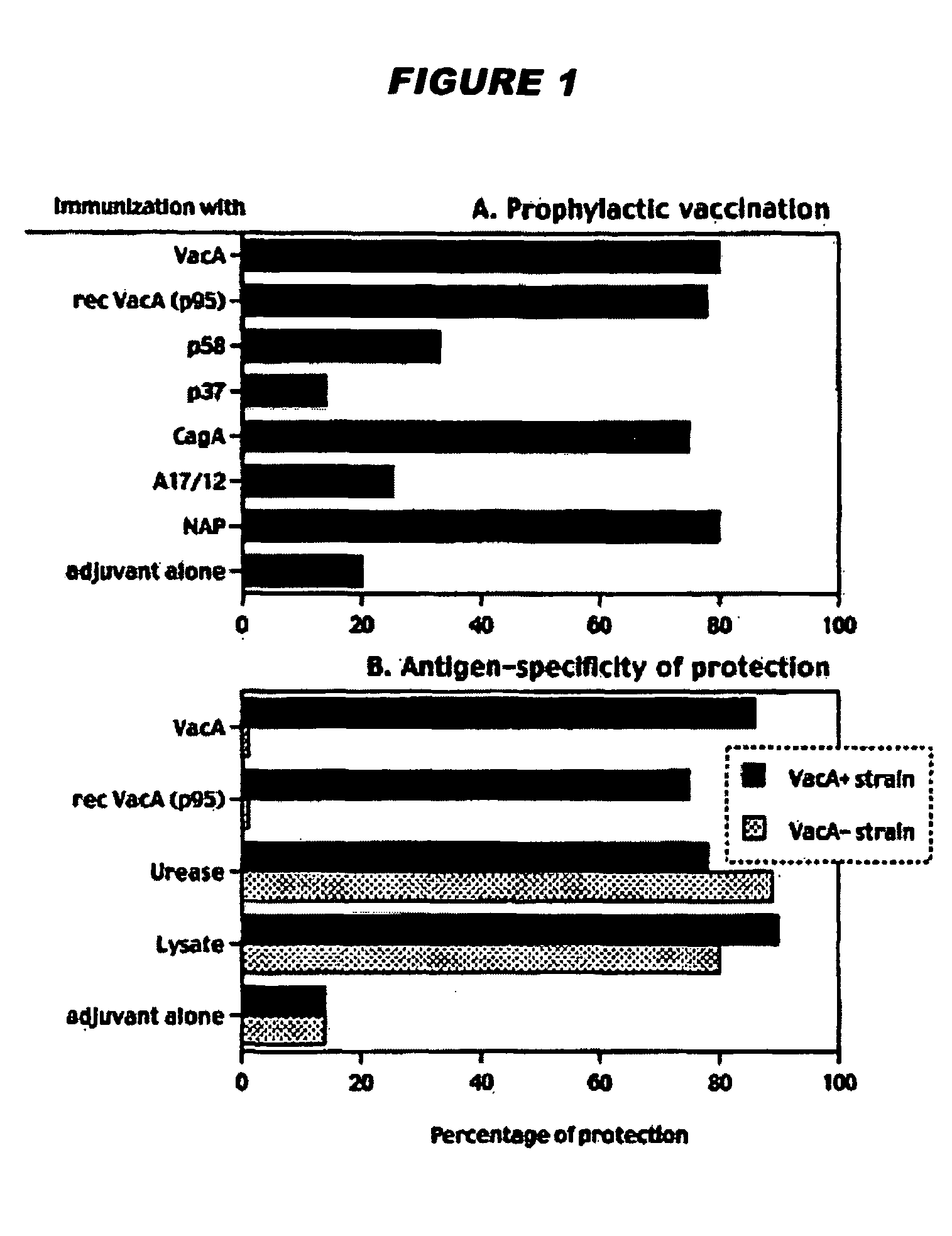
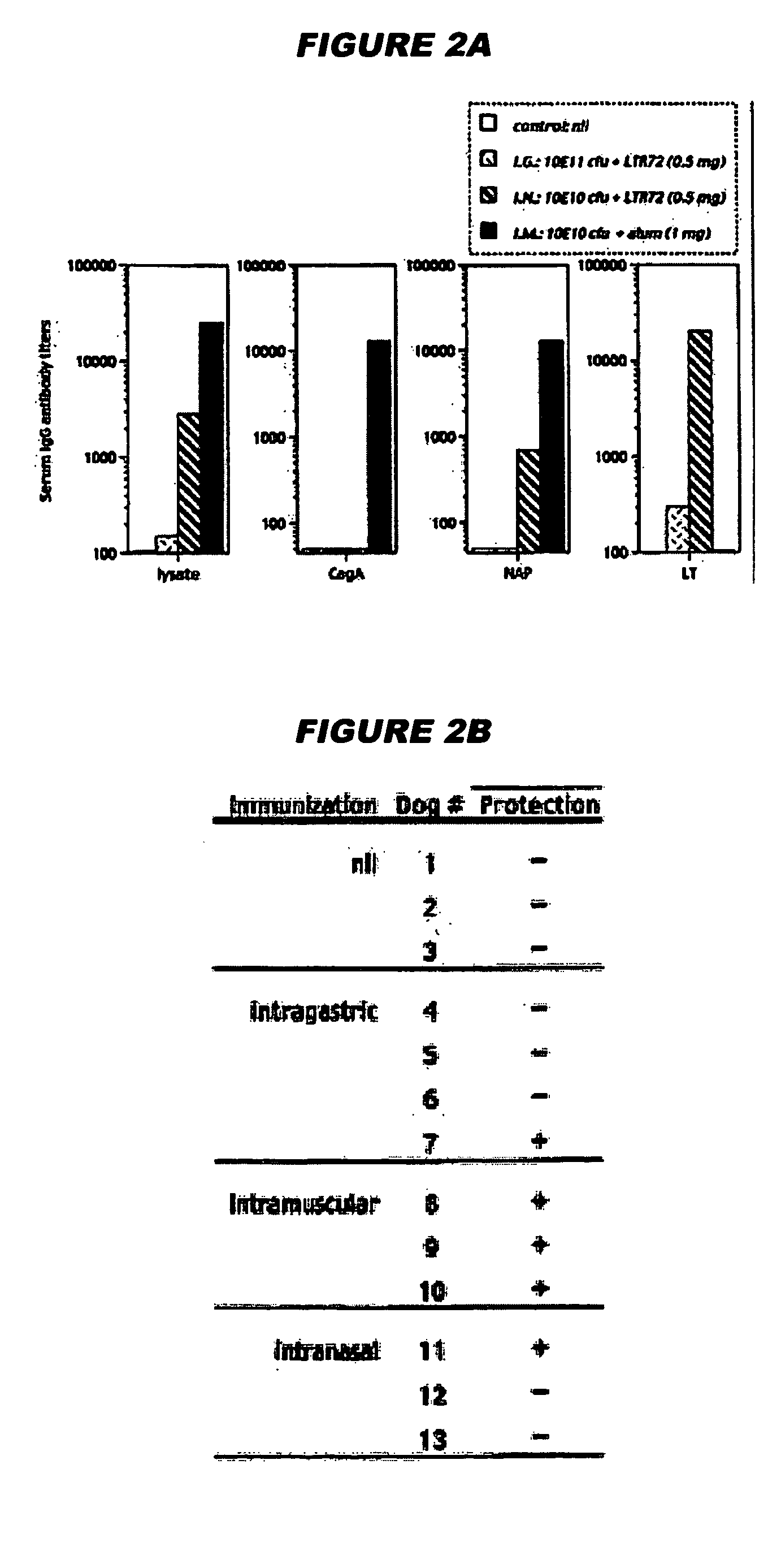
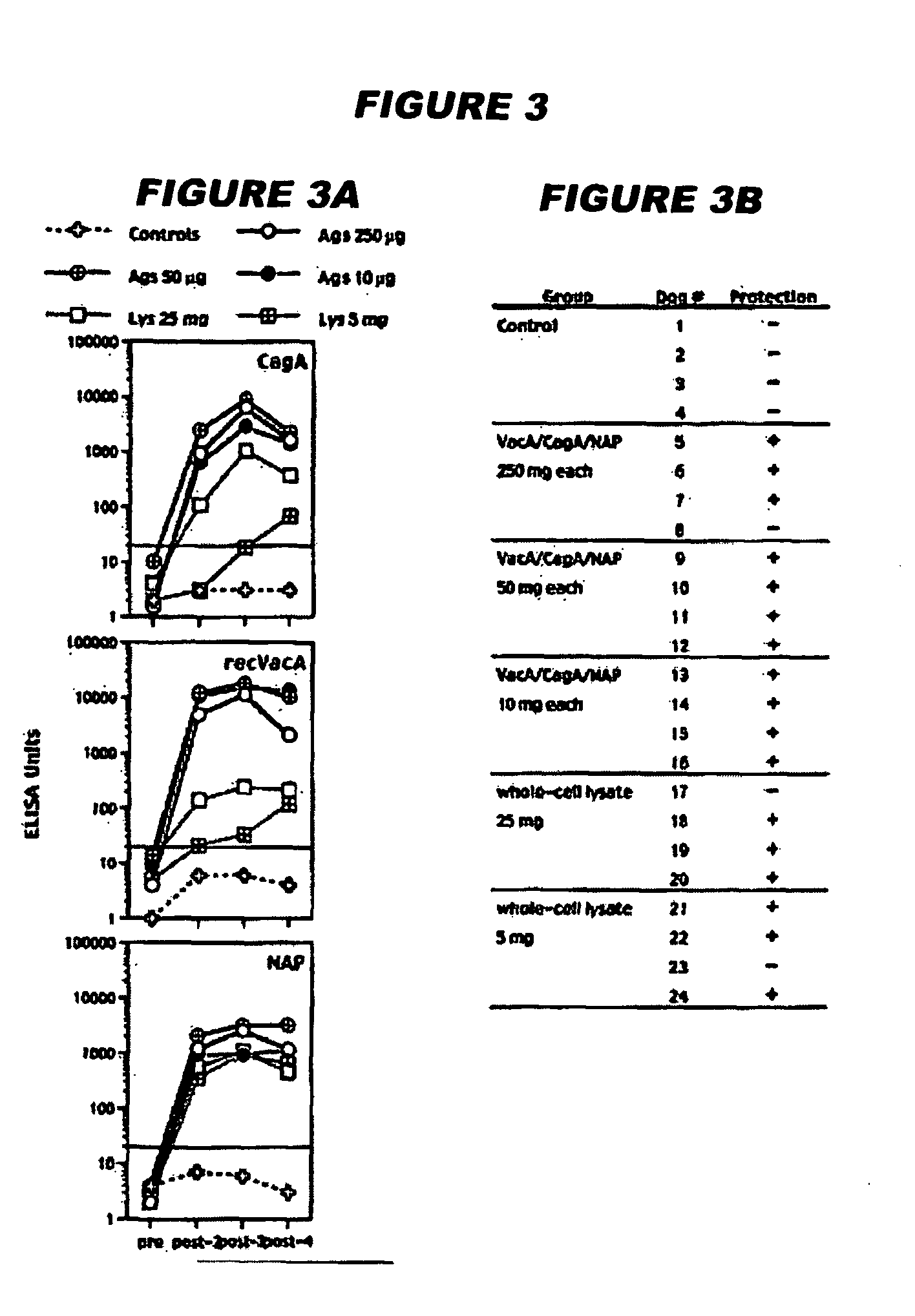
A sterile immunogenic preparation of three purified H. pylori antigens (CagA, VacA and NAP) adjuvanted with alum in an isotonic buffer solution for intramuscular injection. The antigens may be administered in conjunction with antibiotics and / or antisecretories. Urease breath testing, stool antigen testing, and / or immunological analysis may be used as correlate(s) of protection against H. pylori infection. Urea may be used to improve VacA solubility.
Owner:CHIRON CORP
Helicobacter pylori IgG antibody enzyme linked immunosorbent assay (ELISA) diagnostic kit
ActiveCN106802345AHigh sensitivityStrong specificityMaterial analysisPositive controlImmunodiagnostics
The invention belongs to the technical field of medical biology, and in particular relates to a helicobacter pylori IgG antibody enzyme linked immunosorbent assay (ELISA) diagnostic kit. The kit consists of a helicobacter pylori antigen-coated plate, sample diluent, enzyme-labeled liquid, a cleaning solution, a developing solution, a negative control substance, a positive control substance and stop buffer. The kit is high in sensitivity, strong in specificity and high in accuracy, and has good stability; the shelf life of the kit is one year under the condition of room temperature, and the shelf life of the kit reaches three years under the condition of 4 DEG C, so that the shelf life of the kit is obviously prolonged.
Owner:UNIMED BIOTECH SHANGHAI CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com