Helicobacter pylori antigen hla-restricted immunodominant epitope peptide and its application
A Helicobacter pylori and dominant epitope technology, applied in the field of medical biology, can solve problems such as low accuracy, missed screening, and treatment failure, and achieve the goal of avoiding missed and wrong screening, reducing the risk of use, and expanding the width Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] HpaA is a subunit of Helicobacter pylori flagellin protein A, and its sequence is conserved among various strains, with a total length of 260 amino acids, of which 1-27 amino acids are signal peptides. The recombinantly constructed rHpaA is a 28-260 polypeptide derived from the 11637 international standard strain. Therefore, the HpaA protein sequence (No. P55969) derived from Helicobacter pylori 11637 was retrieved in the UniProt protein database, and a short peptide with 18 amino acid steps overlapping was synthesized from the 28th amino acid (synthesized with the assistance of Shanghai Gil Biochemical Company), a total of 37 ( The last one is a 17 amino acid short peptide). The purity is greater than 90%. The synthetic peptide information is shown in Table 1. The synthesized peptides were dissolved in DMSO to a storage concentration of 5 mM, and 10 μL of each of 37 short peptides were mixed together to form a peptide library. Store at -70°C after aliquoting.
[00...
Embodiment 2
[0054] 2.1 Collection and storage of PBMC from positive Hp infection patients
[0055] The peripheral blood of Hp positive patients was obtained from the Chongqing Blood Station of the People’s Liberation Army. 13 For those positive for Helicobacter pylori infection screened by urease breath test, buffy coat cells were collected after blood collection, and then PBMCs were separated with Ficoll-diatrizoate lymphocyte separation medium (Tianjin TBD Company) according to the actual instructions. The isolated PBMCs were resuspended to a cell density of 1×10 7 / mL, add 1mL / tube into a cryopreservation tube, put it in a freezer box at -70°C for overnight, and then transfer it to liquid nitrogen for cryopreservation.
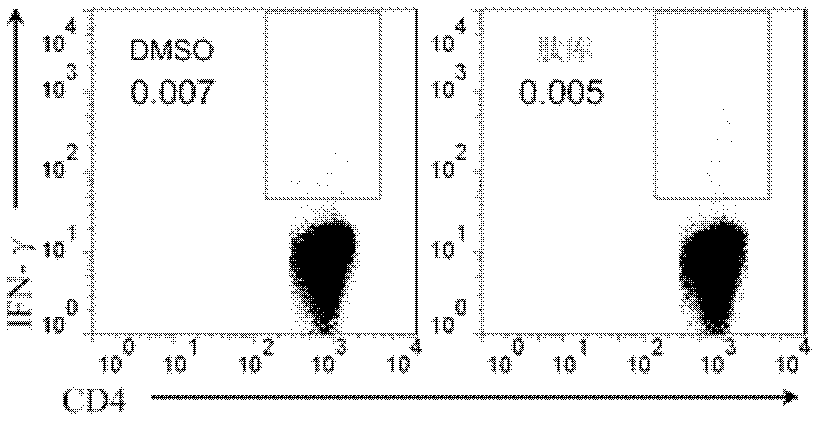
[0056] 2.2 ICS method to detect the frequency of HpaA-specific CD4+ T cells in PBMC of Hp positive patients
[0057] Resuscitate a tube of PBMCs positive for Hp infection, resuspend the cells with RPMI-1640 complete medium to 5×106 / ml, and spread 0.2ml of cell suspen...
Embodiment 3
[0062] 3.1 Methods for expanding antigen-specific T cells in vitro
[0063] Adjust the concentration of PBMC cells in patients with positive Hp infection to 2.5×10 6 / ml, inoculate in 48-well cell culture plate (1ml / well), add appropriate amount of HpaA antigen, mix well and incubate at 37°C, 5% CO 2 cultivated under conditions. On the fifth day, a low dose of recombinant human IL-2 (rhIL-2) (final concentration 25 U / ml) was added. On the 8th day, the culture medium began to turn yellow, and half of the medium was changed (the culture medium replaced contained 25 U / ml rhIL-2, and the cells were subcultured in different wells in due course.
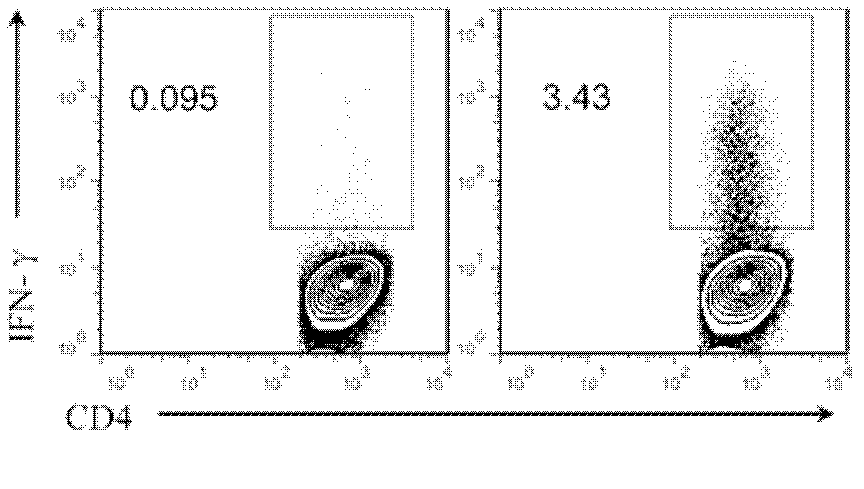
[0064] 3.2 Detection of antigen-specific T cells in vitro
[0065] After the effective expansion of antigen-specific CD4+ T cells in vitro, the frequency has reached the detection range of ICS, and the frequency of antigen-specific CD4+ T cells was detected by ICS method. Collect the cultured cells, centrifuge to remove the medium cont...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com