Helicobacter pylori antibody detection kit, detection method and application
A technology for detecting Helicobacter pylori and antibodies, which is applied to measuring devices, analyzing materials through chemical reactions, and instruments. Accuracy of results, faster detection speed, and simplified detection process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
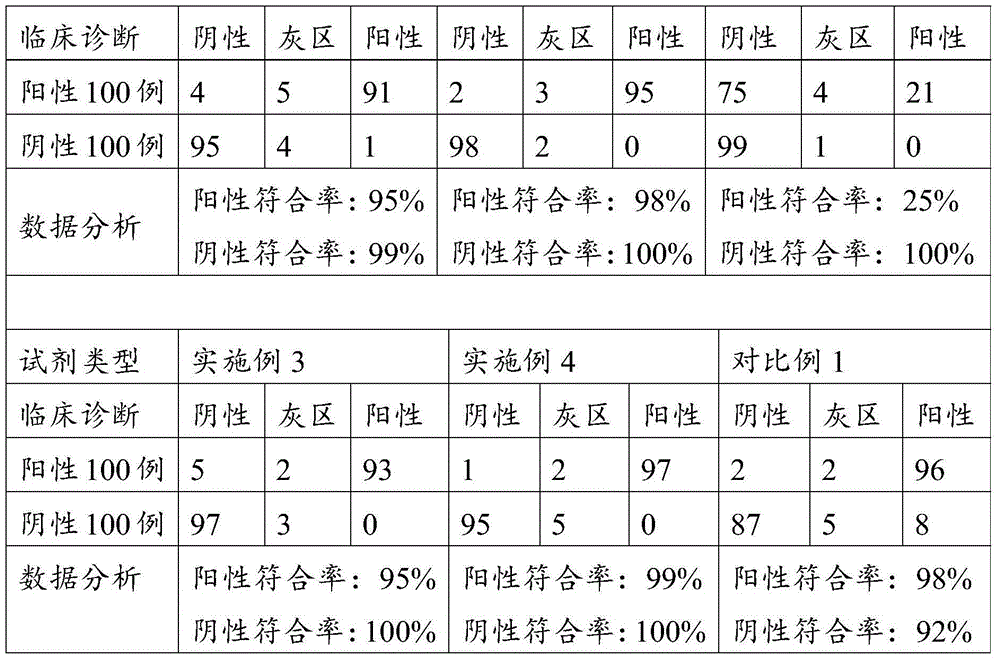
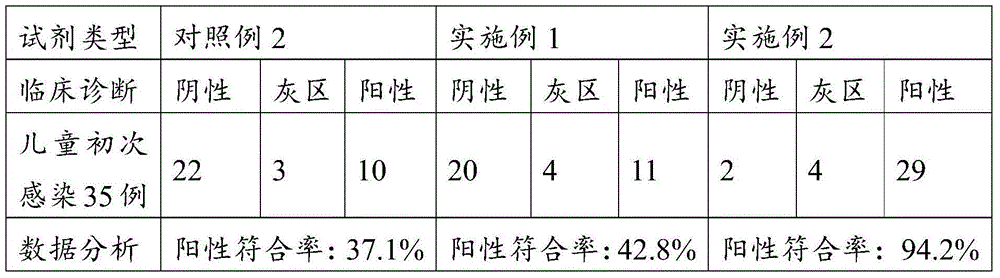
Examples
Embodiment 1
[0247] A detection kit for measuring H.pylori IgG, comprising the following components:
[0248] Magnetic microsphere solution coated with HP antigen
[0249] HP antigen concentration: 50μg / l Magnetic microsphere concentration: 1.2mg / ml
[0250] ABEI solution of labeled anti-human IgG antibody
[0251] Anti-human IgG antibody concentration: 50μg / l ABEI concentration: 0.5mg / l
[0252] Low point calibrator containing HP IgG, where HP IgG concentration: 10.573EIU
[0253] High-point calibrator containing HP IgG, wherein HP IgG concentration: 68.766EIU.
[0254] In actual implementation, each of the above components may contain BSA and preservatives, the concentration of BSA is 0.05g / ml, and sodium azide is used as the preservative, and the concentration is 0.02g / ml.
[0255] A detection method for H.pylori IgG, comprising the steps of:
[0256] Add samples and calibrators to the cuvette;
[0257] Add 100 μl of buffer solution, 20 μl of HP antigen-coated magnetic microsphere...
Embodiment 2
[0264] A detection kit for measuring H.pylori IgM, comprising the following components:
[0265] Magnetic microsphere solution coated with HP antigen
[0266] HP antigen concentration: 50μg / l Magnetic microsphere concentration: 1.2mg / ml
[0267] ABEI solution of labeled anti-human IgM antibody
[0268] Anti-human IgM antibody concentration: 50μg / l ABEI concentration: 0.5mg / l
[0269] Low point calibrator containing HP IgM, where HP IgM concentration: 10.573EIU
[0270] High point calibrator containing HP IgM, where HP IgM concentration: 68.766EIU.
[0271] In actual implementation, each of the above components may contain BSA and preservatives, the concentration of BSA is 0.05g / ml, and sodium azide is used as the preservative, and the concentration is 0.02g / ml.
[0272] A detection method for H.pylori IgM, comprising the steps of:
[0273] Add samples and calibrators to the cuvette;
[0274] Add 100 μl of buffer solution, 20 μl of HP antigen-coated magnetic microsphere s...
Embodiment 3
[0281] A detection kit for measuring H.pylori IgA, comprising the following components:
[0282] Magnetic microsphere solution coated with HP antigen
[0283] HP antigen concentration: 50μg / l Magnetic microsphere concentration: 1.2mg / ml
[0284] ABEI solution of labeled anti-human IgA antibody
[0285] Anti-human IgA antibody concentration: 50μg / l ABEI concentration: 0.5mg / l
[0286] Low point calibrator containing HP IgA, where HP IgA concentration: 10.573EIU
[0287] High point calibrator containing HP IgA, HP IgA concentration: 68.766EIU.
[0288] In actual implementation, each of the above components may contain BSA and preservatives, the concentration of BSA is 0.05g / ml, and sodium azide is used as the preservative, and the concentration is 0.02g / ml.
[0289] A detection method for H.pylori IgA, comprising the steps of:
[0290] Add samples and calibrators to the cuvette;
[0291] Add 100 μl of buffer solution, 20 μl of HP antigen-coated magnetic microsphere solutio...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com