Helicobacter pylori antigen epitope polypeptide and application thereof
A technology of Helicobacter pylori and antigenic epitopes, applied in the direction of application, virus antigen components, peptides, etc., can solve the problems of not being able to completely prevent the colonization of Helicobacter pylori, and not being able to well inhibit the activity of urease, so as to prevent and control infection , enhanced inhibitory and clearance effects, good immunogenicity effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
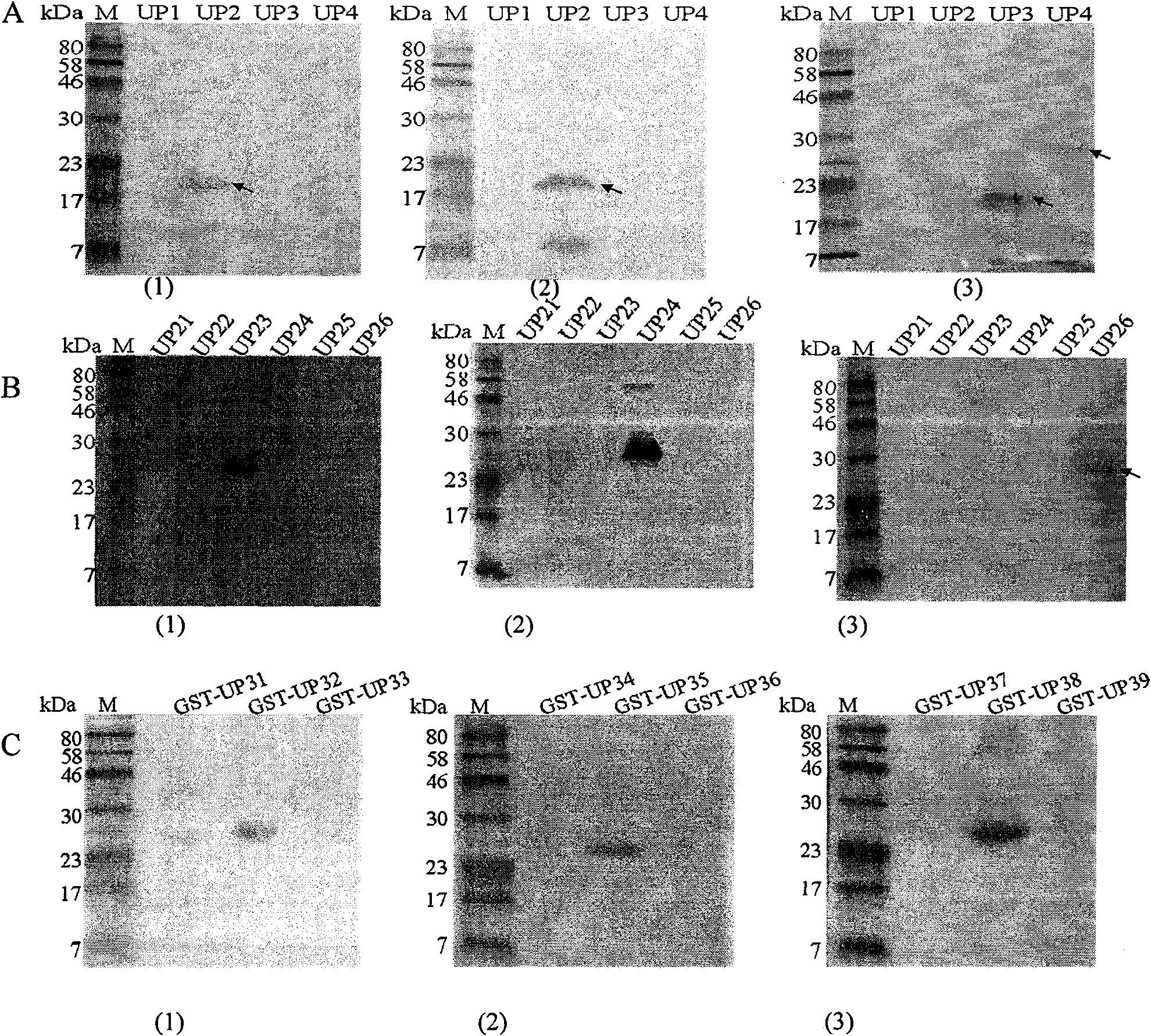
[0049] Example 1. Identification of Antigen Recognition Epitopes of Anti-Helicobacter Pylori Urease B Subunit Monoclonal Antibodies A1H10, B3D9, and A3C10
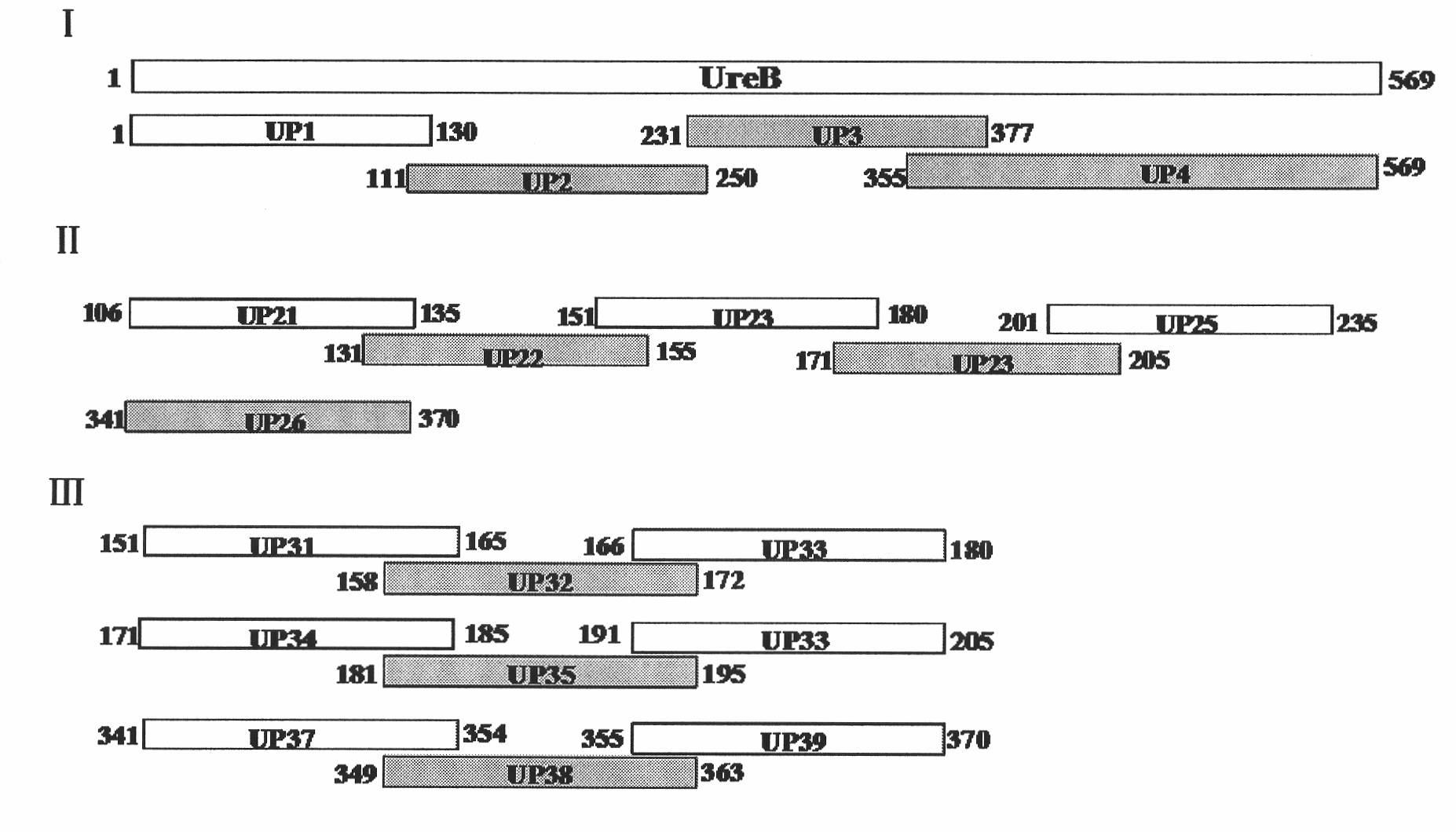
[0050] Antigen epitopes were screened by expressing urease B fragments by multiple truncations, and the positional relationship of each truncated fragment in the Helicobacter pylori urease B subunit is as follows figure 1 shown.
[0051] 1. The first segmented expression of UreB truncated protein and the preliminary identification of antigenic epitope
[0052] 1. Recombinant expression of truncated Helicobacter pylori UreB segmented antigens UP1 (sequence 5), UP2 (sequence 6), UP3 (sequence 7), UP4 (sequence 8):
[0053] 1) Culture of Helicobacter pylori
[0054] Helicobacter pylori (Helicobacter pylori) 26695 (ATCC No.700392) adopts solid culture, culture medium is: Campylobacter jejuni agar base (China Diarrheal Disease Control Shanghai Reagent Supply Research Center) 43g / L, defibrinated sheep blood (purchased by the m...
Embodiment 2
[0151] Example 2. Synthesis of epitope polypeptides to further confirm the epitopes targeted by monoclonal antibodies A1H10, B3D9, and A3C10
[0152] 1. Synthesis of epitope peptides
[0153] For the monoclonal antibody recognition epitope determined in Example 1, epitope polypeptides were synthesized by chemical synthesis (sent to Beijing Zhongke Yaguang Biological Co., Ltd. for synthesis), and named respectively as UP32 (the amino acid sequence is sequence 31 in the sequence listing), The purity of UP35 (the amino acid sequence is sequence 34 in the sequence listing) and UP38 (the amino acid sequence is sequence 37 in the sequence listing) is above 90%, and the synthesis amount is 4 mg.
[0154] 2. Determine the epitopes targeted by monoclonal antibodies A1H10, B3D9, and A3C10 by ELISA
[0155] The three synthetic epitope polypeptides UP32, UP35, and UP38 (10 μg / ml) were respectively coated on ELISA plates (100 ul / well) to detect the epitopes targeted by the A1H10, B3D9, an...
Embodiment 3
[0157] Embodiment 3, the recognition experiment of fusion epitope peptide and synthetic epitope peptide and patient's serum
[0158] In order to detect whether specific antibodies against the antigenic epitopes identified in the present invention are produced in the serum of clinically infected H. pylori patients, we prepared fusion epitope peptides (3 μg / ml) GST-UP32, GST-UP35, Epitope peptide (10 μ g / ml) UP32, UP35, UP38 synthesized by GST-UP38 and embodiment 2 are respectively coated with ELISA plates (100ul / hole), and are collected with this laboratory and use S001 Helicobacter pylori urease detection kit (colloid Gold method, Beijing Kangmei Tianhong Biotechnology Co., Ltd.) identified as UreB-positive 3 parts of patient serum and 2 parts of normal human serum as the primary antibody (1:100) for ELISA analysis, the specific operation steps are the same as step 4 of Example 1.
[0159] The result of the reaction is as Figure 7 As shown, the fusion epitope protein and the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com