Immunologic adjuvant-Helicobacter pylori antigen fused protein oral vaccine and preparation method thereof
An immunoadjuvant and protein technology, applied in the field of biomedicine, can solve the problems of high treatment costs, extensive drug resistance, and large side effects, and achieve the effects of promoting oral absorption, solving bottlenecks and alleviating pain.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
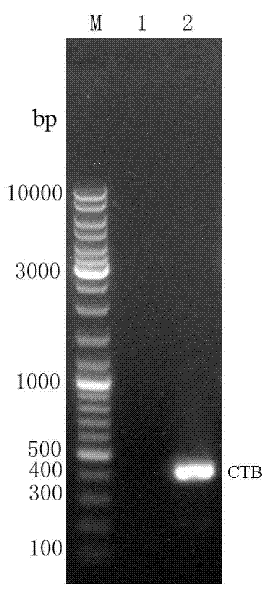
[0038] Example 1: CTB-Linker- ure B Construction of fusion gene (as shown in SEQ ID NO: 1)
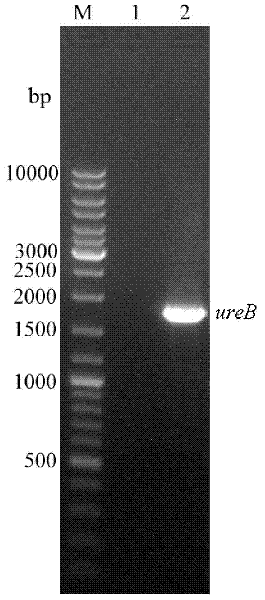
[0039] Firstly, the oral immune adjuvant CTB gene and Helicobacter pylori ure B In order to ensure that the space structure of the two protein subunits is not affected after the fusion gene is expressed, the CTB gene and ure B connected in between
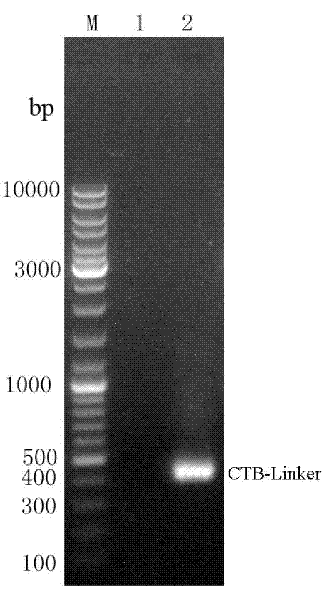
[0040] A connecting peptide sequence (Linker) (as shown in SEQ ID NO: 6):
[0041] AGATCCGCCGCCACCAGATCCACCACCGCCGGATCCACCGCCACC encodes a flexible peptide segment of (Gly4Ser)3, ensuring that the spatial structure of the two protein subunits in the fusion protein is not affected. This connecting peptide was originally designed by Husotn et al. in 1988 based on the X-ray diffraction analysis data of Fab fragments. Later, it was found that the peptide was longer and softer, which could reduce the steric hindrance between each protein subunit in the fusion protein, thereby making it more It is beneficial to the correct folding of each dom...
Embodiment 2
[0057] Example 2: Recombinant transfer plasmid pBacPAK8-(CTB-Linker- ure B ) construction
[0058] To embodiment 1 gained CTB-Linker- ure B The 5' and 3' ends of the fusion gene were double-digested with BamH I enzyme and EcoR I enzyme (Fermentas company), respectively, and the digested fragment was recovered by agarose gel electrophoresis; at the same time, the pBacPAK8 vector was digested with BamH I enzyme and EcoR I enzyme (Invitrogen Company) for enzyme digestion and use agarose gel electrophoresis to recover the digested fragment; then use T4 ligase (Fermentas Company) to connect the recovered fusion gene to the digested pBacPAK8 vector, so that the recombinant gene is placed in the polyhedrin Under the control of the gene promoter, transforming TG 1 Escherichia coli competent cells (Invitrogen Company), positive clones were screened to obtain the recombinant transfer plasmid pBacPAK8-(CTB-Linker- ure B ) (See Figure 5 ), it was subjected to gene sequencing, and it ...
Embodiment 3
[0059] Embodiment 3: Bombyx mori recombinant baculovirus BmNPV-(CTB-Linker- ure B ) construction
[0060] 1. Obtaining Linearized Viral DNA
[0061] BmN cells (Invitrogen) were inoculated with silkworm baculovirus Bm-BacPAK6 (Clontech). After culturing at 27°C for 72 h, the virus culture medium was collected, and the genomic DNA of Bm-BacPAK6 virus was extracted, and used Bsu36 The endonuclease digests the Bm-BacPAK6 viral genome to linearize it.
[0062] 2. Co-transfection of recombinant transfer vector plasmid and linearized viral DNA
[0063] (1) Place well-growing BmN cells in a 50 ml culture flask and culture at 27°C for 4-12 hours to allow the cells to adhere to the wall;
[0064] (2) Take A centrifuge tube and add the recombinant transfer vector plasmid pBacPAK8-(CTB-Linker- ure B ) 5 μl, 20 μl (about 1 μg) of linearized viral DNA, and make up the total volume with HBS (20mM HEPES, N-(2-hydroxyethyl)piperazine-N'-2-ethanesulfonic acid; 15mM NaCl) to 50 μl, mix wel...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com